Abstract
Vertebrate cells require a very narrow pH range for survival. Cells accordingly possess sensory and defense mechanisms for situations where the pH deviates from the viable range. Although the monitoring of acidic pH by sensory neurons has been attributed to several ion channels, including transient receptor potential vanilloid 1 channel (TRPV1) and acid-sensing ion channels (ASICs), the mechanisms by which these cells detect alkaline pH are not well understood. Here, using Ca2+ imaging and patch-clamp recording, we showed that alkaline pH activated transient receptor potential cation channel, subfamily A, member 1 (TRPA1) and that activation of this ion channel was involved in nociception. In addition, intracellular alkalization activated TRPA1 at the whole-cell level, and single-channel openings were observed in the inside-out configuration, indicating that alkaline pH activated TRPA1 from the inside. Analyses of mutants suggested that the two N-terminal cysteine residues in TRPA1 were involved in activation by intracellular alkalization. Furthermore, intraplantar injection of ammonium chloride into the mouse hind paw caused pain-related behaviors that were not observed in TRPA1-deficient mice. These results suggest that alkaline pH causes pain sensation through activation of TRPA1 and may provide a molecular explanation for some of the human alkaline pH–related sensory disorders whose mechanisms are largely unknown.
Introduction
Living things survive in a narrow pH range to maintain delicate biological functions. Thus, it is likely that cells have sensitive systems for monitoring pH. It is well accepted that monitoring of acidic pH can be attributed to several ion channels, including transient receptor potential vanilloid 1 (TRPV1) and acid-sensing ion channels (ASICs) (1, 2). However, alkaline pH monitoring mechanisms are not well characterized, except for CatSper1 in sperm (3), the activity of which is potentiated by intracellular alkalization during ejaculation.
There are many biological conditions that give rise to alkalinity, some of which are associated with pain sensations. Respiratory alkalosis due to hyperventilation, for instance, is known to cause tingling sensation in the extremities and to lower peripheral nerve thresholds (4, 5). High urinary pH and high blood ammonia concentrations were observed in patients with irritable bowel symptoms and urinary tract problems. These patients have recurrent vascular headaches, and some complain of tingling sensations in the hands and feet (6). Moreover, chest pain is frequently experienced by patients having a pancreatic-pleural fistula with effusion of high-pH pancreatic fluids (7). Alkaline environmental insults to the cornea or nasal mucosa also cause defensive behaviors associated with pain (8–10) that are mediated by the trigeminal nerve (11).
Ammonium chloride (NH4Cl) increases intracellular pH without raising extracellular pH (12), and it has been reported that alkaline buffer or NH4Cl induces an increase in intracellular Ca2+ concentration ([Ca2+]i) in some trigeminal neurons (13). The intracellular pH increase by ammonium salts is thought to be induced by ammonia’s rapid diffusion into the cell, where most of it combines with protons (14). Meanwhile, ammonium salts were introduced for the relief of nerve root pain and intercostal neuralgia (15, 16), although pain intensity increased immediately after the injection of ammonium salts for approximately 30 minutes (16). Moreover, ammonia-induced intracellular alkalization was demonstrated with a microelectrode in barnacle muscle fiber and the squid axon (17, 18). However, molecular mechanisms for these abnormalities are poorly understood.
Transient receptor potential (TRP) channels were first described in Drosophila (19). In mammals, they now comprise 6 related protein families (TRPC, TRPV, TRPM, TRPA, TRPML, and TRPP) (20–22). TRP channels are well recognized for their contributions to sensory transduction, responding to a wide variety of stimuli, including temperature, nociceptive stimuli, touch, osmolarity, and pheromones. In particular, the involvement of TRP channels in nociception has been extensively studied following the cloning of the capsaicin (CAP) receptor TRPV1 (23, 24). TRPA1 belongs to the TRPA subfamily and has attracted attention for its potential role in nociception (25–27). There are increasing numbers of reports about the stimuli causing TRPA1 activation (25, 28–40). The fact that structurally unrelated compounds have the ability to activate TRPA1 led two groups to conclude that some of the TRPA1 agonists share the ability to covalently bind cysteine residues of TRPA1 to activate it (41, 42). Moreover, intracellular Ca2+ was found to directly activate TRPA1 via binding to its putative EF hand–like motif in the cytoplasmic N-terminal region (38, 39).
We hypothesized that alkaline pH might act on TRP channels detecting nociceptive stimuli and speculated that TRPA1 might be the target, since a structurally similar nociceptive receptor, TRPV1, responds to acidic pH (2). In this study, we used Ca2+ imaging and patch-clamp methods to examine the ability of alkaline pH to activate TRPA1. We demonstrate that intracellular alkalization can activate TRPA1 in both heterologous expression systems and native sensory neurons.
Results
Intracellular alkalization activates TRPA1 in HEK293 cells expressing TRPA1.
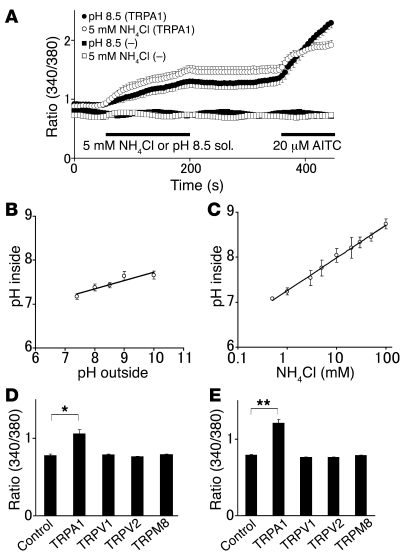
We first used a Ca2+ imaging method to examine whether alkaline pH could activate TRPA1 in vitro. An increase in [Ca2+]i was caused not only by a pH 8.5 Tris buffer solution (n = 37), but also by 5 mM ammonium chloride solution with a pH of 7.4 (n = 32) (Figure 1A). The latter had a greater effect than did Tris buffer solution in HEK293 cells expressing mouse TRPA1 in the presence of extracellular Ca2+. TRPA1 activity was confirmed upon application of allyl isothiocyanate (AITC; 20 μM). Such a [Ca2+]i increase was never observed in the vector-transfected cells in response to either pH 8.5 Tris buffer solution or NH4Cl solution, suggesting that the [Ca2+]i increase was mediated by TRPA1 activation.
Figure 1. TRPA1 mediates increases in [Ca2+]i following intracellular alkalization in HEK293 cells.
(A) Tris buffer solution (pH 8.5) caused an increase in [Ca2+]i in HEK293 cells expressing mouse TRPA1 in the presence of extracellular Ca2+ (filled circles, n = 37), but not in cells expressing vector alone (filled squares, n = 22). Moreover, 5 mM NH4Cl also caused a [Ca2+]i increase (open circles, n = 32), but not in cells expressing vector alone (open squares, n = 23). Horizontal bars indicate the duration of applied stimulus. Ratio 340/380, ratio of fluorescence intensities of fura-2 emissions at 340 nm and 380 nm; sol., solution. (B) Tris buffer solution increased intracellular pH in HEK293 cells loaded with BCECF in a dose-dependent manner (n = 14–31). Tris buffer solution (pH 8.5) caused intracellular alkalization of pH 7.43. (C) NH4Cl increased intracellular pH in HEK293 cells loaded with BCECF in a dose-dependent manner (n = 12–38). NH4Cl (5 mM) caused intracellular alkalization of pH 7.76. The data were fitted with the least-squares linear regression in B and C. (D) Tris buffer solution (pH 8.5) increased [Ca2+]i in HEK293 cells expressing TRPA1 (n = 35) but not in cells expressing TRPV1 (n = 123), TRPV2 (n = 165), TRPM8 (n = 184), or vector alone (control, n = 61). (E) NH4Cl (5 mM) increased [Ca2+]i in HEK293 cells expressing TRPA1 (n = 48), but not in cells expressing TRPV1 (n = 172), TRPV2 (n = 154), TRPM8 (n = 196), or vector alone (n = 67). *P < 0.05, **P < 0.01.
NH4Cl is known to cause intracellular alkalization without affecting extracellular pH through ammonia’s binding with protons in the cytosol (14, 17, 18), suggesting that the observed [Ca2+]i increase was caused by intracellular alkalization. Therefore, we examined intracellular pH changes upon application of high-pH Tris buffer solution or NH4Cl solution using a fluorescent pH dye, BCECF (a representative trace in response to 30 mM NH4Cl or alkaline extracellular solution is shown in Supplemental Figure 1; supplemental material available online with this article; doi: 10.1172/JCI35957DS1). Intracellular pH was slightly low under basal conditions, and it rose with increasing outside pH values or increasing amounts of NH4Cl (Figure 1, B and C, respectively). Extracellular NH4Cl caused a greater intracellular pH increase than did extracellular high-pH solutions in HEK293 cells, as previously reported in other cell types (17, 18, 43, 44). The intracellular pH value in the pH 7.4 extracellular solution was 7.17, and it increased to 7.66 in the pH 10 extracellular solution (Figure 1B). The intracellular pH value in the presence of 0.5 mM NH4Cl was 7.08, and it increased to 8.74 by addition of 100 mM NH4Cl (Figure 1C). The intracellular pH increases were 0.26 U for the pH 8.5 Tris buffer solution and 0.68 U for the 5 mM NH4Cl solution. These results explain the greater [Ca2+]i increase in cells exposed to 5 mM NH4Cl solution and further suggest that TRPA1 is activated by intracellular alkalization. To address whether increases in [Ca2+]i induced by intracellular alkalization were specific to TRPA1, we examined the effects of pH 8.5 Tris buffer solution or 5 mM NH4Cl solution on [Ca2+]i in HEK293 cells expressing other TRP channels (TRPV1, TRPV2, or TRPM8). These channels are expressed in sensory neurons and are thought to be involved in nociception or pain relief (25, 26, 45–50). Both conditions caused a [Ca2+]i increase in HEK293 cells expressing TRPA1 (n = 48 and n = 35 for pH 8.5 Tris buffer solution and 5 mM NH4Cl solution, respectively), but not in cells expressing TRPV1, TRPV2, or TRPM8 (n = 123–196; Figure 1, D and E), indicating the specific action of intracellular alkalization on TRPA1.
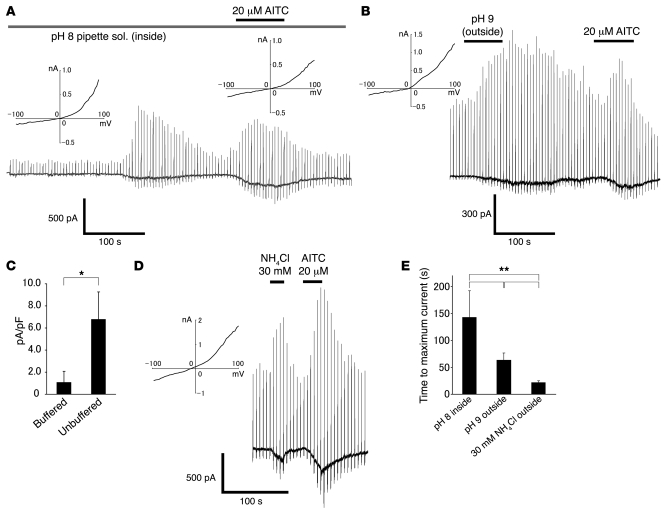
In order to confirm that intracellular alkalization activates TRPA1 channels, we performed whole-cell patch-clamp experiments in HEK293 cells expressing TRPA1. Intracellular alkalization with a pH 8 pipette solution evoked inward currents (at –60 mV) with an outwardly rectifying current-voltage (I-V) relationship revealed by ramp pulses from –100 to +100 mV every 5 seconds (n = 4; Figure 2A). A few minutes delay of current activation upon making a whole-cell configuration can probably be explained by the time necessary for diffusion of the pipette solution into the cytosol. Extracellular pH 9 Tris buffer solution evoked delayed small currents with an outwardly rectifying I-V relationship using the unbuffered pipette solution (6.7 ± 2.4 pA/pF, n = 8; Figure 2, B and C), but not using the pipette solution buffered with HEPES (1.1 ± 1.4 pA/pF, n = 8; Figure 2C), further indicating the importance of intracellular alkaline pH for TRPA1 activation.
Figure 2. Alkaline pH solution and NH4Cl activate TRPA1 in HEK293 cells.
(A) A representative whole-cell current trace activated by pH 8 pipette solution or AITC (20 μM) in the presence of extracellular Ca2+ in HEK293 cells expressing TRPA1. The insets indicate representative I-V curves of pH 8 pipette solution– or AITC-activated currents showing an outward rectification. Holding potential (Vh), –60 mV. Horizontal bars indicate the duration of compound application. (B) A representative current trace activated by pH 9 Tris buffer solution or AITC (20 μM) using the unbuffered pipette solution in the presence of extracellular Ca2+. The inset indicates a representative I-V curve of pH 9 Tris buffer solution–activated current showing an outward rectification. (C) Comparison of current densities activated by pH 9 Tris buffer solution using buffered (n = 8) or unbuffered (n = 8) pipette solution. *P < 0.05. (D) A representative current trace activated by 30 mM NH4Cl or AITC (20 μM) in the presence of extracellular Ca2+. The inset indicates a representative I-V curve of NH4Cl-activated current showing an outward rectification. (E) Comparison of times to maximum current responses evoked by pH 8 pipette solution (n = 4), pH 9 Tris buffer solution (n = 8), or 30 mM NH4Cl (n = 31) using unbuffered pipette solution. **P < 0.01.
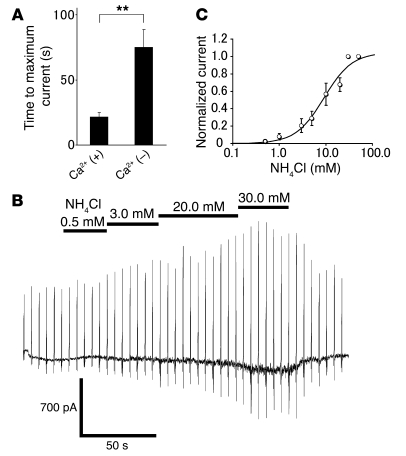
In order to facilitate TRPA1 activation by intracellular alkalization, we used the unbuffered pipette solution in the following experiments. NH4Cl (30 mM) in the pH 7.4 solution, which is known to cause intracellular alkalization, also evoked inward currents with an outwardly rectifying I-V relationship (n = 31; Figure 2D). TRPA1 activation by stimuli causing intracellular alkalization is supported by the similar responses obtained with AITC (20 μM) in the same cells (Figure 2, A, B, and D). The specific effect of intracellular alkalization on TRPA1 was further confirmed by experiments in which NH4Cl or alkaline pipette solution did not activate inward currents in vector alone–transfected HEK293 cells (n = 3 or 3, respectively; data not shown) or HEK293 cells expressing TRPV1 (n = 3 or 8, respectively; data not shown), which is consistent with the Ca2+ imaging data (Figure 1, D and E). The time course of TRPA1 activation in each condition indicates that NH4Cl more rapidly activates TRPA1 than extracellular or intracellular alkaline solution (Figure 2E), which is consistent with the rapid rise of intracellular pH by NH4Cl as shown in Supplemental Figure 1. Intracellular alkalization–induced inward currents were often desensitized during its application in the presence of extracellular Ca2+ (Figure 2, A and B), a property that has been previously reported (51). In light of this, we further characterized the intracellular alkalization–induced TRPA1 currents in the absence of extracellular Ca2+. In this condition, TRPA1 currents were activated more slowly than in the presence of extracellular Ca2+ (Figure 3A). NH4Cl-activated inward currents at –60 mV in the absence of extracellular Ca2+ had an activation threshold of about 1 mM and EC50 of 9.2 mM (Figure 3, B and C). The reversal potential of the I-V curve was 2.5 ± 2.8 mV (n = 4), indicating the involvement of the opening of a nonselective cation channel. These properties are identical to those reported for TRPA1 currents evoked by known stimuli including isothiocyanate and thiosulfinate compounds (28, 29).
Figure 3. Intracellular alkalization activates TRPA1 in HEK293 cells.
(A) Comparison of times to maximum current response evoked by 30 mM NH4Cl using unbuffered pipette solution in the presence (n = 31) or absence (n = 6) of extracellular Ca2+. **P < 0.01. (B) A representative current trace activated by NH4Cl (stepwise increases of concentrations) using unbuffered pipette solution in the absence of extracellular Ca2+. (C) A dose-dependent profile of NH4Cl-activated TRPA1-mediated currents fitted with a Hill equation. The threshold was about 1 mM, and the EC50 value was 9.2 mM. Membrane currents were normalized to the current activated by 30 mM NH4Cl in each cell (n = 6).
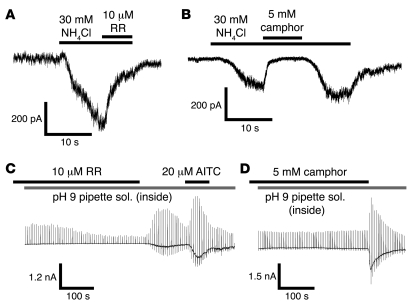
Both ruthenium red (RR; 10 μM) and camphor (5 mM) caused almost complete inhibition of NH4Cl-induced TRPA1 currents (85.8 ± 5.7% inhibition, n = 4 with RR; 98.4 ± 0.5%, n = 4 with camphor; Figure 4, A and B) as previously reported (52, 53). A pH 9 pipette solution did not activate TRPA1 currents in the presence of either RR (n = 5; Figure 4C) or camphor (n = 3; Figure 4D), whereas large current activation was observed upon washout of the compounds. These pharmacological properties further support the notion that TRPA1 is activated by intracellular alkalization.
Figure 4. TRPA1 antagonists inhibit the currents activated by intracellular alkalization in HEK293 cells expressing TRPA1.
(A and B) Representative traces of NH4Cl-activated currents that were inhibited by RR (10 μM, A) or camphor (5 mM, B) in the presence of extracellular Ca2+. (C and D) Representative traces of pH 9.0 pipette solution–activated currents that were inhibited by RR (10 μM; C) or camphor (5 mM; D) in the presence of extracellular Ca2+.
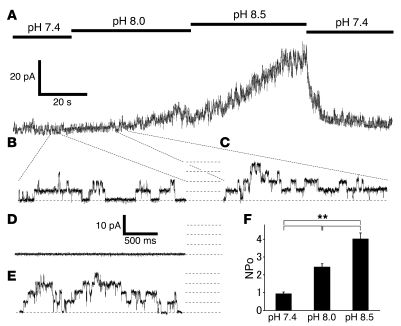
To examine whether intracellular alkaline pH activates TRPA1 in a membrane-delimited fashion, we performed single-channel recordings with an inside-out configuration in HEK293 cells expressing TRPA1. Basal TRPA1 activity was observed when an excised inside-out patch membrane was exposed to a pH 7.4 bath solution at the pipette potential of –60 mV (membrane potential of +60 mV; Figure 5, A and B). A similar property had been previously reported (51). Increasing bath pH to 8.0 or 8.5 increased the channel opening in a pH-dependent manner (Figure 5, A–C), indicating the existence of alkalization-induced activation of the ion channels in the membrane patch excised from HEK293 cells expressing TRPA1. Single-channel activation by alkalization was never observed in the vector-transfected HEK293 cells (Figure 5D), indicating TRPA1 activation by alkaline pH at a single-channel level. The calculated unitary conductance in the KCl bath and NaCl-based pipette solutions was 72 ± 1 picosiemens (pS) at +60 mV (n = 6), and a similar value was observed for AITC-activated currents (73 ± 3 pS, n = 5; Figure 5E). These properties at a single-channel level support the idea that TRPA1 can be activated by alkaline pH directly or through the mechanisms retained even in a small excised patch membrane. Alkaline pH–dependent activation of TRPA1 was more clearly recognized when NPo (channel number × open probability) values were plotted against pH, where the NPo values were significantly increased upon pH increase (Figure 5F).
Figure 5. Properties of single-channel currents of TRPA1 activated by intracellular alkaline pH in the inside-out configuration.
(A) A representative alkaline buffer–activated single-channel current trace in a patch excised from a HEK293 cell expressing TRPA1. Vh (pipette), –60 mV. (B and C) Expanded single-channel current traces from A. Broken lines indicate 0, 1, 2, 3, and 4 channel open levels. (D) A representative single-channel current trace upon exposure to pH 8.0 solution in a patch excised from a HEK293 cell transfected with vector alone. (E) A representative single-channel current trace upon exposure to AITC (20 μM) in a patch excised from a HEK293 cell expressing TRPA1 (NPo, 2.80 ± 0.12; n = 10). (F) NPo values (calculated from data shown in A) plotted against bath pH values (n = 10). NPo values for the currents at pH 7.4, 8.0, and 8.5 were 0.94 ± 0.09, n = 10; 2.44 ± 0.18, n = 10; and 4.01 ± 0.33, n = 10, respectively. **P < 0.01.
Intracellular alkalization activates TRPA1 in sensory neurons.
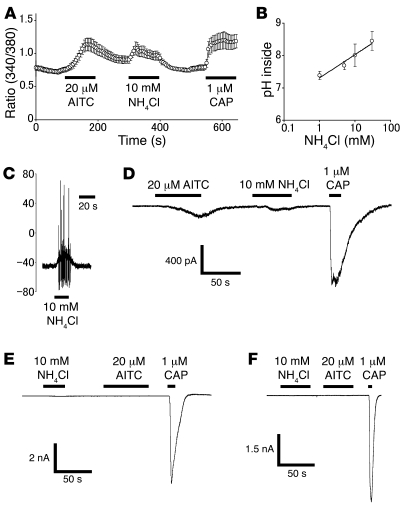
To confirm the effect of intracellular alkalization on TRPA1 activation in native sensory neurons, we performed Ca2+ imaging and whole-cell patch-clamp experiments using isolated mouse dorsal root ganglion (DRG) neurons. NH4Cl (10 mM) induced increases in [Ca2+]i in cells, which responded to both AITC (20 μM) and CAP (1 μM; Figure 6A), as observed above in HEK293 cells (Figure 1A), indicating that cells expressing both TRPA1 and TRPV1 respond to NH4Cl. Intracellular pH rose with increasing amounts of NH4Cl, as determined with the fluorescent pH dye BCECF (Figure 6B), as found above in HEK293 cells (Figure 1C). Moreover, NH4Cl (10 mM) evoked action potentials (n = 5; Figure 6C) and inward currents in some of the small-diameter DRG neurons (18.6 ± 1.2 pF, n = 20; Figure 6D). In the whole-cell patch-clamp experiments, the second responses were often quite small due to desensitization, as we performed the experiments in the presence of extracellular Ca2+ in order to maintain giga-ohm seals. The NH4Cl-sensitive cells also responded to both AITC (20 μM) and CAP (1 μM, n = 5; Figure 6D), suggesting that NH4Cl-sensitive cells express both TRPA1 and TRPV1. These findings are consistent with the notion that TRPV1-positive cells express TRPA1 (27, 54). These results strongly suggest that intracellular alkalization activates TRPA1 in native sensory neurons.
Figure 6. Intracellular alkalization causes an increase in cytosolic Ca2+ concentration and activates inward currents in mouse DRG neurons.
(A) NH4Cl caused an increase in [Ca2+]i in a WT mouse DRG neuron (n = 10). Horizontal bars indicate the duration of applied stimulus. (B) NH4Cl increased intracellular pH in WT mouse DRG neurons loaded with BCECF in a dose-dependent manner (n = 15–24). (C) A representative whole-cell voltage trace upon application of NH4Cl (10 mM) in a WT mouse DRG neuron. (D) A representative whole-cell current trace activated by AITC (20 μM), NH4Cl (10 mM), or CAP (1 μM) in a WT mouse DRG neuron. Vh, –60 mV. Bars indicate duration of the compound application. (E) A representative current trace from a WT mouse DRG neuron responding to CAP but not to NH4Cl or AITC. (F) A representative current trace from a TRPA1-deficient mouse DRG neuron responding to CAP but not to NH4Cl or AITC. In order to maintain giga-ohm seals, patch-clamp experiments on DRG neurons were performed in the presence of extracellular Ca2+ for all experiments.
None of the NH4Cl-insensitive neurons responded to AITC (n = 9), although many did respond to CAP (n = 6, 67%; Figure 6E). Those cells are thought to express TRPV1 but not TRPA1. In these experiments with isolated mouse neurons, 25 of 96 cells (26%) responded to NH4Cl. Moreover, in DRG neurons from TRPA1-deficient mice, none of the CAP-sensitive cells responded to either NH4Cl or AITC (n = 25; Figure 6F). Taken together, these results clearly indicate that intracellular alkalization activates TRPA1 in DRG neurons.
Two cysteine residues of TRPA1 confer sensitivity to alkaline pH.
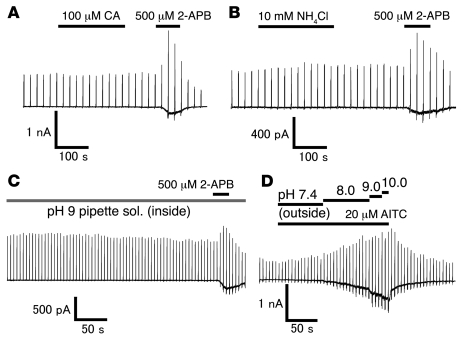
It has been recently shown that TRPA1 is activated by reversible covalent modification of N-terminal cysteine residues by structurally unrelated compounds (41, 42). In order to examine whether TRPA1 activation by intracellular alkalization requires its N-terminal cysteine residues, we mutated 2 cysteines in mouse TRPA1 (C422 and C622) to serines and tested responsiveness of the mutant (TRPA1-2C) to 100 μM cinnamaldehyde (CA) or 20 μM AITC using a patch-clamp method. We first confirmed that the mutant TRPA1-2C responded to 500 μM 2-aminoethoxydiphenyl borate (2-APB) but not to 100 μM CA (n = 3; Figure 7A), which indicated that the 2 cysteine residues were involved in CA-induced but not 2-APB–induced TRPA1 activation. The TRPA1-2C mutant exhibited small current responses to 20 μM AITC (Supplemental Figure 2), suggesting that covalently modified residues might be different depending on the compounds. Intracellular alkalization by 10 mM NH4Cl (n = 4; Figure 7B) or with a pH 9 pipette solution (n = 6; Figure 7C) did not evoke any inward currents at –60 mV in cells expressing TRPA1-2C, whereas 2-APB activated the mutant TRPA1 in the same cells (Figure 7, B and C). These results indicated that the 2 N-terminal cysteine residues were necessary for TRPA1 activation by intracellular alkalization, probably through covalent modification.
Figure 7. Involvement of the 2 N-terminal cysteine residues of mouse TRPA1 in its activation by intracellular alkalization.
(A) A representative whole-cell current trace in response to CA (100 μM) or 2-APB (500 μM) in a cell expressing TRPA1-2C mutant (C422S, C622S) in the presence of extracellular Ca2+. Vh, –60 mV. Bars indicate duration of the compound application. (B) A representative current trace in response to NH4Cl (10 mM) or 2-APB (500 μM) in a cell expressing mutant TRPA1-2C. (C) A representative current trace in response to intracellular pH 9 Tris buffer solution or extracellular 2-APB (500 μM) in a cell expressing the mutant TRPA1-2C. (D) A representative current trace of WT TRPA1 activated by AITC (20 μM) in the bath solution with different pH values.
Note that TRPA1 activation by alkaline pH was observed in the inside-out single-channel recordings (Figure 5, A and C) and that the activation involved 2 cysteine residues (Figure 7, B and C) that were reportedly covalently modified (41, 42). These observations suggest the possibility that there are molecules that have the ability to activate TRPA1 through action on the cysteine residues and are retained even in the excised patch membrane; and that alkalization enhances the molecules’ action. This hypothesis is supported not only by the fact that cysteines can be easily oxidized under alkaline conditions, but also by experiments in which stepwise alkalization potentiates TRPA1 responses evoked by 20 μM AITC (Figure 7D) or 4-hydroxy-2-nonenal (Supplemental Figure 3), both of which are known to act on the cysteine residues activating TRPA1 (41, 42, 55).
NH4Cl causes pain-related behaviors in mice through TRPA1 activation.
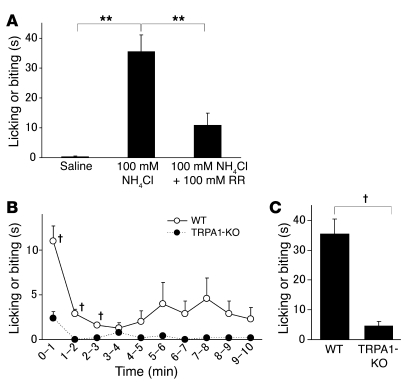
If intracellular alkalization can activate TRPA1, it is reasonable to hypothesize that the condition may also cause pain-related behaviors when administered in vivo. To investigate this, we examined the effects of NH4Cl injected into the mouse hind paw. Intraplantar injection of NH4Cl with pH 7.4 (100 mM, 20 μl) caused licking or biting reactions in mice, which were viewed as pain-related behaviors (Figure 8A). These reactions were significantly reduced by the concomitant application of RR (100 μM, 20 μl) and never observed in mice injected with saline (Figure 8A). When the time courses of these reactions were analyzed, the pain-related behaviors occurred significantly more often in the first 3 minutes after injection (Figure 8B). No apparent swelling was observed in the injected hind paw until 1 hour after injection (data not shown). Furthermore, such NH4Cl-evoked pain-related behaviors were rarely observed in the TRPA1-deficient mice (Figure 8, B and C). These results suggest that NH4Cl evokes acute pain behavior rather than inflammation or hypersensitivity through TRPA1 activation.
Figure 8. Pain-related behaviors induced by NH4Cl injection into mouse hind paws.
(A) The time spent licking or biting induced by injection of NH4Cl (100 mM) with or without RR (100 μM) for 10 minutes in WT mice (n = 9) or with saline as a control (n = 6). **P < 0.01 versus treatment with NH4Cl alone. (B) Time course of licking or biting induced by injection of NH4Cl (100 mM) for 10 minutes in WT (n = 8) or TRPA1-deficient (TRPA1-KO) mice (n = 5). †P < 0.01. (C) The time spent licking or biting induced by injection of NH4Cl (100 mM) for 10 minutes in WT (n = 8) or TRPA1-deficient mice (n = 5). †P < 0.01.
Discussion
In the present study, both Ca2+ imaging and patch-clamp experiments showed that intracellular alkalization could activate TRPA1 in a pH-dependent manner in both HEK293 cells expressing TRPA1 and mouse sensory neurons. Involvement of TRPA1 activation by intracellular alkalization in nociception was confirmed in mice at the behavioral level.
Mechanism of TRPA1 activation by intracellular alkalization.
The properties of intracellular alkalization–evoked responses of TRPA1 were similar to those occurring in response to known chemical stimuli for TRPA1. Although the calculated conductance of the activated currents at the single-channel level was a little smaller than those reported previously (42, 56), the alkalization- and AITC-evoked activation values were not different in our experiments (Figure 5). These similarities indicate that intracellular alkalization activates TRPA1 in the same way as known TRPA1 chemical stimuli. Moreover, considering that the pKa value of the SH in cysteine is 8.37 (57), intracellular alkalization around such a pH range might lead to easy access of cysteine-modifying compounds through its deprotonation. The data shown in Figures 5 and 7 suggest the existence of endogenous activators that can be retained in the excised patch membrane and whose ability to activate TRPA1 is largely enhanced in intracellular alkalization. Candidate molecules are expected to act on intracellular cysteine residues such as 4-hydroxy-2-nonenal, an endogenous aldehyde that has been reported to work as a TRPA1 activator through covalent modification (55). Basal TRPA1 currents observed prior to exposure to alkaline solution in the inside-out configuration (Figure 5) appear to support this idea. The candidate molecules might act on the cysteine-containing motifs through mechanisms other than cysteine modification. Alternatively, pH-dependent release of the membrane lipid-derived substances could act on the cysteines to activate TRPA1.
It is controversial whether single-channel openings of TRPA1 can be observed with some of the reported stimuli, including AITC, in the inside-out configuration. Kim and Cavanaugh reported that inorganic polyphosphates were necessary as intracellular factors for TRPA1 activation and they did not observe any single-channel openings in the inside-out configuration without inorganic polyphosphates (56). However, we consistently observed TRPA1 activities in the inside-out configuration with KCl-based bath solution without addition of polyphosphates, as reported elsewhere (39). We are unable to definitively explain this apparent discrepancy among studies. It is possible that inorganic polyphosphate-like substances, if necessary, might be retained in the excised patch membrane that is pulled in the patch pipette upon making the inside-out configuration.
NH4Cl causes a rapid rise in intracellular pH and then evokes an inward current and an increase in [Ca2+]i in HEK293 cells expressing TRPA1 channels (Supplemental Figure 1; and Figures 1 and 2). This latency raises the possibility that TRPA1 is activated by the increase in [Ca2+]i, as reported previously (38, 39), which may be caused by NH4Cl or alkaline solution. Although an intracellular alkalization induced by NH4Cl is known to increase Ca2+ release from intracellular pools in several cell types (58–60), 5 mM NH4Cl or pH 8.5 Tris buffer solution in the absence of extracellular Ca2+ did not cause any increase in [Ca2+]i in HEK293 cells expressing TRPA1 channels (Supplemental Figure 4). Moreover, the time course of activation and the magnitude of inward currents evoked by 30 mM NH4Cl were not affected by Ca2+ concentration in the pipette solution (Supplemental Figure 5). These results indicate that TRPA1 activation by intracellular alkalization does not involve direct activation by intracellular Ca2+ through binding to a putative EF hand–like motif in the cytoplasmic N-terminal region of TRPA1 (38, 39).
Alkaline pH causes pain sensation through activation of TRPA1.
TRPA1 was initially cloned as a channel activated by cooling below 17°C and was expressed in a subset of TRPV1-positive nociceptive neurons (27). It appeared to be an attractive candidate to act as a thermosensor in the noxious cold range. However, a number of recent conflicting results including the ones using TRPA1-deficient mice suggest that the role of TRPA1 in cold sensation is currently debatable (25, 26, 29, 61). It is well accepted that heterologously expressed TRPA1 channels, apart from their potential ability to be activated by cold temperature, can be activated by several substances, such as isothiocyanate and thiosulfinate compounds, which constitute the pungent ingredients of mustard oil and garlic, respectively (28–31). Furthermore, such TRPA1 activation has been observed in native sensory neurons (28–31). As shown in the behavioral experiments, NH4Cl has the ability to evoke pain-related behaviors through TRPA1 activation (Figure 8). NH4Cl caused intracellular alkalization and evoked action potentials in DRG neurons (Figure 6, B and C). A pH response profile generated by the combination of results in Figure 1C and Figure 3C indicates that the apparent EC50 pH causing TRPA1 activation is around 8.0 (Supplemental Figure 6), a value attainable under pathological conditions (7). These results suggest that intracellular alkalization caused by NH4Cl induces pain-related behaviors in mice.
It is well documented that several pathological conditions (inflammation or sudden cessation of blood flow) cause pain sensation through tissue acidification (62) and that TRPV1 activation is involved in some of these responses (23, 24). Tissues seem to be less frequently exposed to alkaline than acidic conditions. However, there are several alkalization-related situations that might be related to pain sensation. For example, vaporized ammonia causes pain sensation when the cornea and the nasal mucosa are exposed (9, 10). Consistent with the notion that almost all the neurons expressing TRPA1 also express TRPV1 (27, 54), nociceptors in the avian trigeminal mucosa respond to both alkaline (ammonia) and acidic (acetic acid vapor, CO2) stimuli (63), and isolated rat trigeminal ganglion neurons that responded to 5 mM NH4Cl exhibited current activation in response to acidic solution (64). In addition, leakage of pancreatic fluids with a pH of 8.0 reportedly causes pain sensations (7), and respiratory alkalosis and high blood pH caused by urinary tract infection induce tingling sensations (4–6). Furthermore, it is well known that some alkaline compounds induce pain sensation immediately upon intravenous application in the clinical setting. Therefore, it is intriguing to speculate that those pain sensations might be induced through TRPA1 activation.
It has been reported that abdominal pain is often accompanied by a rise in ammonia levels in peritoneal fluid (65). Injection of ammonium salts into peripheral nerve induces a short-term increase in pain intensity resulting in prolonged pain relief, which might be explained by TRPA1 activation and desensitization (15, 16), respectively, a phenomenon that is well documented in TRPV1. These previous studies suggest the possibility that TRPA1 is activated by high ammonia concentration and thereby detects changes in physiological conditions. More detailed analyses using TRPA1-deficient mice should provide insights into the mechanisms of nociception or pain relief caused by alkaline conditions.
Methods
Animals.
Male C57BL/6 mice (4–5 weeks old; SLC) were used. Mutant TRPA1-null mice were generously provided by D. Julius (UCSF, San Francisco, California, USA). They were housed in a controlled environment (12-hour light/12-hour dark cycle; room temperature, 22–24°C; 50%–60% relative humidity) with free access to food and water. All procedures involving the care and use of animals were approved by the National Institute for Physiological Science and carried out in accordance with the NIH Guide for the care and use of laboratory animals (NIH publication no. 85-23. Revised 1985).
Cell culture.
HEK293 cells were maintained in DMEM (supplemented with 10% FBS, penicillin, streptomycin, and l-glutamine) and seeded at a density of 5 × 105 cells per 35-mm dish 24 hours before transfection. Cells were transfected using 1 μg of mouse TRPA1 (a gift from A. Patapoutian, The Scripps Institute, La Jolla, California, USA), rat TRPV1, rat TRPV2, or rat TRPM8 cDNA (a gift from D. Julius) with Lipofectamine and Opti-MEM I Reduced Serum Medium (Invitrogen) as described previously (23). For patch-clamp experiments, cells were cotransfected with these plasmids and GFP in pcDNA3.1 (transfection efficiency was around 50%). Green fluorescence from cells expressing GFP was detected with the aid of a Nikon microscope equipped with a mercury lamp light source and a GFP filter (emission wavelength, 510 nm). Primary cultures of mouse DRG neurons from male C57BL/6 mice (4 weeks of age) were incubated in medium (MEM supplemented with 10% FBS, penicillin, streptomycin, and l-glutamine) containing nerve growth factor (100 ng/ml). Cells were reseeded on coverslips coated with (for DRG neurons) or without (for HEK293 cells) poly-d-lysine. For patch-clamp experiments, small-diameter neurons (16.1 ± 0.6 pF, n = 96) were chosen.
TRPA1 mutants.
A double cysteine mutant of mouse TRPA1 (TRPA1-2C) was generated by cysteine-serine substitutions at C422 and C622, residues that are thought to be covalently modified by several agonists (42). These mutations were made using a modified QuickChange Site-Directed Mutagenesis method (Stratagene). Briefly, PCR was performed using 2 residues of mouse TRPA1 expression vector divided at the ClaI (New England BioLabs) site as templates (the former for C422S, the latter for C622S), 2 synthetic oligonucleotide primers containing specific mutations (C422S-S and AS, 5′-CATTATGCCTCTAGGCAGGGG-3′ and 5′-CCCCTGCCTAGAGGCATAATG-3′; C622S-S and AS, 5′-CCCGAGTCCATGAAAGTTCTT-3′ and 5′-AAGAACTTTCATGGACTCGGG-3′), and primeSTAR HS DNA polymerase (TAKARA). The PCR products were digested with DpnI (New England BioLabs) at 37°C for 1 hour and transformed into DH5α competent cells. The entire sequence including desired substitution in the mutants was confirmed. TRPA1-2C mutant was constructed by combining these 2 mutants at the ClaI site.
Electrophysiology.
Fura-2 or BCECF fluorescence was measured in standard bath solution containing 140 mM NaCl, 5 mM KCl, 2 mM MgCl2, 2 mM CaCl2, 10 mM HEPES, and 10 mM glucose at pH 7.4 (adjusted with NaOH). The ratio of florescence intensities obtained with fura-2 emissions at 340 nm and 380 nm or with BCECF emissions at 488 nm and 440 nm are shown. The calibration of the fluorescence values (BCECF) versus pH was made using nigericin in K+ solution, as described by Thomas et al. (66). The standard bath solutions for patch-clamp experiments were the same as used for fluorescence measurements. To make a Ca2+-free bath solution, CaCl2 was omitted and 5 mM EGTA added. Buffered pipette solution contained 140 mM KCl, 5 mM EGTA, and 10 mM HEPES at pH 7.4 (adjusted with KOH), and unbuffered pipette solution contained 140 mM KCl, 0.5 mM EGTA, and 10 mM mannitol at pH 7.4 (adjusted with KOH). For inside-out single-channel recordings, the bath solution contained 140 mM KCl, 5 mM EGTA, and 10 mM HEPES at pH 7.4 (adjusted with KOH), and the pipette solution was the bath solution used for the fluorescence measurement. Data from whole-cell voltage-clamp recordings were sampled at 10 kHz and filtered at 5 kHz for analysis (Axon 200B amplifier with pCLAMP software; Axon Instruments). Voltage ramp pulses from –100 mV to +100 mV (500 ms) were applied every 5 seconds to generate an I-V curve. Data from whole-cell current-clamp recordings were sampled at 100 Hz and filtered at 5 kHz for analysis. Whole-cell patch-clamp recordings and Ca2+ imaging experiments were performed 1 or 2 days after transfection of HEK293 cells or isolation of DRG neurons. All the experiments were performed at room temperature (25°C).
Behavioral studies.
Mice were placed individually in transparent cages (19.5 × 12 × 12 cm) for 1 hour before experiments. Twenty microliters of NH4Cl (100 mM) was injected intraplantarly into the right hind paw. The time spent licking and biting the injected paw was measured for 10 minutes after injection. In some experiments, RR (100 μM) was coadministered with NH4Cl.
Chemicals.
NH4Cl was obtained from Wako, and 4-hydroxy-2-nonenal was from Calbiochem. The other chemicals were from Sigma-Aldrich.
Statistics.
Data are expressed as mean ± SEM. Student’s t test was used to compare the data between 2 groups, and 1-way ANOVA followed by the 2-tailed multiple t test with Bonferroni correction was used for multiple comparisons of control and treated groups. P values less than 0.05 were considered significant.
Supplementary Material
Acknowledgments
This work was supported by grants to M. Tominaga from the Ministry of Education, Culture, Sports, Science and Technology in Japan and an AstraZeneca Research Grant. We thank N. Fukuta for technical assistance. In this work, experiments using HEK293 cells expressing TRPA1 were performed in collaboration with the Mandom Corp.
Footnotes
Nonstandard abbreviations used: AITC, allyl isothiocyanate; 2-APB, 2-aminoethoxydiphenyl borate; CA, cinnamaldehyde; [Ca2+]i, intracellular Ca2+ concentration; CAP, capsaicin; DRG, dorsal root ganglion; I-V, current-voltage; NPo, channel number × open probability; RR, ruthenium red; TRP, transient receptor potential; TRPA1-2C, the TRPA1 mutant in which cysteines 422 and 622 were changed to serines; TRPV1, transient receptor potential vanilloid 1.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 118:4049–4057 (2008). doi:10.1172/JCI35957
References
- 1.Waldmann R., Champigny G., Bassilana F., Heurteaux C., Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature. 1997;386:173–177. doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- 2.Tominaga M., et al. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/S0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 3.Kirichok Y., Navarro B., Clapham D.E. Whole-cell patch-clamp measurements of spermatozoa reveal an alkaline-activated Ca2+ channel. . Nature. 2006;439:737–740. doi: 10.1038/nature04417. [DOI] [PubMed] [Google Scholar]
- 4. Tenny, S.M., and Lamb, T.W. 1965. Physiological consequences of hypoventilation and hyperventilation. InHandbook of physiology, Section 3. W.O. Fenn, and H. Rahn, editors. American Physiology Society. Washington, DC, USA. 979–1090. [Google Scholar]
- 5.Mogyoros I., Kiernan C., Burke D., Bostock H. Excitability changes in human sensory and motor axons during hyperventilation and ischaemia. Brain. 1997;120:317–325. doi: 10.1093/brain/120.2.317. [DOI] [PubMed] [Google Scholar]
- 6.Cohen P.G. The hypokalemic, bowel, bladder, headache relationship; a new syndrome. The role of the potassium ammonia axis. Med. Hypotheses. 1984;15:135–140. doi: 10.1016/0306-9877(84)90118-X. [DOI] [PubMed] [Google Scholar]
- 7.Greenwald R.A., Deluca R.F., Raskin J.B. Pancreatic-pleural fistula: demonstration by endoscopic retrograde cholangiopancreatography (ERCP) and successful treatment with radiation therapy. Dig. Dis. Sci. 1979;24:240–244. doi: 10.1007/BF01308438. [DOI] [PubMed] [Google Scholar]
- 8.Acosta M.C., Belmonte C., Gallar J. Sensory experiences in humans and single-unit activity in cats evoked by polymodal stimulation of the cornea. J. Physiol. 2001;534:511–525. doi: 10.1111/j.1469-7793.2001.t01-1-00511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Izumi H., Karita K. Reflex vasodilatation in the cat lip elicited by stimulation of nasal mucosa by chemical irritants. Am. J. Physiol. 1993;265:R733–R738. doi: 10.1152/ajpregu.1993.265.4.R733. [DOI] [PubMed] [Google Scholar]
- 10.Lindberg S., Dolata J., Mercke U. Nasal exposure to airway irritants triggers a mucociliary defence reflex in the rabbit maxillary sinus. Acta. Otolaryngol. 1987;104:552–560. doi: 10.3109/00016488709128288. [DOI] [PubMed] [Google Scholar]
- 11.Sekizawa S., Tsubone H. Nasal receptors responding to noxious chemical irritants. Respir. Physiol. 1994;96:37–48. doi: 10.1016/0034-5687(94)90104-X. [DOI] [PubMed] [Google Scholar]
- 12.Roos A., Boron W.F. Intracellular pH. Physiol. Rev. 1981;61:296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- 13.Bryant B.P. Mechanisms of somatosensory neuronal sensitivity to alkaline pH. Chem. Senses. 2005;30(Suppl. 1):i196–i197. doi: 10.1093/chemse/bjh182. [DOI] [PubMed] [Google Scholar]
- 14.Thomas R.C. Intracellular pH of sail neurones measured with a new pH-sensitive glass micro-electrode. J. Physiol. 1974;238:159–180. doi: 10.1113/jphysiol.1974.sp010516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stewart W., Judovich B., Hughes J., Walti A. The effect of ammonium chloride on pain. Am. J. Physiol. 1940;129:474–475. [Google Scholar]
- 16.Davies J.I., Stewart P.B., Fink H.P. Prolonged sensory block using ammonium salts. Anesthesiology. 1967;28:244–245. doi: 10.1097/00000542-196701000-00050. [DOI] [Google Scholar]
- 17.Boron W.F., De Weer P. Intracellular pH transients in squid giant axons caused by CO2, NH3, and metabolic inhibitors. . J. Gen. Physiol. 1976;67:91–112. doi: 10.1085/jgp.67.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boron W.F. Intracellular pH transients in giant barnacle muscle fibers. Am. J. Physiol. 1977;233:C61–C73. doi: 10.1152/ajpcell.1977.233.3.C61. [DOI] [PubMed] [Google Scholar]
- 19.Montell C., Rubin G.M. Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron. 1989;2:1313–1323. doi: 10.1016/0896-6273(89)90069-X. [DOI] [PubMed] [Google Scholar]
- 20.Clapham D.E. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 21.Minke B., Cook B. TRP channel proteins and signal transduction. Physiol. Rev. 2002;82:429–472. doi: 10.1152/physrev.00001.2002. [DOI] [PubMed] [Google Scholar]
- 22.Montell C. The TRP superfamily of cation channels. Sci. STKE. 2005;2005:RE3. doi: 10.1126/stke.2722005re3. [DOI] [PubMed] [Google Scholar]
- 23.Caterina M.J., et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 24.Tominaga M., Caterina M.J. Thermosensation and pain. J. Neurobiol. 2004;61:3–12. doi: 10.1002/neu.20079. [DOI] [PubMed] [Google Scholar]
- 25.Bautista D.M., et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 26.Kwan K.Y., et al. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 27.Story G.M., et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/S0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 28.Bandell M., et al. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/S0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- 29.Jordt S.E., et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 30.Bautista D.M., et al. Pungent products from garlic activate the sensory ion channel TRPA1. Proc. Natl. Acad. Sci. U. S. A. 2005;102:12248–12252. doi: 10.1073/pnas.0505356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Macpherson L.J., et al. The pungency of garlic: activation of TRPA1 and TRPV1 in response to allicin. Curr. Biol. 2005;15:929–934. doi: 10.1016/j.cub.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 32.Fujita F., Moriyama T., Higashi T., Shima A., Tominaga M. Methyl p-hydroxybenzoate causes pain sensation through activation of TRPA1 channels. Br. J. Pharmacol. 2007;151:153–160. doi: 10.1038/sj.bjp.0707219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karashima Y., et al. Bimodal action of menthol on the transient receptor potential channel TRPA1. J. Neurosci. 2007;27:9874–9884. doi: 10.1523/JNEUROSCI.2221-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McNamara C.R., et al. TRPA1 mediates formalin-induced pain. Proc. Natl. Acad. Sci. U. S. A. 2007;104:13525–13530. doi: 10.1073/pnas.0705924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bang S., Kim K.Y., Yoo S., Kim Y.G., Hwang S.W. Transient receptor potential A1 mediates acetaldehyde-evoked pain sensation. Eur. J. Neurosci. 2007;26:2516–2523. doi: 10.1111/j.1460-9568.2007.05882.x. [DOI] [PubMed] [Google Scholar]
- 36.Macpherson L.J., et al. An ion channel essential for sensing chemical damage. J. Neurosci. 2007;27:11412–11415. doi: 10.1523/JNEUROSCI.3600-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu H., Delling M., Jun J.C., Clapham D.E. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat. Neurosci. 2006;9:628–635. doi: 10.1038/nn1692. [DOI] [PubMed] [Google Scholar]
- 38.Zurborg S., Yurgionas B., Jira J.A., Caspani O., Heppenstall P.A. Direct activation of the ion channel TRPA1 by Ca2+. . Nat. Neurosci. 2007;10:277–279. doi: 10.1038/nn1843. [DOI] [PubMed] [Google Scholar]
- 39.Doerner J.F., Gisselmann G., Hatt H., Wetzel C.H. Transient receptor potential channel A1 is directly gated by calcium ions. J. Biol. Chem. 2007;282:13180–13189. doi: 10.1074/jbc.M607849200. [DOI] [PubMed] [Google Scholar]
- 40.Andersson D.A., Gentry C., Moss S., Bevan S. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J. Neurosci. 2008;28:2485–2494. doi: 10.1523/JNEUROSCI.5369-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hinman A., Chuang H., Bautista D.M., David J. TRP channel activation by reversible covalent modification. Proc. Natl. Acad. Sci. U. S. A. 2006;103:19564–19568. doi: 10.1073/pnas.0609598103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Macpherson L.J., et al. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007;445:541–545. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- 43.Adler S., Roy A., Relman A.S. Intracellular acid-base regulation. I. The response of muscle cells to changes in CO2 tension or extracellular bicarbonate concentration. . J. Clin. Invest. 1965;44:8–20. doi: 10.1172/JCI105129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mizuno S., et al. Alkalosis stimulates endothelial nitric oxide synthase in cultured human pulmonary arterial endothelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2002;283:L113–L119. doi: 10.1152/ajplung.00436.2001. [DOI] [PubMed] [Google Scholar]
- 45.Caterina M.J., et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 46.Caterina M.J., Rosen T.A., Tominaga M., Brake A.J., Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- 47.McKemy D.D., Neuhausser W.M., Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 48.Peier A.M., et al. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/S0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 49.Colburn R.W., et al. Attenuated cold sensitivity in TRPM8 null mice. Neuron. 2007;54:379–386. doi: 10.1016/j.neuron.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 50.Proudfoot C.J., et al. Analgesia mediated by the TRPM8 cold receptor in chronic neuropathic pain. Curr. Biol. 2006;16:1591–1605. doi: 10.1016/j.cub.2006.07.061. [DOI] [PubMed] [Google Scholar]
- 51.Nagata K., Duggan A., Kumar G., Garcia-Anoveros J. Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J. Neurosci. 2005;25:4052–4061. doi: 10.1523/JNEUROSCI.0013-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Macpherson L.J., et al. More than cool: promiscuous relationships of menthol and other sensory compounds. Mol. Cell. Neurosci. 2006;32:335–343. doi: 10.1016/j.mcn.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 53.Xu H., Blair N.T., Clapham D.E. Camphor activates and strongly desensitizes the transient receptor potential vanilloid subtype 1 channel in a vanilloid-independent mechanism. J. Neurosci. 2005;25:8924–8937. doi: 10.1523/JNEUROSCI.2574-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kobayashi K., et al. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J. Comp. Neurol. 2005;493:596–606. doi: 10.1002/cne.20794. [DOI] [PubMed] [Google Scholar]
- 55.Trevisani M., et al. 20074-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc. Natl. Acad. Sci. U. S. A. , 10413519–13524. 10.1073/pnas.0705923104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim D., Cavanaugh E.J. Requirement of a soluble intracellular factor for activation of Transient Receptor Potential A1 by pungent chemicals: role of inorganic polyphosphates. J. Neurosci. 2007;27:6500–6509. doi: 10.1523/JNEUROSCI.0623-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dawson, R.M.C., Elliott, D.C., Elliott, W.H., and Jones, K.M. 1986.Data for biochemical research. 3rd edition. Oxford University Press. New York, New York, USA. 496 pp. [Google Scholar]
- 58.Yodozawa S., Speake T., Elliott A. Intracellular alkalinization mobilizes calcium from agonist-sensitive pools in rat lacrimal acinar cells. . J. Physiol. 1997;499:601–611. doi: 10.1113/jphysiol.1997.sp021953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Speake T., Elliott A.C. Modulation of calcium signals by intracellular pH in isolated rat pancreatic acinar cells. J. Physiol. 1998;506:415–430. doi: 10.1111/j.1469-7793.1998.415bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alfonso A., Cabado A.G., Vieytes M.R., Botana L.M. Calcium-pH crosstalks in rat mast cells: cytosolic alkalinization, but not intracellular calcium release, is a sufficient signal for degranulation. Br. J. Pharmacol. 2000;130:1809–1816. doi: 10.1038/sj.bjp.0703490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sawada Y., Hosokawa H., Hori A., Matsumura K., Kobayashi S. Cold sensitivity of recombinant TRPA1 channels. Brain Res. 2007;1160:39–46. doi: 10.1016/j.brainres.2007.05.047. [DOI] [PubMed] [Google Scholar]
- 62.Stevens C.R., Williams R.B., Farrell A.J., Blake D.R. Hypoxia and inflammatory synovitis: observations and speculation. Ann. Rheum. Dis. 1991;50:124–132. doi: 10.1136/ard.50.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mckeegan D.E.F. Mechano-chemical nociceptors in the avian trigeminal mucosa. Brain Res. Rev. 2004;46:146–154. doi: 10.1016/j.brainresrev.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 64.Pidoplichko V.I. Ammonia and proton gated channel populations in trigeminal ganglion neurons. Gen. Physiol. Biophys. 1992;11:39–48. [PubMed] [Google Scholar]
- 65.Mansberger A.R., Jr. Value of peritoneal fluid ammonia levels in the differential diagnosis of the acute abdomen. Ann. Surg. . 1962;155:998–1010. doi: 10.1097/00000658-196206000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thomas J.A., Buchsbaum R.N., Zimniak A., Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979;18:2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.