SUMMARY
The Aurora B kinase is the enzymatic core of the chromosomal passenger complex, which is a critical regulator of mitosis. To identify novel regulators of Aurora B, we performed a genome-wide screen for suppressors of a temperature-sensitive lethal allele of the C. elegans Aurora B kinase AIR-2. This screen uncovered a member of the Afg2/Spaf subfamily of Cdc48-like AAA ATPases as an essential inhibitor of AIR-2 stability and activity. Depletion of CDC-48.3 restores viability to air-2 mutant embryos and leads to abnormally high AIR-2 levels at the late telophase/G1 transition. Furthermore, CDC-48.3 binds directly to AIR-2 and inhibits its kinase activity from metaphase through telophase. While canonical p97/Cdc48 proteins have been assigned contradictory roles in the regulation of Aurora B, our results are the first to identify a member of the Afg2/Spaf AAA ATPases as a critical in vivo inhibitor of this kinase during embryonic development.
INTRODUCTION
Eukaryotes have evolved complex regulatory mechanisms to ensure that the cell cycle progresses in a timely and accurate manner. Key components of these pathways are protein kinases that are critical for the proper timing of each cell cycle phase. Preeminent among these proteins are the cyclin-dependent kinases (Cdks), which upon binding to cyclins, phosphorylate numerous targets to trigger cell cycle progression (Norbury and Nurse, 1991). In addition to Cdk1/cyclin B, members of the Aurora/Ipl1 kinase family are also key regulators of mitosis (Ducat and Zheng, 2004; Vagnarelli and Earnshaw, 2004). These proteins, which include Aurora A and B, are serine/threonine kinases that are essential for cell division events such as spindle assembly, chromosome segregation, and cytokinesis (Andrews et al., 2003).
While Aurora A localizes to mitotic centrosomes and is required for centrosome maturation and the formation of a functional bipolar mitotic spindle (Ducat and Zheng, 2004; Hannak et al., 2001; Vagnarelli and Earnshaw, 2004), Aurora B is the catalytic core of the highly conserved chromosomal passenger complex (CPC) (Ruchaud et al., 2007). The CPC includes, in addition to Aurora B, three regulatory subunits; the inner centromeric protein (INCENP), Survivin, and Borealin/Dasra-B (Ruchaud et al., 2007). Beginning in prophase, the CPC localizes to condensing chromosomes and progressively concentrates at the inner centromere where one function is to correct improper spindle-kinetochore attachments (Vagnarelli and Earnshaw, 2004). At the onset of anaphase, the CPC redistributes to the central spindle and cleavage furrow to regulate the completion of cytokinesis (Vagnarelli and Earnshaw, 2004). Importantly, the other passenger proteins directly influence Aurora B localization (Ruchaud et al., 2007; Jeyaprakash et al., 2007), and phosphorylation of conserved residues in the C-terminus of INCENP substantially increases Aurora B kinase activity (Bishop and Schumacher, 2002; Honda et al., 2003). Aurora B levels peak in early mitosis and then dramatically decline at mitotic exit. In vertebrates, this drop is mediated in part by Aurora B ubiquitination via the anaphase-promoting complex (APC/C-Cdh1), and subsequent degradation by the proteasome (Nguyen et al., 2005; Stewart and Fang, 2005).
Recent reports have linked the Cdc48/p97 AAA ATPase with the regulation of Aurora B and the chromosomal passenger complex. In one study, p97 and its cofactors Npl4 and Ufd1 co-purified with Survivin isolated from Xenopus egg extracts (Vong et al., 2005). Ufd1 was shown to be required for Survivin ubiquitination, and for the localization of Survivin and Aurora B to centromeres. Conversely, the deubiquitinating enzyme hFAM was required for the disassociation of Survivin and Aurora B from anaphase chromosomes (Vong et al., 2005). Hence, this study concluded that p97/Ufd1/Npl4 is a positive regulator of the CPC as it is required for the localization of Survivin and Aurora B to metaphase centromeres (Vong et al., 2005).
Surprisingly, a recent study contradicts these findings, suggesting that p97 is required for the disassociation of Aurora B from chromosomes, which is in turn a prerequisite for nuclear envelope reformation at the end of mitosis (Ramadan et al., 2007). p97 is required for mitotic spindle disassembly and nuclear envelope reformation in Xenopus egg extracts (Cao et al., 2003; Hetzer et al., 2001). However, inhibition or depletion of Aurora B relieved this requirement, suggesting that Aurora B is a key target of p97 in this pathway (Kelly et al., 2007; Ramadan et al., 2007). Indeed, p97 physically interacted with ubiquitinated Aurora B and was required to extract the kinase from chromatin (Ramadan et al., 2007). Chromosome release resulted in a corresponding drop in kinase activity, arguably due to dissemination of the kinase from activating clusters (Kelly et al., 2007). Consistent findings were found upon depletion of the two Cdc48/p97 orthologs in C. elegans (Ramadan et al., 2007). cdc-48.1 and cdc-48.2(RNAi) resulted in defects in chromosome decondensation and nuclear envelope reassembly, as well as the retention of the Aurora B kinase AIR-2 on anaphase chromosomes (Ramadan et al., 2007). In addition, RNAi of either cdc-48.1 or cdc-48.2 partially rescued a hypomorphic temperature-sensitive (ts) allele of air-2, and resulted in an increase in the phosphorylation of histone H3, a conserved target of the Aurora B kinases (Ramadan et al., 2007).
The disparate conclusions reached by these studies raise a number of questions regarding the cellular pathways that control Aurora B kinase activity and functions. To elucidate the regulation of the Aurora B kinase in an unbiased fashion, we undertook a C. elegans genome-wide screen for loss-of-function suppressors of the same air-2 allele used in the study described above, air-2(or207ts) (Severson et al., 2000). Although we did not recover either of the canonical CDC-48 family members in our screen, we did find, among a handful of reproducible suppressors, a member of the Afg2/Spaf subfamily of Cdc48/p97 AAA+ ATPases.
K04G2.3/CDC-48.3, is closely related to yeast Afg2 and mammalian Spaf, which form a distinct subgroup of AAA+ ATPases that also includes an uncharacterized Drosophila protein (Frohlich, 2001). In contrast to canonical Cdc48 and p97, little is known regarding the specific functions of the Afg2/Spaf proteins. The only reported function of S. cerevisiae Afg2 (ATPase family gene) is the release and recycling of nucleolar shuttling factors from pre-60S ribosomal particles (Pertschy et al., 2007). Murine Spaf was first identified due to increased expression in an epidermal chemical carcinogenesis model (Liu et al., 2000). Spaf (spermatogenesis-associated factor) is highly expressed in testis, and is enriched in the cytoplasm of spermatagonia and early spermatocytes (Liu et al., 2000); however the functional role of Spaf in the epidermis or sperm development is not known.
We here report that C. elegans CDC-48.3 is an essential inhibitor of the Aurora B kinase AIR-2. In vitro, CDC-48.3 binds directly to and inhibits AIR-2 kinase activity in an ATPase-dependent manner. In vivo, CDC-48.3 inhibits AIR-2 activity from metaphase through telophase, and is required for the characteristic drop in AIR-2 expression at mitotic exit. Importantly, loss of CDC-48.3 in wild-type embryos results in mitotic spindle and chromosome segregation defects as well as significant delays in mitotic progression. In sum, these results reveal that a member of the highly conserved Afg2/SPAF subfamily of AAA ATPases is essential for timely and accurate cell division and is a critical regulator of the AIR-2 Aurora B kinase.
RESULTS
An RNAi based suppressor screen identifies K04G2.3 as an inhibitor of the AIR-2 kinase
To isolate inhibitors of the C. elegans Aurora B kinase AIR-2, a genome-wide RNAi screen for suppressors of a ts air-2 allele, air-2(or207ts)(Severson et al., 2000), was performed. The or207 mutation replaces a conserved proline (P265) within the predicted kinase domain with lysine, resulting in undetectable kinase activity in vitro (Bishop and Schumacher, 2002). At the permissive temperature, 15°C, air-2(or207ts) embryos are nearly 100% viable and are phenotypically indistinguishable from wild-type (wt). When shifted to restrictive temperatures, air-2(or207ts) hermaphrodites produce dead polyploid one-cell embryos with gross defects in chromosome segregation and cytokinesis, a phenotype highly reminiscent of air-2(RNAi) embryos (Schumacher et al., 1998b; Severson et al., 2000). To identify suppressors of air-2(or207ts) lethality, air-2(or207ts) larvae were fed E. coli transformed with an RNAi feeding library representing 86.9% of all C. elegans open-reading frames (Kamath et al., 2003). To optimize the number of suppressors uncovered, the screen was performed at a semi-permissive temperature, 22°C, which is the lowest temperature that yields ~100% air-2(or207ts) lethality (Figure 1A). Suppressors were identified by the presence of any surviving larvae. 57 candidate suppressors were recovered after screening the entire RNAi library, and retesting confirmed 4 independent and reproducible suppressors. The characterization of the strongest of these suppressors, K04G2.3 is presented here; analysis of the other 3 suppressors will be presented elsewhere. K04G2.3(RNAi) restored air-2(or207ts) embryonic viability to 72.3% versus 1% for controls at 20°C, and 21.3% versus 0% at 22°C. K04G2.3 encodes a homolog of the Afg2/Spaf subfamily of Cdc48-like AAA+ ATPases (Figure S1, Supplemental Data). The closest C. elegans relatives of K04G2.3 encode redundant canonical Cdc48 ATPases, CDC-48.1 and CDC-48.2 (Poteryaev et al., 2005; Sasagawa et al., 2007; Yamauchi et al., 2006). Since the K04G2.3 gene product is closely related to these proteins, we named this gene cdc-48.3 (Accession number: NP_492211).
Figure 1. Loss of C. elegans CDC-48.3 suppresses air-2(or207ts) lethality and mitotic defects.
A) Viability of control and cdc-48.3(RNAi) treated air-2(or207ts) embryos reared at 15, 18, 20, 22, and 25°C. B) Embryos dissected from control-treated air-2(or207ts) and cdc-48.3(RNAi);air-2(or207ts) adult hermaphrodites reared at 22°C were fixed and stained with DAPI (blue), and α-tubulin (green) and AIR-2 (red) antibodies. One-cell embryos at different stages of mitosis are shown. Scale bar =10 μm.
To confirm that cdc-48.3(RNAi) suppression of air-2(or207ts) lethality was specific, we assayed whether cdc-48.3(RNAi) could suppress additional embryonic lethal ts mutants. Indeed, of four mutants examined (Gomes et al., 2001; Meneghini et al., 1999; O’Rourke et al., 2007; Severson et al., 2000), cdc-48.3(RNAi) only restored significant viability to air-2(or207ts) embryos (Figure S2A). To test whether loss of the other Cdc48 homologs could also suppress air-2(or207ts) lethality, RNAi of cdc-48.1 and cdc-48.2 alone or simultaneously (via co-injection of cdc-48.1 and cdc-48.2 dsRNAs) was performed. Neither cdc-48.1(RNAi) nor cdc-48.2(RNAi) alone or in combination could suppress air-2(or207ts) lethality (Table S1). Cdc48 regulates various cellular processes via association with a number of conserved cofactors (Cao et al., 2003; Isaacson et al., 2007; Vong et al., 2005). However, RNAi of the C. elegans homologs of the Cdc48 cofactors Ufd1, Npl4, and Ubx did not suppress air-2(or207ts) lethality (Table S1). Altogether, these data suggest that cdc-48.3 is a specific negative regulator of the air-2 kinase pathway during C elegans embryogenesis, and may act independently of known Cdc48 cofactors.
Loss of CDC-48.3 suppresses mitotic defects in AIR-2-mutant embryos
air-2(or207ts) embryos display defects in chromosome segregation and cytokinesis at restrictive temperatures (Severson et al., 2000). The mutant AIR-2 protein (AIR-2ts) is still expressed at these temperatures but fails to dissociate from anaphase chromosomes and localize to the spindle midzone and midbody. The mutant protein has no detectable kinase activity in vitro (Bishop and Schumacher, 2002); hence kinase activity may potentiate AIR-2 localization dynamics (Kamath et al., 2003). Given that cdc-48.3(RNAi) suppressed air-2(or207ts) lethality, we examined the extent to which cdc-48.3(RNAi) could rescue the localization of the AIR-2ts protein and air-2(or207ts) mitotic defects. At 22°C, AIR-2ts localizes to chromosomes from early prophase through metaphase in both control and cdc-48.3(RNAi) treated air-2(or207ts) embryos (Figure 1B). At anaphase, AIR-2ts remained at least partially localized to chromosomes in the majority of control treated embryos (Figure 1B, Table 1), but was no longer associated with anaphase chromosomes in most cdc-48.3(RNAi) treated embryos. At telophase, AIR-2ts localized around chromosomes in a nuclear envelope-like pattern in control treated embryos, whereas it was associated with the midbody in the majority of cdc-48.3(RNAi) treated embryos. Hence, upon depletion of CDC-48.3, appropriate AIR-2 localization is restored in air-2(or207ts) embryos reared at restrictive temperatures. Furthermore, DAPI staining revealed that while chromosomes segregated properly in approximately 22% of control treated air-2(or207ts) embryos, successful chromosome segregation occurred in approximately 87% of cdc-48.3(RNAi) embryos (Figure 1B, Table 1). Altogether, these findings suggest that suppression of air-2(or207ts) lethality by cdc-48.3(RNAi) is due in part to the restoration of AIR-2 localization, which contributes to increased mitotic fidelity.
Table 1.
Rescue of air-2(or207ts) mitotic defects by CDC-48.3 depletion
| Stage | Midzone/Midbody AIR-2 | Chromosomal/NE* AIR-2 | AIR-2 at Both** | Chromosome Segregation |
|---|---|---|---|---|
| Anaphase | ||||
|
control(RNAi)
(n=23) |
8.7% | 39.1% | 52.2% | 21.7% |
|
cdc-48.3(RNAi)
(n=21) |
47.7% | 19.0% | 33.6% | 85.7% |
| Telophase | ||||
|
control(RNAi)
(n=25) |
12.0% | 68.0% | 20.0% | 28.0% |
|
cdc-48.3(RNAi)
(n=22) |
59.1% | 13.6% | 27.3% | 86.3% |
| Late telophase/G1 | ||||
|
control(RNAi)
(n=23) |
17.4% | 82.6% | N/A | 17.4% |
|
cdc-48.3(RNAi)
(n=26) |
88.5% | 11.5% | N/A | 88.5% |
NE*: Nuclear Envelope
Both**: Chromosomes and midzone/midbody
control(RNAi): T7(RNAi);air-2(or207ts) (22°C)
cdc-48.3(RNAi): cdc-48.3(RNAi);air-2(or207ts) (22°C)
CDC-48.3 regulates AIR-2 protein stability at mitotic exit
One conserved Cdc48 function is to target ubiquitinated proteins to the 26S proteasome for degradation (Cao et al., 2003). Given this and the genetic interaction between cdc-48.3 and air-2, we assayed whether CDC-48.3 regulates AIR-2 stability. Western analysis revealed that AIR-2 levels are significantly upregulated (~3-4 fold) in extracts from cdc-48.3(RNAi) treated embryos as compared to wt and air-2(or207ts) embryos treated with control RNAi (Figure 2A). To assess the affect of CDC-48.3 depletion on the temporal and spatial localization of AIR-2 during the cell cycle, early embryos from control and cdc-48.3(RNAi) treated wt hermaphrodites were immunostained with tubulin and AIR-2-specific antibodies. There were no detectable differences in AIR-2 intensity or localization in cdc-48.3(RNAi) versus control embryos from early prophase through telophase (Figure 2B, 2C). However, at late-telophase/G1, marked accumulation of AIR-2 immunostaining was present at the spindle midbody of cdc-48.3(RNAi) embryos as compared to controls. Note that there is no discernible difference in the length of the mitotic spindle in control vs. cdc-48.3(RNAi) embryos (Figure S2B). A similar pattern was found in subsequent cell cycles and in air-2(or207ts); cdc-48.3(RNAi) vs. control treated air-2(or207ts) embryos (Figures S2C, 2D).
Figure 2. AIR-2 levels are increased at mitotic exit in cdc-48.3(RNAi) embryos.

A) Protein extracts from wt, cdc-48.3(RNAi), air-2(RNAi), air-2(or207ts), and cdc-48.3(RNAi);air-2(or207ts) embryos reared at 22°C were subjected to western analysis with AIR-2 and α-tubulin specific antibodies. Band intensities were measured and normalized to α-tubulin protein levels. Error bars represent standard deviation from three independent experiments. B) Embryos dissected from control and cdc-48.3(RNAi) treated adult hermaphrodites were fixed and stained with DAPI (blue), and α-tubulin (green) and AIR-2 (red) antibodies. One-cell embryos at each mitotic stage are shown. Arrow points to marked accumulation of AIR-2 at the midbody of a cdc-48(RNAi) embryo. Scale bar=10 μm. C, D) The mean fluorescence intensity of chromosomal passenger-localized AIR-2 (prophase and metaphase chromosomes, anaphase central spindle, telophase midzone and midbody) was measured and plotted against spindle length in wt (C) and air-2(or207ts) (D) embryos reared at 22°C and treated with control and cdc-48.3(RNA). Spindle length was measured from the center of one centrosome to the other. Each data point represents one embryo, and colored points mark the start of each mitotic phase as follows: prophase (black), prometaphase (orange), metaphase (purple), anaphase (green), telophase (red), and late telophase/G1 entry (blue). Number of embryos: wt;control(RNAi), n=98; wt;cdc-48.3(RNAi), n=87; air-2(or207ts);control(RNAi), n=91; air-2(or207ts);cdc-48.3(RNAi), n=94.
To visualize the effects of cdc-48.3(RNAi) on AIR-2 dynamics in real time, live imaging of GFP-tagged AIR-2 in early embryos was performed. GFP-AIR-2 intensity and localization were similar in control and cdc-48.3(RNAi) embryos from pronuclear meeting through early telophase of the first mitotic division (Movies S1 and S2, Supplemental Data). In control embryos, the GFP-AIR-2 signal dissipated after cleavage furrow ingression at ~12.5 minutes post pronuclear meeting. However, in all cdc-48.3(RNAi) embryos examined, a robust GFP-AIR-2 signal was present at the spindle midbody following cleavage furrow ingression and persisted into the next mitotic cycle.
CDC-48.3 directly binds and inhibits AIR-2 kinase activity
Cdc48 directly interacts with target proteins to extricate them from protein complexes and cellular structures, as well as for delivery of targets to the 26S proteasome (Ye, 2006). To determine whether AIR-2 and CDC-48.3 physically associate, AIR-2 was immunoprecipitated from extracts made from transgenic animals expressing a GFP-CDC-48.3 fusion protein. This tagged line was used since attempts at creating CDC-48.3 antibodies have failed (T.R.H. unpublished). GFP-CDC-48.3 is present throughout the cytoplasm in small puncta and is greatly reduced upon cdc-48.3(RNAi) (Figure S2D). GFP-CDC-48.3 is present in AIR-2 immunocomplexes isolated from control RNAi treated animals, but not from air-2(RNAi) or cdc-48.3(RNAi) treated animals (Figure 3A). To determine whether AIR-2 and CDC-48.3 directly interact, in vitro binding assays were conducted (Figure 3B). This analysis revealed that AIR-2 readily interacts with full-length CDC-48.3 but not with CDC-48.1 or glutathione beads.
Figure 3. CDC-48.3 directly interacts with and inhibits AIR-2 kinase activity in an ATPase dependent manner.
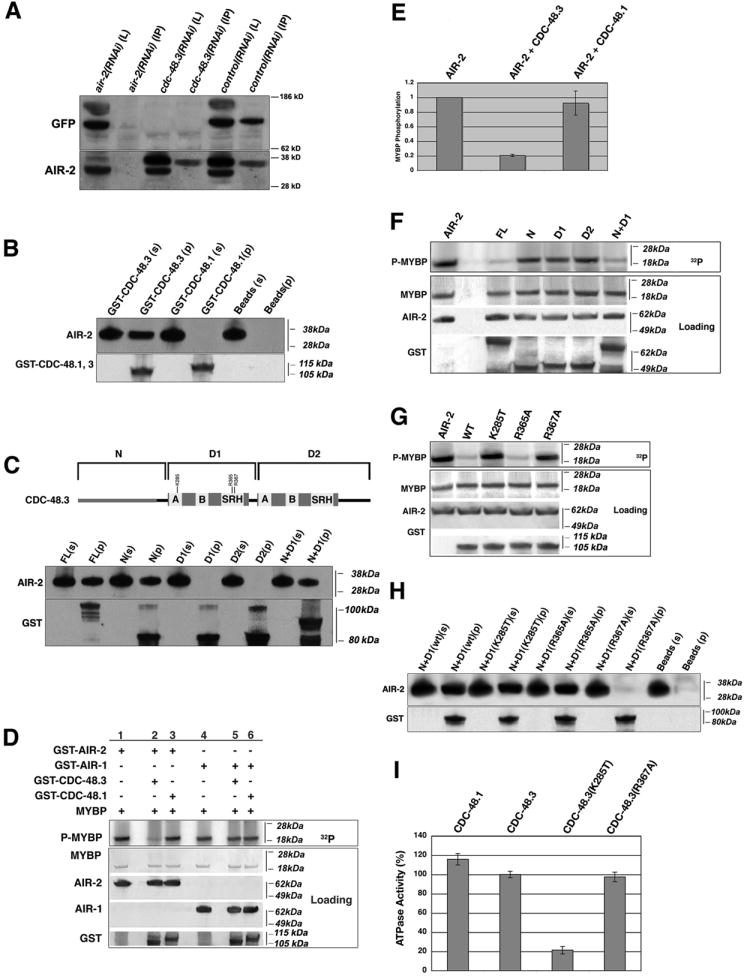
A) AIR-2 was immunoprecipitated from protein extracts of control, cdc-48.3, and air-2(RNAi) treated GFP-CDC-48.3 transgenic animals, and subjected to western blotting with AIR-2 (bottom panel) and GFP specific (top panel) antibodies. L: 10% of protein extract loaded in each IP. IP: immunoprecipitated pellet. B) Recombinant AIR-2 was mixed with glutathione beads incubated with buffer, GST-CDC-48.3, or GST-CDC-48.1. The beads were pelleted, washed and subjected to western analysis with AIR-2 and GST antibodies. s=25% of supernatant left after pelleting beads, p=washed glutathione bead pellet. C) Schematic illustrating CDC-48.3 protein fragments used in binding and kinase assays (Figure 3C, 3F, 3G); Recombinant AIR-2 was mixed with full-length GST-CDC-48.3 or the depicted GST-tagged fragments bound to glutathione beads, and treated as described in (A). D) GST-AIR-2 or GST-AIR-1 were mixed with either GST-CDC-48.3 (lanes 2 and 5) or GST-CDC-48.1 (lanes 3 and 6) in kinase buffer supplemented with [γ32P]-ATP and MYBP as an exogenous substrate (lanes 1-6). [γ32P]-ATP incorporation was visualized by phosphoimaging, MYBP loading by Ponceau S staining, and AIR-2, AIR-1, GST-CDC-48.1, and GST-CDC-48.3 loading by western analysis with AIR-2, AIR-1, and GST antibodies respectively. E) Quantitation of 32P incorporation into MYBP. Error bars represent standard deviation from three independent experiments. MYBP phosphorylation by AIR-2 did not change in the presence of GST-CDC-48.1 (p = 0.43). 32P incorporation into MYBP in the presence of AIR-2 was normalized to 1. F) GST-AIR-2 was mixed with full-length GST-CDC-48.3 or the GST-tagged fragments depicted in (C) in kinase buffer with MYBP as an exogenous substrate. Proteins were visualized as in (D). G) GST-CDC-48.3 (N+D1) mutants proteins were assayed for effects on AIR-2 kinase activity. H) Recombinant AIR-2 was mixed with GST-CDC-48.3 (N + D1) mutant proteins bound to glutathione beads, and treated as described in (A). I) GST-CDC-48.1 and wt or mutant GST-CDC-48.3 proteins were assayed for ATPase activity. Error bars represent standard deviation from three independent experiments.
Structural studies have determined that Cdc48 forms a barrel-like hexamer with a substrate/cofactor binding N-domain “lid” followed by two AAA domains (D1 and D2) which form two stacked rings that provide the ATPase activity necessary to drive Cdc48 functions (Rouiller et al., 2002). Having established a direct physical interaction between CDC-48.3 and AIR-2, we determined which CDC-48.3 domain(s) are required. Incubation of recombinant AIR-2 with GST-CDC-48.3 fragments corresponding to individual domains revealed that the N-terminal substrate-binding domain is sufficient for interaction with AIR-2 (Figure 3C).
Since CDC-48.3 and AIR-2 directly interact in vitro, we tested whether AIR-2 kinase activity is affected by the presence of CDC-48.3. AIR-2 kinase activity was strongly inhibited by addition of CDC-48.3 but not CDC-48.1 (Figure 3D, 3E). Importantly, neither protein inhibited the highly related Aurora A kinase AIR-1 (Schumacher et al., 1998a), suggesting that the inhibition of AIR-2 kinase activity is specific (Figure 3D). Interestingly, the CDC-48.3 N-terminal domain was not sufficient for AIR-2 inhibition. Instead, both the CDC-48.3 N-terminus and the D1 AAA ATPase domain are necessary for a marked reduction in AIR-2 kinase activity (Figure 3F).
To identify residues within the CDC-48.3 N+D1 fragment that are required for AIR-2 inhibition, site-directed mutations were created at conserved residues in the D1 AAA domain. Conserved lysine and arginine residues within the AAA Walker A motif mediate ATP binding, while ATP hydrolysis is dependent on a conserved DEXX sequence in the Walker B motif (Pye et al., 2006; Song et al., 2003). In addition, conserved arginine residues in the SRH domain promote communication between the Cdc48 hexamer subunits (Hishida et al., 2004; Wang et al., 2005). Recombinant GST-CDC-48.3 (N+D1) mutant proteins were assayed for effects on AIR-2 kinase activity. AIR-2 inhibition required lysine 285 (K285) of the D1 Walker A domain and arginine 367 (R367), but not R365, of the SRH domain (Figure 3G). Binding assays with these same mutants revealed that R367 is also required for AIR-2 binding, whereas the K285 mutant protein still binds, but cannot inhibit AIR-2 (Figure 3H, 3G).
To determine whether K285T and R367A affect CDC-48.3 ATPase activity, the mutations were made in the full-length CDC-48.3 protein and assayed for in vitro activity. Wt CDC-48.3 had measurable activity, and was similar to that of CDC-48.1 (Figure 3I). Interestingly, the K285T mutant reduced CDC-48.3 ATPase activity by 80%, whereas the R367A mutation had no effect. Altogether, these results suggest that residues in the SRH domain (R367) can affect the conformation of the N-terminal substrate binding domain, leading to a loss of AIR-2 binding and inhibition, while the Walker A mutation K285T does not affect binding, but is required for CDC-48.3 ATPase activity and AIR-2 inhibition. Importantly, the ATPase activity of the R367A mutant and the ability of the K285T mutant to bind AIR-2 suggest that these mutations do not cause gross defects in CDC-48.3 folding. In sum, inhibition of the AIR-2 kinase is dependent on a direct physical interaction between AIR-2 and the CDC-48.3 N-terminus as well as CDC-48.3 ATPase activity.
CDC-48.3 inhibits AIR-2 kinase activity in vivo
To determine whether CDC-48.3 regulates AIR-2 activity in vivo, the phosphorylation and activation state of AIR-2 was monitored in control and cdc-48.3(RNAi) treated air-2(or207ts) embryos using a commercial phospho-specific pAurora antibody (pAur) that recognizes Aurora A and B autophosphorylation and kinase activation (Ohashi et al., 2006; Yasui et al., 2004). Immunostaining revealed strong AIR-1 dependent mitotic centrosome staining (pAIR-1) and an AIR-2 dependent chromosomal passenger complex staining pattern (pAIR-2 CPC)(Figure 4A and data not shown). In both control and cdc-48.3(RNAi) treated air-2(or207ts) embryos (reared at 22°C), similar levels of pAIR-2 CPC staining were present on condensing chromosomes from early prophase to prometaphase (Figure 4A). However, from metaphase through late telophase, there were increased levels of pAIR-2 CPC staining in cdc-48.3(RNAi) embryos as compared to controls (Figure 4A, 4B). The same trend was found for pAUR levels throughout the entire embryo (Figure 4C), and for pAIR-2 CPC immunostaining in embryos reared at temperatures ranging from 15°-22°C (Figure 4D). As pAIR-2 levels drop in control air-2(or207ts) embryos with increasing temperature, cdc-48.3(RNAi) embryos maintain pAIR-2 levels that exceed or are comparable to those in wt embryos reared at 25°C (white bars, Figure 4D) or air-2(or207ts) embryos reared at 15°C (Figure 4D). A similar increase in pAIR-2 levels was found in wt embryos treated with control and cdc-48.3(RNAi), indicating that the kinase activity of wt AIR-2 is also subject to CDC-48.3 regulation (Figure S3).
Figure 4. pAIR-2 levels are upregulated from metaphase through telophase in cdc-48.3(RNAi);air-2(or207ts) embryos.
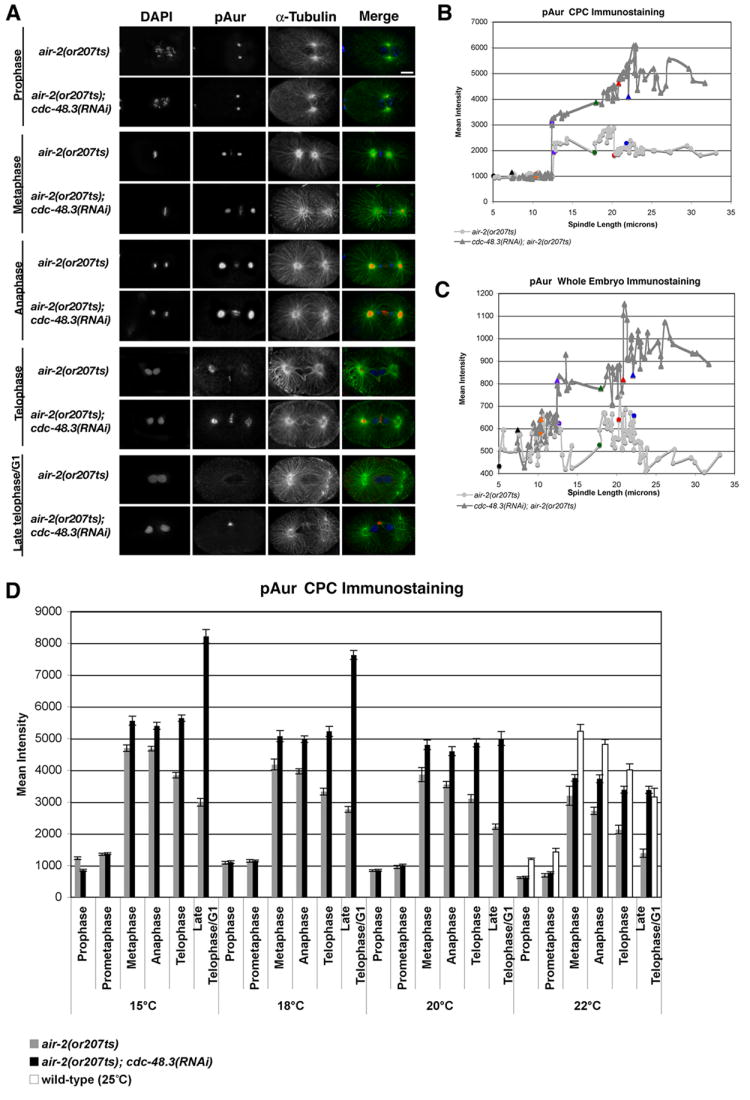
A) Embryos dissected from control and cdc-48.3(RNAi) treated air-2(or207ts) hermaphrodites reared at 22°C were fixed and stained with DAPI (blue), and α-tubulin (green) and pAurora (pAur) (red) antibodies. One-cell embryos at different mitotic stages are shown. Centrosome pAur staining reflects phosphorylated AIR-1. Scale bar = 10 μm. B) Mitotic chromosomal passenger (CPC) localized pAur immunostaining was measured in control (n=89) and cdc-48.3(RNAi) (n=92) treated one-cell air-2(or207ts) embryos (22°C) and plotted against spindle length. C) pAur immunostaining throughout the entire embryo was measured in control (n=89) and cdc-48.3(RNAi) (n=92) treated one-cell air-2(or207ts) embryos (22°C) and plotted against spindle length. D) CPC localized pAur immunostaining was measured in control (n=267) (gray bars) and cdc-48.3(RNAi) (n=269) (black bars) treated one-cell air-2(or207ts) embryos reared at the indicated temperatures. CPC pAur immunostaining measured in wt embryos reared at 25°C is included as a control (white bars). Spindle length and morphology were used to delineate the stages of mitosis.
To confirm these results, the phosphorylation of ICP-1, a substrate and potent activator of the AIR-2 kinase (Bishop and Schumacher, 2002), was monitored by immunostaining wt and air-2(or207ts) embryos treated with control and cdc-48.3(RNAi) (22°C) with a phospho-specific antibody (pICP-1) that recognizes the AIR-2 phosphorylation site (Burrows et al., 2006). In all conditions, pICP-1 localized to chromosomes in early mitosis, and to the spindle midzone and midbody in late mitosis (Figure S4A and data not shown). Centrosome and p-granule pICP-1 staining was not abolished by icp-1 or air-2(RNAi) and thus was not specific (Figure S4B). In both control and cdc-48.3(RNAi) embryos, pICP-1 faintly stained condensing chromosomes from early prophase to prometaphase (Figure S4A). However, as above, from metaphase through late telophase, there were increased levels of pICP-1 staining on chromosomes and spindle midzone/midbody microtubules in cdc-48.3(RNAi) embryos as compared to controls (Figure S4A, S4C, S4D). A similar trend was found when pICP-1 levels were measured throughout the entire embryo (Figure S4E, S4F).
In sum, these findings reveal that in the absence of CDC-48.3, AIR-2 kinase activity is upregulated in C. elegans embryos from metaphase through late telophase/G1. Importantly, this increase in AIR-2 kinase activity does not correlate with the stabilization of AIR-2 in late mitosis (Figure 2), suggesting that CDC-48.3 may inhibit AIR-2 kinase activity and protein levels via distinct mechanisms.
CDC-48.3 depleted embryos display prolonged cell division and mitotic defects
Live imaging of GFP-AIR-2 transgenic animals revealed significant delays in chromosome alignment, anaphase onset and cleavage furrow formation in cdc-48.3(RNAi) embryos (Movies S1 and S2; Figure S5A), consistent with the slow growth phenotype of cdc-48.3(RNAi) embryos (Table S1). Imaging of control and cdc-48.3(RNAi) one-cell embryos from a GFP-α-tubulin; mCherry-Histone H2B transgenic line (OD57) confirmed these mitotic delays (Figure 5; Movies S3 and S4). Since these experiments and the suppression assays were performed by the feeding method of RNAi (Timmons and Fire, 1998) which can often be less robust than microinjection of dsRNA, cdc-48.3 dsRNA was directly injected into the gonads of wt, air-2(or207ts), and OD57 transgenic L4 hermaphrodites. Unlike cdc-48.3(RNAi) feeding, cdc-48.3 dsRNA microinjection resulted in 70-75% embryonic lethality and did not suppress the 95-100% lethality of air-2(or207ts) embryos at 22°C (Figure 5C). Live imaging of the F1 progeny of cdc-48.3 dsRNA injected OD57 animals revealed a variety of mitotic defects including failures in mitotic spindle formation, multi-polar spindles, chromosome segregation errors, and significant delays (Movies S5-S7; Figure 5D, 5E). Similar results were found in immunostained embryos from cdc-48.3 dsRNA injected mothers (Figure S5B and Table 2). Altogether, these results suggest that a partial loss of CDC-48.3 is necessary and sufficient to suppress air-2(or207ts) lethality, but that a minimum amount of CDC-48.3 is required to maintain timely and accurate cell division.
Figure 5. cdc-48.3(RNAi) leads to mitotic delays and spindle defects.

A) Embryos from OD57 (mCherry∷Histone H2B; GFP∷α-tubulin) L4 hermaphrodites fed control and cdc-48.3(RNAi) were subjected to live imaging. Time 0 (panels c and b’) corresponds to metaphase chromosome alignment. a, a’) pronuclear meeting (-390 and -450 seconds respectively from metaphase), b) prophase, b’) metaphase chromosome alignment, c) metaphase chromosome alignment and completion of spindle rotation, c’) completion of spindle rotation, d, d’) anaphase, e, e’) telophase, f, f’) metaphase of AB cell division. Scale bar=10 μm. B) Graph of mitotic progression in control (n=4) and cdc-48.3(RNAi) (n=4) treated OD57 embryos undergoing the first mitotic division. AB Metaphase: metaphase of the AB cell (two-cell embryo). C) Wt and air-2(or207ts) L4 hermaphrodites were subjected to mock or cdc-48.3 dsRNA microinjection, and reared at 15°C or 22°C. Embryonic viability was scored 48 hours post-injection. Wt control 15°C, n=992, 22°C, n=1002; wt cdc-48.3(RNAi) 15°C, n=1123, 22°C, n=1099; air-2(or207ts) control 15°C, n=1055, 22°C, n=1048; air-2(or207ts);cdc-48.3(RNAi) 15°C, n=1068, 22°C, n=1063. D) Live imaging of embryos from OD57 hermaphrodites subjected to mock or cdc-48.3 dsRNA microinjection was performed (control, n=3 embryos; cdc-48.3(RNAi), n=7 embryos). Time 0 (c and c’): metaphase chromosome alignment. a, a’) pronuclear meeting (-330 and -630 seconds respectively from metaphase), b) prophase, b’) metaphase chromosome alignment, c) metaphase chromosome alignment and completion of spindle rotation, c’) completion of spindle rotation, d, d’) anaphase, e, e’) telophase, f, f’) metaphase of AB cell division. Scale bar=10 μm. E) Graph of mitotic progression in one-cell embryos from control (n=3) and cdc-48.3 dsRNA (n=5) injected OD57 hermaphrodites. AB Metaphase: metaphase of the AB cell (two-cell embryo).
Table 2.
Microinjection of cdc-48.3 dsRNA leads to mitotic defects
| Stage* | >2 Centrosomes | Unattached chromosomes | Chromosome congression defects | Chromosome segregation defects |
|---|---|---|---|---|
| Prophase | ||||
|
control(RNAi)
(n=11) |
0.00% | 0.00% | 0.00% | N/A |
|
cdc-48.3(RNAi)
(n=13) |
15.38% | 7.69% | 30.77% | N/A |
| Prometaphase | ||||
|
control(RNAi)
(n=14) |
0.00% | 0.00% | 0.00% | N/A |
|
cdc-48.3(RNAi)
(n=10) |
20.00% | 10.00% | 30.00% | N/A |
| Metaphase | ||||
|
control(RNAi)
(n=7) |
0.00% | 0.00% | 0.00% | N/A |
|
cdc-48.3(RNAi)
(n=7) |
14.29% | 28.57% | 57.14% | N/A |
| Anaphase | ||||
|
control(RNAi)
(n=26) |
0.00% | 0.00% | N/A | 0.00% |
|
cdc-48.3(RNAi)
(n=22) |
22.73% | 9.09% | N/A | 45.45% |
| Telophase | ||||
|
control(RNAi)
(n=16) |
0.00% | 0.00% | N/A | 0.00% |
|
cdc-48.3(RNAi)
(n=17) |
11.76% | 17.65% | N/A | 41.18% |
| Late Telophase/G1 | ||||
|
control(RNAi)
(n=24) |
0.00% | 0.00% | N/A | 0.00% |
|
cdc-48.3(RNAi)
(n=23) |
26.09% | 17.39% | N/A | 56.52% |
Embryos from mock and cdc-48.3 dsRNA microinjected L4 hermaphrodites were fixed and stained with DAPI and α-tubulin antibodies. Mitotic defects in one-cell embryos at various mitotic stages were tabulated. Similar defects were also present in older embryos.
DISCUSSION
Here, we report that C. elegans CDC-48.3, an Afg2/Spaf-related AAA ATPase, regulates the stability, activity, and localization of the Aurora B kinase AIR-2 during embryonic development. Partial depletion of CDC-48.3 rescues the lethality of an air-2(or207ts) mutant, restoring both AIR-2 localization and chromosome segregation to wt patterns. CDC-48.3 appears to regulate AIR-2 via two potentially distinct mechanisms, 1) the regulation of AIR-2 stability at mitotic exit, and 2) direct inhibition of AIR-2 kinase activity from metaphase through late telophase, which requires CDC-48.3 binding and ATPase activity. Inappropriately high levels of AIR-2 activity are likely to contribute to the mitotic delays that are apparent in both partially and more fully depleted cdc-48.3(RNAi) embryos. Hence, one function of the highly conserved Afg2/Spaf family of AAA ATPases is the inhibition of Aurora B kinase activity and stability, which contributes to chromosome segregation and mitotic progression.
CDC-48.3 binds to and inhibits the AIR-2 kinase
AIR-2 physically associates with CDC-48.3, and directly binds the N-terminus in vitro, consistent with studies that have identified this region as the substrate/cofactor binding domain of Cdc48 ATPases (Dai and Li, 2001; Ogura and Wilkinson, 2001; Rouiller et al., 2002). CDC-48.3 inhibits AIR-2 kinase activity in vivo, and the N-terminus and D1 domain are necessary and sufficient for inhibition in vitro. Within the SRH motif of D1, arginine 367 (R367) is highly conserved, and is required for the binding and inhibition of AIR-2. R367 lies within the predicted arginine finger motif, and a recent study revealed that the corresponding residue in p97, R362, is required for binding poly-ubiquitinated substrates (Wang et al., 2005). The authors suggested that this mutation results in a conformational change that alters substrate binding by the N domain. Our findings are consistent with this model, suggesting that this residue is also functionally required in Afg2/Spaf family members. CDC-48.3 K285 is also highly conserved and required for inhibition of AIR-2 kinase activity. The corresponding p97 Walker A residue K524 is essential for ATPase activity (Wang et al., 2005), as is CDC-48.3 K285. Given this, and that catalytically inactive CDC-48.3 K285T retains AIR-2 binding, but does not affect kinase activity, we conclude that CDC-48.3 ATPase activity is required for AIR-2 inhibition.
CDC-48.3 regulates AIR-2 behavior during mitosis
cdc-48.3(RNAi) restores the characteristic chromosomal passenger protein localization pattern to the AIR-2ts protein at a restrictive temperature (22°C), and suppresses the chromosome segregation and cytokinesis defects to the point of viability. AIR-2 kinase activity is significantly upregulated in these embryos at the same temperature. Notably, cdc-48.3(RNAi) had no apparent affect on the AIR-2ts localization pattern, mitotic defects, or lethality of air-2(or207ts) embryos at a higher temperature (25°C). This is likely due to severe defects in AIR-2 activity at this temperature that cannot be overcome by loss of CDC-48.3 inhibition.
Cdc48/p97 AAA ATPases and the Chromosomal Passenger Complex
Two reports have presented drastically different roles for canonical p97/Cdc48 AAA ATPases in the regulation of Aurora B and the chromosomal passenger complex. One found that p97 is required for the localization of Survivin and Aurora B to mitotic chromosomes (Vong et al., 2005), while the second found that p97 and its orthologs in C. elegans are essential for the removal of Aurora B from mitotic chromosomes, subsequent chromosome decondensation, and nuclear envelope assembly (Ramadan et al., 2007). Importantly, they also reported that loss of either C. elegans CDC-48.1 or CDC-48.2 could suppress air-2(or207ts) lethality (Ramadan et al., 2007). In contrast, we found no evidence that depletion of CDC-48.1, CDC-48.2, or any of their predicted cofactors could suppress air-2(or207ts) lethality, even when using identical RNAi protocols and constructs (Ramadan et al., 2007). In addition, we found no changes in AIR-2 localization or activity in embryos depleted of CDC-48.1 and CDC-48.2 singly or together (via mixed feeding or microinjection of dsRNAs)(T.R.H., unpublished observations). Although these differences are striking, they reveal that our cdc-48.3(RNAi) observations are not likely to be due to unintended effects on CDC-48.1 or CDC-48.2 expression. A detailed analysis of AIR-2 activity and functionality vis-à-vis CDC-48.1 and CDC-48.2 will be presented elsewhere (T.R.H. and J.M.S., manuscript in preparation).
We have discovered that a member of the Afg2/Spaf branch of the Cdc48 family is an inhibitor of the Aurora B kinase in vitro and in vivo. However, our findings differ considerably from the reported mode of p97-dependent inhibition (Ramadan et al., 2007). Our in vitro studies revealed that CDC-48.3 binds directly to and inhibits recombinant AIR-2 in the absence of ubiquitination. We have failed to detect AIR-2 ubiquitination in extracts or by immunostaining; hence whether ubiquitination is involved in CDC-48.3-dependent regulation of AIR-2 in vivo is not clear. Nevertheless, depletion of CDC-48.3 does not affect the localization of wt AIR-2 (or any of the other CPC proteins (T.R.H., unpublished observations)), at any stage of the cell cycle and does not appear to affect nuclear envelope reformation. Hence, CDC-48.3 is not required to localize or extract wt AIR-2 from chromosomes, and thus appears to be functioning in a pathway that is independent of canonical Cdc48.
Very little is known about the specific functions of the Afg2/Spaf subfamily of AAA ATPases. Yeast Afg2 is required for the release of ribosomal proteins from nucleolar shuttling proteins, and no functional assays have been reported for mammalian Spaf (Liu et al., 2000; Pertschy et al., 2007). Here, we conclude that the C. elegans member of this family, CDC-48.3, is essential for accurate and timely progression through mitosis. In addition to or perhaps tied to its role in the regulation of AIR-2 activity and stability, CDC-48.3 clearly affects centrosome duplication, spindle assembly, and cell cycle progression. The identification of additional targets of CDC-48.3 and whether the regulation of Aurora B/Ipl1 is a conserved function of Afg2/Spaf AAA ATPase family members in other organisms are important questions for the future.
EXPERIMENTAL PROCEDURES
C. elegans strains
C. elegans strains were maintained at 15°C as described previously (Brenner, 1974; Han et al., 2005). The following strains were used: N2 (wt), EU630 [air-2(or207ts) I] (Severson et al., 2000), EU828 [dhc-1(or195) I] (O’Rourke et al., 2007), EU923 [spn-4(or191ts) IV] (Gomes et al., 2001), EU603 [lit-1(or131ts) III; him-8(e1469) V] (Meneghini et al., 1999), WH371 [unc-119(ed3) ojIs50 [Ppie-1∷GFP∷air-2 unc-119 (+)], JS803 [unc-119(ed3) vw346 [Ppie-1:LAP∷CDC-48.3 unc-119 (+)], OD57 (mCherry∷Histone H2B; GFP∷α-tubulin) (McNally et al., 2006).
To create the WH371 (GFP-AIR-2) and JS803 (LAP/GFP-CDC-48.3) transgenic lines, the full-length AIR-2 and CDC-48.3 cDNA were PCR amplified, sequenced, and subcloned into different vectors. AIR-2 was cloned into the Gateway donor plasmid pDONR201 (Invitrogen, Carlsbad, CA) and then recombined with the pID3.01B destination vector (a gift from G. Seydoux, Johns Hopkins School of Medicine (Stitzel et al., 2006)) to create an in-frame N-terminal GFP fusion protein. CDC-48.3 was cloned into the pIC113 plasmid (a gift from I. Cheeseman, Ludwig Institute, San Deigo (Cheeseman and Desai, 2005)) to create a LAP (GFP-TEV-S-tag)-CDC-48.3 fusion protein. Both transgenes are regulated by the PIE-1 promoter and were introduced into unc-119(ed3) animals by microparticle bombardment (Praitis et al., 2001).
RNAi Screen
Individual clones of the C. elegans RNAi feeding library (MRC Geneservice, Cambridge, UK) (Kamath and Ahringer, 2003) were grown to log phase and then spotted onto NG media plus 50 μg/μl ampicillin and 1 mM IPTG in 24-well dishes. Each well was seeded with 5-10 air-2(or207ts) hypochlorite-synchronized L2 larvae using a multichannel pipette, and incubated at 15°C for 24 hours. Plates were then incubated at 22°C for 3-4 days, and wells assayed for embryo hatching on day 5. Suppressing RNAi constructs uncovered in the initial screen were retested as above except using 60 mm plates at 20°C and 22°C. The identity of each suppressing RNAi construct was confirmed by DNA sequencing.
RNA-Mediated Interference (RNAi)
The feeding method of RNAi delivery was used to inhibit expression of AIR-2, CDC-48.3, ICP-1, CDC-48.1, CDC-48.2 and other candidate proteins identified from the RNAi screen unless otherwise indicated (Timmons, 2006). The entire coding regions of AIR-2, CDC-48.3 and ICP-1 were used as templates for RNAi as previously described (Han et al. 2005). The L4440 RNAi vector was used as an RNAi control. For cdc-48.1 and cdc-48.2(RNAi) suppression assays, L1 larvae were seeded onto nematode growth (NG) plates supplemented with 50 μg/μl ampicillin and 3 mM IPTG (Ramadan et al., 2007). For cdc-48.1/cdc-48.2 double RNAi and cdc-48.3 injected RNAi, sense and antisense mRNAs corresponding to the entire coding regions of each gene were transcribed from linearized plasmid templates using a T7 in vitro transcription kit (Ambion, Austin, TX) and annealed at room temperature overnight. cdc-48.3 dsRNA was singly injected, and cdc-48.1 and cdc-48.2 dsRNAs were co-injected into the gonads of L4 larvae. Injected animals were incubated at 15°C for 2-4 hrs prior to shifting to 20°C and 22°C overnight.
Immunostaining
Immunostaining experiments were performed using RNAi-treated N2 and air-2(or207ts) gravid hermaphrodites (L4 + 24 hours) reared at 20°C unless otherwise indicated. Control and cdc-48.3(RNAi) treated LAP/GFP-CDC-48.3 animals were reared at 25°C. Embryo fixation and antibody application were performed as previously described (Seydoux and Dunn, 1997). Primary antibodies: anti-AIR-2 (Schumacher et al., 1998b), anti-ICP-1 and anti-phospho-ICP-1 (Burrows et al., 2006), anti-phospho-Aurora (pAur) (Cell Signaling, Danvers, MA), monoclonal anti-α-tubulin (Sigma, St. Louis, MO), and monoclonal anti-GFP (Covance, Princeton, NJ). Secondary antibodies: Alexa Fluor® 488 goat anti-mouse IgG and rhodamine-conjugated goat anti-rabbit IgG (Invitrogen Molecular Probes, Eugene, OR).
Live Imaging
Embryos from control and cdc-48.3(RNAi) treated (by feeding or direct dsRNA microinjection) OD57 and WH371 strains were mounted on agarose pads and imaged using a spinning disk confocal (Perkin Elmer, Waltham, MA) attached to Nikon TE2000U inverted microscope. Images were acquired using an ORCA-ER digital camera (Hamamatsu, Bridgewater, NJ) and a 60x 1.2 NA Plan Apo VC lens. The confocal, microscope, and camera were controlled by Ultraview software (Perkin Elmer).
Image Analysis/Immunoquantitation
Immunofluorescent images were acquired on a Nikon 2000U inverted microscope equipped with a Photometrics Coolsnap HQ camera. All functions were controlled through Metamorph software. For all embryos, 26 z-sections were acquired at 0.2-μm steps using a 60x/1.45NA objective. Z stacks were projected and imported into Autodeblur (Autoquant Media Cybernetics, Bethesda MD) and deconvolved for 60 iterations. Deconvolved images were then imported into Imaris x64 software (Bitplane, St. Paul, MN) for quantitation and spindle measurements. For quantitation, 3D isosurfaces were generated based on minimum threshold values within the experimental set, and corresponding mean voxel intensity values were collected for each embryo within the data set. All images were captured using identical exposure times within each experimental set, and all processing steps were identical. Figures were prepared using Adobe Photoshop CS3 (Adobe Systems Inc., San Jose, CA).
Recombinant Protein Production
GST-CDC-48.1 and GST-CDC-48.3 (full-length (FL), amino acids 1-724; N: 1-225; D1: 236-441; D2: 463-724; N+D1: 1-441) were created by PCR amplifying the CDC-48.1 (C06A1.1) and CDC-48.3 (K04G2.3) cDNAs using primers with appropriate restriction enzyme sites for in-frame fusion with the GST moiety of pGEX-6P-1 (Amersham-Pharmacia, Piscataway, NJ). Point mutations in GST-CDC-48.3 (FL and N+D1) were introduced by PCR-based site-directed mutagenesis (Stratagene). All constructs were confirmed by DNA sequencing. Construction of GST-AIR-2 and GST-AIR-1 has been described previously (Bishop and Schumacher, 2002; Rogers et al., 2002). Recombinant GST proteins were expressed in E. coli strain BL21 (DE3) pLys S by 24 hr induction with 1mM IPTG. Proteins were then purified and eluted using previously described procedures (Han et al., 2003).
Kinase Assays and Quantitation
For AIR-2 kinase assays, GST-AIR-2 was mixed with GST-CDC-48.3 or GST-CDC-48.1 in kinase buffer (20mM HEPES, 1 mM DTT, 25 mM β-glycerophosphate, 7.5 mM magnesium chloride, 10 mM ATP, 30 μci [32P]-γ-ATP) supplemented with myelin basic protein (MYBP) (Sigma) for 15 min at room temperature. Reactions were separated by SDS-PAGE, transferred to nitrocellulose, and [32P]-γ-ATP incorporation was determined by phosphoimaging. Protein loading was visualized by Ponceau-S staining (Sigma) or by probing with GST- (Bishop et al., 2005), AIR-1-, or AIR-2-specific antibodies (Schumacher et al., 1998a, 1998b).
KodakID 3.1 quantification software (Eastman Kodak, Rochester, NY) was used to measure protein loading and [32P] incorporation. Phosphorylation of MYBP by AIR-2 or AIR-1 kinases in the presence or absence of CDC-48.1 or CDC-48.3 was calculated as ([32P] incorporation into MYBP/MYBP loading/kinase loading)(average CDC-48 loading/CDC-48 loading in lane) whereas AIR-2 or AIR-1 autophosphorylation in the presence or absence of CDC-48.1 or CDC-48.3 was calculated as ([32P] incorporation into kinase/kinase loading)(average CDC-48 loading/CDC-48 loading in lane).
Immunoprecipitation, Western Analysis, and Binding Assays
Embryos were collected from C. elegans hermaphrodites (N2, air-2(or207ts), and LAP/GFP-CDC-48.3) treated with control, air-2, or cdc-48.3(RNAi) and reared at 22°C (unless otherwise indicated) as described previously (Schumacher et al., 1998b). Embryos were washed and resuspended in lysis buffer (1X PBS, 20 mM HEPES, 1% NP-40, 50 μM β-glycerophosphate, 1 mM Na3VO4, 1 mM dithiothreitol [DTT], 1 mM EDTA + complete protease inhibitors (Roche Diagnostics, Indianapolis, IN)) and sonicated over ice. Following centrifugation, clarified lysates were frozen in liquid nitrogen and stored at -80°C. Protein concentration was determined by Bradford assay (Bio-Rad, Hercules, CA).
For immunoprecipitations, 400 μg embryo extract was incubated with 5 μl affinity-purified AIR-2 antibody (Schumacher et al., 1998b) for 3 hr at 4°C. 20 μl protein G-Sepharose beads (Amersham Biosciences) were added and the extract incubated at 4°C for an additional hour. The beads were pelleted by low-speed centrifugation and washed three times in lysis buffer-minus NP-40. Samples were separated by SDS/PAGE, transferred to nitrocellulose, and the membranes probed with AIR-2 and GFP-specific antibodies. Western analysis was performed as previously described (Schumacher et al., 1998b).
For the in vitro binding assays, 400 mM GST-AIR-2 was treated with Prescission Protease (Amersham-Pharmacia) to remove the GST tag. The cleaved AIR-2 protein was then mixed with GST-CDC-48.3 or GST-CDC-48.1 bound to glutathione beads and rocked over ice for 3 hr. Beads were washed by rocking in PBS+20 mM HEPES, 0.2% Triton-X-100 at 4°C for 5 min and pelleted. Samples were separated by SDS-PAGE, transferred to nitrocellulose, and the membranes probed with GST- and AIR-2-specific antibodies.
ATPase Assays
To perform in vitro ATPase assays, 0.5 μM GST-CDC-48.3, GST-CDC-48.1 and various GST-CDC-48.3 mutant proteins were mixed with ATP and 100 μ assay buffer (Innova Biosciences, Cambridge, UK) + 20 mM MgCl2, and incubated at 37°C for 15 minutes. Absorbance at 630 nm was measured using a spectrophotometer as described by the manufacturer. Activity in control reactions without ATP was subtracted from experimental reactions. Enzyme activity was calculated based on a standard curve generated from adding increasing amounts of inorganic phosphate (Pi) to the assays. Relative ATPase activity was calculated from three independent experiments.
Supplementary Material
Acknowledgments
We thank present and former members of the Schumacher Lab for guidance, King Foundation Summer students M. Post, M. Goldberg, D. Banerjee, and K. Keith for assisting with the RNAi screen, R. Haynes for media preparation, R. Behringer for providing the spinning disk confocal, and S. O’Rourke, J. Audya, K. Oegema, I. Cheeseman, and G. Seydoux for strains and reagents. DNA sequencing was performed at the M.D. Anderson Cancer Center sequencing core (supported by NCI grant CA-16672).Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources (NCRR). This research was supported by NIH R01 GM62181 (J.M.S) and the George and Barbara Bush Foundation for Innovative Cancer Research (J.M.S). T.R.H. was supported by Training Programs in Biochemistry & Molecular Biology (T32 HD007325 21) and Molecular Genetics of Cancer (T32 CA009299 29). The authors have no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrews PD, Knatko E, Moore WJ, Swedlow JR. Mitotic mechanics: the auroras come into view. Curr Opin Cell Biol. 2003;15:672–683. doi: 10.1016/j.ceb.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Bishop JD, Schumacher JM. Phosphorylation of the carboxyl terminus of inner centromere protein (INCENP) by the Aurora B Kinase stimulates Aurora B kinase activity. J Biol Chem. 2002;277:27577–27580. doi: 10.1074/jbc.C200307200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows AE, Sceurman BK, Kosinski ME, Richie CT, Sadler PL, Schumacher JM, Golden A. The C. elegans Myt1 ortholog is required for the proper timing of oocyte maturation. Development. 2006;133:697–709. doi: 10.1242/dev.02241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao K, Nakajima R, Meyer HH, Zheng Y. The AAA-ATPase Cdc48/p97 regulates spindle disassembly at the end of mitosis. Cell. 2003;115:355–367. doi: 10.1016/s0092-8674(03)00815-8. [DOI] [PubMed] [Google Scholar]
- Cheeseman IM, Desai A. A combined approach for the localization and tandem affinity purification of protein complexes from metazoans. Sci STKE. 2005;2005:l1. doi: 10.1126/stke.2662005pl1. [DOI] [PubMed] [Google Scholar]
- Dai RM, Li CC. Valosin-containing protein is a multi-ubiquitin chain-targeting factor required in ubiquitin-proteasome degradation. Nat Cell Biol. 2001;3:740–744. doi: 10.1038/35087056. [DOI] [PubMed] [Google Scholar]
- Ducat D, Zheng Y. Aurora kinases in spindle assembly and chromosome segregation. Exp Cell Res. 2004;301:60–67. doi: 10.1016/j.yexcr.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Frohlich KU. An AAA family tree. J Cell Sci. 2001;114:1601–1602. doi: 10.1242/jcs.114.9.1601. [DOI] [PubMed] [Google Scholar]
- Gomes JE, Encalada SE, Swan KA, Shelton CA, Carter JC, Bowerman B. The maternal gene spn-4 encodes a predicted RRM protein required for mitotic spindle orientation and cell fate patterning in early C. elegans embryos. Development. 2001;128:4301–4314. doi: 10.1242/dev.128.21.4301. [DOI] [PubMed] [Google Scholar]
- Han Z, Riefler GM, Saam JR, Mango SE, Schumacher JM. The C. elegans Tousled-like kinase contributes to chromosome segregation as a substrate and regulator of the Aurora B kinase. Curr Biol. 2005;15:894–904. doi: 10.1016/j.cub.2005.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannak E, Kirkham M, Hyman AA, Oegema K. Aurora-A kinase is required for centrosome maturation in Caenorhabditis elegans. J Cell Biol. 2001;155:1109–1116. doi: 10.1083/jcb.200108051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetzer M, Meyer HH, Walther TC, Bilbao-Cortes D, Warren G, Mattaj IW. Distinct AAA-ATPase p97 complexes function in discrete steps of nuclear assembly. Nat Cell Biol. 2001;3:1086–1091. doi: 10.1038/ncb1201-1086. [DOI] [PubMed] [Google Scholar]
- Hishida T, Han YW, Fujimoto S, Iwasaki H, Shinagawa H. Direct evidence that a conserved arginine in RuvB AAA+ ATPase acts as an allosteric effector for the ATPase activity of the adjacent subunit in a hexamer. Proc Natl Acad Sci U S A. 2004;101:9573–9577. doi: 10.1073/pnas.0403584101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda R, Korner R, Nigg EA. Exploring the functional interactions between Aurora B, INCENP, and survivin in mitosis. Mol Biol Cell. 2003;14:3325–3341. doi: 10.1091/mbc.E02-11-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson RL, Pye VE, Simpson P, Meyer HH, Zhang X, Freemont PS, Matthews S. Detailed structural insights into the p97-Npl4-Ufd1 interface. J Biol Chem. 2007;282:21361–21369. doi: 10.1074/jbc.M610069200. [DOI] [PubMed] [Google Scholar]
- Jeyaprakash AA, Klein UR, Lindner D, Ebert J, Nigg EA, Conti E. Structure of a Survivin-Borealin-INCENP core complex reveals how chromosomal passengers travel together. Cell. 2007;131:271–285. doi: 10.1016/j.cell.2007.07.045. [DOI] [PubMed] [Google Scholar]
- Kamath RS, Ahringer J. Genome-wide RNAi screening in Caenorhabditis elegans. Methods. 2003;30:313–321. doi: 10.1016/s1046-2023(03)00050-1. [DOI] [PubMed] [Google Scholar]
- Kamath RS, Fraser AG, Dong Y, Poulin G, Durbin R, Gotta M, Kanapin A, Le Bot N, Moreno S, Sohrmann M, et al. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature. 2003;421:231–237. doi: 10.1038/nature01278. [DOI] [PubMed] [Google Scholar]
- Kelly AE, Sampath SC, Maniar TA, Woo EM, Chait BT, Funabiki H. Chromosomal enrichment and activation of the aurora B pathway are coupled to spatially regulate spindle assembly. Dev Cell. 2007;12:31–43. doi: 10.1016/j.devcel.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Black J, Kisiel N, Kulesz-Martin MF. SPAF, a new AAA-protein specific to early spermatogenesis and malignant conversion. Oncogene. 2000;19:1579–1588. doi: 10.1038/sj.onc.1203442. [DOI] [PubMed] [Google Scholar]
- McNally K, Audhya A, Oegema K, McNally FJ. Katanin controls mitotic and meiotic spindle length. J Cell Biol. 2006;175:881–891. doi: 10.1083/jcb.200608117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneghini MD, Ishitani T, Carter JC, Hisamoto N, Ninomiya-Tsuji J, Thorpe CJ, Hamill DR, Matsumoto K, Bowerman B. MAP kinase and Wnt pathways converge to downregulate an HMG-domain repressor in Caenorhabditis elegans. Nature. 1999;399:793–797. doi: 10.1038/21666. [DOI] [PubMed] [Google Scholar]
- Nguyen HG, Chinnappan D, Urano T, Ravid K. Mechanism of Aurora-B degradation and its dependency on intact KEN and A-boxes: identification of an aneuploidy-promoting property. Mol Cell Biol. 2005;25:4977–4992. doi: 10.1128/MCB.25.12.4977-4992.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norbury C, Nurse P. Cyclins and cell cycle control. Curr Biol. 1991;1:23–24. doi: 10.1016/0960-9822(91)90116-e. [DOI] [PubMed] [Google Scholar]
- O’Rourke SM, Dorfman MD, Carter JC, Bowerman B. Dynein modifiers in C. elegans: light chains suppress conditional heavy chain mutants. PLoS Genet. 2007;3:e128. doi: 10.1371/journal.pgen.0030128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura T, Wilkinson AJ. AAA+ superfamily ATPases: common structure--diverse function. Genes Cells. 2001;6:575–597. doi: 10.1046/j.1365-2443.2001.00447.x. [DOI] [PubMed] [Google Scholar]
- Ohashi S, Sakashita G, Ban R, Nagasawa M, Matsuzaki H, Murata Y, Taniguchi H, Shima H, Furukawa K, Urano T. Phospho-regulation of human protein kinase Aurora-A: analysis using anti-phospho-Thr288 monoclonal antibodies. Oncogene. 2006;25:7691–7702. doi: 10.1038/sj.onc.1209754. [DOI] [PubMed] [Google Scholar]
- Pertschy B, Saveanu C, Zisser G, Lebreton A, Tengg M, Jacquier A, Liebminger E, Nobis B, Kappel L, van der Klei I, et al. Cytoplasmic recycling of 60S preribosomal factors depends on the AAA protein Drg1. Mol Cell Biol. 2007;27:6581–6592. doi: 10.1128/MCB.00668-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poteryaev D, Squirrell JM, Campbell JM, White JG, Spang A. Involvement of the actin cytoskeleton and homotypic membrane fusion in ER dynamics in Caenorhabditis elegans. Mol Biol Cell. 2005;16:2139–2153. doi: 10.1091/mbc.E04-08-0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praitis V, Casey E, Collar D, Austin J. Creation of low-copy integrated transgenic lines in Caenorhabditis elegans. Genetics. 2001;157:1217–1226. doi: 10.1093/genetics/157.3.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pye VE, Dreveny I, Briggs LC, Sands C, Beuron F, Zhang X, Freemont PS. Going through the motions: the ATPase cycle of p97. J Struct Biol. 2006;156:12–28. doi: 10.1016/j.jsb.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Ramadan K, Bruderer R, Spiga FM, Popp O, Baur T, Gotta M, Meyer HH. Cdc48/p97 promotes reformation of the nucleus by extracting the kinase Aurora B from chromatin. Nature. 2007;450:1258–1262. doi: 10.1038/nature06388. [DOI] [PubMed] [Google Scholar]
- Rogers E, Bishop JD, Waddle JA, Schumacher JM, Lin R. The aurora kinase AIR-2 functions in the release of chromosome cohesion in Caenorhabditis elegans meiosis. J Cell Biol. 2002;157:219–229. doi: 10.1083/jcb.200110045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouiller I, DeLaBarre B, May AP, Weis WI, Brunger AT, Milligan RA, Wilson-Kubalek EM. Conformational changes of the multifunction p97 AAA ATPase during its ATPase cycle. Nat Struct Biol. 2002;9:950–957. doi: 10.1038/nsb872. [DOI] [PubMed] [Google Scholar]
- Ruchaud S, Carmena M, Earnshaw WC. The chromosomal passenger complex: one for all and all for one. Cell. 2007;131:230–231. doi: 10.1016/j.cell.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Sasagawa Y, Yamanaka K, Nishikori S, Ogura T. Caenorhabditis elegans p97/CDC-48 is crucial for progression of meiosis I. Biochem Biophys Res Commun. 2007;358:920–924. doi: 10.1016/j.bbrc.2007.05.022. [DOI] [PubMed] [Google Scholar]
- Schumacher JM, Ashcroft N, Donovan PJ, Golden A. A highly conserved centrosomal kinase, AIR-1, is required for accurate cell cycle progression and segregation of developmental factors in Caenorhabditis elegans embryos. Development. 1998a;125:4391–4402. doi: 10.1242/dev.125.22.4391. [DOI] [PubMed] [Google Scholar]
- Schumacher JM, Golden A, Donovan PJ. AIR-2: An Aurora/Ipl1-related protein kinase associated with chromosomes and midbody microtubules is required for polar body extrusion and cytokinesis in Caenorhabditis elegans embryos. J Cell Biol. 1998b;143:1635–1646. doi: 10.1083/jcb.143.6.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severson AF, Hamill DR, Carter JC, Schumacher J, Bowerman B. The aurora-related kinase AIR-2 recruits ZEN-4/CeMKLP1 to the mitotic spindle at metaphase and is required for cytokinesis. Curr Biol. 2000;10:1162–1171. doi: 10.1016/s0960-9822(00)00715-6. [DOI] [PubMed] [Google Scholar]
- Seydoux G, Dunn MA. Transcriptionally repressed germ cells lack a subpopulation of phosphorylated RNA polymerase II in early embryos of Caenorhabditis elegans and Drosophila melanogaster. Development. 1997;124:2191–2201. doi: 10.1242/dev.124.11.2191. [DOI] [PubMed] [Google Scholar]
- Song C, Wang Q, Li CC. ATPase activity of p97-valosin-containing protein (VCP). D2 mediates the major enzyme activity, and D1 contributes to the heat-induced activity. J Biol Chem. 2003;278:3648–3655. doi: 10.1074/jbc.M208422200. [DOI] [PubMed] [Google Scholar]
- Stewart S, Fang G. Destruction box-dependent degradation of aurora B is mediated by the anaphase-promoting complex/cyclosome and Cdh1. Cancer Res. 2005;65:8730–8735. doi: 10.1158/0008-5472.CAN-05-1500. [DOI] [PubMed] [Google Scholar]
- Stitzel ML, Pellettieri J, Seydoux G. The C. elegans DYRK Kinase MBK-2 Marks Oocyte Proteins for Degradation in Response to Meiotic Maturation. Curr Biol. 2006;16:56–62. doi: 10.1016/j.cub.2005.11.063. [DOI] [PubMed] [Google Scholar]
- Timmons L. Delivery methods for RNA interference in C. elegans. Methods Mol Biol. 2006;351:119–125. doi: 10.1385/1-59745-151-7:119. [DOI] [PubMed] [Google Scholar]
- Timmons L, Fire A. Specific interference by ingested dsRNA. Nature. 1998;395:854. doi: 10.1038/27579. [DOI] [PubMed] [Google Scholar]
- Vagnarelli P, Earnshaw WC. Chromosomal passengers: the four-dimensional regulation of mitotic events. Chromosoma. 2004;113:211–222. doi: 10.1007/s00412-004-0307-3. [DOI] [PubMed] [Google Scholar]
- Vong QP, Cao K, Li HY, Iglesias PA, Zheng Y. Chromosome alignment and segregation regulated by ubiquitination of survivin. Science. 2005;310:1499–1504. doi: 10.1126/science.1120160. [DOI] [PubMed] [Google Scholar]
- Wang Q, Song C, Irizarry L, Dai R, Zhang X, Li CC. Multifunctional roles of the conserved Arg residues in the second region of homology of p97/valosin-containing protein. J Biol Chem. 2005;280:40515–40523. doi: 10.1074/jbc.M509636200. [DOI] [PubMed] [Google Scholar]
- Yamauchi S, Yamanaka K, Ogura T. Comparative analysis of expression of two p97 homologues in Caenorhabditis elegans. Biochem Biophys Res Commun. 2006;345:746–753. doi: 10.1016/j.bbrc.2006.04.160. [DOI] [PubMed] [Google Scholar]
- Yasui Y, Urano T, Kawajiri A, Nagata K, Tatsuka M, Saya H, Furukawa K, Takahashi T, Izawa I, Inagaki M. Autophosphorylation of a newly identified site of Aurora-B is indispensable for cytokinesis. J Biol Chem. 2004;279:12997–13003. doi: 10.1074/jbc.M311128200. [DOI] [PubMed] [Google Scholar]
- Ye Y. Diverse functions with a common regulator: ubiquitin takes command of an AAA ATPase. J Struct Biol. 2006;156:29–40. doi: 10.1016/j.jsb.2006.01.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



