Abstract
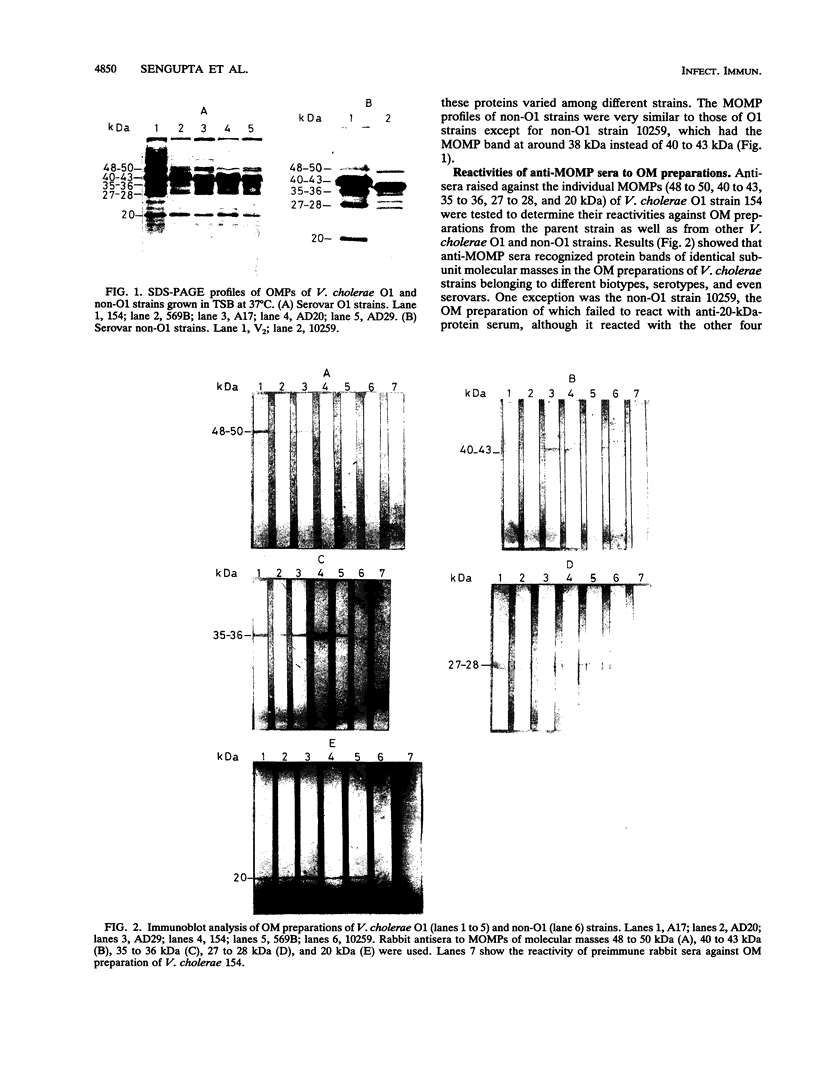
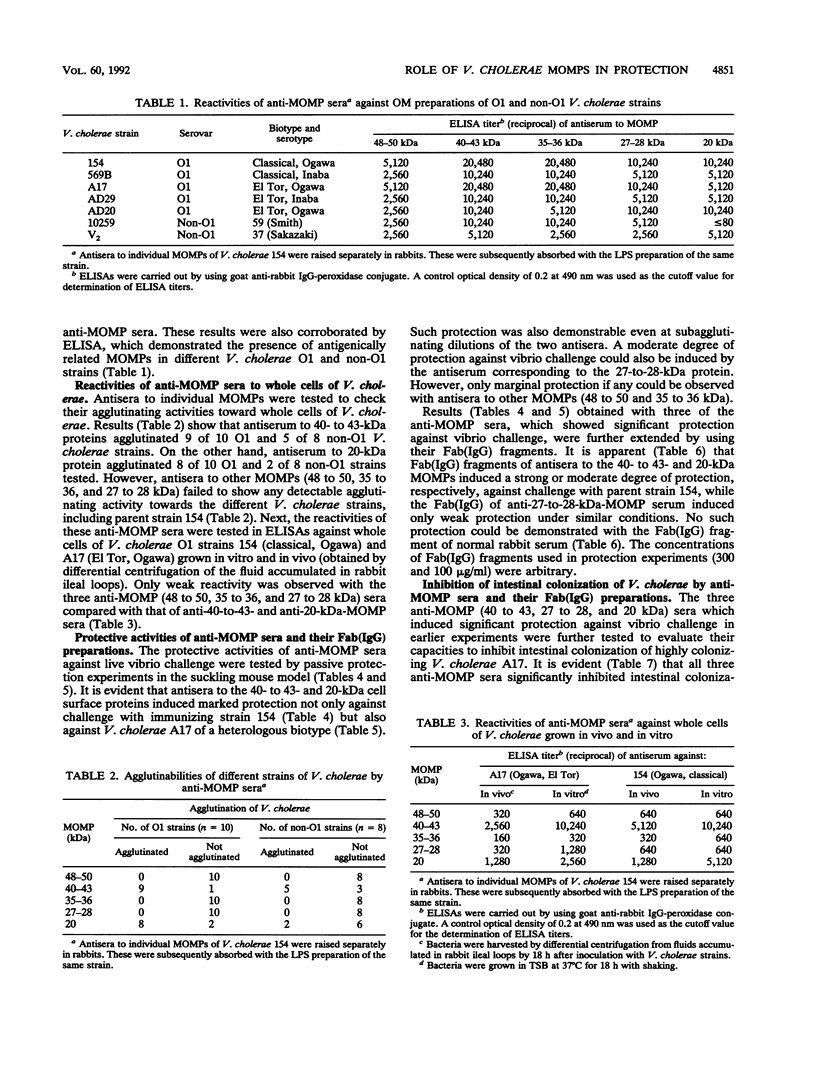
Vibrio cholerae O1 organisms belonging to different biotypes and serotypes were shown to express major outer membrane proteins (MOMPs) with subunit molecular masses of 48 to 50, 40 to 43, 35 to 36, 27 to 28, and 20 kDa. Antisera raised against individual MOMPs of a V. cholerae O1 strain recognized MOMPs of corresponding molecular masses in other O1 and non-O1 strains. Serological data also suggested possible differences in the cell surface exposition of these MOMPs. However, no marked differences between V. cholerae cells grown in vitro and in vivo could be noted in respect to the expression or surface exposition of these MOMPs. Of five MOMPs studied in this work, 40- to 43- and 20-kDa cell surface proteins were shown to be of considerable importance, as antisera to these proteins induced significant protection against V. cholerae challenge in the suckling mouse model. Similar protection, although to a lesser extent, was demonstrable with the antiserum to the 27- to 28-kDa protein. These results were corroborated with the Fab (immunoglobulin G) [Fab(IgG)] fragments of the antisera, thereby suggesting that the observed protection induced by anti-MOMP antibodies did not arise as a result of bacterial clumping. Subsequent studies demonstrated that these antisera as well as their Fab (IgG) fragments induced significant inhibition of intestinal colonization of V. cholerae. The 40- to 43- and 27- to 28-kDa proteins appeared to be porinlike, while the 20-kDa protein was found to be antigenically related to TcpA (subunit A of toxin-coregulated pilus). All these results demonstrate the involvement of more than one cell surface antigen of V. cholerae in the induction of protective immunity through inhibition of intestinal colonization of vibrios.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attridge S. R., Rowley D. Prophylactic significance of the nonlipopolysaccharide antigens of Vibrio cholerae. J Infect Dis. 1983 Nov;148(5):931–939. doi: 10.1093/infdis/148.5.931. [DOI] [PubMed] [Google Scholar]
- Banerjee K. K., Ghosh A. N., Dutta-Roy K., Pal S. C., Ghose A. C. Purification and characterization of a novel hemagglutinin from Vibrio cholerae. Infect Immun. 1990 Nov;58(11):3698–3705. doi: 10.1128/iai.58.11.3698-3705.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitnis D. S., Sharma K. D., Kamat R. S. Role of somatic antigen of Vibrio cholerae in adhesion to intestinal mucosa. J Med Microbiol. 1982 Feb;15(1):53–61. doi: 10.1099/00222615-15-1-53. [DOI] [PubMed] [Google Scholar]
- Cloeckaert A., de Wergifosse P., Dubray G., Limet J. N. Identification of seven surface-exposed Brucella outer membrane proteins by use of monoclonal antibodies: immunogold labeling for electron microscopy and enzyme-linked immunosorbent assay. Infect Immun. 1990 Dec;58(12):3980–3987. doi: 10.1128/iai.58.12.3980-3987.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryz S. J., Jr, Fürer E., Germanier R. Development of an enzyme-linked immunosorbent assay for studying Vibrio cholerae cell surface antigens. J Clin Microbiol. 1982 Jul;16(1):41–45. doi: 10.1128/jcm.16.1.41-45.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta-Roy K., Banerjee K., De S. P., Ghose A. C. Comparative study of expression of hemagglutinins, hemolysins, and enterotoxins by clinical and environmental isolates of non-O1 Vibrio cholerae in relation to their enteropathogenicity. Appl Environ Microbiol. 1986 Oct;52(4):875–879. doi: 10.1128/aem.52.4.875-879.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eubanks E. R., Guentzel M. N., Berry L. J. Evaluation of surface components of Vibrio cholerae as protective immunogens. Infect Immun. 1977 Feb;15(2):533–538. doi: 10.1128/iai.15.2.533-538.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip C., Fletcher G., Wulff J. L., Earhart C. F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973 Sep;115(3):717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilleland H. E., Jr, Gilleland L. B., Matthews-Greer J. M. Outer membrane protein F preparation of Pseudomonas aeruginosa as a vaccine against chronic pulmonary infection with heterologous immunotype strains in a rat model. Infect Immun. 1988 May;56(5):1017–1022. doi: 10.1128/iai.56.5.1017-1022.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M. B., DiRita V. J., Calderwood S. B. Identification of an iron-regulated virulence determinant in Vibrio cholerae, using TnphoA mutagenesis. Infect Immun. 1990 Jan;58(1):55–60. doi: 10.1128/iai.58.1.55-60.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall R. H., Losonsky G., Silveira A. P., Taylor R. K., Mekalanos J. J., Witham N. D., Levine M. M. Immunogenicity of Vibrio cholerae O1 toxin-coregulated pili in experimental and clinical cholera. Infect Immun. 1991 Jul;59(7):2508–2512. doi: 10.1128/iai.59.7.2508-2512.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall R. H., Vial P. A., Kaper J. B., Mekalanos J. J., Levine M. M. Morphological studies on fimbriae expressed by Vibrio cholerae 01. Microb Pathog. 1988 Apr;4(4):257–265. doi: 10.1016/0882-4010(88)90086-1. [DOI] [PubMed] [Google Scholar]
- Holmgren J. Actions of cholera toxin and the prevention and treatment of cholera. Nature. 1981 Jul 30;292(5822):413–417. doi: 10.1038/292413a0. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Svennerholm A. M. Mechanisms of disease and immunity in cholera: a review. J Infect Dis. 1977 Aug;136 (Suppl):S105–S112. doi: 10.1093/infdis/136.supplement.s105. [DOI] [PubMed] [Google Scholar]
- Jonson G., Holmgren J., Svennerholm A. M. Epitope differences in toxin-coregulated pili produced by classical and El Tor Vibrio cholerae O1. Microb Pathog. 1991 Sep;11(3):179–188. doi: 10.1016/0882-4010(91)90048-f. [DOI] [PubMed] [Google Scholar]
- Jonson G., Sanchez J., Svennerholm A. M. Expression and detection of different biotype-associated cell-bound haemagglutinins of Vibrio cholerae O1. J Gen Microbiol. 1989 Jan;135(1):111–120. doi: 10.1099/00221287-135-1-111. [DOI] [PubMed] [Google Scholar]
- Jonson G., Svennerholm A. M., Holmgren J. Vibrio cholerae expresses cell surface antigens during intestinal infection which are not expressed during in vitro culture. Infect Immun. 1989 Jun;57(6):1809–1815. doi: 10.1128/iai.57.6.1809-1815.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabir S. Composition and immunochemical properties of outer membrane proteins of Vibrio cholerae. J Bacteriol. 1980 Oct;144(1):382–389. doi: 10.1128/jb.144.1.382-389.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Kaper J. B., Black R. E., Clements M. L. New knowledge on pathogenesis of bacterial enteric infections as applied to vaccine development. Microbiol Rev. 1983 Dec;47(4):510–550. doi: 10.1128/mr.47.4.510-550.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., Nalin D. R., Craig J. P., Hoover D., Bergquist E. J., Waterman D., Holley H. P., Hornick R. B., Pierce N. P., Libonati J. P. Immunity of cholera in man: relative role of antibacterial versus antitoxic immunity. Trans R Soc Trop Med Hyg. 1979;73(1):3–9. doi: 10.1016/0035-9203(79)90119-6. [DOI] [PubMed] [Google Scholar]
- Majumdar A. S., Dutta P., Dutta D., Ghose A. C. Antibacterial and antitoxin responses in the serum and milk of cholera patients. Infect Immun. 1981 Apr;32(1):1–8. doi: 10.1128/iai.32.1.1-8.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar A. S., Ghose A. C. Evaluation of the biological properties of different classes of human antibodies in relation to cholera. Infect Immun. 1981 Apr;32(1):9–14. doi: 10.1128/iai.32.1.9-14.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar A. S., Ghose A. C. Protective properties of anticholera antibodies in human colostrum. Infect Immun. 1982 Jun;36(3):962–965. doi: 10.1128/iai.36.3.962-965.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning P. A. Involvement of cell envelope components in the pathogenesis of Vibrio cholerae: targets for cholera vaccine development. Vaccine. 1987 Jun;5(2):83–87. doi: 10.1016/0264-410x(87)90051-x. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Nelles L. P., Bamburg J. R. Rapid visualization of protein--dodecyl sulfate complexes in polyacrylamide gels. Anal Biochem. 1976 Jun;73(2):522–531. doi: 10.1016/0003-2697(76)90202-5. [DOI] [PubMed] [Google Scholar]
- Richardson K., Kaper J. B., Levine M. M. Human immune response to Vibrio cholerae O1 whole cells and isolated outer membrane antigens. Infect Immun. 1989 Feb;57(2):495–501. doi: 10.1128/iai.57.2.495-501.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson K., Parker C. D. Identification and characterization of Vibrio cholerae surface proteins by radioiodination. Infect Immun. 1985 Apr;48(1):87–93. doi: 10.1128/iai.48.1.87-93.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears S. D., Richardson K., Young C., Parker C. D., Levine M. M. Evaluation of the human immune response to outer membrane proteins of Vibrio cholerae. Infect Immun. 1984 May;44(2):439–444. doi: 10.1128/iai.44.2.439-444.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta D. K., Sengupta T. K., Ghose A. C. Antibodies to outer membrane proteins of Vibrio cholerae induce protection by inhibition of intestinal colonization of vibrios. FEMS Microbiol Immunol. 1992 Jul;4(5):261–266. doi: 10.1111/j.1574-6968.1992.tb05004.x. [DOI] [PubMed] [Google Scholar]
- Sengupta D., Datta-Roy K., Banerjee K., Ghose A. C. Identification of some antigenically related outer-membrane proteins of strains of Vibrio cholerae O1 and non-O1 serovars involved in intestinal adhesion and the protective role of antibodies to them. J Med Microbiol. 1989 May;29(1):33–39. doi: 10.1099/00222615-29-1-33. [DOI] [PubMed] [Google Scholar]
- Sharma D. P., Attridge S., Hackett J., Rowley D. Nonlipopolysaccharide protective antigens shared by classical and El Tor biotypes of Vibrio cholerae. J Infect Dis. 1987 Apr;155(4):716–723. doi: 10.1093/infdis/155.4.716. [DOI] [PubMed] [Google Scholar]
- Sharma D. P., Thomas C., Hall R. H., Levine M. M., Attridge S. R. Significance of toxin-coregulated pili as protective antigens of Vibrio cholerae in the infant mouse model. Vaccine. 1989 Oct;7(5):451–456. doi: 10.1016/0264-410x(89)90161-8. [DOI] [PubMed] [Google Scholar]
- Sun D. X., Mekalanos J. J., Taylor R. K. Antibodies directed against the toxin-coregulated pilus isolated from Vibrio cholerae provide protection in the infant mouse experimental cholera model. J Infect Dis. 1990 Jun;161(6):1231–1236. doi: 10.1093/infdis/161.6.1231. [DOI] [PubMed] [Google Scholar]
- Taylor R. K., Miller V. L., Furlong D. B., Mekalanos J. J. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc Natl Acad Sci U S A. 1987 May;84(9):2833–2837. doi: 10.1073/pnas.84.9.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ley P., de Graaff P., Tommassen J. Shielding of Escherichia coli outer membrane proteins as receptors for bacteriophages and colicins by O-antigenic chains of lipopolysaccharide. J Bacteriol. 1986 Oct;168(1):449–451. doi: 10.1128/jb.168.1.449-451.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]