Abstract
An understanding of the influence of climate change on Ixodes scapularis, the main vector of Lyme disease in North America, is a fundamental component in assessing changes in the spatial distribution of human risk for the disease. We used a climate suitability model of I. scapularis to examine the potential effects of global climate change on future Lyme disease risk in North America. A climate-based logistic model was first used to explain the current distribution of I. scapularis in North America. Climate change scenarios were then applied to extrapolate the model in time and produce forecasts of vector establishment. The spatially modeled relationship between I. scapularis presence and large-scale environmental data generated the current pattern of I. scapularis across North America with an accuracy of 89% (p<0.0001). Extrapolation of the model revealed a significant expansion of I. scapularis north into Canada with an increase in suitable habitat of 213% by the 2080’s. Climate change will also result in a retraction of the vector from southern United States, and movement into the central United States. This report predicts the effect of climate change on Lyme disease risk and specifically forecasts the emergence of a tick-borne infectious disease in Canada. Our modeling approach could thus be used to outline where future control strategies and prevention efforts need to be applied.
Keywords: Autologistic model, Global Climate Model, Geographic Information Systems, Ixodes scapularis, Greenhouse Effect, Spatial model
Introduction
Global climate change has been implicated in having a potentially serious impact on the future spatial and temporal distribution of vector-borne diseases. While local abundance of vectors may be guided by density dependent factors such as competition, predation and parasitism, the geographic range of arthropod species’ habitat are controlled by large scale density independent factors. In particular, climate factors can be used in the prediction of habitat type (Holdridge 1971) and thus suitability for arthropods (Rogers, Hay et al. 1996; Duchateau, Kruska et al. 1997; Robinson, Rogers et al. 1997; Cumming 2002). Because climate has a well-documented role in the maintenance of vectors and pathogens in nature, some studies have predicted that the present warming phase of the Earth will result in the redistribution of many vector-borne diseases (Reeves, Hardy et al. 1994; Patz, Epstein et al. 1996). For instance, warming temperatures have been predicted to both enhance transmission intensity and extend the distribution of diseases such a malaria and dengue (Martens, Niessen et al. 1995; Lindsay and Martens 1998; Patz, Martens et al. 1998; Hales, de Wet et al. 2002; Tanser, Sharp et al. 2003). In particular, climate change may open up previously uninhabitable territory for arthropod vectors as well as increase reproductive and biting rates, and shorten the pathogen incubation period (Shope 1991; Patz, Epstein et al. 1996). There is therefore an imperative need for further studies on the effect of climate change on a broad range of vectorborne disease systems.
The tick, Ixodes scapularis, the primary vector of Lyme disease in North America (Keirans, Hutcheson et al. 1996; Dennis, Nekomoto et al. 1998), is highly dependent on climate patterns (Stafford 1994; Lindsay, Barker et al. 1995; Bertrand and Wilson 1996). The abiotic environment plays a vital role in the survival of I. scapularis with both water stress and temperature regulating off-host mortality (Needham and Teel 1991; Bertrand and Wilson 1996). As 98% of the two-year life cycle takes place off the host, climate should act as an essential determinant of distribution of established tick populations across North America (Fish 1993). The impact of climate on I. scapularis population maintenance suggests the potential for climate change to alter the current vector distribution. Though recent emergence of Lyme disease throughout the northeastern and mid-Atlantic states has been linked to reforestation (Barbour and Fish), additional influence of environmental change can be expected considering the anticipated shifts in climate
A shift in the distribution is a public health concern in the United States where Lyme disease is the most prevalent vector-borne disease with over 100,000 cases reported since 1982 (Orloski, Hayes et al. 2000). In Canada, established populations of I. scapularis are currently limited to a small number of foci in southern Ontario as current climate conditions are likely to preclude the expansion of the tick into wider distribution in eastern Canada (Lindsay, Artsob et al. 1998; Barker and Lindsay 2000).
The ideal climate-driven model of a disease system would involve a biological approach where the dynamics of both vector and pathogen are modeled explicitly. The successful application of such a model depends on an accurate estimate of the relationships of climatic factors and disease cycle parameters (Rogers and Randolph 2000). However, the complex relationship between the tick vector and the environment hinders a detailed understanding of the ecological constraints to its maintenance, precluding the current use of biological models in climate change projections. Statistical climate-matching approaches have, therefore, been proposed as a substitute for quantitative biological models (Randolph and Rogers 2000; Brownstein, Holford et al. 2003).
We, therefore, used a previously validated spatially predictive logistic model for I. scapularis to predict current climate-based habitat suitability in North America (Brownstein, Holford et al. 2003). This model relies on both seasonal temperature and humidity data to identify the climate constraints on vector distribution in North America. A Global Circulation Model (GCM) was then used to quantify the impact of climate change on the future distribution of I. scapularis.
METHODS
Climate Model Overview
We used a climate-based habitat suitability model for I. scapularis to forecast the effect of global climate change (Brownstein, Holford et al. 2003). This spatially explicit model matched seasonal climate data with the current reported distribution of I. scapularis in the United States (Dennis, Nekomoto et al. 1998). Climate was shown to successfully predict the current distribution of I. scapularis with an accuracy of 95% (p< 0.0001) (Brownstein, Holford et al. 2003). Field sampling at locations of varying probability in the Northeast United States subsequently confirmed the validity of the environmental model.
The suitability model was extrapolated to North America using seasonal climate information. The climate data was derived from a 0.5 × 0.5-degree global dataset of 30-year average monthly climate surfaces derived from interpolation of station data from 1961-1990 (New, Hulme et al. 1999). Climate variables selected for analysis include minimum, maximum and mean monthly temperature, and monthly vapor pressure. Suitable I. scapularis land cover was also included in the model as the proportion of deciduous forest per 0.5-degree pixel. Deciduous forest cover was selected from the Global Land Cover Chacteristics database for North America derived from classification of 1-km resolution Advanced Very High Resolution Radiometer (AVHRR) imagery from 1992 and1993. We chose the International Geosphere Programme Land Cover Classification for extraction of deciduous forest cover (Loveland, Reed et al. 2000). The analysis involved extrapolating the original logistic model for the relationship between environment and known established I. scapularis populations in the United States. As for the national scale model, spatial autocorrelation was accounted for in the model by applying an autologistic approach (Augustin, Mugglestone et al. 1996). This methodology incorporates a smoothing filter, called the autologistic term, as an additional covariate in the logistic model (Augustin, Mugglestone et al. 1996; Osborne, Alonso et al. 2001). Because vector population status in many locations in the United States is unknown, and because we did not include any surveillance data from Canada or Mexico, the model incorporated the modified Gibbs sampler to estimate the distribution in unknown areas (Augustin, Mugglestone et al. 1996; Augustin, Mugglestone et al. 1998). This Monte Carlo-type method involves iterating the procedure of fitting the autologistic model, deriving the probability surface for all locations, and then recalculating the autologistic term until stability is achieved. This procedure was implemented with a program written with Microsoft Visual C++ and produced a final probability surface for the current suitability of I. scapularis in North America. We selected a probability cutoff point for suitability by determining the best combination of sensitivity and specificity. This threshold value was used to decide whether a given cell could support an established vector population.
Climate Model Forecast
The derived relationship between climate and I. scapularis establishment was then used to predict climate-based habitat suitability in future years by applying global climate change forecasting. As a large number of climate change experiments have been completed with varying results, the modeling center of choice was selected based on a set of reliability criteria determined by the Intergovernmental Panel on Climate Change, Task Group on Scenarios for Climate Impact Assessment (IPCC-TGCIA) (IPCC-TGCIA 1999). Based on the criteria of vintage, resolution, and validity, we selected the Canadian Global Coupled Model (CGCM1) produced by the Canadian Centre for Climate Modelling and Analysis (Flato, Boer et al. 2000).
The CGCM1 provided change data for each of the four climatic variables used in the suitability model. Two historically forced integrations were considered. The first was an IS92a-type forcing, which projects an increase in greenhouse gas emissions at a rate of 1% per annum. The second combines both greenhouse gas and sulfate aerosol changes. These integrations forced with and without sulfate produce global mean temperature increases of 3.85°C and 4.91°C by 2080, respectively. The reliability of these climate projections is reflected in the model’s reproduction of present-day climate and historical variation. The model accurately predicts the observed 0.6°C increase in global mean temperature over the past century (Flato, Boer et al. 2000). Climate change data for each variable and integration were obtained for three time points, the 2020’s (2010-2039), the 2050’s (2040-2069) and the 2080’s (2070-2099), from the IPCC Data Distribution Centre. The data were imported, processed and geocoded by a program written in Microsoft Visual C++. For each simulation output, a gridded surface, which contains the monthly climate information, was produced.
Because the CGCM1 dataset has a lower spatial resolution (3.75×3.75-degrees) than the observed present climate data (0.5×0.5-degrees) used to build the current model, the change data was resampled by interpolation using cubic spline. Spline interpolation is considered the appropriate method for downscaling environmental data where there are a large number of data points and the surface is expected to vary smoothly. Monthly change surfaces for each climate variable, time step and GCM scenario were generated (n=288).
We then applied zonal analysis to associate current monthly climate information for North America with the resampled climate change layers to yield future climate predictions. Processing was accomplished by a batch script written for ERDAS Imagine (Atlanta, GA). Similar to the current model, future climate data was summarized by calculating the cell statistics for each climate variable, including mean, maximum, minimum and standard deviation. The autologistic model was then rerun to predict the probability of I. scapularis populations in North America over three time points according to both climate change scenarios. The six new probability surfaces were classified according to the probability threshold for suitability.
Output Quantification
The classified suitability maps were used to assess the impact of climate change on I. scapularis distribution. The number of suitable pixels for each surface was used to calculate the net percent change in suitable area for each integration. Integrating the current suitability map with the forecasted maps revealed the expansion and contraction of suitable area. The amount of change in each direction was then quantified. To estimate the effect of climate on human exposure to I. scapularis in the United States, we converted the habitat suitability surfaces to county maps by zonal analysis. By associating these new suitability maps with US 2000 Census population data (US Census Bureau 2000), we predicted future change in human exposure.
RESULTS
The extrapolated autologistic model produced a current probability surface for the distribution of I. scapularis in North America. The regression coefficients converged after 5 iterations to produce the final probability surface (p<0.0001) with all four climate variables remaining in the model. The landcover variable did not provide additional fit to the model. The probability threshold was set to 21%, predicting the current distribution of I. scapularis in the United States with an overall accuracy of 89%, a sensitivity of 88% and a specificity of 89%.
The cutoff probability was used to develop a current distribution map for North America (Figure 1). We classified 10.1% of the pixels (n=11779) as suitable for I. scapularis. In the United States (n=3351) and Canada (n=6804), 28.9% and 3.7% of the area was above the critical threshold, respectively. Many areas not yet shown to be colonized were classified as having suitable climatic conditions for population maintenance. These areas of suitability were identified in Virginia, North Carolina, Georgia, Minnesota, Iowa, and northern Michigan. In addition, southern Ontario, southern Quebec, New Brunswick, Nova Scotia, Prince Edward Island and eastern Newfoundland were given a high probability of I. scapularis establishment. Suitable climate was not found in Mexico.
Figure 1.
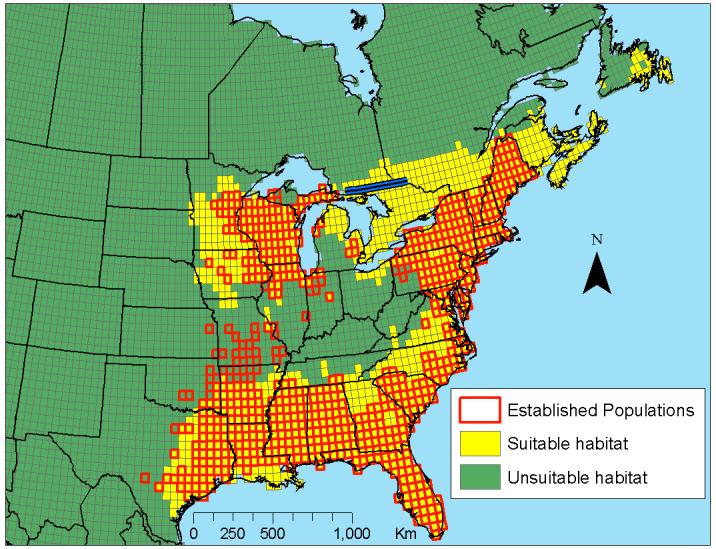
Distribution of climate-based habitat suitability for Ixodes scapularis, as predicted by the climate-based autologistic model. Suitable area (in yellow) represents 10.1% of North America and predicts the current distribution of I. scapularis (in red) with an accuracy of 89%. Non-overlapped yellow pixels represent suitable areas that have yet to be colonized. The blue line across Ontario represents the northern limit of habitat suitability predicted by Lindsay et. al., 1995.
The autologistic model for I. scapularis climate-based suitability was extrapolated to 3 future time points according to GCM scenarios forced with either greenhouse gases alone or combined with the effects of sulfate aerosols. The probability surfaces were classified according to the sensitivity analysis threshold, and subtracted from the current suitability map (Figure 2). The addition of sulfate aerosols to the climate model yielded an overall slower rate of change, but this effect disappears by the 2080s (Table 1).
Figure 2.
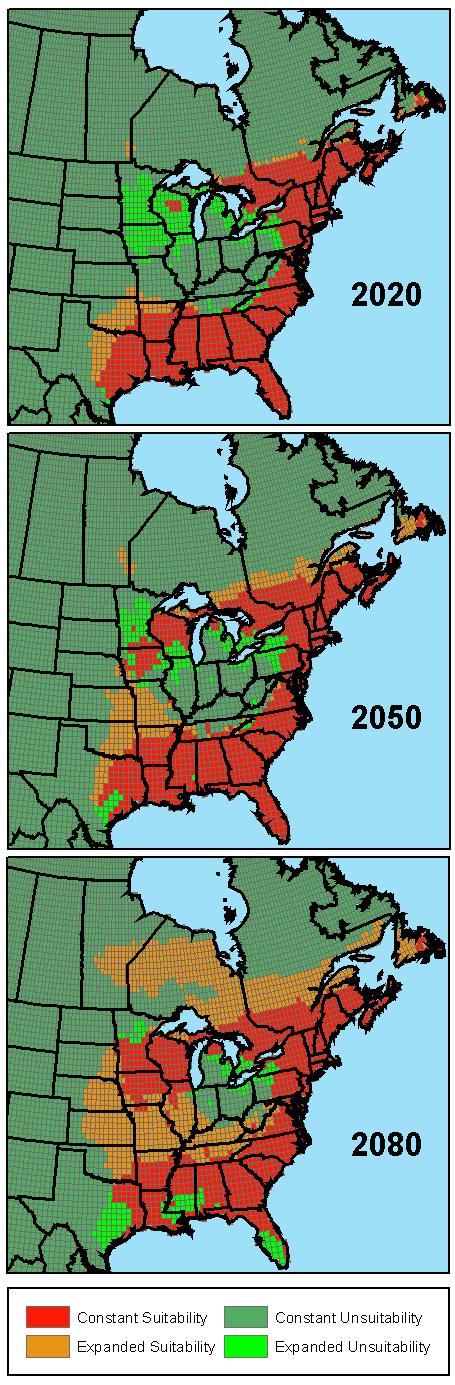
Projected distribution of climate-based habitat suitability for Ixodes scapularis during three future time periods: the 2020’s, the 2050’s and the 2080’s. The simulation is based on climate change predictions using the Canadian Global Coupled Model (CGCM1) integration forced with anticipated increases in both greenhouse gas and sulfate aerosols. Areas of expansion and contraction from the current distribution of I. scapularis are displayed.
Table 1.
Future projected changes in Ixodes scapularis habitat in North America during three future time periods *†
| Net Change — NA (%) |
Net Change — US (%) |
Net Change — CAN (%) |
Expansion (%) |
Retraction (%) |
Population Exposed (%) |
|
|---|---|---|---|---|---|---|
| GG | ||||||
| 2020s | 7.2 | -1.6 | 37.3 | 28.6 | 21.5 | -12.2 |
| 2050s | 30.2 | 8.7 | 105.1 | 51.9 | 21.7 | -11.0 |
| 2080s | 68.1 | 20.0 | 236.5 | 95.0 | 26.9 | 5.6 |
| GG+S | ||||||
| 2020s | -12.0 | -18.5 | 12.9 | 12.8 | 24.7 | -28.0 |
| 2050s | 10.3 | -1.0 | 49.8 | 27.2 | 16.9 | -12.7 |
| 2080s | 68.9 | 28.0 | 212.9 | 82.7 | 13.8 | -1.9 |
Percent change was calculated by subtracting the current predicted distribution fromthe future scenarios
NA, North America; CAN, Canada; GG, simulations forced with greenhouse gas changes alone; GG+S, simulations forced with greenhouse gas and sulfate aerosol changes.
Considering the effects of both greenhouse gas and sulfate aerosols, the area with suitable climate decreases by 12% in the 2020s, as retraction (24.7%) overpowers expansion (12.8%). This decrease is solely attributed to reduction in suitable area in the United States (-18.5%) focused in the Midwest. However, expansion does occur in both southern Canada (12.9%) and the southern United States, including Texas, Oklahoma and Arkansas. The 2050s reverses the trend of decreasing suitability with an increase in area of 10.3%. In the United States, suitability returns to the Midwest and expansion into the central states of Missouri and Kansas takes place. Extension into Canada continues with an additional 49.8% suitable area. The 2080s reveals the most pronounced effect of climate change, with a net increase in suitable area of 68.9%. Contraction of suitable area is confined to the southern states, especially in Texas, Mississippi, and Florida. Encroachment continues in the central United States, filling in previously unsuitable areas and closing the gap between southern and northern populations of I. scapularis. Canada experiences a major expansion of suitability in the 2080’s with a 212.9% increase. In particular, northern Ontario and Manitoba become accessible for I. scapularis colonization.
The potential effect of climate change on human exposure was examined by combining the climate-based suitability surfaces with US 2000 Census population data. Counties suitable for I. scapularis establishment at each time point were revealed (Figure 3). In the 2020s and 2050s, the model predicts a decrease in human exposure (Table 1). By the 2080s, despite the major burst in suitability, there is very little effect of climate change on net human exposure (-1.9%).
Figure 3.
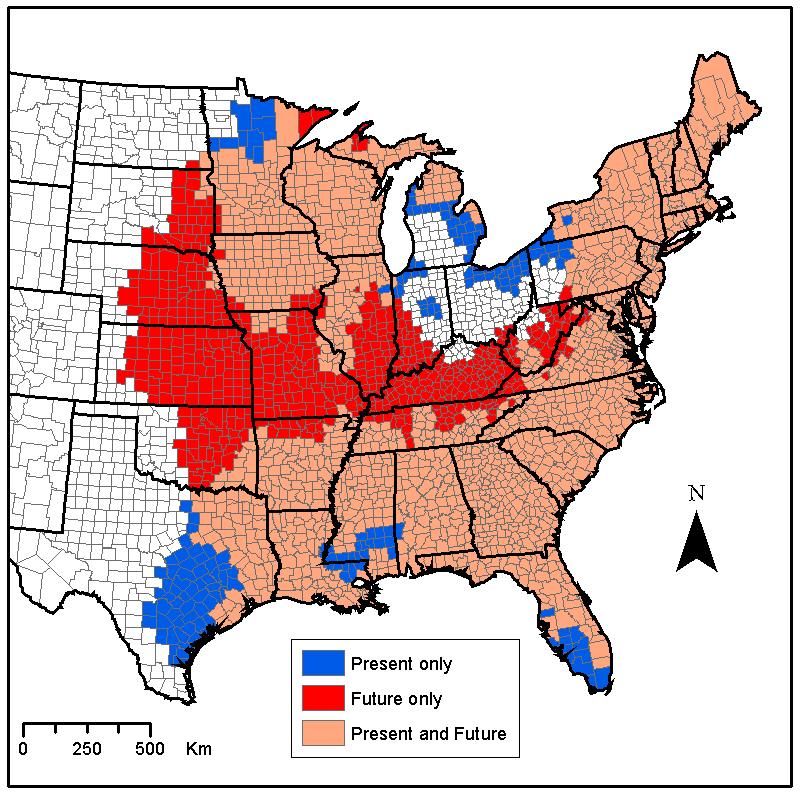
Change in county-based distribution of I. scapularis from present to the 2080s. The future distribution based on climate change data, which considers the effects of both greenhouse gas and sulfate aerosols, was overlaid on the current predicted distribution. The map reveals future suitable (in red) and unsuitable (in blue) counties. Counties that remain suitable over time (in pink) are also displayed.
DISCUSSION
Our findings provide a quantitative assessment of the impact of climate change on the future distribution of the most important vector of Lyme disease in North America. The robustness of our model was confirmed through an 89% accurate prediction of the current distribution of I. scapularis populations in the United States (Figure 1). In Canada, the model predicts high probabilities of establishment in southern areas of Ontario, Quebec, and New Brunswick. This finding is validated by Lindsay et. al. (Lindsay, Barker et al. 1995) who found that suitable habitat for I. scapularis establishment in Ontario occurs south of a line through North Bay, Ontario (46° 2’ N; 79° 3’ W). The limit of establishment according to our model lies within 130 km of North Bay.
Climate change is expected to cause a complicated redistribution of the vector, which reveals two major trends (Figure 2). First, the redistribution is dominated by expansion. The rise in minimum temperature results in the expansion into higher latitudes, which is explained by the inverse relationship between tick survival and the degree of subfreezing temperature exposure (Vandyk, Bartholomew et al. 1996). This trend is clearly shown by the spreading of suitable area north into Canada. Though I. scapularis has been collected from a variety of locations in Canada (Keirans, Hutcheson et al. 1996; Scott, Fernando et al. 2001), establishment has only been shown for a limited number of locations in southern Ontario (Lindsay, Artsob et al. 1998; Barker and Lindsay 2000). Climate change may provide the conditions necessary to yield reproducing populations of I. scapularis either by the systematic advancement from south of the border by movement on mammal hosts or by adventitious introductions from attachment to bird hosts (Klich, Lankester et al. 1996). Similar expansion has been shown for Ixodes ricinus in Sweden where the movement north was predicted by an increase in milder daily temperature (Lindgren, Talleklint et al. 2000). Minimum temperature increase also results in the extension of suitability into higher altitudes. Elevation is an important limiting factor for I. scapularis populations as it indirectly affects population establishment through its influence on the complex interaction between climate, physical factors, and biota (Schulze, Lakat et al. 1984). As a result of increasing temperatures, the model predicts advancement of suitability into the southern Appalachian Mountains.
Second, climate change results in the contraction of suitable area. Because the rise in maximum temperature yields unfavorable conditions for off-host survival of I. scapularis (Needham and Teel 1991), we predict that this will result in the retraction of the vector from the lower latitudes of the United States. This effect is exemplified in the 2080s, where major portions of Texas, Mississippi, and Florida have become uninhabitable for I. scapularis. A comparable simulation of the effect of climate change on I. ricinus seasonal dynamics predicted the rise in temperature would clear the risk of tick-borne encephalitis from much of its present distribution in Europe (Randolph and Rogers 2000). Increasing temperatures also produced temporary contraction in the Midwest in the 2020s. However, the combination of covariates in the autologistic model, including the coupling of increases in temperature with increases in relative humidity, will reproduce suitable conditions in the 2050s. It is impossible to assess whether this regional variability will actually lead to a temporary extinction from the area followed by re-establishment of the tick.
Despite the predicted redistribution, most of the current I. scapularis habitat remains suitable. This stability is especially reflected in the Northeast United States, the main focus of Lyme disease on the continent, where the vector, given a static landscape, will remain established over the next 80 years. The level of human exposure to I. scapularis will also remain approximately constant even though some changes in populations at risk may occur (Table 1). In fact, future population growth in the United States will be most evident in the South, including Texas and Florida (Campbell 1996). With the projected net population change concentrated in areas of future unsuitability, climate change may actually contribute to a decrease in the proportion of the population exposed to I. scapularis in the United States even though its distribution will expand.
Other factors besides climate shifts will likely influence vector distribution and abundance, particularly on a local level. Although incorporating landcover at the continental scale did not increase model fit, our model of suitability is still contingent on the presence of a suitable physical landscape. Previously, landscape features such as deciduous forest and sandy soils that are correlated with I. scapularis presence (Kitron, Bouseman et al. 1991; Glass, Amerasinghe et al. 1994; Bertrand and Wilson 1996) were used to develop a habitat suitability model for I. scapularis (Guerra, Walker et al. 2002). For instance, though our model predicts large areas of climate suitability, the distribution of I. scapularis within these areas is discontinuous as a result of landscape variability (e.g.: agricultural and residential patchiness) (Glass, Amerasinghe et al. 1994; Nicholson and Mather 1996; Walker, McLean et al. 1996; Dister, Fish et al. 1997). Therefore, the application of this model at a higher resolution should be accompanied by landcover data. Due to the importance of landscape in the habitat suitability of I. scapularis, landcover change resulting directly through landscape modification and indirectly through climate change should also be examined for its impact on the future distribution of I. scapularis.
Landscape structure may also play an indirect role in the presence of I. scapularis through its influence on the abundance of the white-tailed deer, its main reproductive host. Although the current range of the white-tailed deer contains the entire expected distribution of I. scapularis with the exception of Newfoundland (Wilson and Ruff 1999), it is host population density that will determine whether an introduction of I. scapularis can result in population maintenance (Spielman, Wilson et al. 1985). Subsequently, white-tailed deer is more likely influenced by shifts in vegetation distribution rather than by thermal conditions due to their physiological tolerance to heat load (Johnston and Schmitz 1997). Changes in landscape structure may therefore play an additional role in dictating future tick distribution.
Further, environmental factors may also be responsible for controlling the enzootic maintenance of the Lyme disease agent, Borrelia burgdorferi. Climate change may exert an indirect impact on infection prevalence via its relationship with host species composition. Increase in temperature may result in the northward expansion of the southern hosts of I. scapularis. In the South, host composition is believed to be dominated by lizard species (Oliver, Cummins et al. 1993), which are either inefficient or incompetent reservoirs of infection for immature ticks resulting in overall low infection rates (Spielman, Wilson et al. 1985). The movement of these hosts northward could result in the disruption of the enzootic cycle of B. burgdorferi in the North reducing the public health
CONCLUSIONS
Our model provides a climate based prediction for the the future distribution of Lyme disease in North America and highlights the probable public health impact of climate change in Canada. Projected changes in tick distribution could be used to form health policy and guide intervention measures for Lyme disease (Hayes and Piesman 2003). Spatially explicit environmental models that predict disease emergence can form valuable tools for strengthening public health preparedness.
ACKNOWLEDGMENTS
The authors thank Brandon Brei, Nita Madhav, and David Skelly for their helpful input. J.S. Brownstein was supported by NASA Headquarters under the Earth Science Fellowship Grant NGT5-01-0000-0205 and the National Science and Engineering Research Council of Canada. This work was also supported by The Harold G. and Leila Y. Mathers Charitable Foundation (DF) and a USDA-ARS Cooperative Agreement 58-0790-2-072 (DF).
REFERENCES
- Augustin NH, Mugglestone MA, et al. An autologistic model for the spatial distribution of wildlife. Journal of Applied Ecology. 1996;33(2):339–347. [Google Scholar]
- Augustin NH, Mugglestone MA, et al. The role of simulation in modelling spatially correlated data. Environmetrics. 1998;9(2):175–196. [Google Scholar]
- Barker IK, Lindsay LR. Lyme borreliosis in Ontario: determining the risks.[comment]. Canadian Medical Association Journal. 2000;162(11):1573–4. [PMC free article] [PubMed] [Google Scholar]
- Bertrand MR, Wilson ML. Microclimate-dependent survival of unfed adult Ixodes scapularis (Acari:Ixodidae) in nature: life cycle and study design implications. Journal of Medical Entomology. 1996;33(4):619–27. doi: 10.1093/jmedent/33.4.619. [DOI] [PubMed] [Google Scholar]
- Brownstein JS, Holford TR, et al. A climate-based model predicts the spatial distribution of the Lyme disease vector Ixodes scapularis in the United States. Environmental Health Perspectives. 2003;111(9):1152–1157. doi: 10.1289/ehp.6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell PR. Population projections for states- by age, sex, race, and hispanic origin, U.S. Bureau of the Census, Population Division, PPL-47. 1996.
- Cumming GS. Comparing climate and vegetation as limiting factors for species ranges of African ticks. Ecology. 2002;82(1):255–268. [Google Scholar]
- Dennis DT, Nekomoto TS, et al. Reported distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the United States. Journal of Medical Entomology. 1998;35(5):629–38. doi: 10.1093/jmedent/35.5.629. [DOI] [PubMed] [Google Scholar]
- Dister SW, Fish D, et al. Landscape characterization of peridomestic risk for Lyme disease using satellite imagery. American Journal of Tropical Medicine and Hygiene. 1997;57(6):687–92. doi: 10.4269/ajtmh.1997.57.687. [DOI] [PubMed] [Google Scholar]
- Duchateau L, Kruska RL, et al. Reducing a spatial database to its effective dimensionality for logistic-regression analysis of incidence of livestock disease. Preventive Veterinary Medicine. 1997;32(34):207–18. doi: 10.1016/s0167-5877(97)00019-6. [DOI] [PubMed] [Google Scholar]
- Fish D. Population ecology of Ixodes damini. In: Ginsberg H, editor. Ecology and Environmental Management of Lyme Disease. Rutgers University Press; New Brunswick, NJ: 1993. pp. 25–42. [Google Scholar]
- Flato GM, Boer GJ, et al. The Canadian Centre for Climate Modelling and Analysis Global Couple Model and its Climate. Climate Dynamics. 2000;16:451–467. [Google Scholar]
- Glass GE, Amerasinghe FP, et al. Predicting Ixodes scapularis abundance on white-tailed deer using geographic information systems. American Journal of Tropical Medicine & Hygiene. 1994;51(5):538–44. doi: 10.4269/ajtmh.1994.51.538. [DOI] [PubMed] [Google Scholar]
- Guerra M, Walker E, et al. Predicting the risk of Lyme disease: habitat suitability for Ixodes scapularis in the north central United States. Emerging Infectious Diseases. 2002;8(3):289–97. doi: 10.3201/eid0803.010166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales S, de Wet N, et al. Potential effect of population and climate changes on global distribution of dengue fever: an empirical model. Lancet. 2002;360(9336):830–4. doi: 10.1016/S0140-6736(02)09964-6. [DOI] [PubMed] [Google Scholar]
- Hayes EB, Piesman J. How can we prevent Lyme disease? N Engl J Med. 2003;348(24):2424–30. doi: 10.1056/NEJMra021397. [DOI] [PubMed] [Google Scholar]
- Holdridge LR. Forest environments in tropical life zones: a piot study. Perganon Press; New York: 1971. [Google Scholar]
- IPCC-TGCIA. Carter TR, Hulme M, Lal M, Intergovernmental Panel on Climate Change, Task Group on Scenarios for Climate Impact Assessment Guidelines on the Use of Scenario Data for Climate Impact and Adaptation Assessment. 1999.
- Johnston KM, Schmitz OJ. Wildlife and climate change: Assessing the sensitivitiy of selected species to simulated doubling of atmospheric CO2. Global Change Biology. 1997;3(6):531–544. [Google Scholar]
- Keirans JE, Hutcheson HJ, et al. Ixodes scapularis (Acari:Ixodidae): redescription of all active stages, distribution, hosts, geographical variation, and medical and veterinary importance. Journal of Medical Entomology. 1996;33(3):297–318. doi: 10.1093/jmedent/33.3.297. [DOI] [PubMed] [Google Scholar]
- Kitron U, Bouseman JK, et al. Use of ARC/INFO GIS to study the distribution of Lyme disease ticks in Illinois. Prev. Vet. Med. 1991;11:243–248. [Google Scholar]
- Klich M, Lankester MW, et al. Spring migratory birds (Aves) extend the northern occurrence of blacklegged tick (Acari:Ixodidae) Journal of Medical Entomology. 1996;33(4):581–5. doi: 10.1093/jmedent/33.4.581. [DOI] [PubMed] [Google Scholar]
- Lindgren E, Talleklint L, et al. Impact of climatic change on the northern latitude limit and population density of the disease-transmitting European tick Ixodes ricinus. Environmental Health Perspectives. 2000;108(2):119–23. doi: 10.1289/ehp.00108119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay LR, Barker IK, et al. Survival and development of Ixodes scapularis (Acari:Ixodidae) under various climatic conditions in Ontario, Canada. Journal of Medical Entomology. 1995;32(2):143–52. doi: 10.1093/jmedent/32.2.143. [DOI] [PubMed] [Google Scholar]
- Lindsay R, Artsob H, et al. Distribution of Ixodes pacificus and Ixodes scapularis re concurrent babesiosis and Lyme disease. Canada Communicable Disease Report. 1998;24(15):121–2. [PubMed] [Google Scholar]
- Lindsay SW, Martens WJM. Malaria in the African highlands: Past, present and future. Bull. WHO. 1998;76(1):33–34. [PMC free article] [PubMed] [Google Scholar]
- Loveland TR, Reed BC, et al. Development of a global land cover characteristics database and IGBP DISCover from 1 km AVHRR data International Journal of Remote Sensing 20001521(6-7)): 1303–1330. [Google Scholar]
- Martens WJ, Niessen LW, et al. Potential impact of global climate change on malaria risk. Environmental Health Perspectives. 1995;103(5):458–64. doi: 10.1289/ehp.95103458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham GR, Teel PD. Off-host physiological ecology of ixodid ticks. Annual Review of Entomology. 1991;36:659–681. doi: 10.1146/annurev.en.36.010191.003303. [DOI] [PubMed] [Google Scholar]
- New M, Hulme M, et al. Representing Twentieth-Century Space-Time Climate Variability. Part I: Development of a 1961-1990 Mean Monthly Terrestrial Climatology. Journal of Climate. 1999;12:829–856. [Google Scholar]
- Nicholson MC, Mather TN. Methods for evaluating Lyme disease risks using geographic information systems and geospatial analysis. Journal of Medical Entomology. 1996;33(5):711–20. doi: 10.1093/jmedent/33.5.711. [DOI] [PubMed] [Google Scholar]
- Oliver JH, Jr., Cummins GA, et al. Immature Ixodes scapularis (Acari: Ixodidae) parasitizing lizards from the southeastern U.S.A. J Parasitol. 1993;79(5):684–9. [PubMed] [Google Scholar]
- Orloski KA, Hayes EB, et al. Surveillance for Lyme disease--United States, 1992-1998. Morbidity and Mortality Weekly Report. 2000;49(3):1–11. [PubMed] [Google Scholar]
- Osborne PE, Alonso JC, et al. Modelling landscape-scale habitat use using GIS and remote sensing: A case study with great bustards. Journal of Applied Ecology. 2001;38(2):458–471. [Google Scholar]
- Patz JA, Epstein PR, et al. Global climate change and emerging infectious diseases. JAMA. 1996;275(3):217–23. [PubMed] [Google Scholar]
- Patz JA, Martens WJ, et al. Dengue fever epidemic potential as projected by general circulation models of global climate change. Environmental Health Perspectives. 1998;106(3):147–53. doi: 10.1289/ehp.98106147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph SE, Rogers DJ. Fragile transmission cycles of tick-borne encephalitis virus may be disrupted by predicted climate change. Proceedings of the Royal Society of London. Series B: Biological Sciences. 2000;267(1454):1741–4. doi: 10.1098/rspb.2000.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves WC, Hardy JL, et al. Potential effect of global warming on mosquito-borne arboviruses. Journal of Medical Entomology. 1994;31(3):323–32. doi: 10.1093/jmedent/31.3.323. [DOI] [PubMed] [Google Scholar]
- Robinson T, Rogers D, et al. Mapping tsetse habitat suitability in the common fly belt of southern Africa using multivariate analysis of climate and remotely sensed vegetation data. Med Vet Entomol. 1997;11(3):235–45. doi: 10.1111/j.1365-2915.1997.tb00401.x. [DOI] [PubMed] [Google Scholar]
- Rogers DJ, Hay SI, et al. Predicting the distribution of tsetse flies in West Africa using temporal Fourier processed meteorological satellite data. Ann Trop Med Parasitol. 1996;90(3):225–41. doi: 10.1080/00034983.1996.11813049. [DOI] [PubMed] [Google Scholar]
- Rogers DJ, Randolph SE. The global spread of malaria in a future, warmer world. Science. 2000;289(5485):1763–6. doi: 10.1126/science.289.5485.1763. [DOI] [PubMed] [Google Scholar]
- Schulze TL, Lakat MF, et al. Ixodes dammini (Acari: Ixodidae) and other ixodid ticks collected from white-tailed deer Odocoileus virginianus in New Jersey USA 1. Geographical Distribution and Its Relation to Selected Environmental and Physical Factors. Journal of Medical Entomology. 1984;21(6):741–749. doi: 10.1093/jmedent/21.6.741. [DOI] [PubMed] [Google Scholar]
- Scott JD, Fernando K, et al. Birds disperse ixodid (Acari: Ixodidae) and Borrelia burgdorferi-infected ticks in Canada. Journal of Medical Entomology. 2001;38(4):493–500. doi: 10.1603/0022-2585-38.4.493. [DOI] [PubMed] [Google Scholar]
- Shope R. Global climate change and infectious diseases. Environmental Health Perspectives. 1991;96:171–4. doi: 10.1289/ehp.9196171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielman A, Wilson ML, et al. Ecology of Ixodes dammini-borne human babesiosis and Lyme disease. Annual Review of Entomology. 1985;30:439–60. doi: 10.1146/annurev.en.30.010185.002255. [DOI] [PubMed] [Google Scholar]
- Stafford KC., 3rd Survival of immature Ixodes scapularis (Acari: Ixodidae) at different relative humidities. Journal of Medical Entomology. 1994;31(2):310–4. doi: 10.1093/jmedent/31.2.310. [DOI] [PubMed] [Google Scholar]
- Tanser FC, Sharp B, et al. Potential effect of climate change on malaria transmission in Africa. Lancet. 2003;362(9398):1792–8. doi: 10.1016/S0140-6736(03)14898-2. [DOI] [PubMed] [Google Scholar]
- US Census Bureau . Washington, DC: 2000. Redistricting Census 2000 TIGER/Line Files [machine-readable data files] [Google Scholar]
- Vandyk JK, Bartholomew DM, et al. Survival of Ixodes scapularis (Acari: Ixodidae) exposed to cold. Journal of Medical Entomology. 1996;33(1):6–10. doi: 10.1093/jmedent/33.1.6. [DOI] [PubMed] [Google Scholar]
- Walker ED, McLean RG, et al. Borrelia burgdorferi-infected Ixodes scapularis (Acari: Ixodidae) and Peromyscus leucopus in northeastern Wisconsin. Journal of Medical Entomology. 1996;33(1):165–8. doi: 10.1093/jmedent/33.1.165. [DOI] [PubMed] [Google Scholar]
- Wilson DE, Ruff S. The Smithsonian Book of North American Mammals. Smithsonian Institution Press; Washington, DC: 1999. [Google Scholar]


