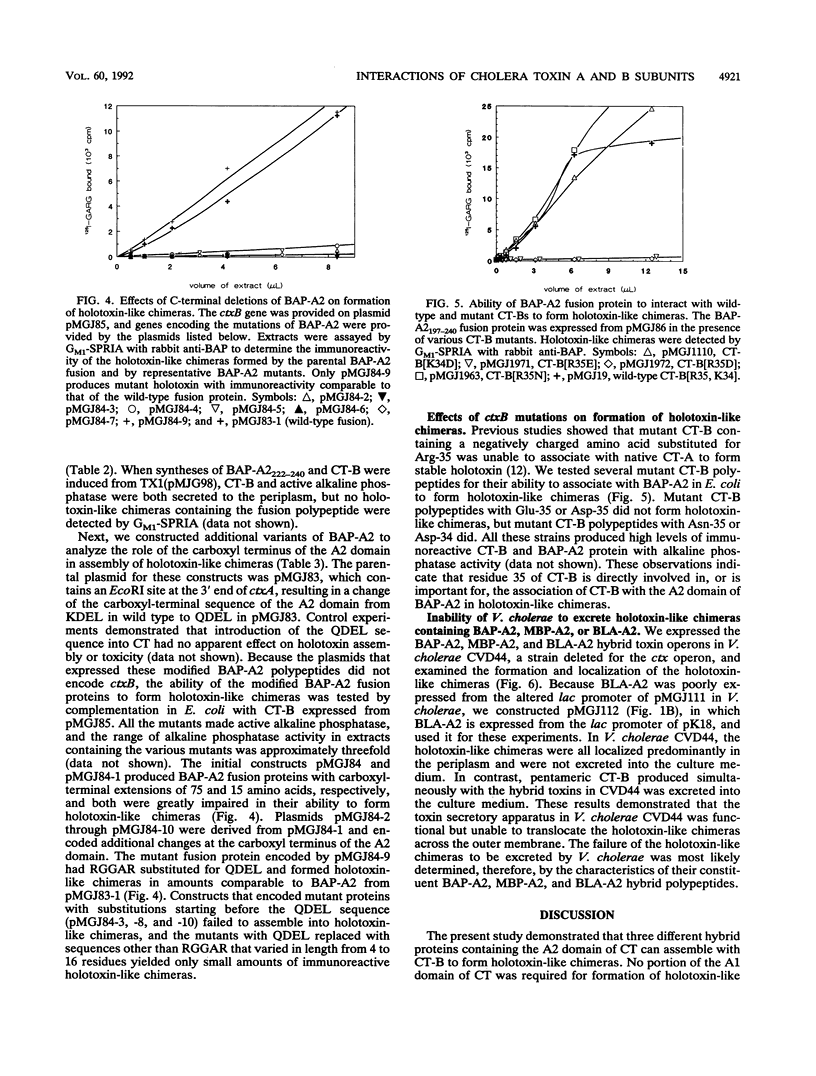
Abstract
Cholera enterotoxin (CT) is produced by Vibrio cholerae and excreted into the culture medium as an extracellular protein. CT consists of one A polypeptide and five B polypeptides associated by noncovalent bonds, and CT-B interacts with CT-A primarily via the A2 domain. Treatment of CT with trypsin cleaves CT-A into A1 and A2 fragments that are linked by a disulfide bond. CT-B binds to ganglioside GM1, which functions as the plasma membrane receptor for CT, and the enzymatic activity of A1 causes the toxic effects of CT on target cells. We constructed translational fusions that joined foreign proteins via their carboxyl termini to the A2 domain of CT-A, and we studied the interactions of the fusion proteins with CT-B. The A2 domain was necessary and sufficient to enable bacterial alkaline phosphatase (BAP), maltose-binding protein (MBP) or beta-lactamase (BLA) to associate with CT-B to form stable, immunoreactive, holotoxin-like chimeras. Each holotoxin-like chimera was able to bind to ganglioside GM1. Holotoxin-like chimeras containing the BAP-A2 and BLA-A2 fusion proteins had BAP activity and BLA activity, respectively. We constructed BAP-A2 mutants with altered carboxyl-terminal sequences and tested their ability to assemble into holotoxin-like chimeras. Although the carboxyl-terminal QDEL sequence of the BAP-A2 fusion protein was not required for interaction with CT-B, most BAP-A2 mutants with altered carboxyl termini did not form holotoxin-like chimeras. When holotoxin-like chimeras containing BAP-A2, MBP-A2, or BLA-A2 were synthesized in V. cholerae, they were found predominantly in the periplasm. The toxin secretory apparatus of V. cholerae was not able, therefore, to translocate these holotoxin-like chimeras across the outer membrane.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boissinot M., Levesque R. C. Nucleotide sequence of the PSE-4 carbenicillinase gene and correlations with the Staphylococcus aureus PC1 beta-lactamase crystal structure. J Biol Chem. 1990 Jan 15;265(2):1225–1230. [PubMed] [Google Scholar]
- Duffy L. K., Kurosky A., Lai C. Y. Cholera toxin A subunit: functional sites correlated with regions of secondary structure. Arch Biochem Biophys. 1985 Jun;239(2):549–555. doi: 10.1016/0003-9861(85)90724-6. [DOI] [PubMed] [Google Scholar]
- Finkelstein R. A., Burks M. F., Zupan A., Dallas W. S., Jacob C. O., Ludwig D. S. Epitopes of the cholera family of enterotoxins. Rev Infect Dis. 1987 May-Jun;9(3):544–561. doi: 10.1093/clinids/9.3.544. [DOI] [PubMed] [Google Scholar]
- Fukuta S., Magnani J. L., Twiddy E. M., Holmes R. K., Ginsburg V. Comparison of the carbohydrate-binding specificities of cholera toxin and Escherichia coli heat-labile enterotoxins LTh-I, LT-IIa, and LT-IIb. Infect Immun. 1988 Jul;56(7):1748–1753. doi: 10.1128/iai.56.7.1748-1753.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons A. New 3-D protein structures revealed. The shape of cholera. Science. 1991 Jul 26;253(5018):382–383. doi: 10.1126/science.1862340. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hardy S. J., Holmgren J., Johansson S., Sanchez J., Hirst T. R. Coordinated assembly of multisubunit proteins: oligomerization of bacterial enterotoxins in vivo and in vitro. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7109–7113. doi: 10.1073/pnas.85.19.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst T. R., Sanchez J., Kaper J. B., Hardy S. J., Holmgren J. Mechanism of toxin secretion by Vibrio cholerae investigated in strains harboring plasmids that encode heat-labile enterotoxins of Escherichia coli. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7752–7756. doi: 10.1073/pnas.81.24.7752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst T. R., Welch R. A. Mechanisms for secretion of extracellular proteins by gram-negative bacteria. Trends Biochem Sci. 1988 Jul;13(7):265–269. doi: 10.1016/0968-0004(88)90160-0. [DOI] [PubMed] [Google Scholar]
- Jobling M. G., Holmes R. K. Analysis of structure and function of the B subunit of cholera toxin by the use of site-directed mutagenesis. Mol Microbiol. 1991 Jul;5(7):1755–1767. doi: 10.1111/j.1365-2958.1991.tb01925.x. [DOI] [PubMed] [Google Scholar]
- Jobling M. G., Holmes R. K. Construction of vectors with the p15a replicon, kanamycin resistance, inducible lacZ alpha and pUC18 or pUC19 multiple cloning sites. Nucleic Acids Res. 1990 Sep 11;18(17):5315–5316. doi: 10.1093/nar/18.17.5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaper J. B., Lockman H., Baldini M. M., Levine M. M. Recombinant nontoxinogenic Vibrio cholerae strains as attenuated cholera vaccine candidates. Nature. 1984 Apr 12;308(5960):655–658. doi: 10.1038/308655a0. [DOI] [PubMed] [Google Scholar]
- Kohl J., Rüker F., Himmler G., Mattanovich D., Katinger H. Engineered gene for Escherichia coli alkaline phosphatase for the construction of translational fusions. Nucleic Acids Res. 1990 Feb 25;18(4):1069–1069. doi: 10.1093/nar/18.4.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathe R., Kieny M. P., Skory S., Lecocq J. P. Linker tailing: unphosphorylated linker oligonucleotides for joining DNA termini. DNA. 1984;3(2):173–182. doi: 10.1089/dna.1984.3.173. [DOI] [PubMed] [Google Scholar]
- Lee C. M., Chang P. P., Tsai S. C., Adamik R., Price S. R., Kunz B. C., Moss J., Twiddy E. M., Holmes R. K. Activation of Escherichia coli heat-labile enterotoxins by native and recombinant adenosine diphosphate-ribosylation factors, 20-kD guanine nucleotide-binding proteins. J Clin Invest. 1991 May;87(5):1780–1786. doi: 10.1172/JCI115197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoil C., Beckwith J. TnphoA: a transposon probe for protein export signals. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8129–8133. doi: 10.1073/pnas.82.23.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus H., Ketley J. M., Kaper J. B., Holmes R. K. Effects of DNase production, plasmid size, and restriction barriers on transformation of Vibrio cholerae by electroporation and osmotic shock. FEMS Microbiol Lett. 1990 Mar 1;56(1-2):149–154. doi: 10.1111/j.1574-6968.1990.tb04139.x. [DOI] [PubMed] [Google Scholar]
- Neill R. J., Ivins B. E., Holmes R. K. Synthesis and secretion of the plasmid-coded heat-labile enterotoxin of Escherichia coli in Vibrio cholerae. Science. 1983 Jul 15;221(4607):289–291. doi: 10.1126/science.6857285. [DOI] [PubMed] [Google Scholar]
- Pridmore R. D. New and versatile cloning vectors with kanamycin-resistance marker. Gene. 1987;56(2-3):309–312. doi: 10.1016/0378-1119(87)90149-1. [DOI] [PubMed] [Google Scholar]
- Sanchez J., Hirst T. R., Uhlin B. E. Hybrid enterotoxin LTA::STa proteins and their protection from degradation by in vivo association with B-subunits of Escherichia coli heat-labile enterotoxin. Gene. 1988 Apr 29;64(2):265–275. doi: 10.1016/0378-1119(88)90341-1. [DOI] [PubMed] [Google Scholar]
- Sanchez J., Svennerholm A. M., Holmgren J. Genetic fusion of a non-toxic heat-stable enterotoxin-related decapeptide antigen to cholera toxin B-subunit. FEBS Lett. 1988 Dec 5;241(1-2):110–114. doi: 10.1016/0014-5793(88)81041-x. [DOI] [PubMed] [Google Scholar]
- Sanchez J., Uhlin B. E., Grundström T., Holmgren J., Hirst T. R. Immunoactive chimeric ST-LT enterotoxins of Escherichia coli generated by in vitro gene fusion. FEBS Lett. 1986 Nov 24;208(2):194–198. doi: 10.1016/0014-5793(86)81016-x. [DOI] [PubMed] [Google Scholar]
- Schlesinger M. J. Formation of a defective alkaline phosphatase subunit by a mutant of Escherichia coli. J Biol Chem. 1967 Apr 10;242(7):1604–1611. [PubMed] [Google Scholar]
- Schödel F., Will H., Johansson S., Sanchez J., Holmgren J. Synthesis in Vibrio cholerae and secretion of hepatitis B virus antigens fused to Escherichia coli heat-labile enterotoxin subunit B. Gene. 1991 Mar 15;99(2):255–259. doi: 10.1016/0378-1119(91)90135-x. [DOI] [PubMed] [Google Scholar]
- Sixma T. K., Pronk S. E., Kalk K. H., Wartna E. S., van Zanten B. A., Witholt B., Hol W. G. Crystal structure of a cholera toxin-related heat-labile enterotoxin from E. coli. Nature. 1991 May 30;351(6325):371–377. doi: 10.1038/351371a0. [DOI] [PubMed] [Google Scholar]
- Sixma T. K., Pronk S. E., Kalk K. H., van Zanten B. A., Berghuis A. M., Hol W. G. Lactose binding to heat-labile enterotoxin revealed by X-ray crystallography. Nature. 1992 Feb 6;355(6360):561–564. doi: 10.1038/355561a0. [DOI] [PubMed] [Google Scholar]
- Spangler B. D., Westbrook E. M. Crystallization of isoelectrically homogeneous cholera toxin. Biochemistry. 1989 Feb 7;28(3):1333–1340. doi: 10.1021/bi00429a059. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. New pUC-derived cloning vectors with different selectable markers and DNA replication origins. Gene. 1991 Apr;100:189–194. doi: 10.1016/0378-1119(91)90365-i. [DOI] [PubMed] [Google Scholar]



