Abstract
The p16INK4a-Rb tumour suppressor pathway is required for the initiation and maintenance of cellular senescence, a state of permanent growth arrest that acts as a natural barrier against cancer progression. Senescence can be overcome if the pathway is not fully engaged, and this may occur when p16INK4a is inactivated. p16INK4a is frequently altered in human cancer and germline mutations affecting p16INK4a have been linked to melanoma susceptibility. To characterize the functions of melanoma-associated p16INK4a mutations, in terms of promoting proliferative arrest and initiating senescence, we utilized an inducible expression system in a melanoma cell model. We show that wild-type p16INK4a promotes rapid cell cycle arrest that leads to a senescence programme characterized by the appearance of chromatin foci, activation of acidic β-galactosidase activity, p53 independence and Rb dependence. Accumulation of wild-type p16INK4a also promoted cell enlargement and extensive vacuolization independent of Rb status. In contrast, the highly penetrant p16INK4a variants, R24P and A36P failed to arrest cell proliferation and did not initiate senescence. We also show that overexpression of CDK4, or its homologue CDK6, but not the downstream kinase, CDK2, inhibited the ability of wild-type p16INK4a to promote cell cycle arrest and senescence. Our data provide the first evidence that p16INK4a can initiate a CDK4/6-dependent autonomous senescence programme that is disabled by inherited melanoma-associated mutations.
Keywords: CDK4, CDK6, melanoma, naevi, p16INK4a, senescence
Introduction
The INK4a/ARF locus, situated on chromosome band 9p21, is one of the most frequently altered sequences in human cancer and germline mutations affecting this locus have been linked to melanoma incidence in approximately 39% of melanoma-prone families (Goldstein etal., 2006b). The lifetime risk of melanoma in p16INK4a germline mutation carriers ranges from 58% in Europe to 91% in Australia by the age of 80 (Bishop etal., 2002). This locus encodes two potent, but distinct tumour suppressor proteins; the cyclin-dependent kinase inhibitor, p16INK4a (Serrano etal., 1993) and the p53 activator p14ARF (Quelle etal., 1995). Both proteins are critically important in the regulation of cell cycle progression and senescence (reviewed in Sharpless, 2005; Collado etal., 2007). p14ARF blocks proliferation by inhibiting the p53 ubiquitin ligase hdm2, to stabilize and activate p53 (Pomerantz etal., 1998; Stott etal., 1998; Zhang etal., 1998) and ARF-null mouse embryonic fibroblasts do not senesce (Kamijo etal., 1997). p16INK4a promotes cell cycle arrest by inhibiting the kinase activities of the cyclin D-dependent kinases, CDK4 and CDK6, to maintain the retinoblastoma protein, Rb in its hypophosphorylated, antiproliferative state (Serrano etal., 1993). The progressive accumulation of p16INK4a is associated with the onset of replicative senescence in primary human epithelial cells (Alcorta etal., 1996; Brenner etal., 1998) and ectopic p16INK4a expression induces growth arrest that phenotypically resembles cellular senescence in human diploid fibroblasts (Zhu etal., 1998; McConnell etal., 1999) and in INK4a/ARF-deficient murine melanocytes (Sviderskaya etal., 2002). Furthermore, p16INK4a-deficient human diploid fibroblasts and melanocytes, isolated from melanoma-prone individuals with inactivating mutations affecting both INK4a alleles, undergo delayed senescence (Sviderskaya etal., 2003; Brookes etal., 2004; Jones etal., 2007) and are readily immortalized by the introduction of the telomerase reverse transcriptase (Sviderskaya etal., 2003).
Cellular senescence can be triggered by multiple mechanisms including induction of the INK4a/ARF locus, telomere attrition, DNA damage, oxidative damage and the aberrant proliferative signals of oncogenes (reviewed in Collado & Serrano, 2006). Once established, senescence permanently limits cellular proliferation and protects against the development of malignant cancer. Accordingly, senescent cells are abundant in premalignant lesions of the skin, the lung and the pancreas whereas they are almost completely absent in malignant tumours (Collado etal., 2005). Senescent cells have been identified, both in vitro and in vivo, using a series of markers (reviewed in Collado & Serrano, 2006; Campisi & d’Adda di Fagagna, 2007). Increased activity of acidic β-galactosidase, termed senescence-associated β-galactosidase (SA-β-gal) is the most widely accepted marker of senescence cells (Dimri etal., 1995). More recently, the appearance of DAPI-stained heterochromatic regions, known as senescence-associated heterochromatic foci, which result in the stable repression of some E2F target genes are involved in the irreversible growth arrest associated with senescence (Narita etal., 2003). These foci are enriched for histone H3 modified at lysine 9 as well as its binding partner heterochromatin protein-1γ (HP-1γ) (Narita etal., 2003). Several other markers of senescence have also been described and validated, including the CDK inhibitor p15INK4b, an anti-apoptotic bcl-2 member, Mcl-1 and the transcription factor, Dec1. Morphological changes such as cell enlargement, vacuolization and cell flattening are also typical of senescent cells (Collado etal., 2005).
Although the role of p16INK4a in initiating senescence is well documented (Vogt etal., 1998; McConnell etal., 1999; Dai & Enders, 2000; Sviderskaya etal., 2002), there is a paucity of data on the mechanisms underlying p16INK4a-induced senescence. Moreover, little is known regarding the ability of melanoma-associated p16INK4a germline mutations to initiate and maintain cellular senescence. This is particularly important as individuals carrying p16INK4a mutations have increased susceptibility to melanoma, and usually display larger, more numerous and dysplastic naevi (Gruis etal., 1995; Bennett & Medrano, 2002). There is persuasive evidence that melanocytic naevi are growth-arrested, senescent lesions, and it is likely that p16INK4a, which is widely expressed in naevi, contributes to establishing cellular senescence (Michaloglou etal., 2005; Gray-Schopfer etal., 2006). This permanent growth arrest would be an efficient barrier to melanoma development that may not be triggered by melanoma-associated p16INK4a variants.
To examine the autonomy and mechanism of p16INK4a-induced senescence, we utilized a melanoma cell model with inducible, physiological levels of p16INK4a expression and compared the impact of wild-type p16INK4a expression to that of two functionally distinct p16INK4a melanoma-associated mutants. The R24P mutation alters a highly conserved residue in the first ankyrin repeat, is closely linked with familial melanoma in at least 11 melanoma-prone kindreds (Della Torre etal., 2001; Mantelli etal., 2002; Goldstein etal., 2006a) but behaved as wild-type p16INK4a in CDK6-binding assays (Harland etal., 1997; Jones etal., 2007). This mutant was shown to be defective in binding CDK4 (Harland etal., 1997) and inducing senescence in human fibroblasts (Jones etal., 2007), but has not previously been analysed in cells of melanocytic lineage. The A36P variant identified in an Australian family (Holland etal., 1999) is impaired in promoting cell cycle arrest (Becker etal., 2001), but there is currently no data on its interaction with CDK4 and CDK6 in vivo. We show that wild-type p16INK4a induced a rapid proliferative arrest that was associated with cell enlargement, vacuolization and appearance of heterochromatic foci. This senescence programme was not triggered by stress to the endoplasmic reticulum or DNA damage and was not activated by the mis-sense melanoma-associated mutants, R24P and A36P. Transient expression of CDK4 or CDK6, but not the downstream kinase CDK2 overcame p16INK4a-induced senescence programme, and although Rb was critical to p16INK4a-mediated arrest, it was not required for p16INK4a-induced cell enlargement and vacuolization. This work confirms p16INK4a-driven senescence is intimately linked to CDK4/6-inhibition and cell cycle arrest, and indicates that the melanoma-associated mis-sense p16INK4a mutants are unable to initiate an effective CDK4/6-dependent senescence programme.
Results
Impact of induced wild-type p16INK4a expression
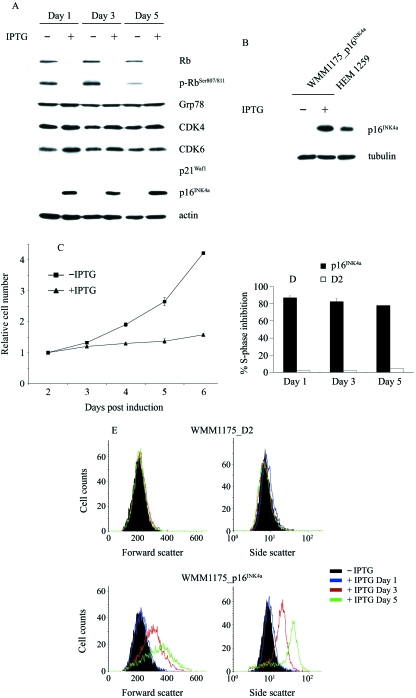
To evaluate the influence of wild-type p16INK4a accumulation on cell proliferation and senescence, the WMM1175 melanoma cell line, which is INK4a/ARF- and p53-null (Rizos etal., 1999) was engineered to express wild-type p16INK4a. In this WMM1175_p16INK4a cell line, p16INK4a expression was induced with 4 mm isopropyl β-D-1-thiogalactopyranoside (IPTG). Accumulation of p16INK4a was detected in the WMM1175_p16INK4a cells 24 h post-induction, and this was maintained for the 5 days of continuous IPTG exposure (Fig. 1A). The level of p16INK4a accumulation in the WMM1175_p16INK4a clone was comparable to p16INK4a expression in normal, actively proliferating human epidermal melanocytes at passage 10 (Fig. 1B). The accumulation of p16INK4a led to the decreased levels of phosphorylated Rb (p-RbSer807/811), the loss of total Rb protein expression (Fang etal., 1998), and a slight increase in the accumulation of CDK4 and CDK6. The p53-target p21Waf1 was not detectable and the endoplasmic reticulum-stress response, which is required for H-RAS-induced senescence (Denoyelle etal., 2006) was not activated by p16INK4a, as determined by the lack of induction of the endoplasmic reticulum-stress sensor Grp78 (BiP) (reviewed in Gething, 1999) (Fig. 1A). Furthermore, the DNA damage checkpoint, which mediates oncogene-induced senescence (Bartkova etal., 2006; Di Micco etal., 2006), was not induced by p16INK4a induction as there was no evidence of increased DNA damage foci in p16INK4a-expressing cells, as marked by H2AX phosphorylation (data not shown).
Fig. 1.
Induced expression of p16INK4a inhibits Rb phosphorylation, limits cell proliferation and alters cell morphology. (A) Expression of the indicated proteins was determined by Western blot at 1, 3 and 5 days after treatment of WMM1175_p16INK4a cells with 4 mm IPTG. (B) Accumulation of p16INK4a in IPTG-treated (5 days) WMM1175_p16INK4a cells compared with levels of endogenous p16INK4a in normal, actively proliferating human neonatal epidermal melanocytes (HEM 1259). (C) The impact of induced p16INK4a expression on the proliferation of the WMM1175_p16INK4a cells was determined over a 5-day induction period using the MTS assay. The results shown are expressed as the average ± standard deviation of at least two independent experiments performed in triplicate. (D) The percentage of cells in S-phase after induction of p16INK4a for up to 5 days was determined by flow cytometry. S-phase inhibition was calculated from at least two independent induction experiments. Percentage S-phase inhibition in the IPTG-treated parental WMM1175_D2 cells, which expresses the lac repressor, but not the p16INK4a transgene, is also shown. (E) The impact of IPTG-exposure on the size (Forward scatter) and granularity (Side scatter) of the WMM1175_p16INK4a melanoma cells and the parental WMM1175_D2 cell line was investigated using flow cytometry on unfixed cells. These results are representative of at least two independent experiments.
As previously demonstrated (Becker etal., 2001), accumulation of p16INK4a potently inhibited the proliferation of the WMM1175 cell line (Fig. 1C), and this was associated with a rapid arrest in the G1-phase of the cell cycle with a concomitant S-phase inhibition that was maintained over the 5-day induction period (Fig. 1D). To ensure that IPTG alone did not affect cell proliferation, the parental WMM1175_D2 cell line, which expresses the lac repressor but not p16INK4a, was treated as the WMM1175_p16INK4a clones and no changes were observed in proliferation (data not shown) or cell cycle distribution (Fig. 1D).
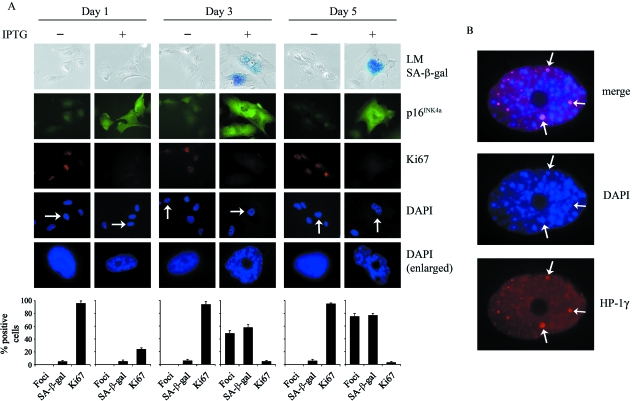
To further examine the p16INK4a-induced arrest, we analysed two key markers of senescence: cell size and vacuolization. Whereas IPTG treatment did not alter the phenotype of the parental WMM1175_D2 cells (Fig. 1E), it caused an obvious increase in the size and granularity, a marker of vacuolization, in the WMM1175_p16INK4a cells (Fig. 1E). These p16INK4a-induced morphological changes were confirmed using microscopy. As shown in Fig. 2A, at 3 days post-induction the cells induced for p16INK4a expression had adopted characteristics of senescent cells, appearing enlarged and flattened. These cells were negative for the proliferation marker Ki67 and acquired SA-β-gal activity (77 ± 3% of p16INK4a-induced cells stained positive for SA-β-gal at 5 days post-induction). These senescence features occurred as late markers of p16INK4a function, and appeared 2 days later than p16INK4a-induced cell cycle arrest, which was evident within 24 h post p16INK4a induction (see Fig. 1D). The uninduced WMM1175_p16INK4a cells (Fig. 2A) and IPTG-treated parental WMM1175_D2 cells (data not shown) did not display a senescence phenotype and the majority of these cells stained positive for Ki67.
Fig. 2.
Impact of induced p16INK4a expression on the cellular senescence programme. (A) WMM1175_p16INK4a cells were exposed to 4 mm IPTG over a 5-day period. The accumulation of p16INK4a, cell proliferation (Ki67), chromatin condensation (DAPI) and the appearance of SA-β-gal was analysed. Cells enlarged to show DAPI-stained chromatin foci are indicated with arrows. Cell counts for each of these markers are shown as histograms, which correspond to the average ± standard deviation of at least two independent induction experiments from a total of at least 500 cells. LM, light microscopy. (B) Representative examples of p16INK4a-induced chromatin condensation (DAPI) and costaining for HP-1γ as surrogates for senescence-associated heterochromatin foci (indicated by arrows).
To further investigate the response of the WMM1175 melanoma cells to p16INK4a we analysed senescence-associated heterochromatic foci by immunostaining with DAPI and HP-1γ. We observed a dramatic increase in the appearance of nuclear foci at 3 and 5 days post-IPTG treatment; 49 ± 5% and 75 ± 5% of p16INK4a-induced cells stained positive for nuclear foci at 3 and 5 days post-induction, respectively, with the appearance of large, prominent, often irregular shaped nuclei (Fig. 2A). The accumulation the HP-1γ, within these nuclear foci confirmed that they are senescence-associated heterochromatic foci (Fig. 2B). Taken together our results confirm that wild-type p16INK4a can induce senescence in WMM1175 melanoma cells in a p53- and p21Waf1-independent manner.
Impact of melanoma-associated p16INK4a mutations on melanoma cell senescence
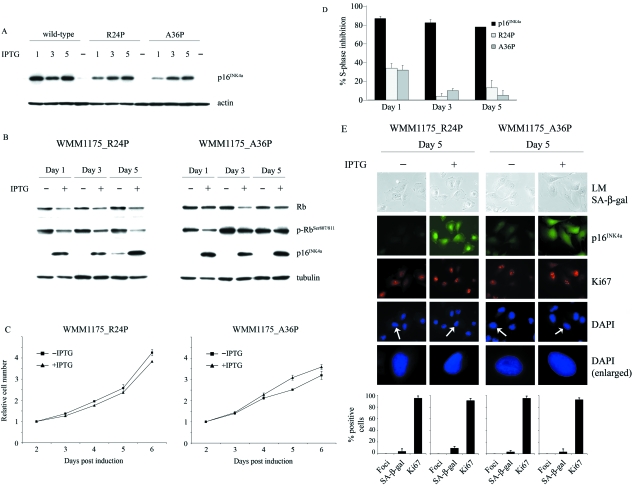
Although it has been shown that wild-type p16INK4a can promote an autonomous senescence programme (Dai & Enders, 2000), there has been no detailed analysis on the impact of melanoma-associated mutations on this programme. This is particularly relevant as there are significant variations in the penetrance of p16INK4a mutations for melanoma (Berwick etal., 2006) and this may relate to loss of specific functions, including the induction of senescence. We analysed two well-defined and common p16INK4a mutants that segregate with disease in high-risk melanoma families. These mutant proteins were also selected because they displayed expression levels comparable to the wild-type p16INK4a protein in the inducible WMM1175 melanoma cell model (Fig. 3A).
Fig. 3.
Melanoma-associated p16INK4a mutants fail to induce cell cycle arrest. (A) Expression of wild-type p16INK4a, R24P and A36P mutant proteins in WMM1175 melanoma cell clones was induced with 4 mm IPTG over a 5-day induction period and compared using immunoblotting. (B) Expression of p16INK4a, total Rb, Ser807/811-phosphorylated Rb and actin was determined 1, 3 and 5 days after treatment of WMM1175_R24P and WMM1175_A36P cells with 4 mm IPTG. (C) The impact of induced mutant p16INK4a on the proliferation of the WMM1175_R24P and WMM1175_A36P cells was determined over the 5-day induction period using the MTS assay. The results shown are expressed as the average ± standard deviation of at least two independent experiments performed in triplicate. (D) The percentage of cells in S-phase after induction of p16INK4a or a melanoma-associated p16INK4a mutant in the WMM1175 cells was determined by flow cytometry. The percentage S-phase inhibition was calculated from at least two independent induction experiments. (E) Expression of the melanoma-associated variants R24P or A36P was induced in the WMM1175 melanoma cells and the impact on cell morphology (LM), proliferation (Ki67), chromatin condensation (DAPI) and SA-β-gal activity was analysed over a 5-day period. Representative examples of the 5-day IPTG induction point are shown. Cells enlarged to show DAPI-stained chromatin foci are indicated with arrows. Cell counts for the cell cycle markers are shown as histograms, which correspond to the average ± standard deviation of at least two independent induction experiments from a total of at least 500 cells. LM, light microscopy.
All clones expressing mutant p16INK4a proteins were analysed as the wild-type WMM1175_p16INK4a clone in cell proliferation and senescence assays. The R24P mutant, which retains CDK6 inhibitory activity, partially inhibited Rb phosphorylation and slightly diminished the levels of total Rb over the 5-day induction period (Fig. 3B). In contrast, the A36P mutant had no consistent impact on Rb levels or its phosphorylation status (Fig. 3B). Expression of the p16INK4a mutations (R24P and A36P) had no long-term inhibitory effect on WMM1175 cell proliferation (Fig. 3C) and this correlated closely with the consistently weaker inhibition of S-phase induced by the mutants when compared with the sustained S-phase inhibition and G1 arrest observed when the wild-type p16INK4a protein was induced (Fig. 3D). Furthermore, these mutant p16INK4a proteins produced no detectable changes in cell size and morphology (data not shown), had no impact on heterochromatic foci and did not induce SA-β-gal activity (Fig. 3E).
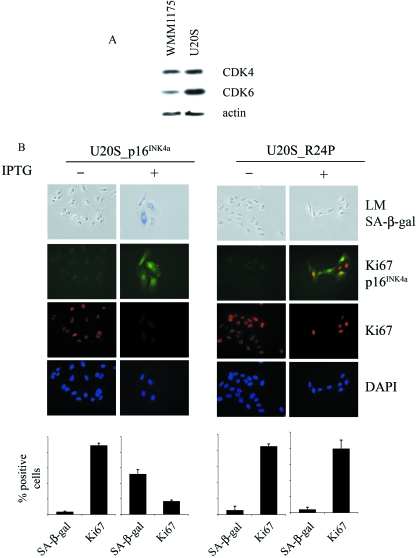
p16INK4a-induced arrest and senescence requires inhibition of CDK4 and CDK6
Given that the R24P variant, which binds and inhibits CDK6, but not CDK4 (Jones etal., 2007), was still incapable of promoting arrest, we hypothesized that inhibition of both CDK4 and CDK6 was required for p16INK4a-induced arrest and senescence. After screening a panel of human cancer cell lines for CDK4 and CDK6 expression (data not shown), the U20S cell line was selected, as it accumulated approximately threefold higher levels of CDK6 compared to the WMM1175 cell line (Fig. 4A). U20S cells were engineered to inducibly express the wild-type p16INK4a or the R24P mutant protein. As expected, expression of wild-type p16INK4a, but not R24P, arrested U20S cells and promoted their senescence. p16INK4a-induced senescence in the U20S cells was associated with cell enlargement and SA-β-gal activity, but not DNA heterochromatic foci (Fig. 4B).
Fig. 4.
The melanoma-associated p16INK4a R24P mutant fails to induce cell cycle arrest or senescence in U20S cells. (A) Expression of CDK4, CDK6 and actin was compared in the U20S and WMM1175 cells. Band intensities were determined by densitometric measurements using a phosphoimager (Molecular Dynamics). (B) Stable pools of U20S cells expressing inducible forms of wild-type p16INK4a or R24P were exposed to 4 mm IPTG over a 5-day period. Representative examples of the 5-day IPTG induction time point are shown. The accumulation of p16INK4a, cell proliferation (Ki67) and the appearance of SA-β-gal was analysed. Cell counts for these markers are shown as histograms, which correspond to the average ± standard deviation of at least two independent induction experiments from a total of at least 500 cells. LM, light microscopy.
Furthermore, when either CDK4 or CDK6 was ectopically expressed in the WMM1175_p16INK4a cell line, induced expression of p16INK4a failed to inhibit cell proliferation and did not induce senescence. In particular, in the presence of ectopic CDK4 or CDK6 expression, p16INK4a did not promote cell enlargement, heterochromatic foci or SA-β-gal activity (Fig. 5). In contrast, WMM1175_p16INK4a cells transiently transfected with vector only and induced for wild-type p16INK4a expression showed all the characteristic markers of senescence (Fig. 5).
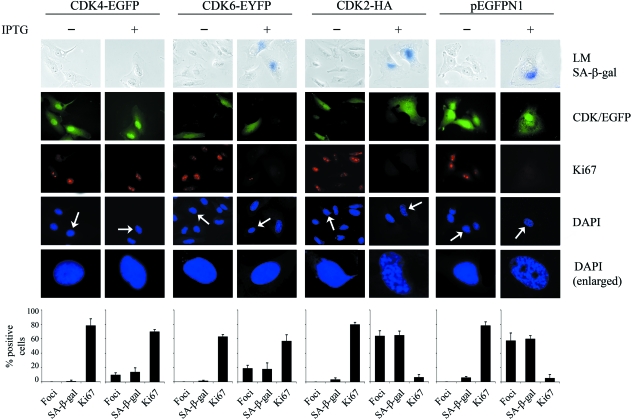
Fig. 5.
CDK4 and CDK6 inhibition is critical to p16INK4a-induced senescence. WMM1175_p16INK4a cells were transfected with CDK4-EGFP, CDK6-EYFP, CDK2-HA or vector DNA (only the pEGFPN1 vector control is shown here), as indicated. Approximately 6 h post-transfection, cells were treated with PBS (–) or induced for p16INK4a expression with 4 mm IPTG (+). At 72 h post-induction, cells were stained for transgene expression (CDK/GFP), markers of senescence (SA-β-gal, DAPI) and proliferation (Ki67), as indicated. Cell counts for each of these markers are shown as histograms, which correspond to the average ± standard deviation of at least two independent induction experiments from a total of at least 300 cells.
To ensure that p16INK4a-mediated cell cycle arrest was specifically overcome by expression of its CDK4 and CDK6 binding partners, we also transiently introduced CDK2, a kinase that accelerates and augments CDK4/6-initiated Rb hyperphosphorylation (reviewed in Johnson & Walker, 1999; Sherr, 1993). Ectopically expressed CDK2 did not overcome the ability of p16INK4a to induce cell cycle arrest or senescence (Fig. 5). These data confirm that the inhibition of both CDK4 and CDK6 kinase activity is required for p16INK4a-mediated cell cycle arrest and senescence. More importantly, they suggest that all known functions of p16INK4a, including the induction of chromatin condensation and p16INK4a-mediated changes in cell morphology and size, depend on CDK4/6 binding and inhibition.
The Rb protein is the critical downstream target of p16INK4a
It is well established that the downstream impact of p16INK4a-mediated inhibition of CDK4 and CDK6 activity is the hypophosphorylation and activation of Rb, as shown in Fig. 1A. It has also been recognized that p16INK4a accumulation promotes the rapid disappearance of Rb (Serrano etal., 1997; Fang etal., 1998; Ausserlechner etal., 2005), and we observed Rb loss in both the WMM1175 (Fig. 1A) and U20S cells (data not shown). Considering that Rb loss coincided with cell cycle arrest and occurred earlier than the onset of senescence (Rb loss and arrest were detected 24 h post p16INK4a induction, whereas senescence was detected 72 h after p16INK4a expression was induced; see Figs 1A and 2A), it was important to establish whether Rb depletion alone (with no p16INK4a expression) promoted cell cycle arrest and senescence. Silencing of Rb with an Rb-specific silencing molecule, 72 h or 96 h post-transduction (Fig. 6A), did not in itself promote cell cycle arrest or senescence as judged by the continued proliferation of Rb shRNA-transduced WMM1175 (data not shown) and WMM1175_p16INK4a cells (Fig. 6B). These Rb-null cells did not stain positive for SA-β-gal activity, did not form DNA foci (Fig. 6B) and did not enlarge (Fig. 6C). Thus, down-regulation of Rb expression alone does not inhibit cell cycle proliferation nor does it promote senescence. More importantly, induced p16INK4a could only promote cell cycle arrest and cellular senescence, as judged by acquired Ki67 staining and SA-β-gal activity and DNA foci formation, in the presence of Rb (Fig. 6B).
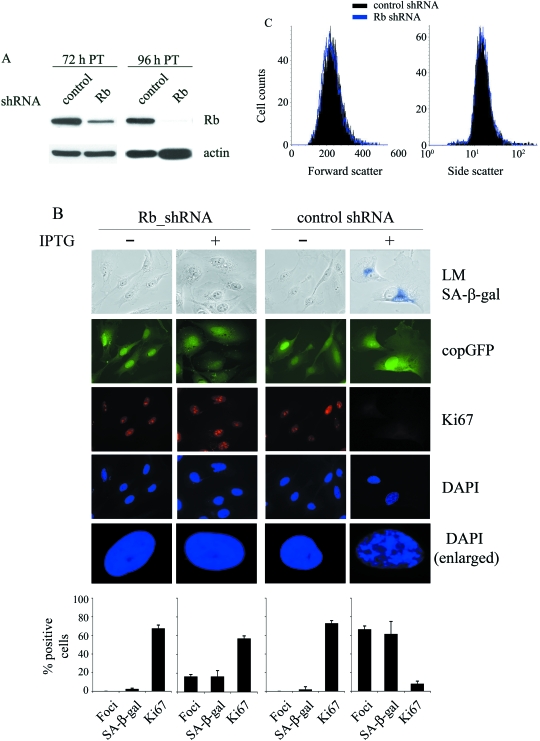
Fig. 6.
Silencing Rb expression does not promote an arrest or senescence response. (A) WMM1175 melanoma cells were transduced with a control shRNA or an Rb-specific silencing molecule, as indicated. The efficiency of transduction was controlled with co-expression of copGFP and was consistently above 90%. At 72 h and 96 h post-transduction (PT), cells were harvested and protein expression analysed using SDS-PAGE with the indicated antibodies. (B) WMM1175_p16INK4a cells were transduced with a control or a Rb-specific shRNA molecule and approximately 96 h post-transduction the cells were treated for 3 days with IPTG (+) or PBS (–) and stained for markers of transduction (copGFP), senescence (SA-β-gal, DAPI) and proliferation (Ki67), as indicated. Cell counts for each of these markers are shown as histograms, which correspond to the average ± standard deviation of at least two independent induction experiments from a total of at least 300 cells. (C) The impact of Rb silencing on the size (Forward scatter) and granularity (Side scatter) of the WMM1175 melanoma cells was investigated, 96 h post-transduction, using flow cytometry on paraformaldehyde fixed cells. These results are representative of at least two independent experiments.
Although loss of Rb did not initiate senescence and Rb was required for p16INK4a-mediated senescence it was important to clarify whether Rb was the critical downstream target for all p16INK4a functions. This was particularly relevant as it has been suggested that CDK4 and CDK6 may phosphorylate as yet unidentified substrates (Ruas etal., 2007), and we have now shown that these binding partners are critical for p16INK4a-induced senescence. Thus, the WMM1175_p16INK4a cells were transduced with a control shRNA or an Rb-specific silencing molecule and 96 h post-transduction the cells were induced for wild-type p16INK4a expression. Analysis of these cells, 72 h after p16INK4a induction, revealed that Rb expression remained effectively silenced and the cells remained inducible for p16INK4a expression (Fig. 7A). Intriguingly, the ability of p16INK4a to increase cell size and granularity did not require Rb, and p16INK4a induced these distinctive changes in cell morphology regardless of Rb status (Fig. 7B).
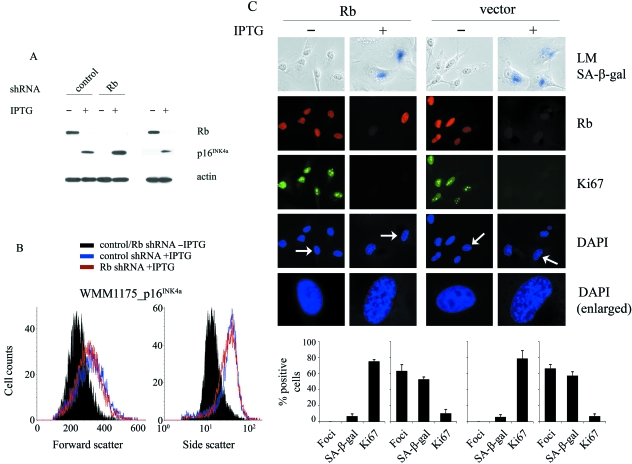
Fig. 7.
p16INK4a-induced arrest and senescence requires the expression of Rb.WMM1175_p16INK4a melanoma cells were transduced with a control shRNA or an Rb-specific silencing molecule, as indicated. The efficiency of trasduction was controlled with the co-expression of copGFP and was consistently above 90%. At 96 h post-transduction cells were treated with PBS (–) or 4 mm IPTG (+) and 72 h post-induction cells were analysed. (A) Cells were harvested and protein expression analysed using SDS-PAGE with the indicated antibodies. (B) The impact of 72 h of p16INK4a induction on the size (Forward scatter) and granularity (Side scatter) of the WMM1175_p16INK4a melanoma cells was investigated, 96 h post-transduction with a control or Rb-specific shRNA molecule, using flow cytometry on paraformaldehyde fixed cells. These results are representative of at least two independent experiments. (C) WMM1175_p16INK4a cells were transiently transfected with an Rb or empty expression plasmid, and approximately 6 h post-transfection the cells were treated for three days with IPTG (+) or PBS (–) and stained for Rb, markers of senescence (SA-β-gal, DAPI) and proliferation (Ki67), as indicated. Cell counts for each of these markers are shown as histograms, which correspond to the average ± standard deviation of at least two independent induction experiments from a total of at least 300 cells.
Considering that depletion of Rb occurs after the onset of p16INK4a-induced arrest but prior to the onset of p16INK4a-induced senescence (see Fig. 1), it was possible that reinstating the expression of Rb could influence p16INK4a-induced senescence. As shown in Fig. 7(C), however, when Rb expression was transiently reintroduced into the WMM1175_p16INK4a cells, p16INK4a retained its activity and effectively promoted cell cycle arrest followed by senescence. Thus, p16INK4a-induced cell cycle arrest and senescence requires the complete inhibition of CDK4 and CDK6 activity and the transient activation of Rb. The subsequent loss of Rb expression appears incidental to p16INK4a-mediated arrest and senescence, although we are investigating the precise mechanism of Rb loss. Furthermore, the p16INK4a-driven inhibition of CDK4 and CDK6 promotes changes in cell morphology independent of Rb, suggesting additional kinase targets may contribute to the activity of p16INK4a.
Discussion
p16INK4a is a highly penetrant melanoma tumour suppressor that regulates cell cycle progression by inhibiting the kinase activities of cyclin D-associated CDK4 and CDK6 (Serrano etal., 1993). Active binary cyclin D-CDK complexes initiate Rb phosphorylation driving cells towards DNA replication in S-phase. Although the CDK inhibitory functions of p16INK4a are well described, the mechanisms underlying p16INK4a-mediated senescence are poorly understood and the relative contribution of p16INK4a-induced senescence to its role as a tumour suppressor has not been addressed.
A few studies have examined the impact of p16INK4a on the senescence of human dermal fibroblasts and melanocytes derived from rare melanoma-prone individuals carrying germline mutations in both INK4a/ARF alleles. These p16INK4a-deficient cells were resistant to oncogenic RAS-induced senescence (Huot etal., 2002; Jones etal., 2007) and had an extended, but finite lifespan that terminated with senescence (Sviderskaya etal., 2003; Brookes etal., 2004). Interpretation of these results is complicated, however, by the possible contribution of defective p14ARF (Huot etal., 2002; Sviderskaya etal., 2003; Brookes etal., 2004), which is known to confer a growth advantage when silenced (Voorhoeve & Agami, 2003). Moreover, there is considerable variability in the different cell strains with regard to their lifespan (Brookes etal., 2004; Jones etal., 2007), p16INK4a expression (Beausejour etal., 2003), chromosomal stability (Sviderskaya etal., 2003) and inducibility of p16INK4a by the RAS oncogene (Jones etal., 2007). To avoid some of these confounding effects, silencing molecules have been applied to deliberately and specifically ablate p16INK4a. Nevertheless, the data remain inconclusive; in most, but not all reports, p16INK4a deficiency modestly extended the replicative lifespan of cells but did not impair senescence (Bond etal., 1999; Voorhoeve & Agami, 2003; Denoyelle etal., 2006). p16INK4a was also not essential for H-RAS-induced melanocyte senescence (Denoyelle etal., 2006), although it was required for RAS-induced fibroblast senescence (Bond etal., 1999; Huot etal., 2002).
As an alternative strategy, we applied an inducible melanoma cell model to thoroughly characterize the p16INK4a senescence pathway, with a particular emphasis on the analysis of well-established markers of senescence. We then examined the impact of two melanoma-associated mis-sense p16INK4a mutations on the senescence of this melanoma cell model. By utilizing an inducible cell clone we eliminated cell-related variations and manipulated the induction of p16INK4a and melanoma-associated p16INK4a variants, to obtain near-physiological expression levels. As expected, the wild-type p16INK4a protein promoted rapid cell cycle arrest that was associated at later time points with the onset of senescence and the appearance of classic senescence markers, including enlarged cells with heterochromatic foci and SA-β-gal activity. As expected, these markers proved useful in combination, as none are specific or persistent in all senescence cells (Collado & Serrano, 2006). In fact, U20S cells induced to express wild-type p16INK4a acquired SA-β-gal activity, showed a large increase in cellular size but did not feature condensed chromatin. Although senescence-associated heterochromatic foci are associated with the silencing of E2F-1 genes (Narita etal., 2003), it is evident that they are late markers of senescence, occur later than E2F-1 target gene silencing (data not shown) and can be absent in highly vacuolized and arrested cells (Denoyelle etal., 2006).
The mis-sense p16INK4a variants, R24P and A36P, failed to inhibit proliferation and to initiate senescence. The R24P mutation was able to slightly reduce the hyperphopshorylation of Rb, presumably because it retains CDK6 inhibitory activity but this was not sufficient to maintain a G1 arrest. This is consistent with data indicating that this is a highly penetrant melanoma-susceptibility mutant that has been identified in at least eight melanoma-prone families worldwide (Goldstein etal., 2006b). Furthermore, it reinforces that CDK4, rather than CDK6 (Shennan etal., 2000), is the critical kinase in melanoma. CDK4 germline mutations have been identified in eight melanoma-prone families worldwide (Zuo etal., 1996; Soufir etal., 1998; Molven etal., 2005; Pjanova etal., 2007; Soufir etal., 2007) and these disrupt the interaction between p16INK4a and CDK4 (Zuo etal., 1996). Mouse embryonic fibroblasts derived from CDK4R24C/R24C mice (CDK4R24C is resistant to p16INK4a inhibition) (Rane etal., 2002) and human diploid fibroblasts overexpressing CDK4 have an extended lifespan (Morris etal., 2002; Ramirez etal., 2003) and carcinogen-treated mice carrying oncogenic CDK4 are highly susceptible to melanoma development (Sotillo etal., 2001). Our results demonstrate that ectopic expression of wild-type CDK4 overcame p16INK4a-induced arrest and senescence, and it is not surprising that overexpression of its homologue, CDK6, but not the downstream kinase CDK2, would abrogate p16INK4a activity. Thus, the ability of p16INK4a to bind and inhibit CDK4 and CDK6 is directly linked not only to cell cycle regulation but also to initiating the senescence programme.
The critical downstream target of the p16INK4a-CDK4/6 complex is Rb, which is strictly required for p16INK4a-mediated cell cycle arrest. Moreover, we now show that Rb is central to the p16INK4a-induced senescence programme, and that in the absence of Rb p16INK4a does not promote SA-β-gal activity or chromatin condensation. Surprisingly, the status of Rb did not affect the ability of p16INK4a to induce large increases in cellular size and extensive vacuolization, reminiscent of the morphological changes induced by various oncogenes (Denoyelle etal., 2006). The mechanism and impact of this p16INK4a activity remains to be defined, although it is dependent on CDK4/6 inhibition and is presumably associated with the ability of p16INK4a to elevate protein synthesis and ATP levels (Ausserlechner etal., 2005).
Although p16INK4a-induced cellular senescence provides an important brake to human cell transformation in culture its contribution to the tumour suppressor functions of p16INK4a has been poorly defined. Our data confirm that senescence induction is tightly linked to the cell cycle inhibitory actions of p16INK4a, and importantly that both these functions are disabled by highly penetrant melanoma-associated variants. Furthermore, our data identify CDK4 and CDK6 as the central kinase targets of p16INK4a in the regulation of senescence. Our results provide the first evidence that p16INK4a can initiate an autonomous senescence programme that is disabled by inherited melanoma-associated mutations. This is consistent with the notion that the senescence programme limits the development of tumours and the inability to initiate and maintain senescence is an important contributor to melanoma development.
Experimental procedures
Cell culture and transfections
Human WMM1175 melanoma cells (ARF-null, p53-null, Rb+/+; Rizos etal., 1999) and HEK293T cells were grown in Dulbecco's modified Eagle's medium (DMEM, Gibco BRL, Carlsbad, CA, USA) supplemented with 10% foetal bovine serum and glutamine. Human epidermal melanocytes (HEM1259) were obtained from Cell Applications (San Diego, CA, USA) and grown in HAM's F10 media, supplemented with ITS premix (Becton Dickinson, Franklin Lakes, NJ, USA), TPA, IBMX, cholera toxin, 20% foetal bovine serum and glutamine (modified from Halaban etal., 1986). All cells were cultured in a 37 °C incubator with 5% CO2.
The WMM1175_p16INK4a cell clones carrying the stably integrated p16INK4a(wild-type or mutant) gene under IPTG-inducible expression control has been described previously (Becker etal., 2001). The U20S_p16INK4a cell clones were generated as previously described (Becker etal., 2001), except that a pooled population of transfected cells was analysed. p16INK4a inducible cells were maintained in DMEM/10% foetal bovine serum supplemented with 250 µg mL−1 hygromycin and 500 µg mL−1 geneticin (Gibco). Stable cells were seeded 24 h prior to induction in the absence of antibiotics and were induced with 4 mm IPTG.
For CDK2, CDK4, CDK6 and Rb transfections, cells (1 × 105) were seeded on coverslips in six-well plates and transfected with 2 µg CDK4-pEGFPN1, CDK6-EYFPN1, CDK2-HA, Rb, pEGFPN1 (Clontech, Mountain View, CA, USA) or pCMV-HA vector using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA).
Lentivirus transductions
Lentiviruses were produced in HEK293T cells using the pSIH1-H1-copGFP (Copepod green fluorescent protein) shRNA expression vector (Systems Biosciences, Mountain View, CA, USA) encased in viral capsid encoded by three packaging plasmids as described previously (Dull etal., 1998). Viruses were concentrated as described previously (Reiser, 2000). Viral titres were determined using 1 × 105 U2OS cells/well in six-well plates, transduced with serial dilutions of the concentrated viral stocks in the presence of Polybrene (8 µg mL−1; Sigma, St. Louis, MO, USA). Cells were harvested 48 h post-transduction, analysed by flow cytometry for GFP expression and viral titre calculated.
For Rb silencing experiments, cells were transduced at an MOI of 10 with either a virus encoding Rb shRNA or a control shRNA, with no homology to any human gene. Cells were incubated for 72–96 h prior to analysis to allow expression of shRNA constructs and efficient silence of Rb.
Constructs
CDK6-EYFP was constructed by subcloning the CDK6 insert from CDK6-PVL1292(a gift from B. Sarcevic) into pEYFPC1 (Clontech). The Rb-directed shRNA sequence corresponds to nucleotides 662–680 (GenBank accession number NM_000321.1). The control shRNA sequence 5′-TTAGAGGCGAGCAAGACTA-3′ showed no homology to any known human transcript.
Western blotting
Total cellular proteins were extracted at 4 °C using RIPA lysis buffer containing protease inhibitors (Roche, Basel, Switzerland). Proteins (30–50 µg) were resolved on 12% sodium dodecyl sulfate–polyacrylamide gels and transferred to Immobilon-P membranes (Millipore, Bedford, MA, USA). Western blots were probed with antibodies against p16INK4a (N20, Santa Cruz Biotechnology, Santa Cruz, CA, USA), p21Waf1 (C-19, Santa Cruz), Grp 78 (H129, Santa Cruz), total Rb (aa 332-344, Becton Dickinson), phosphorylated p-RbSer807/811 (#9308, Cell Signalling, Danvers, MA, USA), α-tubulin (236-10501, Invitrogen), β-actin (AC-74, Sigma-Aldrich), CDK4 (DCS-31, Sigma-Aldrich) and CDK6 (K6.90+K6.83, Neomarkers, Fremont, CA, USA).
Proliferation assays
Cells were seeded at 1000 cells per well in a 96-well plate, with or without 4 mm IPTG. Number of viable cells was determined daily over 5-day induction period using the MTS assay (Promega, Madison, WI, USA) and analysed with the VICTOR2 1420 Multilabel Counter (PerkinElmer, Waltham, MA, USA).
Flow cytometry
For cell cycle analysis, cells were fixed in 70% ethanol at 4 °C for at least 1 h, washed in PBS and stained with propidium iodide (50 ng µL−1) containing ribonuclease A (50 ng µL−1). DNA content from at least 6000 cells was analysed using ModFIT software (Verity Software House, Topsham, ME, USA). The percentage of S-phase inhibition was calculated using the following formula: [(percentage of S-phase cells in uninduced cells) – (percentage of S-phase cells in induced cells)/(percentage of S-phase cells in uninduced cells)] × 100. Cell size and granularity was determined using flow cytometry on unfixed cells or cells fixed in 1% paraformaldehyde/PBS and analysed with CellQuest Pro (BD Biosciences)
Indirect immunofluorescence
Cultured cells (3–4 × 104) seeded on coverslips in 12-well plates were washed in PBS and fixed in 2% formaldehyde, 0.2% glutaraldehyde, 7.4 mm Na2HPO4, 1.47 mm KH2PO4, 137 mm NaCl, and 2.68 mm KCl. Cells were then rinsed three times with PBS and SA-β-gal activity was detected as previously described (Dimri etal., 1995). The same cells were immunostained for 50 min with primary antibodies followed by a 50-min exposure to Alexa Fluor 488- or Alexa Fluor 594-conjugated secondary IgG (Molecular Probes, Carlsbard, CA, USA). Nuclear DNA was stained with 1 µg mL−1 DAPI for 10–15 min.
Acknowledgments
CDK2 and CDK6 clones were kindly provided by Drs Brian Gabrielli and Boris Sarcevic, respectively. The Rb expression plasmid was a kind gift of Dr Eiji Hara. This work is supported by Program Grant 402761 of the National Health and Medical Research Council (NHMRC) of Australia and an infrastructure grant to Westmead Millennium Institute by the Health Department of New South Wales through Sydney West Area Health Service. Westmead Institute for Cancer Research is the recipient of capital grant funding from the Australian Cancer Research Foundation. H.R. is an RD Wright Fellow of the NHMRC and L.S. is a Cameron Melanoma Research Fellow, Melanoma and Skin Cancer Research Institute, University of Sydney. S.H. is a Cancer Institute of New South Wales Scholar and is supported by a PhD scholarship provided by the German Academic Exchange Service (DAAD).
References
- Alcorta DA, Xiong Y, Phelps D, Hannon G, Beach D, Barrett JC. Involvement of the cyclin-dependent kinase inhibitor p16 (INK4a) in replicative senescence of normal human fibroblasts. Proc. Natl Acad. Sci. USA. 1996;93:13742–13747. doi: 10.1073/pnas.93.24.13742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausserlechner MJ, Obexer P, Geley S, Kofler R. G1 arrest by p16 (INK4A) uncouples growth from cell cycle progression in leukemia cells with deregulated cyclin E and c-Myc expression. Leukemia. 2005;19:1051–1057. doi: 10.1038/sj.leu.2403729. [DOI] [PubMed] [Google Scholar]
- Bartkova J, Rezaei N, Liontos M, Karakaidos P, Kletsas D, Issaeva N, Vassiliou LV, Kolettas E, Niforou K, Zoumpourlis VC, Takaoka M, Nakagawa H, Tort F, Fugger K, Johansson F, Sehested M, Andersen CL, Dyrskjot L, Orntoft T, Lukas J, Kittas C, Helleday T, Halazonetis TD, Bartek J, Gorgoulis VG. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature. 2006;444:633–637. doi: 10.1038/nature05268. [DOI] [PubMed] [Google Scholar]
- Beausejour CM, Krtolica A, Galimi F, Narita M, Lowe SW, Yaswen P, Campisi J. Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J. 2003;22:4212–4222. doi: 10.1093/emboj/cdg417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker TM, Rizos H, Kefford RF, Mann GJ. Functional impairment of melanoma-associated p16INK4a mutants in melanoma cells despite retention of cyclin-dependent kinase 4 binding. Clin. Cancer Res. 2001;7:3282–3288. [PubMed] [Google Scholar]
- Bennett DC, Medrano EE. Molecular regulation of melanocyte senescence. Pigment Cell Res. 2002;15:242–250. doi: 10.1034/j.1600-0749.2002.02036.x. [DOI] [PubMed] [Google Scholar]
- Berwick M, Orlow I, Hummer AJ, Armstrong BK, Kricker A, Marrett LD, Millikan RC, Gruber SB, Anton-Culver H, Zanetti R, Gallagher RP, Dwyer T, Rebbeck TR, Kanetsky PA, Busam K, From L, Mujumdar U, Wilcox H, Begg CB. The prevalence of CDKN2A germ-line mutations and relative risk for cutaneous malignant melanoma: an international population-based study. Cancer Epidemiol. Biomarkers Prev. 2006;15:1520–1525. doi: 10.1158/1055-9965.EPI-06-0270. [DOI] [PubMed] [Google Scholar]
- Bishop DT, Demenais F, Goldstein AM, Bergman W, Bishop JN, Bressac-de Paillerets B, Chompret A, Ghiorzo P, Gruis N, Hansson J, Harland M, Hayward N, Holland EA, Mann GJ, Mantelli M, Nancarrow D, Platz A, Tucker MA, Consortium MG. Geographical variation in the penetrance of CDKN2A mutations for melanoma. J. Natl. Cancer Inst. 2002;94:894–903. doi: 10.1093/jnci/94.12.894. [DOI] [PubMed] [Google Scholar]
- Bond JA, Haughton MF, Rowson JM, Smith PJ, Gire V, Wynford-Thomas D, Wyllie FS. Control of replicative life span in human cells: barriers to clonal expansion intermediate between M1 senescence and M2 crisis. Mol. Cell. Biol. 1999;19:3103–3114. doi: 10.1128/mcb.19.4.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner AJ, Stampfer MR, Aldaz CM. Increased p16 expression with first senescence arrest in human mammary epithelial cells and extended growth capacity with p16 inactivation. Oncogene. 1998;17:1993–205. doi: 10.1038/sj.onc.1201919. [DOI] [PubMed] [Google Scholar]
- Brookes S, Rowe J, Gutierrez Del Arroyo A, Bond J, Peters G. Contribution of p16(INK4a) to replicative senescence of human fibroblasts. Exp. Cell Res. 2004;298:549–559. doi: 10.1016/j.yexcr.2004.04.035. [DOI] [PubMed] [Google Scholar]
- Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130:223–233. doi: 10.1016/j.cell.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Collado M, Gil J, Efeyan A, Guerra C, Schuhmacher AJ, Barradas M, Benguria A, Zaballos A, Flores JM, Barbacid M, Beach D, Serrano M. Tumour biology: senescence in premalignant tumours. Nature. 2005;436:642. doi: 10.1038/436642a. [DOI] [PubMed] [Google Scholar]
- Collado M, Serrano M. The power and the promise of oncogene-induced senescence markers. Nat. Rev. Cancer. 2006;6:472–476. doi: 10.1038/nrc1884. [DOI] [PubMed] [Google Scholar]
- Dai CY, Enders GH. p16 INK4a can initiate an autonomous senescence program. Oncogene. 2000;19:1613–1622. doi: 10.1038/sj.onc.1203438. [DOI] [PubMed] [Google Scholar]
- Della Torre G, Pasini B, Frigerio S, Rovini D, Delia D, Peters G, Huot T, Bianchi-Scarra G, Lantieri F, Rodolfo M, Parmiani G, Pierotti M. CDKN2A and CDK4 mutation analysis in Italian melanoma-prone families: functional characterization of a novel CDKN2A germ line mutation. Br. J. Cancer. 2001;85:836–844. doi: 10.1054/bjoc.2001.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denoyelle C, Abou-Rjaily G, Bezrookove V, Verhaegen M, Johnson TM, Fullen DR, Pointer JN, Gruber SB, Su LD, Nikiforov MA, Kaufman RJ, Bastian BC, Soengas MS. Anti-oncogenic role of the endoplasmic reticulum differentially activated by mutations in the MAPK pathway. Nat. Cell Biol. 2006;8:1053–1063. doi: 10.1038/ncb1471. [DOI] [PubMed] [Google Scholar]
- Di Micco R, Fumagalli M, Cicalese A, Piccinin S, Gasparini P, Luise C, Schurra C, Garre M, Nuciforo PG, Bensimon A, Maestro R, Pelicci PG, d’Adda di Fagagna F. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature. 2006;444:638–642. doi: 10.1038/nature05327. [DOI] [PubMed] [Google Scholar]
- Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl Acad. Sci. USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D, Naldini L. A third generation lentivirus vector with a conditional packaging system. J. Virol. 1998;72:8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Jin X, Xu HJ, Liu L, Peng HQ, Hogg D, Roth JA, Yu Y, Xu F, Bast RC, Jr, Mills GB. Expression of p16 induces transcriptional downregulation of the RB gene. Oncogene. 1998;16:1–8. doi: 10.1038/sj.onc.1201525. [DOI] [PubMed] [Google Scholar]
- Gething MJ. Role and regulation of the ER chaperone BiP. Semin. Cell Dev. Biol. 1999;10:465–472. doi: 10.1006/scdb.1999.0318. [DOI] [PubMed] [Google Scholar]
- Goldstein AM, Chan M, Harland M, Gillanders EM, Hayward NK, Avril MF, Azizi E, Bianchi-Scarra G, Bishop DT, Bressac-de Paillerets B, Bruno W, Calista D, Cannon Albright LA, Demenais F, Elder DE, Ghiorzo P, Gruis NA, Hansson J, Hogg D, Holland EA, Kanetsky PA, Kefford RF, Landi MT, Lang J, Leachman SA, Mackie RM, Magnusson V, Mann GJ, Niendorf K, Newton Bishop J, Palmer JM, Puig S, Puig-Butille JA, de Snoo FA, Stark M, Tsao H, Tucker MA, Whitaker L, Yakobson E. High-risk melanoma susceptibility genes and pancreatic cancer, neural system tumors, and Uveal melanoma across GenoMEL. Cancer Res. 2006a;66:9818–9828. doi: 10.1158/0008-5472.CAN-06-0494. [DOI] [PubMed] [Google Scholar]
- Goldstein AM, Chan M, Harland M, Hayward NK, Demenais F, Bishop DT, Azizi E, Bergman W, Bianchi-Scarra G, Bruno W, Calista D, Cannon-Albright LA, Chaudru V, Chompret A, Cuellar F, Elder DE, Ghiorzo P, Gillanders EM, Gruis N, Hansson J, Hogg D, Holland EA, Kanetsky PA, Kefford RF, Landi MT, Lang JM, Leachman S, Mackie RM, Magnusson V, Mann G, Newton-Bishop J, Palmer JM, Puig S, Puig-Butille JA, Stark M, Tsao H, Tucker MA, Whitaker L, Yakobson E, Genomel MG, Study Group LM Features associated with germline CDKN2A mutations: a GenoMEL study of melanoma-prone families from three continents. J. Med. Genet. 2006b;44:99–106. doi: 10.1136/jmg.2006.043802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray-Schopfer VC, Cheong SC, Chong H, Chow J, Moss T, Abdel-Malek ZA, Marais R, Wynford-Thomas D, Bennett DC. Cellular senescence in naevi and immortalisation in melanoma: a role for p16? Br. J. Cancer. 2006;95:496–505. doi: 10.1038/sj.bjc.6603283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruis NA, van der Velden PA, Sandkuijl LA, Prins DE, Weaver-Feldhaus J, Kamb A, Bergman W, Frants RR. Homozygotes for CDKN2 (p16) germline mutation in Dutch familial melanoma kindreds. Nat. Genet. 1995;10:351–353. doi: 10.1038/ng0795-351. [DOI] [PubMed] [Google Scholar]
- Halaban R, Ghosh S, Duray P, Kirkwood JM, Lerner AB. Human melanocytes cultured from nevi and melanomas. J. Invest. Dermatol. 1986;87:95–101. doi: 10.1111/1523-1747.ep12523594. [DOI] [PubMed] [Google Scholar]
- Harland M, Meloni R, Gruis N, Pinney E, Brookes S, Spurr NK, Frischauf A-M, Bataille V, Peters G, Cuzick J, Selby P, Bishop DT, Bishop JN. Germline mutations of the CDKN2 gene in UK melanoma families. Hum. Mol. Genet. 1997;6:2061–2067. doi: 10.1093/hmg/6.12.2061. [DOI] [PubMed] [Google Scholar]
- Holland EA, Schmid H, Kefford RF, Mann GJ. CDKN2A (p16INK4a) and CDK4 mutation analysis in 131 Australian melanoma probands: effect of family history and multiple primary melanomas. Genes Chromosomes Cancer. 1999;25:339–348. [PubMed] [Google Scholar]
- Huot TJ, Rowe J, Harland M, Drayton S, Brookes S, Gooptu C, Purkis P, Fried M, Bataille V, Hara E, Newton-Bishop J, Peters G. Biallelic mutations in p16(INK4a) confer resistance to Ras- and Ets-induced senescence in human diploid fibroblasts. Mol. Cell. Biol. 2002;22:8135–8143. doi: 10.1128/MCB.22.23.8135-8143.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DG, Walker CL. Cyclins and cell cycle checkpoint. Annu. Rev. Pharmacol. Toxicol. 1999;39:295–312. doi: 10.1146/annurev.pharmtox.39.1.295. [DOI] [PubMed] [Google Scholar]
- Jones R, Ruas M, Gregory F, Moulin S, Delia D, Manoukian S, Rowe J, Brookes S, Peters G. A CDKN2A mutation in familial melanoma that abrogates binding of p16INK4a to CDK4 but not CDK6. Cancer Res. 2007;67:9134–9141. doi: 10.1158/0008-5472.CAN-07-1528. [DOI] [PubMed] [Google Scholar]
- Kamijo T, Zindy F, Roussel MF, Quelle DE, Downing JR, Ashmun RA, Grosveld G, Sherr CJ. Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell. 1997;91:649–659. doi: 10.1016/s0092-8674(00)80452-3. [DOI] [PubMed] [Google Scholar]
- Mantelli M, Barile M, Ciotti P, Ghiorzo P, Lantieri F, Pastorino L, Catricala C, Torre GD, Folco U, Grammatico P, Padovani L, Pasini B, Rovini D, Queirolo P, Rainero ML, Santi PL, Sertoli RM, Goldstein AM, Bianchi-Scarra G. High prevalence of the G101W germline mutation in the CDKN2A (P16ink4a) gene in 62 Italian malignant melanoma families. Am. J. Med. Genet. 2002;107:214–221. [PubMed] [Google Scholar]
- McConnell BB, Gregory FJ, Stott FJ, Hara E, Peters G. Induced expression of p16INK4a inhibits both CDK4- and CDK2-associated kinase activity by reassortment of cyclin-CDK-inhibitor complexes. Mol. Cell. Biol. 1999;19:1981–1989. doi: 10.1128/mcb.19.3.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaloglou C, Vredeveld LC, Soengas MS, Denoyelle C, Kuilman T, van der Horst CM, Majoor DM, Shay JW, Mooi WJ, Peeper DS. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature. 2005;436:720–724. doi: 10.1038/nature03890. [DOI] [PubMed] [Google Scholar]
- Molven A, Grimstvedt MB, Steine SJ, Harland M, Avril MF, Hayward NK, Akslen LA. A large Norwegian family with inherited malignant melanoma, multiple atypical nevi, and CDK4 mutation. Genes Chromosomes Cancer. 2005;44:10–18. doi: 10.1002/gcc.20202. [DOI] [PubMed] [Google Scholar]
- Morris M, Hepburn P, Wynford-Thomas D. Sequential extension of proliferative lifespan in human fibroblasts induced by over-expression of CDK4 or 6 and loss of p53 function. Oncogene. 2002;21:4277–4288. doi: 10.1038/sj.onc.1205492. [DOI] [PubMed] [Google Scholar]
- Narita M, Nunez S, Heard E, Lin AW, Hearn SA, Spector DL, Hannon GJ, Lowe SW. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell. 2003;113:703–716. doi: 10.1016/s0092-8674(03)00401-x. [DOI] [PubMed] [Google Scholar]
- Pjanova D, Engele L, Randerson-Moor JA, Harland M, Bishop DT, Newton Bishop JA, Taylor C, Debniak T, Lubinski J, Kleina R, Heisele O. CDKN2A and CDK4 variants in Latvian melanoma patients: analysis of a clinic-based population. Melanoma Res. 2007;17:185–191. doi: 10.1097/CMR.0b013e328014a2cd. [DOI] [PubMed] [Google Scholar]
- Pomerantz J, Schreiber-Agus N, Liégeois NJ, Silverman A, Alland L, Chin L, Potes J, Chen K, Orlow I, Lee H-W, Cordon-Cardo C, DePinho RA. The Ink4a tumor suppressor gene product, p19Arf, interacts with MDM2 and neutralizes MDM2's inhibition of p53. Cell. 1998;92:713–723. doi: 10.1016/s0092-8674(00)81400-2. [DOI] [PubMed] [Google Scholar]
- Quelle DE, Zindy F, Ashmun RA, Sherr CJ. Alternative reading frames of the INK4a tumor suppressor gene encode two unrelated proteins capable of inducing cell cycle arrest. Cell. 1995;83:993–1000. doi: 10.1016/0092-8674(95)90214-7. [DOI] [PubMed] [Google Scholar]
- Ramirez RD, Herbert BS, Vaughan MB, Zou Y, Gandia K, Morales CP, Wright WE, Shay JW. Bypass of telomere-dependent replicative senescence (M1) upon overexpression of Cdk4 in normal human epithelial cells. Oncogene. 2003;22:433–444. doi: 10.1038/sj.onc.1206046. [DOI] [PubMed] [Google Scholar]
- Rane SG, Cosenza SC, Mettus RV, Reddy EP. Germline transmission of the CDK4(R24C) mutation facilitates tumorigenesis and escape from cellular senescence. Mol. Cell Biol. 2002;22:644–656. doi: 10.1128/MCB.22.2.644-656.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser J. Production and concentration of pseudotyped HIV-1-based gene transfer vectors. Gene Ther. 2000;7:910–913. doi: 10.1038/sj.gt.3301188. [DOI] [PubMed] [Google Scholar]
- Rizos H, Darmanian AP, Indsto JO, Shannon JA, Kefford RF, Mann GJ. Multiple abnormalities of the p16INK4a-pRb regulatory pathway in cultured melanoma cells. Melanoma Res. 1999;9:10–19. doi: 10.1097/00008390-199902000-00003. [DOI] [PubMed] [Google Scholar]
- Ruas M, Gregory F, Jones R, Poolman R, Starborg M, Rowe J, Brookes S, Peters G. CDK4 and CDK6 delay senescence by kinase-dependent and p16INK4a-independent mechanisms. Mol. Cell. Biol. 2007;27:4273–4282. doi: 10.1128/MCB.02286-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano M, Hannon GJ, Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993;366:704–707. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;85:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- Sharpless NE. INK4a/ARF: a multifunctional tumor suppressor locus. Mutat. Res. 2005;576:22–38. doi: 10.1016/j.mrfmmm.2004.08.021. [DOI] [PubMed] [Google Scholar]
- Shennan MG, Badin AC, Walsh S, Summers A, From L, McKenzie M, Goldstein AM, Tucker MA, Hogg D, Lassam N. Lack of germline CDK6 mutations in familial melanoma. Oncogene. 2000;19:1849–1852. doi: 10.1038/sj.onc.1203507. [DOI] [PubMed] [Google Scholar]
- Sherr CJ. Mammalian G1 cyclins [Review] Cell. 1993;73:1059–1065. doi: 10.1016/0092-8674(93)90636-5. [DOI] [PubMed] [Google Scholar]
- Sotillo R, Garcia JF, Ortega S, Martin J, Dubus P, Barbacid M, Malumbres M. Invasive melanoma in Cdk4-targeted mice. Proc. Natl Acad. Sci. USA. 2001;98:13312–13317. doi: 10.1073/pnas.241338598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soufir N, Avril M-F, Chompret A, Demenais F, Bombled J, Spatz A, Stoppa-Lyonnet D, Bénard J, Bressac-de Paillerets B, the French Familial Melanoma Group Prevalence of p16 and CDK4 germline mutations in 48 melanoma-prone families in France. Hum. Mol. Genet. 1998;7:209–216. doi: 10.1093/hmg/7.2.209. [DOI] [PubMed] [Google Scholar]
- Soufir N, Ollivaud L, Bertrand G, Lacapere JJ, Descamps V, Vitoux D, Lebbe C, Wolkenstein P, Dupin N, Saiag P, Basset-Seguin N, Grandchamp B. A French CDK4-positive melanoma family with a co-inherited EDNRB mutation. J. Dermatol. Sci. 2007;46:61–64. doi: 10.1016/j.jdermsci.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Stott FJ, Bates S, James MC, McConnell BB, Starborg M, Brookes S, Palmero I, Ryan K, Hara E, Vousden KH, Peters G. The alternative product from the human CDKN2A locus, p14ARF, participates in a regulatory feedback loop with p53 and MDM2. EMBO J. 1998;17:5001–5014. doi: 10.1093/emboj/17.17.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sviderskaya EV, Hill SP, Evans-Whipp TJ, Chin L, Orlow SJ, Easty DJ, Cheong SC, Beach D, DePinho RA, Bennett DC. p16Ink4a in melanocyte senescence and differentiation. J. Natl Cancer Inst. 2002;94:446–454. doi: 10.1093/jnci/94.6.446. [DOI] [PubMed] [Google Scholar]
- Sviderskaya EV, Gray-Schopfer VC, Hill SP, Smit NP, Evans-Whipp TJ, Bond J, Hill L, Bataille V, Peters G, Kipling D, Wynford-Thomas D, Bennett DC. p16/cyclin-dependent kinase inhibitor 2A deficiency in human melanocyte senescence, apoptosis, and immortalization: possible implications for melanoma progression. J. Natl Cancer Inst. 2003;95:723–732. doi: 10.1093/jnci/95.10.723. [DOI] [PubMed] [Google Scholar]
- Vogt M, Haggblom C, Yeargin J, Christiansen-Weber T, Haas M. Independent induction of senescence by p16INK4a and p21CIP1 in spontaneously immortalized human fibroblasts. Cell Growth Differ. 1998;9:139–146. [PubMed] [Google Scholar]
- Voorhoeve PM, Agami R. The tumor-suppressive functions of the human INK4A locus. Cancer Cell. 2003;4:311–319. doi: 10.1016/s1535-6108(03)00223-x. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Xiong Y, Yarbrough WG. ARF promotes MDM2 degradation and stabilizes p53: ARF-INK4a locus deletion impairs both the Rb and p53 tumor suppression pathways. Cell. 1998;92:725–734. doi: 10.1016/s0092-8674(00)81401-4. [DOI] [PubMed] [Google Scholar]
- Zhu J, Woods D, McMahon M, Bishop JM. Senescence of human fibroblasts induced by oncogenic Raf. Genes Dev. 1998;12:2997–3007. doi: 10.1101/gad.12.19.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo L, Weger J, Yang Q, Goldstein AM, Tucker MA, Walker GJ, Hayward N, Dracopoli NC. Germline mutations in the p16ink4a binding domain of CDK4 in familial melanoma. Nat. Genet. 1996;12:97–99. doi: 10.1038/ng0196-97. [DOI] [PubMed] [Google Scholar]