Abstract
Interspecific differences in foraging behavior may help to determine whether the outcome of interspecific competition is coexistence or exclusion. Mosquitoes in the genus Culex are commonly described as foraging primarily by filtering the water column. This behavior contrasts with that of other container-dwelling genera, such as Aedes and Ochlerotatus, that are thought to forage primarily by browsing on container and detritus surfaces. We compared the feeding behavior of Cx. pipiens, Ae. albopictus, and Oc. triseriatus in a laboratory experiment in which we monitored behavior of individual mosquitoes in two different food environment treatments: food suspended in the water column only and food attached to leaf surfaces only. For each mosquito in each food environment, we quantified the time allocated by larvae to one of four positions and to one of three activities. The effect of treatment was significant, with individuals in Fluid Only environments spending more time resting-filtering at the surface, and individuals in Leaf Only environments spending more time browsing on walls. There were significant differences among species, with Cx. pipiens spending more time at the surface than the other species, which spent more time thrashing below the surface. There was no significant interaction of species and treatment, indicating that all three species modify their behavior in similar ways in these environments. Contrary to current understanding, our data suggest that Cx. pipiens browse as frequently as do these potential competitors but show a greater concentration of foraging effort at the top of a container.
Keywords: Culex pipiens, Aedes albopictus, Ochlerotatus triseriatus, foraging
INTRODUCTION
Container-dwelling mosquitoes inhabit both man-made (e.g., discarded automobile tires, cemetery vases) and natural (e.g., tree holes, bamboo internodes) vessels. Within these habitats, multiple mosquito species may be present simultaneously, so that in food-limiting environments, individuals may experience interspecific resource competition (Juliano 1998, Juliano et al. 2004). Competition for resources is thought to be an important determinant of mosquito success (e.g., Ho et al. 1989, Juliano 1998, Lounibos et al. 2002, Juliano et al. 2004).
Mosquito nutritional requirements are met through the consumption of both dead and living organic material (Merritt et al. 1992). The largest portion of the larval diet is composed of heterotrophic microorganisms (i.e., bacteria, fungi, protozoans) that grow on container or detritus surfaces or are suspended in fluid (Merritt et al. 1992, Clements 1999). An important way of categorizing larvae of different species is by how they obtain their food (Clements 1999, Merritt et al. 1992). Larvae use their mouth parts to browse on hard surfaces and to filter particles from fluid (Merritt et al. 1992). General categories of feeding mode include filter feeding, browsing, and predation (Surtees 1959), with more specific categories including collecting-gathering, collecting-filtering, scraping, shredding, and predation (Merritt et al. 1992, Clements 1999). These classifications are useful and enable us to relate feeding behavior, resource type and availability, and larval success to adult production, which is of considerable medical importance (Hawley 1985). Larval behavior patterns, including those associated with feeding, have been suggested to influence competitive ability among mosquito species (Ho et al. 1989, Juliano et al. 1993, Grill and Juliano 1996, Yee et al. 2004).
Three common container mosquito species in Illinois are Aedes albopictus (Skuse), Ochlerotatus triseriatus (formerly in the genus Aedes, Reinert 2000) (Say), and Culex pipiens (L). Ae. albopictus is an invasive species that was introduced into North America in the mid-1980s (Hawley et al. 1987). Aedes albopictus is a potential vector of dengue, LaCrosse encephalitis, and eastern equine encephalitis (Hawley 1988, Mitchell et al. 1993). In addition, Ae. albopictus is known to have negative ecological effects on resident mosquito species (Juliano 1998, Lounibos et al. 2001, 2002). Ochlerotatus triseriatus predominantly occupies containers within forested areas (Bradshaw and Holzapfel 1985, 1988). Adult female Oc. triseriatus are the primary vectors of LaCrosse encephalitis, especially in the Great Lakes region and the Carolinas (Grimstad et al. 1977, Szumlas et al. 1996). Culex pipiens occupies both natural and man-made containers and, among other diseases, has recently been implicated as a vector of West Nile Virus (Truell et al. 2001, Goddard et al. 2003). Although all these species combine filtering and browsing to obtain food resources, species in the genus Culex have been categorized as “collector-filterers” that feed in the water column (Dahl et al. 1988, Merritt et al. 1992), whereas Oc. triseriatus and Ae. albopictus have been classified as “collector-gatherers” and “shredders”, obtaining resources from organic surfaces and sediments (Merritt et al. 1992).
We tested the hypotheses that: 1) larvae of these species display different feeding behaviors in different food environments; and 2) Cx. pipiens would feed primarily by filtering in the water column, whereas Ae. albopictus and Oc. triseriatus would feed primarily by removing particles from surfaces via browsing. In order to test these hypotheses, we video recorded feeding behaviors in two distinct food environments where gains from different feeding modes would likely differ.
MATERIALS AND METHODS
Rearing
Individuals used in this experiment were first-generation progeny of field-collected larvae obtained from tree holes in central Illinois (Oc. triseriatus) and from discarded automobile tires in East St. Louis, IL (Ae. albopictus, Cx. pipiens). In East St. Louis, Cx. pipiens co-occurs with Cx. quinquefasciatus (Say), and these species are known to produce hybrids in regions of cohabitation (Brogdon 1984, Vinogradova 2000). Field-collected larvae were sorted based on a siphonal index, which can typically separate pure populations of these species (Brogdon 1984, Vinogradova 2000). We cannot, however, separate hybrids reliably, hence our laboratory population probably included individuals carrying genes of Cx. quinquefasciatus. About 90% of field-collected individuals from our East St. Louis site were morphologically consistent with Cx. pipiens. We regard our laboratory population as a representative sample of the interbreeding members of the Cx. pipiens complex from our field site. In this paper, we will refer to them as Cx. pipiens for simplicity. Larvae of Ae. albopictus and Oc. triseriatus were raised to adults on bovine liver powder (ICN Biochemicals, Cleveland, OH), and then released into 0.6 m2 cages. Larvae of Cx. pipiens were raised to adulthood on an artificial diet comprised of blood meal, powdered milk, yeast, and wheat flour (see Copeland 1987 for details). Adult females of all species were blood fed on anesthetized laboratory mice in order to obtain eggs used to produce larvae for this study. Adult female Cx. pipiens were provided with a 500 ml black cup filled with 400 ml of de-ionized (DI) water and several blades of a foxtail grass (Setaria faberi Herrm.), which provided an oviposition stimulant. Egg rafts from Cx. pipiens were collected within 24 h of being laid and larvae were hatched in pans before being transferred to individual 20 ml vials. Eggs of Ae. albopictus and Oc. triseriatus were synchronously hatched in 5 × 40 mm glass tubes filled with a solution of 0.33 g nutrient broth in 750 ml DI water. After 24 h, hatched larvae were rinsed and transferred to new individual 20 ml glass vials with 20 ml DI water. Larvae were housed in an environmental chamber at 27°C and a 14:10 L:D cycle. The newly hatched larvae were fed an artificial diet suspension that was prepared by mixing 0.30 g of an artificial diet in 1000 ml of deionized (DI) water (see Copeland 1987 for details). This food suspension was dispensed with a pipette from a beaker held on a stirring plate to ensure homogeneous delivery of food to larvae (Juliano and Gravel 2002). Larvae were fed 500 μl of the artificial diet suspension on the first day and 1000 μl every other day thereafter. Water in each vial was changed every other day. Larvae were reared under these conditions for approximately six d by which time they had grown to late 3rd to early 4th instars. Twenty-four h before behavioral observations, all larvae were transferred into individual 50 ml plastic beakers filled with 50 ml DI water without food in order to standardize hunger for all individuals.
Observation environments
A foxtail grass (S. faberi) was collected at the margin of railroad tracks near the Illinois State University (Normal, IL) campus and stored at room temperature. Leaf blades were cut into strips (1 cm × 5 cm) and placed individually into 50 ml beakers of DI water and maintained at 26 °C in the dark, which prevented algal growth but allowed growth of bacteria, fungi, and protozoans. After five d, the soaked leaf blades were used to create two separate food environments: Food on leaf surfaces only and food in fluid only. Food on leaf only (hereafter “Leaf Only”) was created by transferring the leaf blade from its original beaker to a new 50 ml beaker with fresh DI water. In that new beaker, the leaf was positioned so that it adhered to the beaker wall. Food in fluid only (hereafter “Fluid Only”) was created by transferring the remaining 50 ml of leaf blade leachate (i.e., suspended particulate matter and microorganisms present in the fluid due to the soaking of the grass leaf blades) from the same beaker into a new 50 ml beaker. Transferring the contents in this manner eliminated any microorganisms that likely grew on the sides of the beaker during the 5 d preparation period. Within 5 min after setting up the food environments, each replicate received an individually-reared larva of one of the three species. This short time until measurement would have limited the microorganism colonization to the undesired locations (e.g., in the fluid in the ‘Leaf Only’ treatment). In all, 10 replicates of each species in each food environment were used for a total of 60 observations.
Behavior measurement and analysis
We recorded behaviors of larvae in each treatment for 30 min using a Panasonic Digital Video Camera connected to a personal computer using Digital Video Creator (Dazzle Multimedia, Inc., Fremont CA). Each larva was given a five min acclimation period in the observation beaker before initiating the recording of behavior. Recordings were made with the camera positioned directly above the treatment beakers in an isolated room with no observers present. A single 30 min video clip had only images of six treatment beakers at a time due to resolution constraints. However, each video clip represented a single replicate of all treatment combinations (three species × two food environments).
From each video clip, the activity and position of each larva were recorded every minute for 30 min in instantaneous scan censuses (Martin and Bateson 1986, Juliano and Gravel 2002, Kesavaraju and Juliano 2004, Yee et al. 2004). Activities were classified into three categories, 1) Browsing: larva moving along a surface propelled by feeding movements of the mouth parts; 2) Resting-Filtering: larva completely still or larva drifting through the water column, propelled by feeding movements of mouth parts; and 3) Thrashing: larva propelling itself through the water by energetic lateral flexion of the body (Juliano and Reminger 1992, Grill and Juliano 1996, Juliano and Gravel 2002, Kesavaraju and Juliano 2004). In past behavioral studies (Juliano and Reminger 1992, Grill and Juliano 1996, Juliano and Gravel 2002, Kesavaraju and Juliano 2004, Yee et al. 2004) we have been able to separate filtering and resting behaviors. We were unable to do so in this study as Cx. pipiens were observed to filter while remaining essentially motionless. The resolution of our camera was insufficient for us to discriminate filtering and resting, hence we treated these behaviors as a single activity. Positions were classified into four categories, 1) Surface: larva’s spiracular siphon in contact with the surface; 2) Bottom: larva within 1 mm of the bottom of the beaker; 3) Wall: larva within 1 mm of the sides of the beaker or leaf surface; and 4) Middle: larva not in contact with the surface and more than 1 mm from the beaker’s or leaf’s surfaces. We predicted that larval feeding mode (Merritt et al. 1992) would vary with food environment, so that larvae would show high frequencies of filtering-resting in the Fluid Only environment and high frequencies of browsing in the Leaf Only environment. Based on previous review of foraging behavior (Merritt et al. 1992), we predicted also that Cx. pipiens would spend more time filtering-resting and less time browsing than either Ae. albopictus or Oc. triseriatus regardless of the food environment.
In order to meet assumptions of normality and homogeneous variance, proportions of observations of activities and positions were arcsine-square root transformed. To reduce the number of variables and to obtain uncorrelated descriptors of behavior, we used principal component analysis on transformed proportions of activities and positions (Juliano and Gravel 2002, PROC FACTOR SAS Institute Inc, 1990). Principal components (PC’s) with eigenvalues > 1.0 were retained for further analysis (Hatcher and Stepanski 1994). Principal component scores were analyzed using Multivariate Analysis of Variance (MANOVA, PROC GLM,SAS Institute Inc., 1990) with species and food environment treatments as independent variables and retained PC’s as dependent variables. Significant MANOVA effects were interpreted using standardized canonical coefficients (Scheiner 2001), which quantify the magnitude of the contributions of the individual PC’s to significant multivariate effects. Significant effects were analyzed further using multivariate pair-wise contrasts (Scheiner 2001) with Bonferroni adjustment to control for experimental-wise error rate.
RESULTS
Principal Component Analysis reduced the three activities and four positions to two PC axes, which summarized 85.2% of the variation in these measures (Table 1). We interpreted PC1 as a scale on which individuals with large positive PC scores spent considerable time browsing at walls, whereas individuals with large negative PC scores spent considerable time resting-filtering at the surface (Table 1). Individuals with high positive scores on PC2 thrashed frequently and occupied the bottom and middle, whereas individuals with large negative scores occupied the surface (Table 1).
Table 1.
Principal Component Analysis for larval feeding behavior. Values ≥ 40 are listed in boldface and indicate strong loadings on each principal component.
| Response Variable | PC1 | PC2 |
|---|---|---|
| Resting-Filtering | − 98 | − 16 |
| Thrashing | + 8 | + 80 |
| Browsing | + 98 | − 6 |
| Surface | − 78 | − 52 |
| Bottom | + 3 | + 90 |
| Wall | + 92 | − 33 |
| Middle | − 54 | + 67 |
|
| ||
| Interpretation | Resting-Filtering, Surface vs. Browsing Wall | Thrashing, Bottom, Middle vs. Surface |
We detected significant MANOVA effects of species and food environment but no significant interaction (Table 2). The lack of an interaction indicates that behavior differences between treatments were consistent across all species. The food environment effect was strongly associated with PC1, and secondarily associated with PC2 (Table 2). Individuals in Fluid Only environments had large negative scores indicating frequent resting-filtering at the surface (Figure 1), whereas individuals in Leaf Only environments had large positive scores indicating frequent browsing on walls (Figure 1). The species effect was strongly associated with PC2, with the relationship to PC1 very weak (Table 2). PC2 scores for Cx. pipiens were negative, indicating that this species occupied the surface position (Figure 2). PC2 scores for Ae. albopictus and Oc. triseriatus were large and positive, indicating that these species were often moving and not feeding (i.e., thrashing) at the bottom and wall (Figure 2).
Table 2.
Results of two-way (species, food environment) MANOVA on feeding behavior patterns. The magnitude of the standardized canonical coefficients indicate the degree of contribution by each factor to the significant MANOVA effect.
| Standardized Canonical Coefficients | ||||||
|---|---|---|---|---|---|---|
| Source | df | error df | Pillai’s Trace | P- value | PC1 | PC2 |
| Environment (E) | 2 | 53 | 0.5928 | < 0.0001 | + 1.4370 | - 0.5549 |
| Species (S) | 4 | 108 | 0.4130 | < 0.0001 | - 0.1599 | + 1.2130 |
| S × E | 4 | 108 | 0.0780 | 0.3586 | + 1.4677 | - 0.5421 |
Figure 1.
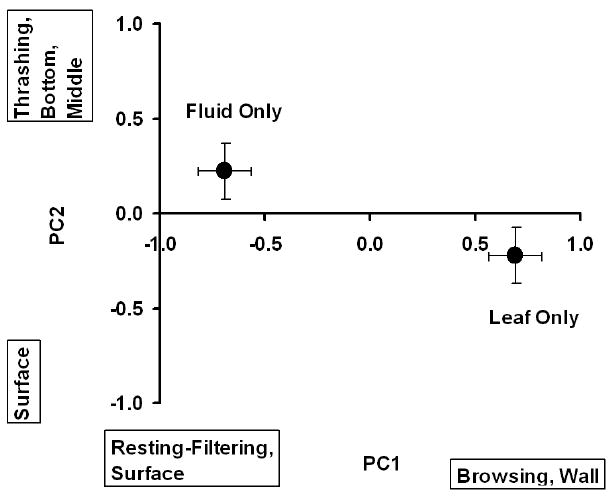
Means of PC1 and PC2 (± 1 SE) for food environments. PC1 was the primary contributor to the significant multivariate effect of environment. Activities and positions most closely associated with large positive or large negative PC scores are indicated parallel to each axis.
Figure 2.
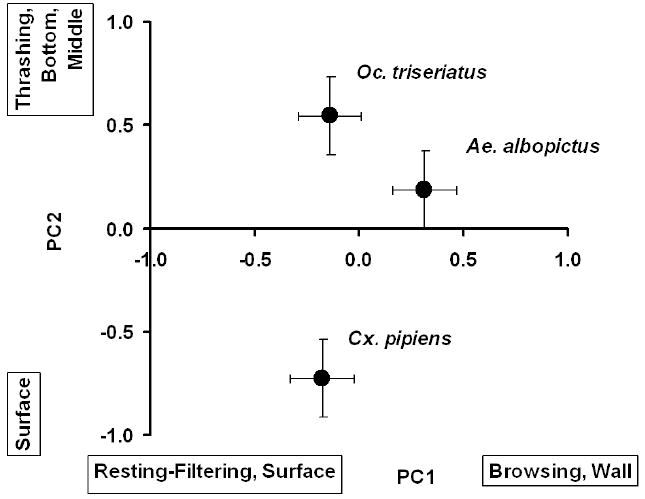
Means of PC1 and PC2 (± 1 SE) for the three species. PC2 was the primary contributor to the significant multivariate effect of species. Activities and positions most closely associated with large positive or large negative PC scores are indicated parallel to each axis. Significant differences were detected between Culex pipiens and each of the other two species based on multivariate pair-wise multiple comparisons. Multivariate comparison of Ae. albopictus and Oc. triseriatus was not significant.
DISCUSSION
In this experiment, larvae displayed consistent feeding behaviors that appeared to be correlated with the food environments. For instance, larvae spent more time browsing on walls with a leaf present but were found at the surface where they rested-filtered when in Fluid Only beakers. This is consistent with studies that have shown that the presence of a leaf elicits greater browsing by Aedes mosquito larvae than when a leaf is absent (Nilsson1, Khawaled et al. 1988, David et al. 2002, Yee et al. 2004). However, our results contrast with those of Walker and Merritt (1991) who showed that larvae of Oc. triseriatus allocated more time to feeding near the surface compared to browsing on leaves when leaves were present. Their study monitored 20 larvae simultaneously in a single container, and it is possible that inter-individual interference limited access to leaf surfaces.
The apparent attractiveness of leaves to larvae of all three species suggests that larvae receive cues (e.g., phagostimulants such as nucleic acids or nucleotides) from microorganisms or organic surfaces that attract them to specific food areas. These cues may stimulate greater foraging effort in areas where more food resources are available (Merritt et al. 1992, Clements 1999). Aggregation of larvae (Aly 1983) cannot explain more time spent by larvae at the wall, as we observed single larvae in each container. Hunger also has been implicated as a factor altering larval feeding patterns. For example, Juliano et al. (1993) demonstrated that hungry Oc. triseriatus alter their allocation of time to feeding by increasing browsing and decreasing filtering. Conversely, larvae of four species of Culex in high-food environments were more sedentary compared to individuals in low-food environments (Workman and Walton 2003). Another behavior, the startle response, decreases in well-fed Aedes aegypti individuals (Olsson and Klowden 1998). It seems likely that food availability and larval hunger can routinely affect larval behavior.
Significant consistent differences in behaviors were detected among species. Culex pipiens tended to occupy the top of containers, whereas Ae. albopictus and Oc. triseriatus spent more time thrashing in the middle or at the bottom. Because these differences are consistent between food environments (i.e., there is no interaction), these species may segregate spatially even though they feed in similar ways. Spatial segregation on similar resources is a common explanation for how species may decrease or avoid competition (Morin 1999). Surprisingly, larvae of Cx. pipiens browsed (65.3 ± 6.3 %) at rates comparable to those of Ae. albopictus (72.7 ± 6.6 %) and Oc. triseriatus (62.0 ± 7.1%) with a leaf present. Thus, our prediction, based on Dahl et al. (1988) and Merritt et al. (1992), that Cx. pipiens should browse less was not supported. Dahl et al. (1988) categorized three species of Culex as either obligate or facultative filter feeders and two species of Aedes as either obligate or facultative brushers (equivalent to browsers) based on head and mouth morphology. Although morphological differences exist, such differences should be interpreted with caution, as it is apparent from our work that feeding behavior is both complex and flexible in response to resource availability. Our results reinforce Walker and Merritt’s (1991) conclusion that classification of larval mosquito species into rigid feeding categories is misleading because mosquito larvae demonstrate considerable behavioral flexibility in feeding mode.
We have shown that larvae of three container mosquito species employ similar feeding modes in similar food environments, but that there exist interspecific differences in spatial distribution in simple laboratory microcosms. Furthermore, these data indicate that Cx. pipiens does not appear to spend less time browsing surfaces. Similarity of the feeding behavior of Cx. pipiens and these aedine mosquitoes suggests that competition for food is possible when they co-occur in a container. It remains to be tested how feeding differences are manifest in more complex field environments and how these differences translate into costs or benefits to larval survival, growth, or adult success.
Acknowledgments
We thank K. S. Costanzo for supplying us with Culex pipiens and for enthusiastic input into this project, R. C. Anderson for identification of plants, and R. King and the staff of East Side Health District, East St. Louis, for assistance in the field. This work was supported by funds from the National Institute of Allergy and Infectious Disease (R01-AI44793) and the Department of Biological Sciences, Illinois State University.
Footnotes
Nilsson, C. 1986. Feeding and food utilization by mosquito larvae. Ph. D. dissertation. Uppsala University, Uppsala, Sweden.
REFERENCES CITED
- Aly C. Feeding behavior of Aedes vexans larvae (Diptera: Culicidae) and its influence on the effectiveness of Bacillus thuringiensis var. israelensis. Bull Soc Vector Ecol. 1983;8:94–100. [Google Scholar]
- Bradshaw WE, Holzapfel CM. The distribution and abundance of treehole mosquitoes in eastern North America: Perspectives from Florida. In: Lounibos LP, Rey JR, Frank JH, editors. Ecology of mosquitoes: proceedings of a workshop. Florida Medical Entomological Laboratory; Vero Beach, FL: 1985. pp. 3–24. [Google Scholar]
- Bradshaw WE, Holzapfel CM. Drought and the organization of tree-hole mosquito communities. Oecologia. 1988;74:507–514. doi: 10.1007/BF00380047. [DOI] [PubMed] [Google Scholar]
- Brogdon WG. The siphonal index. I. A method for evaluating Culex pipiens subspecies and intermediates. Mosq System. 1984;16:144–152. [Google Scholar]
- Clements AN. The Biology of Mosquitoes. I. Chapman and Hall; London, U.K: 1992. [Google Scholar]
- Clements AN. The Biology of Mosquitoes. II. Chapman and Hall; London, U.K: 1999. [Google Scholar]
- Copeland RS. Establishment of a free-mating colony of Anopheles barberi, with notes on development rates. J Am Mosq Contr Assoc. 1987;3:502–503. [PubMed] [Google Scholar]
- Dahl C, Widahl L-E, Nilsson C. Functional analysis of the suspension feeding system in mosquitoes (Diptera: Culicidae) Ann Entomol Soc Am. 1988;81:105–127. [Google Scholar]
- David J-P, Ferran A, Gambier J, Meyran J-C. Taste sensitivity of detritivorous mosquito larvae to decomposed leaf litter. J Chem Ecol. 2002;28:983–995. doi: 10.1023/a:1015257700992. [DOI] [PubMed] [Google Scholar]
- Goddard LB, Roth AE, Reisen WK, Scott TW. Vertical transmission of West Nile Virus by three California Culex (Diptera: Culicidae) species. J Med Entomol. 2003;40:743–746. doi: 10.1603/0022-2585-40.6.743. [DOI] [PubMed] [Google Scholar]
- Grill CP, Juliano SA. Predicting interactions based on behavior: predation and competition in container-dwelling mosquitoes. J Anim Ecol. 1996;65:63–76. [Google Scholar]
- Grimstad PR, Craig GB, Ross QE, Yuill TM. Aedes triseriatus and LaCrosse virus: geographic variation in vector susceptibility and ability to transmit. Am J Trop Med Hyg. 1977;26:990–996. doi: 10.4269/ajtmh.1977.26.990. [DOI] [PubMed] [Google Scholar]
- Hatcher L, Stepanski EJ. A step by step approach to using SAS system for univariate and multivariate analyses. SAS Institute Inc; Cary, NC: 1994. [Google Scholar]
- Hawley WA, Reiter P, Copeland RS, Pumpuni CB, Craig GB., Jr Aedes albopictus in North America: Probable introduction in used tires from northern Asia. Science. 1987;236:1114–1116. doi: 10.1126/science.3576225. [DOI] [PubMed] [Google Scholar]
- Hawley WA. The effect of larval density on adult longevity of a mosquito, Aedes sierrensis: epidemiological consequences. J Anim Ecol. 1985;54:955–964. [Google Scholar]
- Hawley WA. The biology of Aedes albopictus. J Am Mosq Contr Assoc. 1988;4(suppl):1–40. [PubMed] [Google Scholar]
- Ho BC, Ewert A, Chew ALM. Interspecific competition between Aedes aegypti, Aedes albopictus, and Aedes triseriatus (Diptera: Culicidae): Larval development in mixed cultures. J Med Entmol. 1989;26:615–623. doi: 10.1093/jmedent/26.6.615. [DOI] [PubMed] [Google Scholar]
- Juliano SA. Species introduction and replacement among mosquitoes: interspecific resource competition or apparent competition? Ecology. 1998;79:255–268. [Google Scholar]
- Juliano SA, Gravel ME. Predation and the evolution of prey behavior: An experiment with tree hole mosquitoes. Behav Ecol. 2002;13:301–311. [Google Scholar]
- Juliano SA, Hechtel LJ, Waters JR. Behavior and risk of predation in larval tree hole mosquitoes: effects of hunger and population history of predation. Oikos. 1993;68:229–241. [Google Scholar]
- Juliano SA, Lounibos LP, O’Meara GF. A field test for competitive effects of Aedes albopictus on A. aegypti in South Florida: Differences between sites of coexistence and exclusion? Oecologia. 2004;139:583–593. doi: 10.1007/s00442-004-1532-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano SA, Reminger L. The relationship between vulnerability to predation and behavior of larval treehole mosquitoes: geographic and ontogenetic differences. Oikos. 1992;63:465–476. [Google Scholar]
- Kesavaraju B, Juliano SA. Differential behavioral responses to water-borne cues to predation in two container dwelling mosquitoes. Ann Entomol Soc Am. 2004;97:194–201. doi: 10.1603/0013-8746(2004)097[0194:dbrtwc]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khawaled K, Barak Z, Zaritsky A. Feeding behavior of Aedes aegypti larvae and toxicity of dispersed and naturally encapsulated Bacillus thuringiensis var. israelensis. J Invert Pathol. 1988;52:419–426. doi: 10.1016/0022-2011(88)90054-7. [DOI] [PubMed] [Google Scholar]
- Lounibos LP, Suárez S, Menéndez Z, Nishimura N, Escher RL, O’Connell SM, Rey JJ. Does temperature affect the outcome of larval competition between Aedes aegypti and Aedes albopictus? J Vector Ecol. 2002;27:86–95. [PubMed] [Google Scholar]
- Lounibos LP, O’Meara GF, Escher RL, Nishimura N, Cutwa M, Nelson T, Campos RE, Juliano SA. Testing predicted competitive displacement of native Aedes by the invasive Asian Tiger Mosquito Aedes albopictus in Florida, USA. Biol Invas. 2001;3:151–166. [Google Scholar]
- Martin P, Bateson P. Measuring behavior: An introductory guide. Cambridge University Press; Cambridge, U.K: 1986. [Google Scholar]
- Merritt RW, Dadd RH, Walker ED. Feeding behavior, natural food, and nutritional relationships of larval mosquitoes. Annu Rev Entomol. 1992;37:349–376. doi: 10.1146/annurev.en.37.010192.002025. [DOI] [PubMed] [Google Scholar]
- Mitchell CJ, McLean RG, Nasci RS, Crans WJ, Smith GC, Caccamise DF. Susceptibility parameter of Aedes albopictus to per oral infection with Eastern equine encephalitis virus. J Med Entomol. 1993;30:233–235. doi: 10.1093/jmedent/30.1.233. [DOI] [PubMed] [Google Scholar]
- Morin PJ. Community Ecology. Blackwell Science, Inc; Oxford, U.K: 1999. [Google Scholar]
- Olsson B, Klowden MJ. Larval diet affects the alarm response of Aedes aegypti (L.) mosquitoes (Diptera: Culicidae) J Insect Behavior. 1998;11:593–596. [Google Scholar]
- Reinert JF. New classification for the composite genus Aedes (Diptera, Culicidae, Aedini), elevation of subgenus Ochlerotatus to generic rank, reclassification of the other subgenera, and notes on certain subgenera and species. J Am Mosq Contr Assoc. 2000;16:175–188. [PubMed] [Google Scholar]
- SAS Institute Inc. SAS/STAT Users Guide, Version 6. 4. 1 and 2. SAS Institute Inc; Cary NC: 1990. [Google Scholar]
- Scheiner SM. MANOVA. Multiple response variables and multi species interactions. In: Scheiner SM, Gurevitch J, editors. Design and analysis of ecological experiments. 2. Oxford University Press; Oxford, U.K: 2001. pp. 99–133. [Google Scholar]
- Surtees G. Functional and morphological adaptations to the larval mouthparts in the subfamily Culicidae (Diptera) with a review of some related studies by Montschadsky. Proc R Entomol Soc Lond. 1959;34:7–16. [Google Scholar]
- Szumlas DE, Apperson CS, Powell EE. Seasonal occurrence and abundance of Aedes triseriatus and other mosquitoes in a LaCrosse virus-endemic area of western North Carolina. J Am Mosq Contr Assoc. 1996;12:184–193. [PubMed] [Google Scholar]
- Turell M, O’Guinn ML, Dohm DJ, Jones JW. Vector competence of North American mosquitoes (Diptera: Culicidae) for West Nile Virus. J Med Entomol. 2001;38:130–134. doi: 10.1603/0022-2585-38.2.130. [DOI] [PubMed] [Google Scholar]
- Vinogradova EB. Culex pipiens pipiens mosquitoes; taxonomy, distribution, ecology, physiology, genetics, applied importance and control. Pensoft; Sofia, Bulgaria: 2000. [Google Scholar]
- Walker ED, Merritt RW. Behavior of larval Aedes triseriatus (Diptera: Culicidae) J Med Entomol. 1991;28:581–589. doi: 10.1093/jmedent/28.5.581. [DOI] [PubMed] [Google Scholar]
- Workman PD, Walton WE. Larval behavior of four Culex (Diptera: Culicidae) associated with treatment wetlands in the southwestern United States. J Vector Ecol. 2003;28:213–228. [PubMed] [Google Scholar]
- Yee DA, Kesavaraju B, Juliano SA. Interspecific differences in feeding behavior and survival under food-limited conditions for larval Aedes albopictus and Aedes aegypti. (Diptera: Culicidae) Ann Entomol Soc Am. 2004;97:720–728. doi: 10.1603/0013-8746(2004)097[0720:IDIFBA]2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]


