Abstract
FGF signaling is associated with breast cancer and is required for mammary placode formation in the mouse. In this study, we employed a genetic mosaic analysis based on Cre-mediated recombination to investigate FGF receptor 2 (Fgfr2) function in the postnatal mammary gland. Mosaic inactivation of Fgfr2 by the MMTVCre transgene enabled us to compare the behavior of Fgfr2 null and Fgfr2 heterozygous cells in the same gland. Fgfr2 null cells were at a competitive disadvantage to their Fgfr2 heterozygous neighbors in the highly proliferative terminal end buds (TEBs) at the invasion front, owing to a negative effect of loss of Fgfr2 function on cell proliferation. However, Fgfr2 null cells were tolerated in mature ducts. In these genetic mosaic mammary glands, the epithelial network is apparently built by TEBs that over time are composed of a progressively larger proportion of Fgfr2-positive cells. However, subsequently, most cells lose Fgfr2 function, presumably due to additional rounds of Cre-mediated recombination. Using an independent strategy to create mosaic mammary glands, which employed an adenovirus-Cre that acts only once, we confirmed that Fgfr2 null cells were out-competed by neighboring Fgfr2 heterozygous cells. Together, our data demonstrate that Fgfr2 functions in the proliferating and invading TEBs, but it is not required in the mature ducts of the pubertal mammary gland.
Keywords: Cre recombinase, Epithelial-mesenchymal crosstalk, FGF signaling, Mosaic analysis, Terminal end buds
Introduction
Branching morphogenesis is an essential developmental process that is responsible for the formation of a variety of epithelial organs, from the trachea and air sacs in the fly to the mammary gland, lung, kidney and salivary gland in vertebrates (Affolter et al., 2003). Unlike branching in most other vertebrate organs, however, mammary branching morphogenesis occurs primarily during puberty (Sternlicht et al., 2006). Under the influence of pubertal hormones that begin surging in mice at 3 weeks of age, a rudimentary pre-pubertal ductal tree initiates rapid epithelial invasion and bifurcation of the terminal end bud (TEB) at the tip of each primary duct. This process is completed at about 9 weeks of age, when the primary ducts have extended to the distal end of the mammary gland fat pad and the TEBs regress. As the mammary tree extends distally, it is further elaborated by formation of lateral (secondary) branches, which sprout from the trailing primary ducts, as well as tertiary side-branches, until an intricate epithelial network is established in the adult gland (Fig. 1A) (Sternlicht et al., 2006; Wiseman and Werb, 2002).
Fig. 1.
Expression of FGF receptor genes in postnatal mammary glands. (A) Schematic diagrams of the developing mammary gland at the stages indicated. The terminal end buds (TEBs) develop at the onset of puberty (3 weeks after birth) at the distal tip of each primary duct. The TEBs regress when the primary branches have extended to the distal end of the fat pad (stroma). Proximal (Pr) is to the left and distal (Di) is to the right in this and all figures showing mammary glands in wholemount. (B-E′) Expression of Fgfr1 (B and B′), Fgfr2 (C and C′), Fgfr3 (D and D′), and Fgfr4 (E and E′) as detected by in situ hybridization with 35S-labeled probes on paraffin sections of mammary glands from female mice at 5 weeks of age. (B-E) The in situ hybridization signal as viewed in dark-field is displayed in the Photoshop red channel; DAPI staining of nuclei was viewed by fluorescence, and the signal is displayed in the Photoshop blue channel. (B′-E′) In situ hybridization signal in the samples shown in panels B-E as viewed in dark-field. (F-H) Immunofluorescence using anti-FGFR2 antibody (red, F-H) and anti-smooth muscle actin (SMA, green, F′, G′, H′) on frozen sections of mammary glands from females at the stages indicated. Samples were counterstained with nuclear dye To-Pro3 (blue, F″, G″, H″). Arrow in panel F points to cap cells (cc). Note that a few body cells also express SMA (arrowheads in panel F′). (G, G′) Myoepithelium (me, open arrowhead) but not luminal epithelium (le, arrow) expresses smooth muscle actin. (F‴-H‴) The FGFR2 and SMA signals are overlaid, showing co-expression in the cap cells of the TEBs at 5 weeks (F‴) and in the myoepithelium in the ducts at 5 weeks (G‴) and 10 weeks (H‴). Insets in panels F‴-H‴ are high-magnification views of the area in dashed box with FGFR2 and nuclear signals overlaid to show that FGFR2 was mainly nuclear at 5 weeks in both ducts and TEBs (arrowheads in panels F‴ and G‴, respectively) but was mainly cytoplasmic and at the cell surface in 10 week ducts (arrowheads in panel H‴). Scale bars: 100 µm. Abbreviations: bc, body cells; cc, cap cells; lb, lateral branch; le, luminal epithelium; me, myoepithelium.
Understanding the cellular and molecular mechanisms that control mammary branching has been a major focus of mammary gland biology. Several different signaling pathways, including EGF (Luetteke et al., 1999; Sternlicht et al., 2005), IGF (Bonnette and Hadsell, 2001; Ruan and Kleinberg, 1999), TGF-β (Crowley et al., 2005; Joseph et al., 1999), Wnt (Roarty and Serra, 2007) and Hedgehog (Lewis et al., 1999; Moraes et al., 2007) signaling, have been shown to positively or negatively regulate this complex process [reviewed by Hennighausen and Robinson, 2005; Lu et al., 2006b; Wiseman and Werb, 2002]. Another candidate signaling pathway is fibroblast growth factor (FGF) signaling, which has long been associated with breast cancer and mammary development in the mouse. In fact, members of the FGF family, including Fgf3, Fgf4, and Fgf8 were discovered over twenty years ago because breast tumors formed when these genes were overexpressed as a consequence of mouse murine tumor virus (MMTV) insertions (MacArthur et al., 1995; Peters et al., 1983, 1989). Moreover, mice lacking Fgf10 fail to form mammary placodes (Mailleux et al., 2002), thus suggesting that FGF signaling is required for embryonic mammary gland development.
Vertebrates have four FGF receptors, FGFR1-FGFR4, that share several evolutionarily conserved domains: three immunoglobulin (Ig)-like extracellular domains, a transmembrane domain, and an intracellular tyrosine kinase domain (Coumoul and Deng, 2003; Itoh and Ornitz, 2008, 2004). Although mice lacking Fgfr2 die at midgestation (Xu et al., 1998), before the mammary gland develops, those that lack a specific isoform of Fgfr2, Fgfr2IIIb, survive to term but the mammary placode fails to form, as in Fgf10 null mice (Mailleux et al., 2002). These results thus suggest that FGF10-FGFR2IIIb signaling is required for embryonic mammary gland development, and raise the question of whether it is also needed in the postnatal mammary gland. Indeed, Fgf7 and Fgf10 are expressed in the mesenchyme/stroma during both embryonic (Mailleux et al., 2002) and postnatal development (Kouros-Mehr and Werb, 2006; Pedchenko and Imagawa, 2000). In culture, FGF signaling regulates a variety of cell behaviors, including proliferation, survival, polarity, and changes in extracellular matrix deposition that are essential for mammary branching (Fata et al., 2007; Simian et al., 2001; Sternlicht et al., 2005; Xian et al., 2007, 2005). In this study, we have tested the hypothesis that FGF signaling functions during branching morphogenesis in the mammary gland by using the Cre/lox system to bypass the embryonic lethality in mice lacking FGF receptor genes.
Materials and methods
Mouse strains
Mouse lines carrying the Fgfr2Δ and Fgfr2fl alleles (Yu et al., 2003) were kindly provided by Dr. David Ornitz. The R26R Cre-reporter line (Soriano, 1999) was kindly provided by Dr. Philippe Soriano. Mice carrying the MMTV-Cre transgene D line (Wagner et al., 2001) were purchased from the Jackson Laboratory. All mice were maintained on a mixed genetic background. Offspring from crosses of the various lines were genotyped according to methods in the publications describing the mouse lines. Immunologically deficient nude (nu/nu) mice were purchased from Charles River Laboratories (strain code: 088).
Mammary gland wholemount preparation, photography and morphometric analysis
Mammary glands #3 or #4 were harvested and mounted on glass slides, stained with Carmine red, and cleared as described previously (Wiseman et al., 2003). Wholemounts were photographed using a digital camera (Nikon DXM1200) mounted on a stereo microscope (Leica MZFL 111) and accompanying software (Nikon ACT-1). Adobe Photoshop CS2 was used to process images and to measure ductal penetration. Ductal penetration was the mean length of the three longest primary epithelial branches in each mammary gland. This was assessed by measuring the lengths of straight lines from the center of the lymph node to the ends of those three branches (see Figs. 2C, D). The number of branch points per millimeter of duct was the mean number of branch points on those three longest primary ducts divided by their mean length.
Fig. 2.
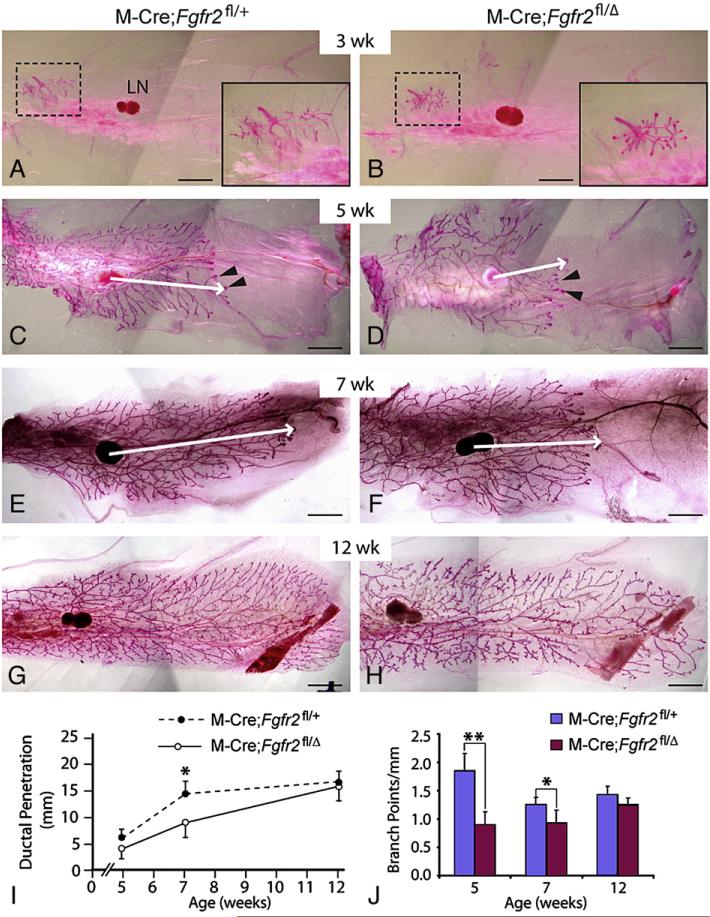
Consequences of MMTV-Cre-mediated inactivation of Fgfr2 in mammary epithelium. (A-H) The mammary branching tree at the postnatal stages indicated, as revealed by Carmine Red staining of glands in wholemount. (A, C, E, G) glands from control (M-Cre;Fgfr2fl/+) mice; (B, D, F, H) glands from mutant (M-Cre;Fgfr2fl/Δ) mice. Insets in panels A and B show high-magnification views of the rudimentary ductal tree (area in dashed box). (C-F) Arrowheads indicate TEBs at the tips of invading mammary epithelium. Arrows indicate the extent of ductal penetration in the fat pad. (I, J) Quantitative comparisons of ductal penetration and branch point formation between control and mutant glands. At 5 weeks, ductal penetration measurements were 6.0±1.4 (control, n=5) and 4.5±1.7 (mutant, n=6); at 7 weeks, the measurements were 14.6±2.2 (control, n=4) and 9.1±3.2 (mutant, n=4); at 12 weeks, they were 16.8±2.2 (control, n=8) and 16.0±2.9 (mutant, n=12). Measurements of branching points were 1.8±0.3 (control) and 0.9±0.2 (mutant) at 5 weeks, 1.3±0.1 (control) and 0.9±0.2 (mutant) at 7 weeks, and 1.4±0.1 (control) and 1.3±0.1 (mutant) at 12 weeks. Values shown are the mean±SD for each data point: **P<0.0005; *P<0.05, unpaired, two-tailed Student's t test. Scale bars: 2.5 mm. Abbreviation: LN, lymph node.
In situ hybridization analysis of mRNA, immunofluorescence analysis and staining for β-galactosidase activity
RNA in situ hybridization analysis on sections of mammary glands was performed according to established protocols (Wiseman et al., 2003). Probes for detection of Fgfr1 (Yamaguchi et al., 1992), Fgfr2 (Peters et al., 1992), Fgfr3 (Peters et al., 1993) and Fgfr4 (Stark et al., 1991) RNA were prepared as reported previously. All probes were tested on positive control samples before being used on paraffin sections of the mammary gland (data not shown).
For immunofluorescence analysis, mammary glands were harvested and fixed in 4% paraformaldehyde overnight at 4 °C. 10-µm frozen sections were cut using a Leica cryostat. Sections were blocked for 1 h at room temperature (RT) in PBS containing 5% bovine serum albumin and 0.5% Tween20, followed by incubation in primary antibodies for 1 h at RT. Primary antibodies used in this study were anti-FGFR2 antibody (Santa Cruz Biotechnology, sc-122, 1:200 dilution), anti-smooth muscle actin antibody (Sigma, 1A4, 1:500 dilution), and anti-β-galactosidase (GAL) antibody (Cappel, 55976, 1:500). Goatanti-rabbit Alexa 560 (Invitrogen, A11010 1:1000) was used as secondary antibody. Samples were counterstained with To-Pro-3 (Invitrogen, T3605, 1:10,000). Confocal microscopy was performed on a Zeiss LSM510 microscope.
To assay for β-GAL activity in wholemount, mammary glands were harvested, fixed for 30 min in 4% paraformaldehyde at room temperature, washed thoroughly in phosphate-buffered saline (PBS), and stained overnight in LacZ (which encodes β-GAL) staining buffer (Roche 11828673001) at 37 °C. When necessary, LacZ-stained wholemount mammary glands were counterstained with the fluorescent nuclear dye Yo-Yo1 (Invitrogen Y3601, 1:10,000).
Cell culture, adenovirus infection and transplantation of primary mammary epithelium
Donor mammary glands were harvested, minced and dissociated in buffer [10 mM Hepes buffer, 5% fetal bovine serum (FBS), RPMI (Invitrogen 61870), Penicillin-Streptomycin 100 U/ml] containing collagenase (Sigma C5138-1G, 2 mg/ml) for 1 h at 37 °C. Primary epithelium was purified by five repetitions of washes in the dissociation buffer containing no collagenase and collected using a swinging-bucket centrifuge at 400 ×g. Purified primary mammary epithelium was then resuspended in growth medium (5 µg/ml insulin, 1 µg/ml hydrocortisone, 10 ng/ml EGF, 10% FBS, Penicillin-Streptomycin 100 U/ml, Gentamicin 50 µg/ml in DMEM/F12) and infected overnight with Adenovirus-Cre-GFP (green fluorescent protein) (He et al., 1998) at a multiplicity of infection of ∼25 particles per cell (Rijnkels and Rosen, 2001). The next day, organoids were washed several times with PBS and placed in fresh growth medium. When examined under a fluorescent stereo microscope (Leica MZFL 111), ∼50-70% primary mammary epithelial cells (MECs) appeared to be infected as indicated by GFP expression. They were cultured for another 24 h to allow for recovery from infection before being transplanted into cleared fat pads of nude mice at 3 weeks of age. Recipient nude mice were sacrificed 8 weeks after transplantation and mammary glands were harvested, stained for LacZ activity, and examined on a Leica stereo microscope (MZFL 111).
Cell proliferation and apoptosis assays
For cell proliferation analysis, mice were injected with bromodeoxyuridine (BrdU) (5 mg/100 g body weight) 2 h prior to euthanasia. Mammary glands were harvested and fixed in 4% paraformaldehyde overnight at 4 °C. 10-µm frozen sections were obtained using a Leica cryostat. Detection of β-GAL protein and BrdU incorporation were performed using the anti-β-GAL antibody cited above and a kit from Roche (1296736), respectively. Antigen retrieval was performed in 10 mM sodium citrate (pH 6.0) in a microwave oven. Samples were counterstained with To-Pro-3. Confocal microscopy was performed on a LSM510 Zeiss confocal. A total of 2500-5000 cells were counted in each mammary gland.
To assess apoptosis, frozen sections of mammary glands were assayed for β-GAL protein and TUNEL according to manufacturer's protocol (Roche 1684817). A total of 3000-5500 cells were counted in each mammary gland. Alternatively, cell death was examined by Lysotracker™ staining using established protocols (Grieshammer et al., 2005; Lu et al., 2006a).
Results
Expression of FGF receptors in postnatal mammary glands
We first surveyed expression of the four FGF receptor tyrosine kinase genes, Fgfr1, Fgfr2, Fgfr3 and Fgfr4, by RNA in situ hybridization to determine which of these genes might function during postnatal branching of the murine mammary gland. In sections through mammary glands of pubertal wild-type mice at 5 weeks of age, we detected both Fgfr1 (Figs. 1B, B′) and Fgfr2 (Figs. 1C, C′) RNA in TEBs and in the ductal epithelium (data not shown). In contrast, no Fgfr3 (Figs. 1D, D′)or Fgfr4 (Figs. 1E, E′) RNA was detected.
Since Fgfr2 appeared to be expressed at a substantially higher level than Fgfr1, we further characterized Fgfr2 expression and aimed our experiments at determining its function in the postnatal mammary gland. Using immunofluorescence microscopy, we detected FGFR2 protein in the mammary epithelium from 2 to 14 weeks of age (Figs. 1F-H‴, data not shown). At 5 weeks, a majority of cells in the TEB, the interior body cells and the surrounding layer of cap cells, were positive for FGFR2 (Fig. 1F). Co-staining with an antibody against smooth muscle actin (SMA), which labels filaments in the cytoplasm of cap cells and a few body cells of the TEB (Fig. 1F′), showed that FGFR2 was primarily located in the nuclei of TEB cells (Fig. 1F‴), as has been observed previously in TEBs and Sertoli cell precursors in the testis (Schmahl et al., 2004). FGFR2 protein was present in the luminal epithelium in mammary glands from pubertal (5 weeks) females, where it was mainly nuclear, and from mature (10 weeks) females, where it was mainly in the cytoplasm and at the cell surface (Figs. 1G, G‴, H, H‴). In addition, FGFR2 was detected in a few basal cells, where it was mostly nuclear and did not overlap with SMA signals (Figs. 1G‴, H‴). Taken together, these expression data suggest that FGFR2 functions during postnatal branching morphogenesis and in the ductal epithelium of the adult mammary gland.
Mammary branching morphogenesis is delayed when Fgfr2 is inactivated by MMTV-Cre
Since mice homozygous for an Fgfr2 null allele die at midgestation (Xu et al., 1998; Yu et al., 2003), we used a conditional knock-out strategy to eliminate Fgfr2 function after birth via Cre-mediated recombination. We found that the MMTV-Cre transgene (M-Cre) we employed (Wagner et al., 2001) functioned in mammary epithelium by at least postnatal day 3 (data not shown). Male mice carrying one copy of M-Cre and heterozygous for an Fgfr2 null allele, Fgfr2Δ (Yu et al., 2003), were crossed with females homozygous for an Fgfr2 conditional allele, Fgfr2fl (Yu et al., 2003) to generate control (M-Cre;Fgfr2fl/+) and mutant (M-Cre;Fgfr2fl/Δ) female mice. We then examined mammary gland development in these animals at several critical stages (Fig. 2).
At 3 weeks after birth, we observed a rudimentary ductal tree in the mutant glands that was not obviously different from that observed in control glands (Figs. 2A, B). However, in pubertal mice at 5 weeks, when vigorous mammary branching is occurring, we noticed a defect in the branching tree in mutant glands (Figs. 2C, D). In comparison to control glands, mammary ducts in mutant glands penetrated 75% of the normal distance into the fat pad (Fig. 2I) and formed half as many branch points (Fig. 2J). Ductal penetration was also delayed in the mutants at 7 weeks (Figs. 2E, F, I, J). Despite the initially retarded invasion, the ducts in mutant glands eventually completely infiltrated the mammary fat pad by 12 weeks, which is ∼3 weeks after branching morphogenesis is normally complete, although ductal branches were slightly sparser than in control glands at this stage (Figs. 2G-J). Together, the above data suggest that branching morphogenesis is delayed, but not blocked, in the mutant glands due to loss of FGFR2 function in mammary epithelium.
Fgfr2 null cells are at a competitive disadvantage to Fgfr2 heterozygous cells during branching morphogenesis in mosaic mammary glands
One potential explanation for the mutant phenotype that we observed may relate to the observation that MMTV-Cre functions incompletely in mammary epithelium (Wagner et al., 2001). As a result, M-Cre;Fgfr2fl/Δ mutant mammary glands may contain a mixture of Fgfr2 null and Fgfr2 heterozygous cells, the latter of which might continue ductal invasion in the pubertal glands. To test this hypothesis, we employed a reporter allele, R26Rfl, that expresses lacZ when it has undergone Cre-mediated recombination (Soriano, 1999). We first produced a line of mice homozygous for both Fgfr2fl and R26Rfl (Fgfr2fl/fl;Rfl/fl), and then crossed females of this line with male mice that were carrying MMTV-Cre and were heterozygous for the Fgfr2Δ allele, to generate control (M-Cre;Fgfr2fl/+;Rfl/+) and mutant (M-Cre;Fgfr2fl/Δ;Rfl/+) progeny.
To determine whether R26Rfl and Fgfr2fl loci are equally susceptible to Cre-mediated recombination and thus whether lacZ expression accurately reports the conversion of Fgfr2fl to an Fgfr2Δ allele, we analyzed the expression of β-GAL and FGFR2 protein by immunofluorescence on frozen sections from both control and mutant adult mammary glands. We found that mammary epithelium in the distal glands was composed of a mixture of β-GAL-positive and β-GAL-negative cells (Figs. 3A, B). As expected, co-staining for FGFR2 protein in control glands showed that FGFR2 was present in all luminal epithelial cells (Figs. 3A′, A″), since even those in which recombination of the Fgfr2fl allele had occurred still carried a wild-type Fgfr2 allele. By contrast, in the mutant glands, we observed mosaicism of FGFR2-negative and FGFR2-positive cells (Fig. 3B′), since recombination of the Fgfr2fl allele should render the cells Fgfr2 null. Importantly, among the luminal epithelial cells all the β-GAL-positive cells were also FGFR2-negative, indicating that recombination of both R26Rfl and Fgfr2fl had occurred in the same cells; conversely, all the β-GAL-negative luminal epithelial cells were also FGFR2-positive (Fig. 3B″), indicating that no Cre-mediated recombination of either allele had occurred in those cells. These results thus indicate that β-GAL-staining is an accurate reporter of Fgfr2 null cells in mutant glands.
Fig. 3.
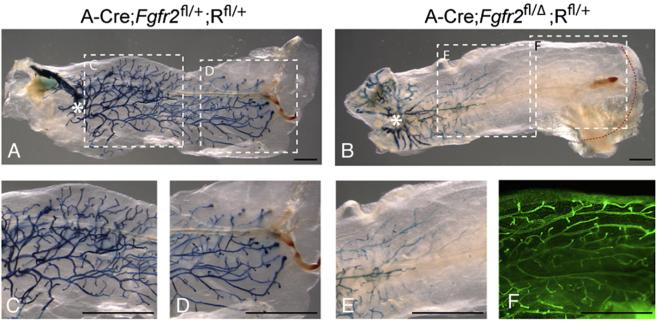
Analysis of Fgfr2 genetic mosaics produced by MMTV-Cre-mediated recombination. Immunofluorescence assays for co-expression of β-GAL and FGFR2 on frozen sections from distal mammary glands from mice at 14 weeks. (A-A″) control (M-Cre;Fgfr2fl/+;Rfl/+) and (B-B″) mutant (M-Cre;Fgfr2fl/Δ;Rfl/+) glands. The green channel (A, B) shows β-GAL expression and the red channel (A′, B′) shows FGFR2 protein expression. Note that FGFR2 expression is ubiquitous in control epithelium (A′), and mosaic in mutant epithelium (B′). β-GAL expression is mosaic in both control (A) and mutant (B) epithelium, and FGFR2 and β-GAL expression are mutually exclusive in the mutant epithelium (B-B″). Seven different sections from control and mutant #4 mammary glands were examined for these experiments. Scale bars: 10 µm. (C-L) Assays for β-GAL activity in wholemounts of glands at the stages indicated. The dashed boxes in panels C-F demarcate the portions of the branching trees that are shown at higher magnification in insets in panels C and D or in separate panels (G-J). In all glands, β-GAL expression marks cells derived from those in which MMTV-Cre-mediated recombination occurred. Only a few β-GAL-positive cells (arrowheads, see also 3P) were present in TEBs of mutant mammary glands at 7 weeks (J, n=10). Note in panel F that at 7 weeks there are few β-GAL-positive cells in the distal epithelium of the mutant glands, whereas panel L shows that at 14 weeks (n=14) many cells in that region are β-GAL-positive. Note that in this series of experiments, ductal penetration was also significantly delayed in the mutant glands at 7 weeks; it was 84% of that in the control glands (8.9 mm±1.7 vs. 10.6 mm±0.6, P<0.01, unpaired, two-tailed Student's t test). 10-15 mammary glands were examined at each stage. Scale bars: 2.5 mm. (M-P) Sections through TEBs in mammary glands at the stages indicated, assayed for β-GAL activity in wholemount and counterstained with nuclear fast red. (M, N) control (M-Cre;Fgfr2fl/+;Rfl/+) and (O, P) mutant (M-Cre;Fgfr2fl/Δ;Rfl/+) glands. At least 5 TEBs were examined for each stage. Note Cre activity was primarily restricted to the body cells at 5 weeks (M, O). Scale bars: 50 µm.
Next, we assessed the mammary glands from control and mutant mice for β-GAL activity at various postnatal stages to determine the distribution of cells in which Cre-mediated recombination had occurred. At 3 weeks, before the onset of pubertal branching, β-GAL-positive cells were evenly distributed throughout the epithelial branching network, including ducts and tips, in both control and mutant mammary glands (Figs. 3C, D). At 5 weeks, there was a similar number of, or perhaps slightly more, β-GAL-positive cells in control than in mutant TEBs, which were primarily body cells, and subtending ducts (immediately adjacent to the TEBs) (Figs. 3M, O). At 6 and 7 weeks, β-GAL-positive cells were evenly dispersed throughout the entire branching network in control glands (Figs. 3E, G, H, N), whereas in mutant glands they were mostly restricted to the proximal region of the branching network (Figs. 3F, I, J). Near the invasion front (red dotted line in Fig. 3F), and at some distance behind it, there were very few β-GAL-positive cells in the mutant gland (Fig. 3J). Analysis of thinner sections showed that the few β-GAL-positive cells present primarily lined the lumen and did not occupy the leading edge of multi-layered TEBs (Fig. 3P). Together, these data indicate that during branching morphogenesis in the mutant glands, the β-GAL-positive/Fgfr2 null cells are at a competitive disadvantage to β-GAL-negative/Fgfr2 heterozygous cells and consequently do not participate in the formation of the distal branching network. By contrast, β-GAL-positive/Fgfr2 heterozygous cells in control mosaic glands are functionally equivalent/similar to and can compete with β-GAL-negative/Fgfr2 wild-type cells to give rise to the whole mammary epithelium.
Interestingly, we found that, by 14 weeks, the distal epithelium of the mutant glands contained β-GAL-positive cells throughout the ductal tree, thus resembling the control glands (Figs. 3K, L). The most plausible explanation of this finding is that there are consecutive round(s) of Cre-mediated recombination in the mature glands. The effect of this would be to convert many of the β-GAL-negative/Fgfr2 heterozygous cells that participated in mammary branching into β-GAL-positive/Fgfr2 null cells. This hypothesis is consistent with the reports that the MMTV promoter driving Cre expression is responsive to progesterone (Otten et al., 1988; von der Ahe et al., 1985) and thus Cre may be expressed cyclically in concert with progesterone surges during the estrus cycle.
We also examined the mutant mammary glands during pregnancy and lactation. For these experiments, adult females older than 14 weeks of age were crossed with wild-type males. Interestingly, fewer alveolar units were found in mutant mammary glands than in control glands at pregnancy day 14 (Supplementary Figs. 1A, B). Assays for β-GAL enzymatic activity showed that β-GAL-positive/Fgfr2 null cells were evenly distributed throughout the mutant glands and were present in the alveoli (Supplementary Figs. 1C, D). The mammary epithelium in mutant glands was able to differentiate and produce milk during lactation despite being considerably less dense than in control glands (Supplementary Figs. 1E-H, data not shown). Consistent with previous reports (Jackson et al., 1997; Parsa et al., 2008), these data suggest that Fgfr2 also plays a role during pregnancy and/or lactation.
Fgfr2 null cells persist in the proximal branches of the mammary tree
One possible explanation for the competitive disadvantage of Fgfr2 null cells is that FGFR2 functions as a survival factor and Fgfr2 null cells are therefore eliminated by cell death during mammary branching. If this is the case, the presence of Fgfr2 null cells in the luminal epithelium of the proximal mammary gland may have resulted from recent recombination events due to consecutive round(s) of MMTV-Cre activity and therefore these cells may have participated in duct formation when they were still Fgfr2 heterozygotes. To test directly whether Fgfr2 null cells can contribute to and survive in luminal epithelium, we performed experiments using a replication incompetent adenovirus encoding the Cre gene, which acts only once (He et al., 1998; Naylor et al., 2005; Rijnkels and Rosen, 2001; Rohlmann et al., 1996; Seagroves et al., 2003). Mammary epithelial cells were harvested from Fgfr2+/+;Rfl/fl and Fgfr2fl/fl;Rfl/fl female mice at 5 weeks of age and infected with the recombinant adenovirus in culture. Cre protein produced in those cells that were successfully infected (∼50-70% of treated cells) should convert the Fgfr2fl allele into an Fgfr2 null allele and activate β-GAL expression from the R26R allele. The mosaic donor cell population was then transplanted into fat pads of immunologically deficient mice at 3 weeks of age, from which endogenous epithelium had been removed. 8 weeks after transplantation, we harvested mammary glands and examined the distribution of β-GAL-positive cells in the regenerated ductal tree.
We found that the donor cells successfully repopulated the fat pad and established a mature mammary tree. In control glands, β-GAL-positive cells were distributed evenly throughout the entire branching tree (Figs. 4A, C, D). In mutant glands, we observed a distribution of β-GAL-positive cells similar to that observed in M-Cre;Fgfr2fl/Δ;Rfl/+ glands at 7 weeks (Fig. 4B, compare with 3F). Thus there were many β-GAL-positive cells in the luminal epithelium in the proximal branching tree, near and distal to the site of donor cell injection (asterisk in Fig. 4B), and their number decreased in progressively more distal regions, with virtually none near the invasion front (Figs. 4B, E). These results indicate that in the mammary gland Fgfr2 null cells are at a competitive disadvantage to Fgfr2 heterozygous cells, and that Fgfr2 null cells are viable for at least 8 weeks.
Fig. 4.
Analysis of Fgfr2 heterozygote/null genetic mosaics produced by Adenovirus Cre-mediated recombination. (A-E) Assays for β-GAL activity in mammary glands in which the ductal tree developed for 8 weeks from donor mammary epithelial cells harvested from (A) control Fgfr2+/+;Rfl/fl and (B) mutant Fgfr2fl/fl;Rfl/fl female mice and infected with adenovirus-Cre (see Materials and methods). Asterisks indicate the transplantation sites in the recipient fat pads from which donor cells grew out. Dashed boxes in panels A and B demarcate the regions shown at higher magnification in the corresponding panels (C-E). (F) The glands were stained with Yo-Yo1 to illuminate the branching tree; This staining, shown only for the region demarcated by the dashed box (F) in panel B, demonstrates that there are many epithelial branches in a region of the gland that contains virtually no β-GAL-positive/Fgfr2 null cells. 10 transplanted glands were examined for each genotype. Scale bars: 2.5 mm.
We investigated whether Fgfr2 null cells were dying by performing a TUNEL assay on mammary glands at 4 weeks after birth (Supplementary Fig. 2). Overall, there were few dying cells at this stage, which was confirmed by an alternative cell death analysis based on Lysotracker™ staining (Grieshammer et al., 2005; Lu et al., 2006a) (data not shown). We did not observe any obvious difference in the frequency of dying β-GAL-negative and β-GAL-positive cells in TEBs of the control (M-Cre;Fgfr2fl/+;Rfl/+, n=3) and mutant (M-Cre;Fgfr2fl/Δ;Rfl/+, n=4) glands, although the very low rate of dying cells increased in the ducts in mutant glands. Together, these data suggest that FGFR2 does not function as a survival factor in TEBs and that Fgfr2 null cells persist in mature ducts.
FGFR2 promotes cell proliferation in TEBs during postnatal branching
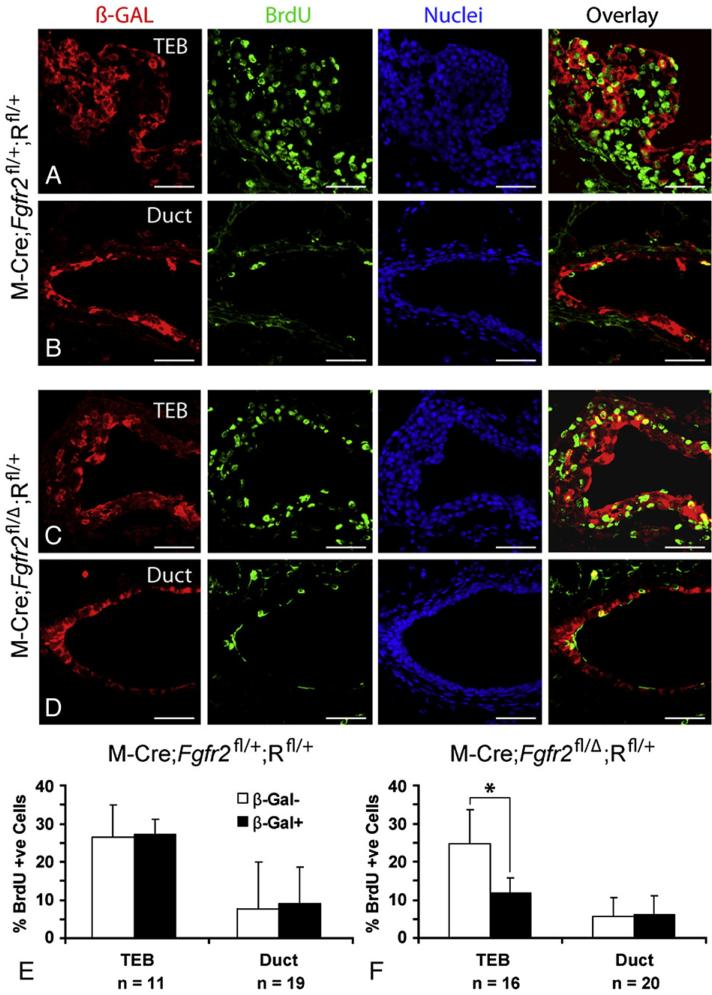
If FGFR2 is necessary for normal cell proliferation in the mammary gland, then this would explain why Fgfr2 null cells are out-competed by neighboring Fgfr2 heterozygous cells. We investigated this by determining the percent BrdU-labeled cells in control (M-Cre;Fgfr2fl/+;Rfl/+, n=3) and mutant (M-Cre;Fgfr2fl/Δ;Rfl/+, n=4) glands at 4 weeks of age (Fig. 5). We found no significant difference in percent BrdU-labeled, β-GAL-negative and BrdU-labeled, β-GAL-positive cells in either TEBs or ducts in control glands (Figs. 5A, B, and E), suggesting that Fgfr2 heterozygous cells are similar to wild-type cells with regard to their proliferative capacity. By contrast, in mutant glands, the frequency of BrdU-labeled, β-GAL-negative cells was twice as high as BrdU-labeled, β-GAL-positive cells in TEBs (Figs. 5C, F). In the ducts, we detected no difference in percent BrdU-labeling between β-GAL-negative and β-GAL-positive cells (Figs. 5D, F). Together, these results demonstrate that Fgfr2 promotes cell proliferation in TEBs during postnatal branching morphogenesis and in its absence, Fgfr2 negative cells proliferate more slowly than their heterozygous neighbors in TEBs.
Fig. 5.
Cell proliferation as assessed by BrdU incorporation assays in Fgfr2 genetic mosaics produced by MMTV-Cre-mediated recombination. (A-D) Immunofluorescent co-staining of β-GAL and BrdU on frozen sections of control and mutant mammary glands at 4 weeks. Samples were counterstained with To-Pro3. (E and F) A quantitative comparison of the percentage of BrdU-positive cells in the β-GAL-negative and β-GAL-positive cell populations in TEBs and ducts of mammary glands from control (E, n=3) and mutant (F, n=4) mice. A total of 1533 cells from 11 TEBs and 1341 cells from 19 ducts were counted in control glands; and 3052 cells from 16 TEBs and 1798 cells from 20 ducts were counted in mutant glands. Values are the mean±SD for each data point. *P<0.00005, paired, two-tailed Student's t test. Note that the only significant difference in percent BrdU-positive cells was observed between β-GAL-negative (FGFR2 heterozygous) and β-GAL-positive (FGFR2 null) cells in TEBs. Scale bars: 50 µm.
Discussion
In this study, we provide direct genetic evidence that FGFR2 functions during mammary branching morphogenesis. By analyzing genetic mosaic mammary glands composed of a mixture of Fgfr2 null and Fgfr2 heterozygous epithelial cells, we show that Fgfr2 null cells are out-competed by their Fgfr2 heterozygous neighbors and are depleted from the TEBs at the epithelial invasion front. This competitive disadvantage is due, at least in part, to reduced proliferation of Fgfr2 null cells. Thus, FGFR2 functions in the highly proliferative TEBs for normal ductal morphogenesis, but is not required in mature ducts. Unlike EGF receptor (EGFR), which primarily functions in the stroma, our study points to the importance of FGFR2 signaling acting in the mammary epithelium to promote epithelial morphogenesis in the postnatal mammary gland.
FGFR2 contributes to mammary branching by stimulating cell proliferation in TEBs
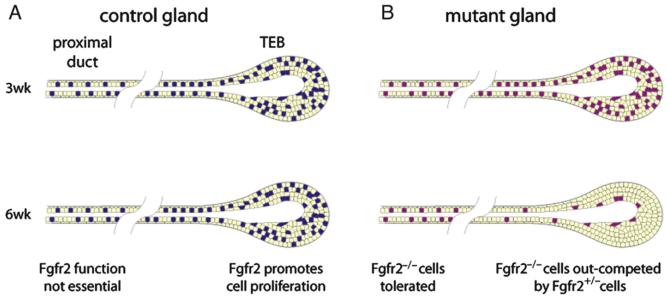
Because complete loss of Fgfr2 function results in midgestation lethality due to a defective placenta (Xu et al., 1998; Yu et al., 2003), we used a conditional gene inactivation approach to assess Fgfr2 function in the postnatal mammary gland. We took advantage of the inefficiency of the MMTV-Cre transgene to produce animals with mammary glands that were mosaic for Fgfr2 null and Fgfr2 heterozygous cells, and in which the Fgf2 null cells were marked by LacZ expression from the recombined R26Rfl reporter allele. We found that pre-pubertal mammary glands had a substantial number of Fgfr2 null cells distributed evenly in the developing ductal tree. However, at the onset of puberty, when branching morphogenesis accelerates and becomes dependent on rapid proliferation of cells in the TEBs, Fgfr2 null cells were rapidly diluted out of the TEBs. This dilution apparently occurred because the Fgfr2 null cells proliferate more slowly than the neighboring heterozygous cells. Once absent from the TEB, Fgfr2 null cells no longer contributed to the distal ductal network (Fig. 6). Together, our data suggest that the delay in ductal penetration in the mutant glands likely resulted from the presence of a substantial number of Fgfr2 null cells early on, whereas the preponderance of Fgfr2 heterozygous cells in the TEBs at later stages resulted in the eventual completion of branching morphogenesis.
Fig. 6.
Model of FGFR2 function during postnatal branching morphogenesis in the mammary gland. (A) Schematic representations of primary branches in control genetic mosaic mammary glands at 3 and 6 weeks. Fgfr2 heterozygous cells are colored yellow and wild-type cells are colored blue. Fgfr2 is expressed in mammary epithelium, including TEBs and ducts, during postnatal development. Fgfr2 promotes cell proliferation of in TEB cells to ensure normal branching morphogenesis but is not required in the proximal ducts. (B) Mutant genetic mosaic mammary glands. Fgfr2 heterozygous cells are colored yellow and Fgfr2 null cells are colored purple. Fgfr2 null cells survive and persist in proximal ducts, but when TEBs undergo rapid cell proliferation and active epithelial invasion following the onset of puberty, Fgfr2 null cells are rapidly depleted. Once diluted out of the TEB, Fgfr2 null cells no longer contribute to the distal ductal network.
Our results show that Fgfr2 null cells that are incorporated in the ductal tree can survive for many weeks. This result is interesting because in other developmental contexts (Grieshammer et al., 2005; Lu et al., 2008; Poladia et al., 2006), including the branching tips of Fgfr2 null kidney epithelium (Zhao et al., 2004), a loss of FGF signaling can lead to cell death. It is possible that in the mammary epithelium, survival of the Fgfr2 null cells is stimulated by signaling via FGFR1, which we have shown is co-expressed with FGFR2 in mammary epithelial cells. Additionally, their survival may depend on other receptor tyrosine kinases (RTKs), including ERBB2 (Jackson-Fisher et al., 2004) and insulin-like growth factor-I receptor (IGF-IR) (Bonnette and Hadsell, 2001), which are expressed in mammary epithelium and are required for ductal branching. Thus, RTKs may have a combinatorial function in regulating epithelial cell survival in the mammary gland.
Our analysis of cell proliferation in mammary glands at 4 weeks showed that in TEBs, where many cells are dividing, the percent Fgfr2 null cells actively engaged in proliferation was approximately half that of their Fgfr2 heterozygous neighbors. Given such a differential, it is easy to understand why the Fgfr2 null cells are rapidly diluted out of the TEBs. In contrast, in ducts, where many fewer cells proliferate, the percent Fgfr2 null cells actively engaged in proliferation was similar to that of their Fgfr2 heterozygous neighbors. Thus, the results of our mosaic analysis indicate that in the normal mammary gland FGF signaling functions in TEB cells to stimulate them to proliferate frequently enough to generate both cells at the tips of branching epithelium and cells in the subtending duct. These results are consistent with a recent report demonstrating, by using a dominant-negative approach, that function of FGF receptors, presumably FGFR2b, is required for cell proliferation and TEB maintenance in the mammary gland (Parsa et al., 2008). These data are also similar to a recent study showing that Fgfr2 inactivation causes a reduction of cell proliferation in prostate epithelium (Lin et al., 2007). Since the Fgfr2 null cells were still able to proliferate in mammary and prostate glands, a conclusion from these studies and ours is that other signaling cascades besides FGF signaling must contribute to the overall proliferation rate. Candidates for these signals include HGF/MET, IGF1/IGFR and WNT pathways, a lack of each of which causes defects in mammary gland development [reviewed in Sternlicht et al., 2006; Wiseman and Werb, 2002].
Our mosaic analysis allowed us to directly compare cell behaviors of Fgfr2 heterozygous cells with wild-type cells in the same control mosaic glands. Unlike in mutant glands, Fgfr2 heterozygous cells in control glands were able to compete with wild-type cells and contribute evenly to the mammary ductal tree along the proximal-distal axis. Indeed, cell proliferation and cell death analyses further demonstrated that Fgfr2 heterozygous cells and wild-type cells are indistinguishable in both aspects. These results are consistent with previous studies showing that Fgfr2 heterozygosity is sufficient to sustain development of multiple vertebrate organs, including embryonic mammary gland (Mailleux et al., 2002), limb (Lu et al., 2008), and kidney (Zhao et al., 2004). Together, we conclude that one copy of Fgfr2 is sufficient to support normal mammary gland branching morphogenesis.
Cre-mediated mosaic analysis facilitates phenotypic analysis
Genetic mosaic analysis is a powerful complement to conventional knock-out and conditional null strategies for studying gene function and for analyzing cell lineages and cell behaviors during development (Ciruna and Rossant, 2001; Shakya et al., 2005; Tam and Rossant, 2003). In mice, this approach has generally involved producing chimeras by combining null with wild-type or heterozygous embryos. However, one limitation of this method is that an early requirement for the gene of interest in a given lineage may result in exclusion of mutant cells from that lineage and thus prevent an assessment of the gene's function at later stages (Rossant and Spence, 1998). This limitation can be overcome by producing chimeras not with null embryos, but with embryos in which the gene of interest will be conditionally inactivated by Cre-mediated recombination. In this study we have used an alternative approach in which we created genetic mosaic embryos using the MMTV-Cre transgene or adenovirus-Cre. This technique combined the advantages of genetic mosaic and conditional knock-out analyses and enabled us to determine that Fgfr2 functions during branching morphogenesis in the postnatal mammary gland. We were also able to demonstrate that although Fgfr2 is expressed in both TEBs and ducts, it is essential in the TEBs, where the majority of cell proliferation is ongoing, but is not required in ducts, where many fewer cells are proliferating.
Our study underscores two features of the MMTV-Cre transgene that limit its usefulness for conventional tissue-specific loss- or gain-of-function analyses in the mammary gland. First, the Cre transgene does not function in all cells. Thus, in situations when mutant cells proliferate more slowly or are at some other competitive disadvantage, like the Fgfr2 null cells in this study, compensation by cells in which Cre-mediated recombination did not occur may obscure the null mutant phenotype. On the other hand, in cases where mutant cells have a significant competitive advantage over cells in which Cre-mediated recombination does not occur, the mutant phenotype should be readily observable (Li et al., 2002). Second, due to progesterone-responsiveness of the promoter, the MMTV-Cre transgene presumably undergoes consecutive round(s) of activation, which can result in Cre-mediated recombination after the process of interest has occurred and thus confound the interpretation. It is important to bear these caveats in mind when the MMTV-Cre transgene is used for tissue-specific analysis of gene function in the mammary gland.
Functions of FGF signaling in vertebrate and invertebrate branching morphogenesis
The data reported here and in other studies demonstrate that a key function of FGFR2 in vertebrate branching morphogenesis is to regulate cell proliferation and/or survival. However, other functions of FGF signaling in this process must also be considered. Of particular interest in this context are the studies of the role of FGF signaling during branching morphogenesis in the Drosophila tracheal and air sac systems, where FGF signaling regulates cell migration, cell rearrangement and cell shape changes (Cabernard et al., 2004; Ghabrial et al., 2003; Metzger and Krasnow, 1999; Uv et al., 2003). Unlike the tracheal system, in which there is no cell proliferation when the cells are undergoing migration and branching, the fly air sac system resembles vertebrate organs in that cell proliferation and cell death are concomitant with branching morphogenesis (Cabernard et al., 2004). Using genetic mosaic analysis, recent studies have shown that cells lacking FGF signaling activities fail to migrate and thus do not stay at the leading edge during branching morphogenesis of the fly trachea (Ghabrial and Krasnow, 2006; Sutherland et al., 1996) and air sacs (Cabernard and Affolter, 2005).
Therefore, it remains a possibility that in genetically mosaic mammary glands, cell behaviors other than proliferation also contribute to FGFR2-dependent cell competition during branching morphogenesis. Indeed, FGF signaling regulates an array of cell behaviors in mammary gland organotypic cultures (Ewald et al., 2008) and in various vertebrate developmental settings (Bottcher and Niehrs, 2005; Ciruna and Rossant, 2001; Coumoul and Deng, 2003; Mariani et al., 2008; Shim et al., 2005; Xian et al., 2005). Future studies should address whether FGFR2 regulates such cell behaviors, especially cell fate determination, cell adhesion and migration, during branching morphogenesis of the mammary gland.
Epithelial-stromal interactions during mammary branching morphogenesis
The Fgfr2 gene produces two receptor isoforms, FGFR2b and FGFR2c, which are generated by alternative splicing (Itoh and Ornitz, 2004). In various vertebrate tissues, FGFR2b has been shown to be primarily expressed in the epithelium and to respond to mesenchyme-derived FGFs, such as FGF7 and FGF10, whereas FGFR2c is expressed in the mesenchyme and responds to epithelium-derived FGFs (Ornitz et al., 1996; Orr-Urtreger et al., 1993; Peters et al., 1992; Yu et al., 2000). As mentioned earlier, FGF10-FGFR2b signaling is required for embryonic mammary gland development (Mailleux et al., 2002). In addition, Fgf10 continues to be expressed in the stroma of the postnatal gland (Kouros-Mehr and Werb, 2006; Pedchenko and Imagawa, 2000). Thus, although our study does not directly address this issue, it is likely that the effects we observed in the mutant mammary glands were due to the absence of the Fgfr2b isoform.
In addition to FGF signaling, several other molecular pathways are involved in epithelium-stroma interactions during branching morphogenesis in the postnatal mammary gland. For example, epithelium-derived amphiregulin (AREG), a member of the EGF family, promotes (Luetteke et al., 1999; Sebastian et al., 1998), and TGF-β1 inhibits (Ewan et al., 2002; Mailleux et al., 2007) mammary branching by targeting the stroma (Cheng et al., 2005; Pollard, 2001; Serra and Crowley, 2005). Interestingly, TGF-β1 has been shown to inhibit Fgf10 expression in several experimental settings, including fibroblast (Beer et al., 1997), lung (Lebeche et al., 1999) and prostate epithelial cultures (Tomlinson et al., 2004). Whether or how TGF-β1 or EGFR regulates production and activity of stromal FGFs during mammary branching morphogenesis remains to be determined.
Acknowledgments
We thank Hilary Beggs for providing the recombinant adenovirus and Hosein Kouros-Mehr for amplifying it. We also thank Yasmine Perdue, Ying Yu, and Helen Capili for technical assistance, and our colleagues in the Martin and Werb laboratories for critical comments on the manuscript. This work was supported by fellowships from an Institutional NIH National Research Award HL07731 (P. L.) and the California Breast Cancer Research Program(11FB-0015) (A. J. E), and by the National Institutes of Health grants HD025331 (G. R. M.), ES012801 and CA057621 (Z. W.).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.ydbio.2008.06.005.
Supplementary Material
References
- Affolter M, Bellusci S, Itoh N, Shilo B, Thiery JP, Werb Z. Tube or not tube: remodeling epithelial tissues by branching morphogenesis. Dev. Cell. 2003;4:11–18. doi: 10.1016/s1534-5807(02)00410-0. [DOI] [PubMed] [Google Scholar]
- Beer HD, Florence C, Dammeier J, McGuire L, Werner S, Duan DR. Mouse fibroblast growth factor 10: cDNA cloning, protein characterization, and regulation of mRNA expression. Oncogene. 1997;15:2211–2218. doi: 10.1038/sj.onc.1201383. [DOI] [PubMed] [Google Scholar]
- Bonnette SG, Hadsell DL. Targeted disruption of the IGF-I receptor gene decreases cellular proliferation in mammary terminal end buds. Endocrinology. 2001;142:4937–4945. doi: 10.1210/endo.142.11.8500. [DOI] [PubMed] [Google Scholar]
- Bottcher RT, Niehrs C. Fibroblast growth factor signaling during early vertebrate development. Endocr. Rev. 2005;26:63–77. doi: 10.1210/er.2003-0040. [DOI] [PubMed] [Google Scholar]
- Cabernard C, Affolter M. Distinct roles for two receptor tyrosine kinases in epithelial branching morphogenesis in Drosophila. Dev. Cell. 2005;9:831–842. doi: 10.1016/j.devcel.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Cabernard C, Neumann M, Affolter M. Cellular and molecular mechanisms involved in branching morphogenesis of the Drosophila tracheal system. J. Appl. Physiol. 2004;97:2347–2353. doi: 10.1152/japplphysiol.00435.2004. [DOI] [PubMed] [Google Scholar]
- Cheng N, Bhowmick NA, Chytil A, Gorksa AE, Brown KA, Muraoka R, Arteaga CL, Neilson EG, Hayward SW, Moses HL. Loss of TGF-beta type II receptor in fibroblasts promotes mammary carcinoma growth and invasion through upregulation of TGF-alpha-, MSP- and HGF-mediated signaling networks. Oncogene. 2005;24:5053–5068. doi: 10.1038/sj.onc.1208685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciruna B, Rossant J. FGF signaling regulates mesoderm cell fate specification and morphogenetic movement at the primitive streak. Dev. Cell. 2001;1:37–49. doi: 10.1016/s1534-5807(01)00017-x. [DOI] [PubMed] [Google Scholar]
- Coumoul X, Deng CX. Roles of FGF receptors in mammalian development and congenital diseases. Birth Defects Res C Embryo Today. 2003;69:286–304. doi: 10.1002/bdrc.10025. [DOI] [PubMed] [Google Scholar]
- Crowley MR, Bowtell D, Serra R. TGF-beta, c-Cbl, and PDGFR-alpha the in mammary stroma. Dev. Biol. 2005;279:58–72. doi: 10.1016/j.ydbio.2004.11.034. [DOI] [PubMed] [Google Scholar]
- Ewald AJ, Brenot A, Duong M, Chan BS, Werb Z. Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Dev. Cell. 2008;14:570–581. doi: 10.1016/j.devcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewan KB, Shyamala G, Ravani SA, Tang Y, Akhurst R, Wakefield L, Barcellos-Hoff MH. Latent transforming growth factor-beta activation in mammary gland: regulation by ovarian hormones affects ductal and alveolar proliferation. Am. J. Pathol. 2002;160:2081–2093. doi: 10.1016/s0002-9440(10)61158-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fata JE, Mori H, Ewald AJ, Zhang H, Yao E, Werb Z, Bissell MJ. The MAPK (ERK-1,2) pathway integrates distinct and antagonistic signals from TGFalpha and FGF7 in morphogenesis of mouse mammary epithelium. Dev. Biol. 2007;306:193–207. doi: 10.1016/j.ydbio.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghabrial A, Luschnig S, Metzstein MM, Krasnow MA. Branching morphogenesis of the Drosophila tracheal system. Annu. Rev. Cell Dev. Biol. 2003;19:623–647. doi: 10.1146/annurev.cellbio.19.031403.160043. [DOI] [PubMed] [Google Scholar]
- Ghabrial AS, Krasnow MA. Social interactions among epithelial cells during tracheal branching morphogenesis. Nature. 2006;441:746–749. doi: 10.1038/nature04829. [DOI] [PubMed] [Google Scholar]
- Grieshammer U, Cebrian C, Ilagan R, Meyers E, Herzlinger D, Martin GR. FGF8 is required for cell survival at distinct stages of nephrogenesis and for regulation of gene expression in nascent nephrons. Development. 2005;132:3847–3857. doi: 10.1242/dev.01944. [DOI] [PubMed] [Google Scholar]
- He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. U. S. A. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennighausen L, Robinson GW. Information networks in the mammary gland. Nat. Rev. Mol. Cell Biol. 2005;6:715–725. doi: 10.1038/nrm1714. [DOI] [PubMed] [Google Scholar]
- Itoh N, Ornitz D. Functional evolutionary history of the mouse Fgf gene family. Developmental Dynamics. 2008;237:18–27. doi: 10.1002/dvdy.21388. [DOI] [PubMed] [Google Scholar]
- Itoh N, Ornitz DM. Evolution of the Fgf and Fgfr gene families. Trends Genet. 2004;20:563–569. doi: 10.1016/j.tig.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Jackson D, Bresnick J, Rosewell I, Crafton T, Poulsom R, Stamp G, Dickson C. Fibroblast growth factor receptor signalling has a role in lobuloalveolar development of the mammary gland. J. Cell Sci. 1997;110(Pt 11):1261–1268. doi: 10.1242/jcs.110.11.1261. [DOI] [PubMed] [Google Scholar]
- Jackson-Fisher AJ, Bellinger G, Ramabhadran R, Morris JK, Lee KF, Stern DF. ErbB2 is required for ductal morphogenesis of the mammary gland. Proc. Natl. Acad. Sci. U. S. A. 2004;101:17138–17143. doi: 10.1073/pnas.0407057101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph H, Gorska AE, Sohn P, Moses HL, Serra R. Overexpression of a kinase-deficient transforming growth factor-beta type II receptor in mouse mammary stroma results in increased epithelial branching. Mol. Biol. Cell. 1999;10:1221–1234. doi: 10.1091/mbc.10.4.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouros-Mehr H, Werb Z. Candidate regulators of mammary branching morphogenesis identified by genome-wide transcript analysis. Dev. Dyn. 2006;235:3404–3412. doi: 10.1002/dvdy.20978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebeche D, Malpel S, Cardoso WV. Fibroblast growth factor interactions in the developing lung. Mech. Dev. 1999;86:125–136. doi: 10.1016/s0925-4773(99)00124-0. [DOI] [PubMed] [Google Scholar]
- Lewis MT, Ross S, Strickland PA, Sugnet CW, Jimenez E, Scott MP, Daniel CW. Defects in mouse mammary gland development caused by conditional haploinsufficiency of Patched-1. Development. 1999;126:5181–5193. doi: 10.1242/dev.126.22.5181. [DOI] [PubMed] [Google Scholar]
- Li G, Robinson GW, Lesche R, Martinez-Diaz H, Jiang Z, Rozengurt N, Wagner KU, Wu DC, Lane TF, Liu X, Hennighausen L, Wu H. Conditional loss of PTEN leads to precocious development and neoplasia in the mammary gland. Development. 2002;129:4159–4170. doi: 10.1242/dev.129.17.4159. [DOI] [PubMed] [Google Scholar]
- Lin Y, Liu G, Zhang Y, Hu YP, Yu K, Lin C, McKeehan K, Xuan JW, Ornitz DM, Shen MM, Greenberg N, McKeehan WL, Wang F. Fibroblast growth factor receptor 2 tyrosine kinase is required for prostatic morphogenesis and the acquisition of strict androgen dependency for adult tissue homeostasis. Development. 2007;134:723–734. doi: 10.1242/dev.02765. [DOI] [PubMed] [Google Scholar]
- Lu P, Minowada G, Martin GR. Increasing Fgf4 expression in the mouse limb bud causes polysyndactyly and rescues the skeletal defects that result from loss of Fgf8 function. Development. 2006a;133:33–42. doi: 10.1242/dev.02172. [DOI] [PubMed] [Google Scholar]
- Lu P, Sternlicht MD, Werb Z. Comparative mechanisms of branching morphogenesis in diverse systems. J. Mammary Gland Biol. Neoplasia. 2006b;11:213–228. doi: 10.1007/sl0911-006-9027-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Yu Y, Perdue Y, Werb Z. The apical ectodermal ridge is a timer for generating distal limb progenitors. Development. 2008;135:1395–1405. doi: 10.1242/dev.018945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luetteke NC, Qiu TH, Fenton SE, Troyer KL, Riedel RF, Chang A, Lee DC. Targeted inactivation of the EGF and amphiregulin genes reveals distinct roles for EGF receptor ligands in mouse mammary gland development. Development. 1999;126:2739–2750. doi: 10.1242/dev.126.12.2739. [DOI] [PubMed] [Google Scholar]
- MacArthur CA, Shankar DB, Shackleford GM. Fgf-8, activated by proviral insertion, cooperates with the Wnt-1 transgene in murine mammary tumorigenesis. J. Virol. 1995;69:2501–2507. doi: 10.1128/jvi.69.4.2501-2507.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailleux AA, Overholtzer M, Schmelzle T, Bouillet P, Strasser A, Brugge JS. BIM regulates apoptosis during mammary ductal morphogenesis, and its absence reveals alternative cell death mechanisms. Dev. Cell. 2007;12:221–234. doi: 10.1016/j.devcel.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailleux AA, Spencer-Dene B, Dillon C, Ndiaye D, Savona-Baron C, Itoh N, Kato S, Dickson C, Thiery JP, Bellusci S. Role of FGF10/FGFR2b signaling during mammary gland development in the mouse embryo. Development. 2002;129:53–60. doi: 10.1242/dev.129.1.53. [DOI] [PubMed] [Google Scholar]
- Mariani FV, Ahn CP, Martin GR. Genetic evidence that FGFs have an instructive role in limb proximal-distal patterning. Nature. 2008 doi: 10.1038/nature06876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger RJ, Krasnow MA. Genetic control of branching morphogenesis. Science. 1999;284:1635–1639. doi: 10.1126/science.284.5420.1635. [DOI] [PubMed] [Google Scholar]
- Moraes RC, Zhang X, Harrington N, Fung JY, Wu MF, Hilsenbeck SG, Allred DC, Lewis MT. Constitutive activation of smoothened (SMO) in mammary glands of transgenic mice leads to increased proliferation, altered differentiation and ductal dysplasia. Development. 2007;134:1231–1242. doi: 10.1242/dev.02797. [DOI] [PubMed] [Google Scholar]
- Naylor MJ, Li N, Cheung J, Lowe ET, Lambert E, Marlow R, Wang P, Schatzmann F, Wintermantel T, Schuetz G, Clarke AR, Mueller U, Hynes NE, Streuli CH. Ablation of beta1 integrin in mammary epithelium reveals a key role for integrin in glandular morphogenesis and differentiation. J. Cell Biol. 2005;171:717–728. doi: 10.1083/jcb.200503144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornitz DM, Xu J, Colvin JS, McEwen DG, MacArthur CA, Coulier F, Gao G, Goldfarb M. Receptor specificity of the fibroblast growth factor family. J. Biol. Chem. 1996;271:15292–15297. doi: 10.1074/jbc.271.25.15292. [DOI] [PubMed] [Google Scholar]
- Orr-Urtreger A, Bedford MT, Burakova T, Arman E, Zimmer Y, Yayon A, Givol D, Lonai P. Developmental localization of the splicing alternatives of Fibroblast Growth Factor Receptor-2 (FGFR2) Developmental Biology. 1993;158:475–486. doi: 10.1006/dbio.1993.1205. [DOI] [PubMed] [Google Scholar]
- Otten AD, Sanders MM, McKnight GS. The MMTV LTR promoter is induced by progesterone and dihydrotestosterone but not by estrogen. Mol. Endocrinol. 1988;2:143–147. doi: 10.1210/mend-2-2-143. [DOI] [PubMed] [Google Scholar]
- Parsa S, Ramasamy SK, De Langhe S, Gupte VV, Haigh JJ, Medina D, Bellusci S. Terminal end bud maintenance in mammary gland is dependent upon FGFR2b signaling. Dev. Biol. 2008;317:121–131. doi: 10.1016/j.ydbio.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Pedchenko VK, Imagawa W. Pattern of expression of the KGF receptor and its ligands KGF and FGF-10 during postnatal mouse mammary gland development. Mol. Reprod. Dev. 2000;56:441–447. doi: 10.1002/1098-2795(200008)56:4<441::AID-MRD1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Peters G, Brookes S, Smith R, Dickson C. Tumorigenesis by mouse mammary tumor virus: evidence for a common region for provirus integration in mammary tumors. Cell. 1983;33:369–377. doi: 10.1016/0092-8674(83)90418-x. [DOI] [PubMed] [Google Scholar]
- Peters G, Brookes S, Smith R, Placzek M, Dickson C. The mouse homolog of the hst/k-FGF gene is adjacent to int-2 and is activated by proviral insertion in some virally induced mammary tumors. Proc. Natl. Acad. Sci. U. S. A. 1989;86:5678–5682. doi: 10.1073/pnas.86.15.5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters K, Ornitz D, Werner S, Williams L. Unique expression pattern of the FGF receptor 3 gene during mouse organogenesis. Dev. Biol. 1993;155:423–430. doi: 10.1006/dbio.1993.1040. [DOI] [PubMed] [Google Scholar]
- Peters KG, Werner S, Chen G, Williams LT. Two FGF receptor genes are differentially expressed in epithelial and mesenchymal tissues during limb formation and organogenesis in the mouse. Development. 1992;114:233–243. doi: 10.1242/dev.114.1.233. [DOI] [PubMed] [Google Scholar]
- Poladia DP, Kish K, Kutay B, Hains D, Kegg H, Zhao H, Bates CM. Role of fibroblast growth factor receptors 1 and 2 in the metanephric mesenchyme. Dev. Biol. 2006;291:325–339. doi: 10.1016/j.ydbio.2005.12.034. [DOI] [PubMed] [Google Scholar]
- Pollard JW. Tumour-stromal interactions. Transforming growth factor-beta isoforms and hepatocyte growth factor/scatter factor in mammary gland ductal morphogenesis. Breast Cancer Res. 2001;3:230–237. doi: 10.1186/bcr301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijnkels M, Rosen JM. Adenovirus-Cre-mediated recombination in mammary epithelial early progenitor cells. J. Cell Sci. 2001;114:3147–3153. doi: 10.1242/jcs.114.17.3147. [DOI] [PubMed] [Google Scholar]
- Roarty K, Serra R. Wnt5a is required for proper mammary gland development and TGF-beta-mediated inhibition of ductal growth. Development. 2007;134:3929–3939. doi: 10.1242/dev.008250. [DOI] [PubMed] [Google Scholar]
- Rohlmann A, Gotthardt M, Willnow TE, Hammer RE, Herz J. Sustained somatic gene inactivation by viral transfer of Cre recombinase. Nat. Biotechnol. 1996;14:1562–1565. doi: 10.1038/nbt1196-1562. [DOI] [PubMed] [Google Scholar]
- Rossant J, Spence A. Chimeras and mosaics in mouse mutant analysis. Trends Genet. 1998;14:358–363. doi: 10.1016/s0168-9525(98)01552-2. [DOI] [PubMed] [Google Scholar]
- Ruan W, Kleinberg DL. Insulin-like growth factor I is essential for terminal end bud formation and ductal morphogenesis during mammary development. Endocrinology. 1999;140:5075–5081. doi: 10.1210/endo.140.11.7095. [DOI] [PubMed] [Google Scholar]
- Schmahl J, Kim Y, Colvin JS, Ornitz DM, Capel B. Fgf9 induces proliferation and nuclear localization of FGFR2 in Sertoli precursors during male sex determination. Development. 2004;131:3627–3636. doi: 10.1242/dev.01239. [DOI] [PubMed] [Google Scholar]
- Seagroves TN, Hadsell D, McManaman J, Palmer C, Liao D, McNulty W, Welm B, Wagner KU, Neville M, Johnson RS. HIF1alpha is a critical regulator of secretory differentiation and activation, but not vascular expansion, in the mouse mammary gland. Development. 2003;130:1713–1724. doi: 10.1242/dev.00403. [DOI] [PubMed] [Google Scholar]
- Sebastian J, Richards RG, Walker MP, Wiesen JF, Werb Z, Derynck R, Hom YK, Cunha GR, DiAugustine RP. Activation and function of the epidermal growth factor receptor and erbB-2 during mammary gland morphogenesis. Cell Growth Differ. 1998;9:777–785. [PubMed] [Google Scholar]
- Serra R, Crowley MR. Mouse models of transforming growth factor beta impact in breast development and cancer. Endocr. Relat. Cancer. 2005;12:749–760. doi: 10.1677/erc.1.00936. [DOI] [PubMed] [Google Scholar]
- Shakya R, Watanabe T, Costantini F. The role of GDNF/Ret signaling in ureteric bud cell fate and branching morphogenesis. Dev. Cell. 2005;8:65–74. doi: 10.1016/j.devcel.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Shim K, Minowada G, Coling DE, Martin GR. Sprouty2, a mouse deafness gene, regulates cell fate decisions in the auditory sensory epithelium by antagonizing FGF signaling. Dev. Cell. 2005;8:553–564. doi: 10.1016/j.devcel.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Simian M, Hirai Y, Navre M, Werb Z, Lochter A, Bissell MJ. The interplay of matrix metalloproteinases, morphogens and growth factors is necessary for branching of mammary epithelial cells. Development. 2001;128:3117–3131. doi: 10.1242/dev.128.16.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Stark KL, McMahon JA, McMahon AP. FGFR-4, a new member of the fibroblast growth factor receptor family, expressed in the definitive endoderm and skeletal muscle lineages of the mouse. Development. 1991;113:641–651. doi: 10.1242/dev.113.2.641. [DOI] [PubMed] [Google Scholar]
- Sternlicht MD, Kouros-Mehr H, Lu P, Werb Z. Hormonal and local control of mammary branching morphogenesis. Differentiation. 2006;74:365–381. doi: 10.1111/j.1432-0436.2006.00105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternlicht MD, Sunnarborg SW, Kouros-Mehr H, Yu Y, Lee DC, Werb Z. Mammary ductal morphogenesis requires paracrine activation of stromal EGFR via ADAM17-dependent shedding of epithelial amphiregulin. Development. 2005;132:3923–3933. doi: 10.1242/dev.01966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland D, Samakovlis C, Krasnow MA. branchless encodes a Drosophila FGF homolog that controls tracheal cell migration and the pattern of branching. Cell. 1996;87:1091–1101. doi: 10.1016/s0092-8674(00)81803-6. [DOI] [PubMed] [Google Scholar]
- Tam PP, Rossant J. Mouse embryonic chimeras: tools for studying mammalian development. Development. 2003;130:6155–6163. doi: 10.1242/dev.00893. [DOI] [PubMed] [Google Scholar]
- Tomlinson DC, Grindley JC, Thomson AA. Regulation of Fgf10 gene expression in the prostate: identification of transforming growth factor-beta1 and promoter elements. Endocrinology. 2004;145:1988–1995. doi: 10.1210/en.2003-0842. [DOI] [PubMed] [Google Scholar]
- Uv A, Cantera R, Samakovlis C. Drosophila tracheal morphogenesis: intricate cellular solutions to basic plumbing problems. Trends Cell Biol. 2003;13:301–309. doi: 10.1016/s0962-8924(03)00083-7. [DOI] [PubMed] [Google Scholar]
- von der Ahe D, Janich S, Scheidereit C, Renkawitz R, Schutz G, Beato M. Glucocorticoid and progesterone receptors bind to the same sites in two hormonally regulated promoters. Nature. 1985;313:706–709. doi: 10.1038/313706a0. [DOI] [PubMed] [Google Scholar]
- Wagner KU, McAllister K, Ward T, Davis B, Wiseman R, Hennighausen L. Spatial and temporal expression of the Cre gene under the control of the MMTV-LTR in different lines of transgenic mice. Transgenic Res. 2001;10:545–553. doi: 10.1023/a:1013063514007. [DOI] [PubMed] [Google Scholar]
- Wiseman BS, Sternlicht MD, Lund LR, Alexander CM, Mott J, Bissell MJ, Soloway P, Itohara S, Werb Z. Site-specific inductive and inhibitory activities of MMP-2 and MMP-3 orchestrate mammary gland branching morphogenesis. J. Cell Biol. 2003;162:1123–1133. doi: 10.1083/jcb.200302090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman BS, Werb Z. Stromal effects on mammary gland development and breast cancer. Science. 2002;296:1046–1049. doi: 10.1126/science.1067431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xian W, Schwertfeger KL, Rosen JM. Distinct roles of fibroblast growth factor receptor 1 and 2 in regulating cell survival and epithelial-mesenchymal transition. Mol. Endocrinol. 2007;21:987–1000. doi: 10.1210/me.2006-0518. [DOI] [PubMed] [Google Scholar]
- Xian W, Schwertfeger KL, Vargo-Gogola T, Rosen JM. Pleiotropic effects of FGFR1 on cell proliferation, survival, and migration in a 3D mammary epithelial cell model. J. Cell Biol. 2005;171:663–673. doi: 10.1083/jcb.200505098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Weinstein M, Li C, Naski M, Cohen R, Ornitz D, Leder P, Deng C. Fibroblast growth factor receptor 2 (FGFR2)-mediated reciprocal regulation loop between FGF8 and FGF10 is essential for limb induction. Development. 1998;125:753–765. doi: 10.1242/dev.125.4.753. [DOI] [PubMed] [Google Scholar]
- Yamaguchi TP, Conlon RA, Rossant J. Expression of the fibroblast growth factor receptor FGFR-1/flg during gastrulation and segmentation in the mouse embryo. Dev. Biol. 1992;152:75–88. doi: 10.1016/0012-1606(92)90157-c. [DOI] [PubMed] [Google Scholar]
- Yu K, Herr AB, Waksman G, Ornitz DM. Loss of fibroblast growth factor receptor 2 ligand-binding specificity in Apert syndrome. Proc. Natl. Acad. Sci. U. S. A. 2000;97:14536–14541. doi: 10.1073/pnas.97.26.14536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K, Xu J, Liu Z, Sosic D, Shao J, Olson EN, Towler DA, Ornitz DM. Conditional inactivation of FGF receptor 2 reveals an essential role for FGF signaling in the regulation of osteoblast function and bone growth. Development. 2003;130:3063–3074. doi: 10.1242/dev.00491. [DOI] [PubMed] [Google Scholar]
- Zhao H, Kegg H, Grady S, Truong HT, Robinson ML, Baum M, Bates CM. Role of fibroblast growth factor receptors 1 and 2 in the ureteric bud. Dev. Biol. 2004;276:403–415. doi: 10.1016/j.ydbio.2004.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.