Abstract
Background:
When venous thromboembolism (VTE) includes deep-vein thrombosis (DVT) and pulmonary embolism (PE), patients with acute traumatic spinal cord injury (SCI) have the highest incidence of VTE among all hospitalized groups, with PE the third most common cause of death. Although low–molecular-weight heparin (LMWH) outperforms low-dose unfractionated heparin (LDUH) in other patient populations, the evidence in SCI remains less robust.
Objective:
To determine whether the efficacy for LMWH shown in previous SCI surveillance studies (eg, routine Doppler ultrasound) would translate into real-world effectiveness in which only clinically evident VTE is investigated (ie, after symptoms or signs present).
Methods:
A retrospective cohort study was conducted of 90 patients receiving LMWH dalteparin (5,000 U daily) or LDUH (5,000 U twice daily) for VTE prophylaxis after acute traumatic SCI. The incidence of radiographically confirmed VTE was primarily analyzed, and secondary outcomes included complications of bleeding and heparin-induced thrombocytopenia.
Results:
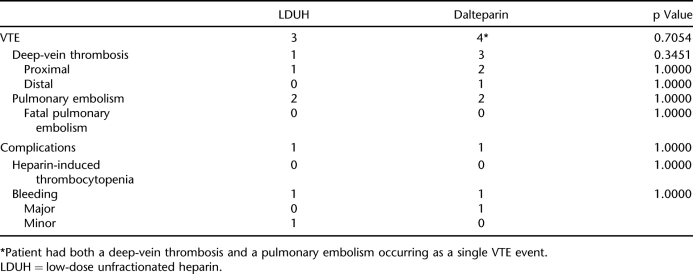
There was no statistically significant association (p = 0.7054) between the incidence of VTE (7.78% overall) and the type of prophylaxis received (LDUH 3/47 vs dalteparin 4/43). There was no significant differences in complications, location of VTE, and incidence of fatal PE. Paraplegia (as opposed to tetraplegia) was the only risk factor identified for VTE.
Conclusions:
There continues to be an absence of definitive evidence for dalteparin (or other LMWH) over LDUH as the choice for VTE prophylaxis in patients with SCI. Novel approaches to VTE prophylaxis are urgently required for this population, whose risk of fatal PE has not decreased over the last 25 years.
Keywords: Dalteparin; Heparin, low–molecular-weight; Pulmonary embolism; Rehabilitation; Spinal cord injuries; Venous thrombosis; Tetraplegia; Paraplegia
INTRODUCTION
When venous thromboembolism (VTE) includes deep-vein thrombosis (DVT) and pulmonary embolism (PE), patients with acute traumatic spinal cord injury (SCI) have the highest incidence of VTE among all hospitalized groups (1). In this population, PE accounts for 37% of all deaths in those not receiving VTE prophylaxis (2) and remains the third most common cause of death (3). The risk of fatal PE has not decreased over the last 25 years (3), being 210 times that in a similar healthy population (4). Depending on the diagnostic investigation, the incidence of asymptomatic DVT is 60% to 100% in acute SCI when surveillance techniques (routine screening) are applied (5–7). In contrast, clinically evident DVT (ie, with apparent symptoms and/or signs) occurs in only 10% and PE in 3% of patients with acute SCI (8), with a blended VTE 91-day incidence of 5.4% (9).
Methods of VTE prophylaxis include mechanical (pressure-graded elastic stockings [GES], external pneumatic compression stockings, venous foot pump, and neuromuscular electrical stimulation), pharmacologic (aspirin, dipyridamole, warfarin, adjusted-dose or low-dose unfractionated heparin [LDUH], low–molecular-weight heparin [LMWH]), and surgical/invasive (inferior vena cava filters) (1,10,11).
There is a significant body of literature presenting the incidence of VTE in SCI using surveillance techniques and comparing LMWH with LDUH. Retrospective trials suggest a correlation between increased use of LMWH and a lower incidence of VTE (12,13). Prospective trials have shown a nonstatistically significant trend towards fewer thrombotic events and a significant reduction in bleeding complications with LMWH (14,15). Randomized controlled trials after major trauma (16) demonstrate fewer VTE events in the LMWH group, but no conclusions could be drawn with the subgroup of patients with SCI. Randomized controlled trials in SCI (7,17) revealed no difference in overall VTE during the acute phase (within 2 weeks), but fewer PEs were recorded in the LMWH group (17). During the rehabilitation phase of patients with SCI (weeks 2–8), LMWH outperformed LDUH with fewer VTE events (5/59 vs 13/60, respectively), although statistical significance was not quite reached (p = 0.052) (7). This latter study was criticized because it included an exclusion rate of >75% and the use of ultrasound as the surveillance diagnostic test, which has been shown not to be highly sensitive in asymptomatic postoperative patients (18–20).
There has only been 1 prospective, randomized trial of clinically evident VTE in patients with SCI comparing LMWH with LDUH (21). Despite not reaching statistical significance, this German study identified trends in the ability of LMWH to prevent VTE compared with LDUH (6/80 vs 12/86, respectively) and promote fewer proximal VTEs (21). The literature is not yet definitive, as evidenced by the aforementioned studies and other descriptive studies (22–27). LMWH has become the standard of care for pharmacologic VTE prophylaxis in acute SCI. This is reflected in various guidelines (1,10,28) and the shift in clinical practice within our center from LDUH to the LMWH dalteparin during the mid to late 1990s. This led to our historical cohort chart review to compare these 2 pharmacologic agents, and we hypothesized that the incidence of VTE and prophylaxis complications (major bleeding and heparin-induced thrombocytopenia [HIT]) would decrease with the use of dalteparin vs LDUH when used in acute traumatic SCI. In essence, the purpose of our study was to determine whether the efficacy of LMWH shown in previous surveillance studies would translate into real-world effectiveness in which only clinically evident VTE is investigated.
METHODS
Study Design and Patients
Ethics approval was received from our institution. All patients who sustained acute traumatic SCI from 1994 to 2004 and received care at our institution were included in the study. However, during 1997 and 1998, there was a transition from the routine use of LDUH to LMWH dalteparin for VTE pharmacologic prophylaxis within our center. The treatment of most of these patients would have been switched from LDUH to dalteparin during the prophylaxis period (usually occurring upon transfer from the acute care facility to the rehabilitation facility). Therefore the patients admitted in 1997 and 1998 were excluded from our study. The acute care (including the use of intravenous methylprednisolone) of patients with acute traumatic SCI was unchanged from 1994–2004.
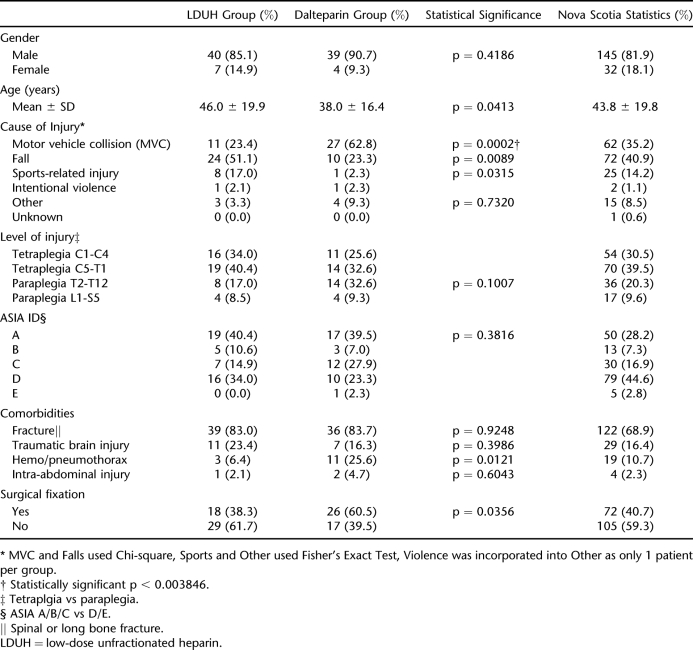
A total of 189 patient charts were reviewed. Ninety-nine patients were excluded (Table 1), with 47 remaining in the LDUH group (heparin 5,000 U subcutaneous twice daily) and 43 in the LMWH group (dalteparin [Fragmin] 5,000 units subcutaneous daily). There were 43 patients who did not receive pharmacologic prophylaxis primarily because they had mild injuries, were ambulatory, and were expected to be discharged within a few days. Epidemiological data have never been published on patients with traumatic SCI who were injured within the Province of Nova Scotia, Canada. Table 2 provides this data for the 9 years that data were collected.
Table 1.
Reasons for Exclusion
Table 2.
Patient Demographics
Outcome Measures
Our primary analysis was the incidence of radiographically confirmed clinically evident VTE (ie, after the development of symptoms or signs). The diagnostic criterion for DVT was failure to compress a deep-venous segment involving the proximal (at or above the popliteal fossa) or distal (calf) veins. PE was diagnosed by ventilation-perfusion lung scan (high probability), computed tomographic scan of the chest, or pulmonary angiography. Secondary outcome measures included the development of HIT and bleeding complications. Bleeding episodes were classified as major or minor (29). Major bleeding required one of the following criteria: a fall in hemoglobin (Hgb) of 20 g/L or more, transfusion of 2 or more units of packed cells, retroperitoneal or intracranial bleeding, or bleeding warranting treatment cessation. Minor bleeding was defined as clinically overt bleeding but not meeting the criteria for major bleeding.
Statistical Analysis
Our primary and secondary analyses required the use of Fisher's exact tests due to small cell counts. A p value <0.05 was considered statistically significant. When comparing demographic variables, a Bonferroni adjusted p value was used to correct for multiple comparisons (p = 0.05/13 variables = 0.003846). Cause of injury used a Fisher's exact test for “sports-related injury” and “other,” whereas “motor vehicle collision” and “fall” categories required a chi-square test. Demographic variables were evaluated for an association with VTE (ie, risk factors) using Fisher's exact test for categorical variables, and the nonparametric Wilcoxon rank sum test was used for continuous variables.
RESULTS
Demographic Variables and VTE Data
The 2 groups were similar except for cause of injury, with more motor vehicle collisions in the dalteparin group (Table 2). Seven VTEs were identified among the 90 patients enrolled in the study (7.78% incidence). There was no statistically significant association between the incidence of VTE and the type of prophylaxis received (Table 3). There was also no association between the location of DVT (proximal vs distal) and the 2 treatment groups (Table 3). The standard of care for all patients was to receive GES upon admission as part of routine nonpharmacologic VTE prophylaxis. Three patients on LDUH and 2 patients on dalteparin were not recorded to have worn GES, but none of these patients developed a VTE. A subgroup analysis was conducted excluding patients with ASIA D and E injuries. When considering only ASIA A/B/C injuries, 3 of 31 patients (9.7%) in the LDUH group and 3 of 32 (9.4%) in the dalteparin developed VTE, a nonstatistically significant association (p = 1.000).
Table 3.
Incidences of Venous Thromboembolism and Complications
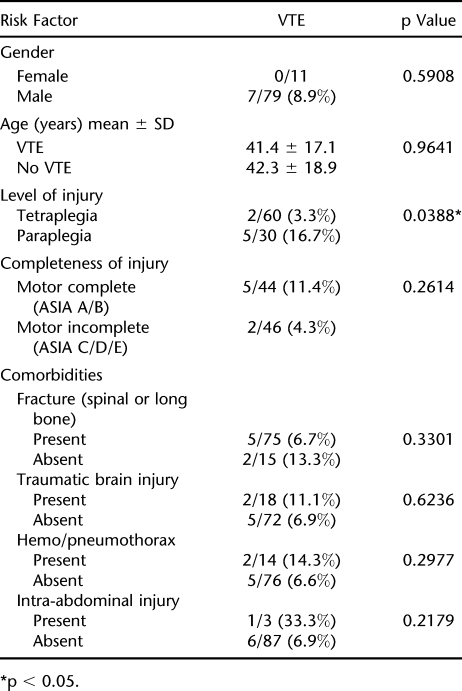
In our study, paraplegia (compared with tetraplegia) was the only risk factor identified to increase the incidence of VTE (Table 4).
Table 4.
Risk Factors for Venous Thromboembolism (VTE)
Complications and Deaths
No patients in this study were diagnosed with HIT (Table 3). There was no statistically significant association between bleeding complications and treatment group (p = 1.0000) (Table 3). The single major bleeding episode was in the dalteparin group. This patient suffered a concomitant severe traumatic brain injury and 22 days after his injury was shown to have an ICU stress ulcer upon investigation with gastroscopy. Temporary dalteparin cessation was required for 9 days, and he did not have a significant drop in Hgb.
Of note, 1 patient receiving dalteparin who was excluded from the study because his initial care was outside of our facility had a gluteal hematoma 50 days after his injury. This required his VTE prophylaxis to be discontinued. Hgb fell to 78 g/L, but he did not require a blood transfusion.
There was a total of 9 deaths, 7 in the LDUH group and 2 in the dalteparin group. None of these was felt to be secondary to a VTE. However, the causes of death were not confirmed by autopsy. Details of each case will therefore be provided below.
There were 7 patient deaths within the LDUH group. (a) A 70-year-old man suffered cardiac arrest on transfer to the acute care facility. He developed acute renal failure secondary to rhabdomyolysis. Seven days after his injury, he died of a respiratory arrest during dialysis. (b) A 26-year-old man with a high cervical ASIA A injury was admitted with hypothermia and hypotension. Four days after his injury, his family withdrew care and his ventilation was discontinued. (c) A 74-year-old man had a very high cervical injury and lower medulla ASIA A injury and wished not to be ventilator dependent. His care was withdrawn 6 days after his injury. (d) A 69-year-old man with end-stage renal disease experienced a high cervical ASIA A injury. He suffered an acute myocardial infarction in hospital with subsequent hypotension. A decision was made to palliate, and he died 11 days after his injury. (e) An 85-year-old man developed a low cervical ASIA A SCI after a fall in addition to an intraventricular hemorrhage. During intubation, he experienced aspiration pneumonia and a cardiac arrest on transfer to the acute care facility. He succumbed to a respiratory arrest 6 days after his injury. (f) A 67-year-old woman with frontal lobe dementia suffered a high cervical ASIA C SCI. She developed respiratory failure 3 days after her injury and possibly aspiration pneumonia. After she did not respond to antibiotics, her care was changed to comfort measures only, and she died 10 days after her injury. An autopsy was performed, but the results were not made available for our study. (g) A 68-year-old man with a very high cervical ASIA C SCI suffered cardiac arrest and subsequent anoxic brain injury on transfer to the acute care facility. His care was withdrawn in the fourth day after his injury.
The 2 deaths within the dalteparin group were reviewed. (a) A 73-year-old man with a C5 ASIA A SCI and a do-not-resuscitate order died secondary to respiratory failure 77 hours after his injury. An autopsy was not performed. (b) A 35-year-old man with a high cervical ASIA A or B SCI suffered a concomitant severe traumatic brain injury. Off of the ventilator, he scored a 3T on Glasgow Coma Score with brainstem reflexes only. His care was withdrawn, and he died 78 hours after injury.
Investigations and Presentation
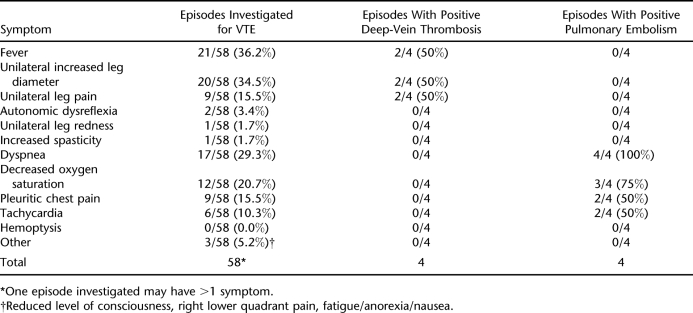
The 4 PEs were diagnosed by ventilation-perfusion scan (2 cases), chest computed tomographic scan (1 case), and pulmonary angiography (1 case). All 4 DVTs were diagnosed by compression ultrasound. The average time from injury to onset of symptoms and/or signs that led to a positive investigation for VTE was 38.1 ± 26.6 days (range 8–98 days) after injury. A total of 58 episodes were investigated (occurrences in which symptoms and/or signs were investigated for VTE), with 8 being positive for VTE (Table 5). Therefore, 0.65 episodes were investigated per patient, of which 8 of 58 (13.8%) led to the diagnosis of a DVT or PE. Of note, screening (ie, surveillance testing) for VTE was not routine but was carried out on 11 patients. Three had a d-dimer, 3 had a compression ultrasound, and 5 had both. None of these screening procedures led to the positive identification of a VTE.
Table 5.
Episodes Leading to Investigations for Venous Thromboembolism*
DISCUSSION
The 7.78% incidence of VTE among the 90 patients enrolled compares similarly to other reports of primarily clinically evident VTE (8,9,21). Our study found no difference in the incidence of overall VTE, proximal VTE, or VTE among more severe injuries (ASIA A/B/C), regardless of the type of heparin used. We may conclude from this that dalteparin may not be as efficacious vs LDUH as it is in other patient populations. Alternatively, given the number of patients, it may be that our study was underpowered to achieve this conclusion. We also failed to demonstrate a significant reduction in bleeding and HIT complications in the dalteparin group, as did other investigators (14,15). The largest randomized study of VTE prophylaxis in patients with SCI also found only trends in reduced bleeding complications with LMWH and did not show a significant difference when comparing with LDUH (7,17). Perhaps, then, LMWH is safer in some clinical populations but not the SCI population.
A review of the pathophysiology of VTE in SCI may help us understand why LMWH does not appear to be as efficacious or safe as in other populations. The etiology of VTE in acute traumatic SCI can be conceptualized by Virchow's triad of venous stasis, endothelial integrity, and hypercoagulability. Venous stasis can be caused by immobilization related to weakness, operating room time, comorbid fractures requiring casting, orthoses (eg, halo, TLSO), and sedatives (30,31). Immobilization and weakness may explain some of the increased VTE risk as noted by a 10-fold increase in the DVT risk of a paretic leg in stroke compared with the nonparetic leg (32). However, in SCI, there is also a loss of sympathetic input to vasoconstrict blood vessels (33). Decreased venous competence may be secondary to decreased venous distensibility/capacity and an increase in venous flow resistance (30,31). Given these changes, some postulate that anticoagulation alone is not effective in patients with SCI and therefore advocate multiple mechanical and pharmacologic methods of VTE prophylaxis (26).
Endothelial integrity may also be compromised by trauma. However, in acute traumatic SCI, researchers have also noted decreased fibrinolytic reactivity (which is closely related to endothelial integrity), increased d-dimer, and impaired rhythmical circadian variations in fibrinolytic parameters possibly secondary to a deregulated autonomic nervous system (30,31). A decrease in fibrinolysis may explain the increased proximal migration of DVTs (31), persistence and recurrence of VTEs despite adequate anticoagulation (34), and low rates of venous recanalization in the SCI population (35,36).
Hypercoagulability represents the last component of Virchow's triad and is especially interesting in patients with SCI. Documented changes to coagulation include an increase in platelets, factor VIII, vWF, platelet aggregation (returns to normal in later stages of injury), fibrinogen, euglobulin clot lysis time, plasma alpha-1 antitrypsin activity, and antigen concentration (30,31). Decreased are plasma alpha-2 antiplasmin antigen concentration and total antiplasmin activity. Although the pathophysiology behind all of these changes has not been fully elucidated, many of them are felt to be related to neurohormonal factors induced by the SCI (30,31).
The aforementioned VTE etiologic risk factors specific to SCI may explain one study's increased incidence of clinically evident VTE in patients with acute SCI (64%) compared with immobilized trauma patients with vertebral fracture but no paralysis (0%) (37). Although there is good evidence for LMWH in other patient groups, including trauma and joint arthroplasty (1), individuals with SCI may need special considerations given these specific etiologic factors.
Numerous variables have been associated with an increased risk of VTE in patients with SCI. These include but are not limited to advanced age (1,10,12,17,38,39), male gender (9,39), level of injury (paraplegia vs tetraplegia) (9,39), completeness of injury (motor complete vs motor incomplete) (21,39), history of thrombosis (10,38), lower extremity fracture (1,10,38,40), dehydration (38), flaccid paralysis (12), obesity (10,12,15,38), delayed thromboprophylaxis (1), estrogen therapy (38), pregnancy (38), heterotopic ossification (41) and various comorbidities, such as cancer (10,12), congestive heart failure (9,10,38), chronic obstructive pulmonary disease (9), and diabetes mellitus (9). The largest epidemiological study of VTE in SCI by Jones et al (9) of 16,240 patients found male sex, African American race, complete paraplegia vs tetraplegia, and the presence of 3 or more (of 30) comorbid conditions vs none to be significant predictors of VTE. Being younger than 14 years of age was predictive of not developing VTE. Paraplegia (vs tetraplegia) was the only risk factor identified in our study to show an association with increased incidence of VTE. However, it is likely that due to the small numbers in our study, we were underpowered to show a significant association with any of the other variables investigated.
Although there were only 7 VTE events found within our study population, a few points of interest may be gleaned from further analysis. Notably, the mean duration to VTE presentation was 38.1 ± 26.6 days (range 8–98 days) after injury. This is consistent with other reports and highlights the fact that most VTEs will occur within the timeframe of pharmacologic prophylaxis proposed within guidelines (10). However, some VTEs may still occur outside of this window, after VTE prophylaxis has been discontinued secondary to an unfavorable risk-benefit ratio. The ideal duration of prophylaxis and stratification of subgroups requiring a longer duration based on VTE risk factors within the SCI population require further study.
Only 13.8% of episodes investigated for VTE actually led to the diagnosis of a VTE in our study. Models have been identified to stratify outpatient individuals to high, medium, or low probability for a positive VTE depending on certain risk factors, symptoms, or signs (42). Within our study, the only symptoms/signs that led to a positive investigation for DVT were fever, unilateral increased leg diameter, and unilateral leg pain. Each of these symptoms/signs was present in 50% of the positive DVT symptom events. For PE, the symptom/signs were dyspnea, decreased oxygen saturation, pleuritic chest pain, and tachycardia. These occurred in 100%, 75%, 50%, and 50% of the positive PE symptom events, respectively. If clinical models are ever developed for inpatients with SCI, these symptoms may be used to increase the pretest probability of VTE diagnostic tests within this population.
Among the notable articles comparing LDUH with LMWH, patients with lumbosacral injuries, ASIA C and/or D classifications, and concomitant traumatic brain injury have been excluded from study (7,14,15,17). These populations represent a significant proportion of SCI patients who are typically provided pharmacologic VTE prophylaxis. One strength of our study is improved generalizability with the inclusion of SCI patients of all spinal and ASIA levels and those who suffer concomitant traumatic brain injury. In addition, our study provided all patients with GES, whereas 1 large randomized controlled trial of patients with SCI in rehabilitation (7) did not use GES in the LMWH group.
Among those excluded from our study, 6 were diagnosed with VTE, each of which raises important issues for VTE prophylaxis. Patients whose injury occurred elsewhere and were later transferred to our facility were excluded because we could not guarantee consistency in their acute care. Of the 12 patients in this category, 3 developed VTE.
Fourteen patients had their prophylaxis started more than 72 hours after their injury. One of these patients was diagnosed with a DVT and a concomitant PE 2 weeks after his injury. His prophylaxis was initiated on day 4, shortly after he developed symptoms of VTE. This reinforces the importance of the guideline to perform an ultrasound of the legs if prophylaxis has not been started within the first 72 hours of injury (10).
Of 12 patients switched from LDUH to dalteparin, 1 patient whose switch occurred on day 17 after injury developed a DVT on day 37. Another patient excluded due to a previous history of DVT and PE developed a DVT. This raises the controversial options of prophylactic anticoagulation or inferior vena cava filter in this unique population of patients with traumatic SCI and a premorbid diagnosis of VTE.
We would argue that compared with surveillance testing, evaluating for clinically evident VTE is more appropriate within both clinical and research settings. This represents the “real-world” situation in which patients are only investigated upon presentation of VTE symptoms or signs. Therefore, research in this context is more generalizable. Moreover, the accuracy of ultrasound in postoperative asymptomatic individuals is poor (1,19,20), and DVTs are more likely to be proximal if they are symptomatic (at or proximal to the popliteal vein) (18). Clinically evident DVTs therefore represent a more significant form of VTE given the increased likelihood of proximal migration/extension (18). Because VTE surveillance detects approximately 4 times as many VTEs (80% vs 20%), perhaps most DVTs in surveillance studies are clinically insignificant or false positives (43). The 2004 American College of Chest Physicians VTE prophylaxis guidelines for patients with acute traumatic SCI suggest the need for future studies to include clinically evident VTE (1).
Although some advocate surveillance testing, the level of evidence has not become a standard of care within VTE prophylaxis guidelines (1,10). However, some advantages of screening for VTE include the inaccuracy of the clinical assessment in the diagnosis of DVT, with a sensitivity of only 30% (44,45). This figure may drop further in patients with SCI due to reduced sensation and the horizontal position decreasing obvious leg swelling. Dyspnea, as a symptom of PE, may often be presumed clinically to represent atelectasis or pneumonia. On the other hand, spasticity and autonomic dysreflexia may act as sensitive (but nonspecific) indicators of VTE. Screening techniques may identify a preclinical DVT, reducing the likelihood of sudden PE (possibly during sleep) and potential mortality. In fact, a 2002 study showed surveillance ultrasound to detect DVT in 27% of SCI patients, and 0.77% of patients in this group suffered nonfatal PE. In contrast, when clinical diagnosis was used in the same population, DVT was only detected in 9%, and 4.3% of patients suffered PE, including 3.5% with fatal PE (24).
Some authors propose surveillance with d-dimer testing upon admission to a rehabilitation center. This has been shown to have 100% sensitivity and 100% negative predictive value in 29 patients with SCI (46). However, a high false-positive rate was associated with comorbidities, such as urinary tract infection, pneumonia, heterotopic ossification, and pressure ulcers (46). Another study found that d-dimer screening can decrease the need for screening ultrasound by 31% if ultrasound is carried out only on patients with a positive d-dimer (47). Studies of VTE surveillance with duplex ultrasound in patients with SCI at rehabilitation admission have found incidences of 8 of 92 (8.7%) (48) and 22 of 189 (11.6%) (49). However, in the latter series, <40% of patients had appropriate VTE prophylaxis at the time of their rehabilitation admission. Surveillance ultrasound at admission to rehabilitation has been shown to be cost effective in the SCI population (50) and has been recommended by some authors to be completed upon changing facility (12).
The main limitations of our study include the lack of causality and biases inherent in retrospective cohort designs. This was most evident in the number of patients excluded secondary to their prophylaxis not being provided within 72 hours of injury and those switched from LDUH to dalteparin partway through their course of VTE prophylaxis. The latter scenario was still present despite the exclusion of the primary 2 years of data in which this clinical shift in practice occurred. The number of patients enrolled provides relatively low support to determine a significant difference in our primary and secondary outcome measures. In addition, subgroup analyses, such as comparison of demographic variables between groups and VTE risk factor identification, were limited by the sample size.
In our series, although there were more deaths in the LDUH group (9 vs 2 in the dalteparin group), none were attributed to VTE.
However, autopsies were not performed given the retrospective nature of our study, whereas a prospective design could include autopsy as a requirement. Our findings are limited to dalteparin and cannot necessarily be generalized to other forms of LMWH, despite the current absence of documented efficacy differences between the various types of LMWH for VTE prophylaxis in the patients with SCI. We may only comment on the use of LDUH at a dose of 5,000 U twice daily. Other authors have used higher doses, such as 5,000 U 3 times daily (7,12–14,17) or 7,500 U twice daily (21).
In fact, the incidence of VTE in the SCI population is still alarmingly high despite our best treatments. Therefore, we urgently need novel approaches (or combinations of approaches) toward VTE prophylaxis in the acute traumatic SCI population. New anticoagulant considerations include direct thrombin inhibitors or indirect factor Xa inhibitors (51). Various combinations of approaches may also be warranted. For example, an Italian study combined LMWH, early mobilization, GES, and external pneumatic compression stockings during the first 30 days after SCI and found that only 2% of 99 patients developed DVT as detected by clinical means or ultrasound surveillance after 30 to 45 days (24). These combinations may or may not include clinical models to alter pretest probability of diagnostic tests and screening investigations, such as d-dimer testing. Head-to-head trials comparing LDUH (perhaps at higher doses) with other forms of LMWH could also be considered.
CONCLUSIONS
Among 90 patients with acute traumatic SCI admitted to our facility over 9 years, there was no significant association between the type of heparin used (dalteparin vs LDUH) and the incidence of VTE (7.78% overall, no fatal PE) or complications of VTE prophylaxis (bleeding and HIT). There continues to be an absence of definitive evidence for LMWH over LDUH in these patients. Paraplegia was associated with an increased incidence of VTE when compared with tetraplegia. Novel approaches to VTE prophylaxis are urgently required for this population, whose risk of fatal PE has not decreased over the last 25 years (3).
REFERENCES
- Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy Chest. 2004126 (suppl 3) S338–S400. [DOI] [PubMed] [Google Scholar]
- Tribe C. Cause of death in the early and late stages of paraplegia. Paraplegia. 1963;1:19–47. doi: 10.1038/sc.1963.3. [DOI] [PubMed] [Google Scholar]
- DeVivo MJ, Krause JS, Lammertse DP. Recent trends in mortality and causes of death among persons with spinal cord injury. Arch Phys Med Rehabil. 1999;80:1411–1419. doi: 10.1016/s0003-9993(99)90252-6. [DOI] [PubMed] [Google Scholar]
- DeVivo MJ, Stover SL. Long-term survival and causes of death. In: Stover SL, DeLisa JL, Whiteneck GG, editors. Spinal Cord Injury: Clinical Outcomes From the Model Systems. Gaithersburg, MD: Aspen Publishers; 1995. pp. 289–316. [Google Scholar]
- Geerts WH, Heit JA, Clagett GP, et al. Prevention of venous thromboembolism Chest. 2001119 (suppl 1) S132–S175. [DOI] [PubMed] [Google Scholar]
- Attia J, Ray JG, Cook DJ, Douketis J, Ginsberg JS, Geerts WH. Deep vein thrombosis and its prevention in critically ill adults. Arch Intern Med. 2001;161:1268–1279. doi: 10.1001/archinte.161.10.1268. [DOI] [PubMed] [Google Scholar]
- Spinal Cord Injury Thromboprophylaxis Investigators. Prevention of venous thromboembolism in the rehabilitation phase after spinal cord injury: prophylaxis with low-dose heparin or enoxaparin. J Trauma. 2003;54:1116–1126. doi: 10.1097/01.TA.0000042159.90102.C2. [DOI] [PubMed] [Google Scholar]
- Chen D, Apple DF, Jr, Hudson LM, Bode R. Medical complications during acute rehabilitation following spinal cord injury: current experience of the model systems. Arch Phys Med Rehabil. 1999;80:1397–1401. doi: 10.1016/s0003-9993(99)90250-2. [DOI] [PubMed] [Google Scholar]
- Jones T, Ugalde V, Franks P, Zhou H, White RH. Venous thromboembolism after spinal cord injury: incidence, time course, and associated risk factors in 16,240 adults and children. Arch Phys Med Rehabil. 2005;86:2240–2247. doi: 10.1016/j.apmr.2005.07.286. [DOI] [PubMed] [Google Scholar]
- Consortium for Spinal Cord Medicine. Prevention of Thromboembolism in Spinal Cord Injury. Washington, DC: Paralyzed Veterans of America; 1999. [Google Scholar]
- Johns JS, Nguyen C, Sing RF.Vena cava filters in spinal cord injuries: evolving technology J Spinal Cord Med. 200629 (3) 183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D, Hartwig D, Chen D, Soltysik RC, Yarnold PR. Spinal cord injury risk assessment for thromboembolism (SPIRATE study) Am J Phys Med Rehabil. 2003;82:950–956. doi: 10.1097/01.PHM.0000098043.88979.BA. [DOI] [PubMed] [Google Scholar]
- Green D, Sullivan S, Simpson J, Soltysik RC, Yarnold PR. Evolving risk for thromboembolism in spinal cord injury (SPIRATE study) Am J Phys Med Rehabil. 2005;84:420–422. doi: 10.1097/01.phm.0000163714.73660.70. [DOI] [PubMed] [Google Scholar]
- Green D, Lee MY, Lim AC, et al. Prevention of thromboembolism after spinal cord injury using low-molecular-weight heparin. Ann Intern Med. 1990;113:571–574. doi: 10.7326/0003-4819-113-8-571. [DOI] [PubMed] [Google Scholar]
- Green D, Chen D, Chmiel JS, et al. Prevention of thromboembolism in spinal cord injury: role of low molecular weight heparin. Arch Phys Med Rehabil. 1994;75:290–292. doi: 10.1016/0003-9993(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Geerts WH, Jay RM, Code KI, et al. A comparison of low-dose heparin with low–molecular-weight heparin as prophylaxis against venous thromboembolism after major trauma. N Engl J Med. 1996;335:701–707. doi: 10.1056/NEJM199609053351003. [DOI] [PubMed] [Google Scholar]
- Spinal Cord Injury Thromboprophylaxis Investigators. Prevention of venous thromboembolism in the acute treatment phase after spinal cord injury: a randomized, multicenter trial comparing low-dose heparin plus intermittent pneumatic compression with enoxaparin. J Trauma. 2003;54:1111–1115. doi: 10.1097/01.TA.0000066385.10596.71. [DOI] [PubMed] [Google Scholar]
- Kearon C, Julian JA, Math M, Newman TE, Ginsberg JS.Noninvasive diagnosis of deep venous thrombosis Ann Intern Med. 1998128 (8) 663–677. [DOI] [PubMed] [Google Scholar]
- Wells PS, Lensing AW, Davidson BL, Prins MH, Hirsh J. Accuracy of ultrasound for the diagnosis of deep venous thrombosis in asymptomatic patients after orthopedic surgery: a meta-analysis. Ann Intern Med. 1995;122:47–53. doi: 10.7326/0003-4819-122-1-199501010-00008. [DOI] [PubMed] [Google Scholar]
- Jongbloets LM, Lensing AW, Kiipman MM, Bűller HR, ten Cate JW. Limitations of compression ultrasound for the detection of symptomless postoperative deep vein thrombosis. Lancet. 1994;343:1142–1144. doi: 10.1016/s0140-6736(94)90240-2. [DOI] [PubMed] [Google Scholar]
- Lohmann U, Glaser E, Braun BE, Botel U.Prevention of thromboembolism in spinal fractures with spinal cord injuries: standard heparin versus low–molecular-weight heparin in acute paraplegia [in German] Zentralbl Chir. 2001126 (5) 385–390. [DOI] [PubMed] [Google Scholar]
- Riklin C, Baumberger M, Wick L, Michel D, Sauter B, Knecht H. Deep vein thrombosis and heterotopic ossification in spinal cord injury: a 3-year experience at the Swiss Paraplegic Centre Nottwil. Spinal Cord. 2003;41:192–198. doi: 10.1038/sj.sc.3101421. [DOI] [PubMed] [Google Scholar]
- Harris S, Chen D, Green D. Enoxaparin for thromboembolism prophylaxis in spinal injury: preliminary report on experience with 105 patients. Am J Phys Med Rehabil. 1996;75:326–327. doi: 10.1097/00002060-199609000-00002. [DOI] [PubMed] [Google Scholar]
- Aito S, Pieri A, D'Andrea M, Marcelli F, Cominelli E. Primary prevention of deep venous thrombosis and pulmonary embolism in acute spinal cord injured patients. Spinal Cord. 2002;40:300–303. doi: 10.1038/sj.sc.3101298. [DOI] [PubMed] [Google Scholar]
- Hebbeler SL, Marciniak CM, Crandall S, Chen D, Nussbaum S, Mendelewski S.Daily vs twice daily enoxaparin in the prevention of thromboembolic disorders during rehabilitation following acute spinal cord injury J Spinal Cord Med. 200427 (3) 236–240. [DOI] [PubMed] [Google Scholar]
- Merli GJ, Crabbe S, Doyle L, Ditunno JF, Herbision GJ. Mechanical plus pharmacological prophylaxis for deep vein thrombosis in acute spinal cord injury. Paraplegia. 1992;30:558–562. doi: 10.1038/sc.1992.115. [DOI] [PubMed] [Google Scholar]
- Frisbie JH, Sasahara AA. Low-dose heparin prophylaxis for deep venous thrombosis in acute spinal cord injury patients: a controlled study. Paraplegia. 1981;19:343–346. doi: 10.1038/sc.1981.65. [DOI] [PubMed] [Google Scholar]
- Hadley MN, Walters BC, Grabb PA, et al. Deep venous thrombosis and thromboembolism in patients with cervical spinal cord injuries Neurosurgery. 200250 (suppl 3) S73–S80. [DOI] [PubMed] [Google Scholar]
- Graafsma YP, Prins MH, Lensing AW, de Haan RJ, Huisman MV, Buller HR. Bleeding classification in clinical trials: observer variability and clinical relevance. Thromb Haemost. 1997;78:1189–1192. [PubMed] [Google Scholar]
- Bravo G, Guizar-Sahagun G, Ibarra A, Centurion D, Villalon CM. Cardiovascular alterations after spinal cord injury: an overview. Curr Med Chem Cardiovasc Hematol Agents. 2004;2:133–148. doi: 10.2174/1568016043477242. [DOI] [PubMed] [Google Scholar]
- Miranda AR, Hassouna HI. Mechanisms of thrombosis in spinal cord injury. Hematol Oncol Clin North Am. 2000;14:401–416. doi: 10.1016/s0889-8588(05)70141-6. [DOI] [PubMed] [Google Scholar]
- Brandstater ME, Roth EJ, Siebens HC.Venous thromboembolism in stroke: literature review and implications for clinical practice Arch Phys Med Rehabil. 199273 (suppl 5) S379–S391. [PubMed] [Google Scholar]
- Guttman L. Spinal Cord Injuries: Comprehensive Management and Research. 2nd ed. Oxford: Blackwell Scientific Publications; 1976:458–460
- Yelnik A, Dizien O, Bussel B, et al. Systematic lower limb phlebography in acute spinal cord injury in 147 patients. Paraplegia. 1991;29:253–260. doi: 10.1038/sc.1991.36. [DOI] [PubMed] [Google Scholar]
- Lim AC, Roth EJ, Green D. Lower limb paralysis: its effect on the recanalization of deep-vein thrombosis. Arch Phys Med Rehabil. 1992;73:331–333. doi: 10.1016/0003-9993(92)90005-h. [DOI] [PubMed] [Google Scholar]
- Kim SW, Charallel JT, Park KW, et al. Prevalence of deep venous thrombosis in patients with chronic spinal cord injury. Arch Phys Med Rehabil. 1994;75:965–968. [PubMed] [Google Scholar]
- Myllynen P, Kammonen M, Rokkanen P, Bostman O, Lalla M, Laasonen E. Deep venous thrombosis and pulmonary embolism in patients with acute spinal cord injury: a comparison with nonparalyzed patients immobilized due to spinal fractures. J Trauma. 1985;25:541–543. doi: 10.1097/00005373-198506000-00013. [DOI] [PubMed] [Google Scholar]
- Hedeman LS, Shellenberger MK, Gordon JH.Studies in experimental spinal cord trauma. 1. Alterations in catecholamine levels J Neurosurg. 197440 (1) 37–43. [DOI] [PubMed] [Google Scholar]
- Ragnarsson K, Hall KM, Wilmot CB, et al. Management of pulmonary, cardiovascular, and metabolic conditions after spinal cord injury. In: Stover S, DeLisa JA, Whiteneck GG, editors. Spinal Cord Injury: Clinical Outcomes From the Model System. Gaithersburg, MD: Aspen Publishers; 1995. pp. 79–99. [Google Scholar]
- Maxwell RA, Chavarria-Aguilar M, Cockerham WT, et al. Routine prophylactic vena cava filtration is not indicated after acute spinal cord injury. J Trauma. 2002;52:902–906. doi: 10.1097/00005373-200205000-00013. [DOI] [PubMed] [Google Scholar]
- Colachis SC, Clinchot DM. The association between deep venous thrombosis and heterotopic ossification in patients with acute traumatic spinal cord injury. Paraplegia. 1993;31:507–512. doi: 10.1038/sc.1993.82. [DOI] [PubMed] [Google Scholar]
- Wells PS, Owen C, Doucette S, Fergusson D, Tran H.Does this patient have deep vein thrombosis JAMA. 2006295 (2) 199–207. [DOI] [PubMed] [Google Scholar]
- Weingarden SI.Deep venous thrombosis in spinal cord injury: overview of the problem Chest. 1992102 (suppl 6) S636–S639. [PubMed] [Google Scholar]
- Wells PS, Hirsh J, Anderson DR, et al. Accuracy of clinical assessment of deep-vein thrombosis. Lancet. 1995;345:1326–1330. doi: 10.1016/s0140-6736(95)92535-x. [DOI] [PubMed] [Google Scholar]
- Hirsh J, Hull RD. Venous Thromboembolism: Natural History, Diagnosis, and Management. Boca Raton, FL: CRC Press; 1987. [Google Scholar]
- Akman MN, Cetin N, Bayramoglu M, Isiklar I, Kilinc S. Value of the D-dimer test in diagnosing deep vein thrombosis in rehabilitation inpatients. Arch Phys Med Rehabil. 2004;85:1091–1094. doi: 10.1016/j.apmr.2003.10.023. [DOI] [PubMed] [Google Scholar]
- Roussi J, Bentolila S, Boudaoud L, et al. Contribution of D-dimer determination in the exclusion of deep venous thrombosis in spinal cord injury patients. Spinal Cord. 1999;37:548–552. doi: 10.1038/sj.sc.3100891. [DOI] [PubMed] [Google Scholar]
- Kadyan V, Clinchot DM, Mitchell GL, Colachis SC. Surveillance with duplex ultrasound in traumatic spinal cord injury on initial admission to rehabilitation. J Spinal Cord Med. 2003;26:231–235. doi: 10.1080/10790268.2003.11753689. [DOI] [PubMed] [Google Scholar]
- Powell M, Kirshblum S, O'Connor KC. Duplex ultrasound screening for deep vein thrombosis in spinal cord injured patients at rehabilitation admission. Arch Phys Med Rehabil. 1999;80:1044–1046. doi: 10.1016/s0003-9993(99)90058-8. [DOI] [PubMed] [Google Scholar]
- Kadyan V, Clinchot DM, Colachis SC. Cost-effectiveness of duplex ultrasound surveillance in spinal cord injury. Am J Phys Med Rehabil. 2004;83:191–197. doi: 10.1097/01.phm.0000113401.47681.a6. [DOI] [PubMed] [Google Scholar]
- Bates SM, Weitz JI. New anticoagulants: beyond heparin, low–molecular-weight heparin and warfarin. Br J Pharmacol. . 2005;144:1017–1028. doi: 10.1038/sj.bjp.0706153. [DOI] [PMC free article] [PubMed] [Google Scholar]