Abstract
Accurate staging of disease is necessary in patients with newly diagnosed esophageal cancer in order to prompt appropriate curative or palliative therapy. Computed tomography (CT) may be used to evaluate for local spread into adjacent structures (T4 disease) and to diagnose distant metastases (M1). Endoscopic ultrasonography (EUS) is the modality of choice for distinguishing T1 tumors from higher stage lesions and for detecting and sampling regional lymph nodes (N1 disease). Positron emission tomography (PET) scanning is most helpful for detecting previously occult distant metastases. Optimal staging generally requires a multimodality approach.
Keywords: Esophageal cancer, neoplasm staging, computed tomography, FDG-PET scanning
Introduction
Accurate staging of disease in patients with esophageal carcinoma is necessary in order to prompt appropriate curative or palliative therapy. Different imaging modalities have different strengths and weaknesses in evaluating the various features of the disease, and a combined imaging approach is usually necessary for optimal assessment.
Anatomy and epidemiology
The esophagus is divided into four anatomic regions for reporting and staging purposes. The cervical portion extends from the cricoid cartilage to the thoracic inlet. The thoracic esophagus extends from the thoracic inlet to the gastroesophageal junction and is divided into three regions: upper, mid and lower. The upper thoracic esophagus extends from the thoracic inlet to the carina; the mid-thoracic esophagus from the carina to the diaphragm; and the lower thoracic and abdominal esophagus (which measures approximately 3 cm in length) from the diaphragm down to and including the gastroesophageal junction[2,3]. The cervical esophagus ends approximately 18 cm from the incisors; the upper, mid and lower thoracic esophagus end at approximately 24, 32, and 40 cm from the incisors, respectively.
The esophageal wall consists of four layers: mucosa, submucosa, muscularis propria, and adventitia. There is no serosa to serve as a barrier between the esophagus and the surrounding structures; lack of a serosa facilitates tumor spread through the esophageal wall into adjacent structures. A rich plexus of lymphatics encircles the entire length of the esophagus, enabling lymphatic spread of tumor to cervical, mediastinal and upper abdominal lymph nodes. An experimental study showed that dye injected into the esophageal wall at one level may drain to lymph nodes at all other levels of the esophagus in some patients, and also frequently drains directly into the thoracic duct, potentially leading to hematogenous metastases[4]. Because of these features, ‘skip metastases’ are not uncommon, meaning that distant sites may be involved without involvement of lymph nodes close to the primary tumor.
Advanced disease is frequently seen at initial clinical presentation. Only about 24% of esophageal cancer cases are diagnosed while the cancer is still confined to the primary site (localized stage); 30% are diagnosed after the cancer has spread to regional lymph nodes or directly beyond the primary site; 30% are diagnosed after the cancer has already metastasized (distant stage); for the remaining 16% the staging information is unknown[5].
Staging classification
The International Union Against Cancer (UICC) and American Joint Committee on Cancer (AJCC) have staged esophageal cancer using the TNM system whereby T categorizes the depth of invasion into or through the esophageal wall, N the status of regional lymph nodes, and M metastases to distant sites (Table 1)[2]. Stage groupings of the TNM system, based on prognosis, are shown in Table 2[2,3].
Table 1.
The TNM system
| Primary tumor (T) | |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ |
| T1 | Tumor confined to mucosa or invades lamina propria or submucosa |
| T2 | Tumor invades muscularis propria |
| T3 | Tumor invades adventitia |
| T4 | Tumor invades adjacent structures |
| Regional lymph nodes (N) | |
| N0 | No regional lymph node metastasis |
| N1 | Regional lymph node metastasis |
| Distant metastasis (M) | |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
Table 2.
Stage grouping
| Stage 0 | Tis | N0 | M0 |
| Stage I | T1 | N0 | M0 |
| Stage IIA | T2 | N0 | M0 |
| T3 | N0 | M0 | |
| Stage IIB | T1 | N1 | M0 |
| T2 | N1 | M0 | |
| Stage III | T3 | N1 | M0 |
| T4 | Any N | M0 | |
| Stage IV | Any T | Any N | M1 |
| Stage IVa | Any T | Any N | M1a |
| Stage IVb | Any T | Any N | M1b |
Regional lymph nodes are determined according to the level of the primary esophageal tumor[2]. For example, regional sites for cervical esophageal primaries include the cervical, internal jugular, scalene, supraclavicular, and periesophageal lymph nodes. The regional sites for upper, middle, and lower intrathoracic esophageal tumors are periesophageal and subcarinal nodes, and for gastroesophageal junction tumors are lower periesophageal, diaphragmatic, pericardial, left gastric, and celiac nodes. Lymph node metastases to any other sites constitute distant metastatic (M1) disease.
For upper thoracic and for lower thoracic primary tumors only, M1 is broken down into M1a and M1b categories (cervical and mid-thoracic primary tumors have no such breakdown) (Table 3). Tumor spread to cervical lymph nodes from an upper thoracic primary neoplasm falls into the M1a category; spread to any other non-regional lymph nodes represents M1b disease. Similarly, celiac axis lymph node metastases confer M1a status upon primary tumors located in the lower thoracic esophagus, whereas spread to any other non-regional lymph nodes represents M1b disease. Patients with M1a disease have a better prognosis than those with M1b disease and are generally felt to represent surgical candidates.
Table 3.
M categories
| Tumors of the upper thoracic esophagus | |
| M1a | Metastasis in cervical nodes |
| M1b | Other distant metastasis |
| Tumors of the mid-thoracic esophagus | |
| M1a | Not applicable |
| M1b | Non-regional lymph nodes and/or other distant metastasis |
| Tumors of the lower thoracic esophagus | |
| M1a | Metastasis in celiac lymph nodes |
| M1b | Other distant metastasis |
Patients with newly diagnosed esophageal cancer often present with distant metastases. In a series by Quint et al., 18% of patients presented with M1 disease. Distant metastases were most commonly diagnosed in upper abdominal lymph nodes (45%), followed by liver (35%) and lung (20%). Less commonly, distant metastases were seen in cervical/supraclavicular lymph nodes, bone, adrenal glands, peritoneum, brain, pericardium, pleura, stomach, pancreas, spleen, soft tissues and kidney[6].
Primary tumor (T) staging
Endoscopic ultrasound (EUS) is the major modality used to stage the primary tumor and is particularly helpful in distinguishing T1 lesions from T2–4 lesions[7]. This distinction may be crucial for treatment planning: at some centers, T1 lesions are treated with endoscopic therapy or with esophagectomy alone, whereas higher stage lesions may be treated with neoadjuvant chemoradiation, followed by esophagectomy.
Unlike EUS, computed tomography (CT) is incapable of distinguishing the layers of the esophageal wall in order to determine the depth of tumor invasion, i.e. T1 vs. T2 disease. Gross tumor invasion into mediastinal fat may be diagnosed at CT (T3 disease), manifesting as abnormal soft tissue in the mediastinal fat, with or without obliteration of fat planes between the esophagus and adjacent mediastinal structures[8]. However, most investigators believe that CT is unreliable in detecting or excluding minimal fat invasion. On the other hand, one recent study found that distention of the esophagus with effervescent granules and post-processing the data with multiplanar reconstructions did lead to accurate assessment of extraesophageal tumor spread[9]. In general, the major role of CT in evaluating the T stage is to detect local tumor invasion into adjacent structures (T4 disease), suggesting inoperability. The presence of a fat plane between an esophageal tumor and an adjacent mediastinal structure (e.g. central airway, aorta, pericardium) is an accurate indicator of lack of invasion of the structure. However, the converse is not necessarily true: lack of a fat plane is not diagnostic for invasion (T4 disease), in either cachectic patients or in those with normal body weight. Moreover, adjacent fat planes are sometimes obscured in patients who have undergone radiation therapy.
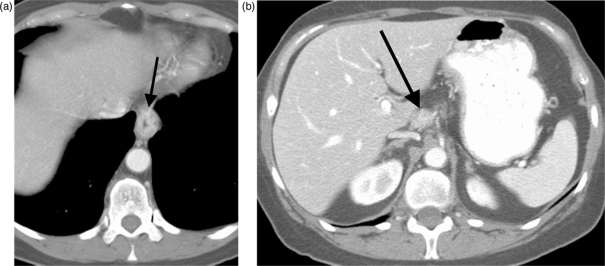
Deep invasion of the aortic wall is unresectable, whereas invasion limited to the adventitia is generally resectable[10]. Various CT techniques and criteria have been suggested to help diagnose or exclude unresectable aortic invasion. An older study reported that contact between the mass and the aorta over more than 90 degrees of the aortic circumference was 80% accurate for diagnosing aortic invasion[11]. Another study found that obliteration of the normal fatty triangle located between the spine, esophagus and aorta showed a sensitivity of 100% and specificity of 82% in this setting[12]. Unfortunately, however, these criteria do not appear to be reliable in clinical practice, and other studies have reported lower accuracies; for example, Lehr et al. found sensitivity, specificity and accuracy figures of 6%, 85% and 58%, respectively[13]. Thus, suspected aortic invasion is difficult to prove preoperatively, and in most cases, this determination is a surgical one (Fig. 1). Fortunately, however, unresectable aortic invasion is a very rare finding.
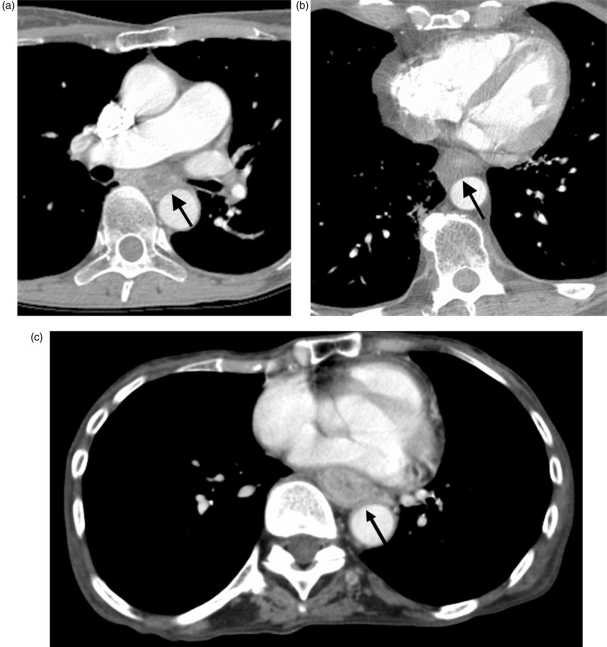
Figure 1.
Three patients with broad loss of the fat plane between the esophagus and the aorta (arrows). At surgery, there was no aortic invasion in (A) (squamous cell carcinoma) or (B) (adenocarcinoma), however aortic invasion was present in (C) (squamous cell carcinoma).
Unlike aortic invasion, tumor spread into the central airways is not rare, and should always be considered in a patient with an upper or mid-thoracic primary tumor. Flattening or indentation of the wall of the trachea or left main stem bronchus by an adjacent esophageal mass (particularly on inspiratory images) is suggestive of invasion, although this finding may be caused by simple mass effect upon the membranous portion of the airway, without invasion (Fig. 2). Frank abnormal soft tissue in the lumen of the airway or a fistula between the esophagus and the airway are specific, if extremely uncommon, findings of airway invasion. Overall the reported sensitivity for tracheobronchial involvement ranges from 31 to 100%, specificity from 68 to 98% and accuracies range from 74 to 97%[14]. Imaging features suggesting invasion should be further evaluated with bronchoscopy and confirmed with biopsy.
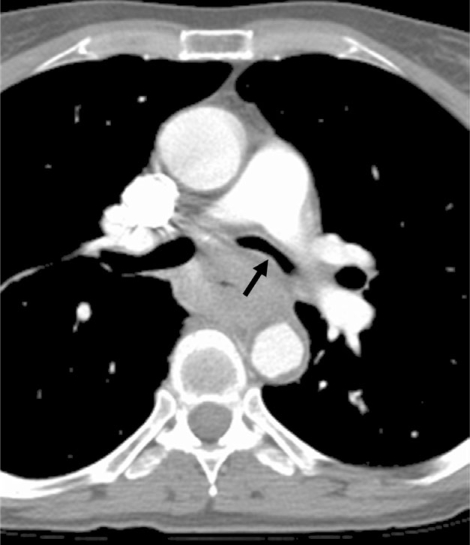
Figure 2.
Esophageal adenocarcinoma causing narrowing of the left mainstem bronchus at CT (arrow). Airway invasion was confirmed bronchoscopically.
Pericardial invasion is suggested by pericardial thickening and/or effusion, obliteration of the fat plane between the tumor and the pericardium, or mass effect upon the pericardium[8,15]. Extensive invasion is unresectable and minimal invasion may be resectable. Similarly, extensive invasion of the diaphragm may also be unresectable.
Although positron emission tomography (PET) is incapable of primary tumor staging, due to its poor spatial resolution, it has been reported that the maximum SUV of the primary tumor correlates well with overall tumor stage and survival; 4-year survival of patients with maximum SUV of ≤6.6 was 89%, compared to 31% for maximum SUV > 6.6[16].
Regional lymph node (N) staging
Regional lymph nodes are best evaluated using EUS. Although sensitivity is high (approximating 80%), specificity is somewhat lower (approximating 70%)[17]. Therefore, patient management should not be determined based solely upon an EUS diagnosis of abnormal appearing regional lymph nodes. Rather, EUS-guided lymph node biopsy should be performed for confirmation of lymph node involvement.
Diagnosing metastatic disease to regional lymph nodes with CT is limited for two major reasons. First, a bulky primary esophageal mass may obscure adjacent, involved lymph nodes[15]. Second, the diagnosis of lymph node disease is based solely on size criteria. However, enlarged nodes may be benign and reactive in nature, whereas small nodes may harbor microscopic metastases. Several size criteria for lymph node enlargement have been suggested. Traditionally 10 mm has been considered the upper normal limit for paraesophageal lymph nodes. For subdiaphragmatic nodes, an upper normal threshold of 8 mm has been used, with nodes between 6 and 8 mm considered as indeterminate[18]. However, more recently, Schroder and colleagues have found these figures to be overestimations[19]. In a histopathological study of specimens from 40 patients with esophageal squamous cell carcinoma, 1196 lymph nodes were analyzed; 129 lymph nodes were found to have metastatic infiltration. Average maximum lymph node diameters were 5.1 mm (±3.8 mm) for tumor-free lymph nodes and 6.7 mm (±4.2 mm) for tumor-containing lymph nodes. Furthermore, only 9.3% of all resected lymph nodes measured 10 mm or more in maximal diameter. In addition, there was no significant correlation between lymph node size and the frequency of nodal metastases[19].
Using varied size thresholds, recently published helical CT studies have demonstrated diverse results in the detection of regional lymph node metastases, with sensitivities of 11–69%, specificities of 71–95%, and accuracies of 66–83%20–22 (Fig. 3).
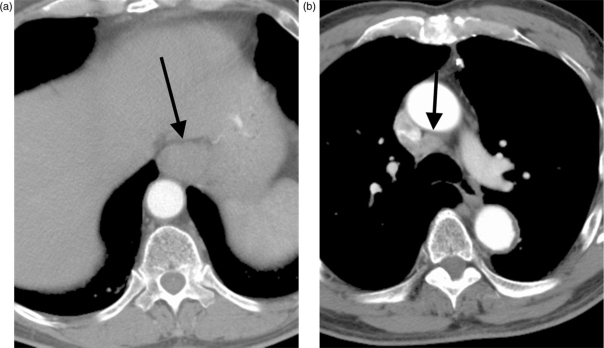
Figure 3.
Distal esophageal adenocarcinoma (arrow, A) and right paratracheal lymph node enlargement (arrow, B), suggesting regional nodal metastatic disease. Lymph node biopsy, however, revealed no malignancy.
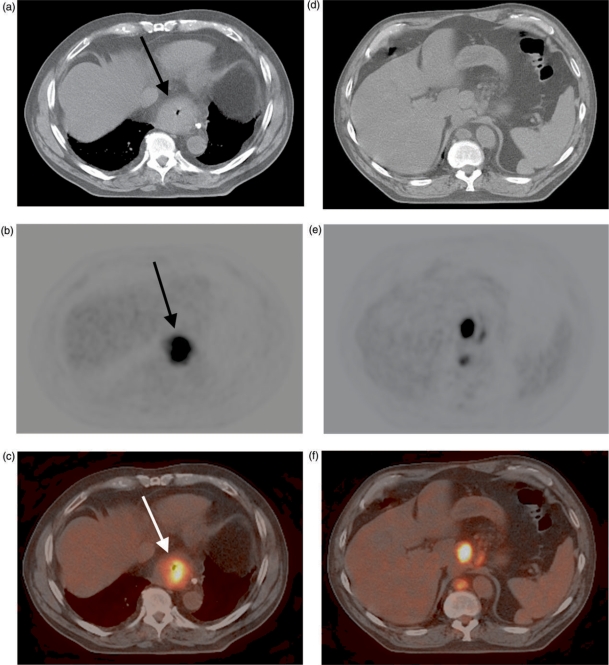
There are conflicting results in the imaging literature regarding the accuracy of PET in staging the regional lymph nodes; a recent meta-analysis revealed a sensitivity of 51% and a specificity of 84% in this setting[23]. The major problem is obscuration of adjacent lymph nodes by uptake in the primary tumor, leading to low sensitivity (Fig. 4). However, PET is useful in detecting regional nodal disease that is not immediately near the esophageal primary. Published studies have found that the use of PET-CT helps in more precisely defining the location of FDG avidity in the region of the primary tumor, thereby improving regional lymph node staging accuracy[24,25 (Fig. 5]). In one study, the sensitivity, specificity and accuracy of PET-CT were 94%, 92% and 92%, respectively, on a nodal group by group basis[25].
Figure 4.
FDG avid distal esophageal adenocarcinoma (arrow) on PET-CT scanning (A–C). Regional nodal (N1) disease (found at surgery) was not diagnosed preoperatively at PET, probably because uptake in the primary tumor obscured the abnormal, adjacent lymph nodes.
Figure 5.
FDG avid distal esophageal adenocarcinoma (arrow, A–C) on PET-CT scanning. The CT image helps to localize nearby foci of FDG avidity to abnormal lymph nodes in the gastrohepatic ligament and retrocrural region (D–F).
Distant metastatic disease (M) staging
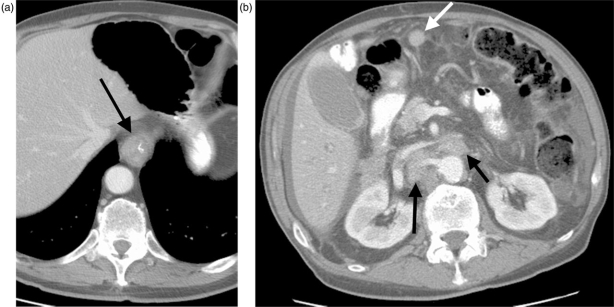
CT scanning is often useful for detection of distant metastatic disease in lymph nodes and non-nodal sites (Figs. 6 and 7). CT scanning of patients with esophageal carcinoma should include an intravenous contrast-enhanced CT of the chest and abdomen with dedicated liver technique to optimize visualization of hepatic metastases. Among 201 CT exams performed for staging purposes, Gollub and colleagues found that none of the pelvic CT scans affected patient management; these authors concluded that it is therefore unnecessary to perform pelvic CT as part of the staging workup[26].
Figure 6.
Primary distal esophageal adenocarcinoma (arrow, A) with extensive distant metastatic disease to sites including retroperitoneal lymph nodes (black arrows, B) and peritoneum (white arrow, B). The findings represent M1b disease.
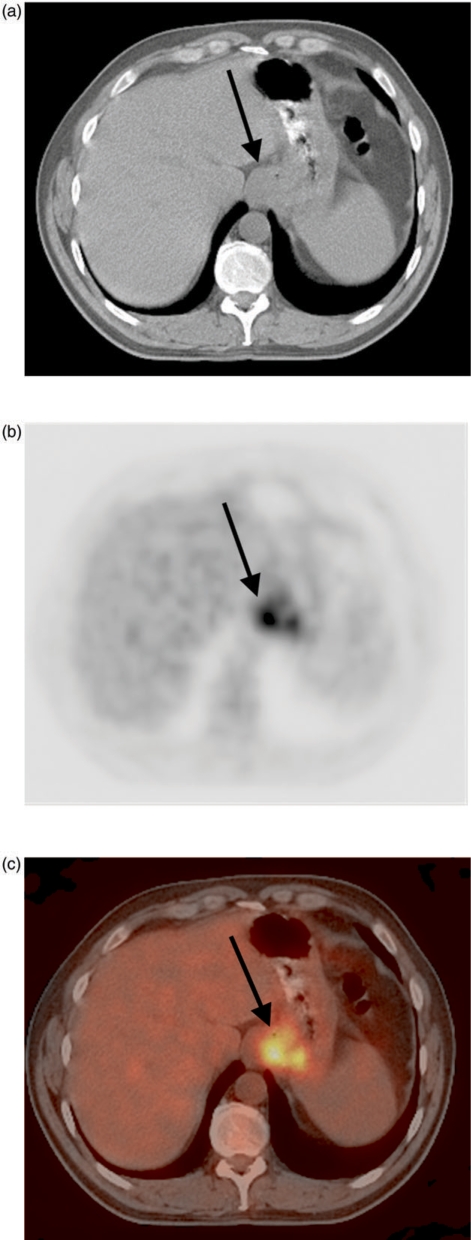
Figure 7.
Distal esophageal adenocarcinoma (arrow, A) with metastasis in a celiac axis lymph node (arrow, B), constituting M1a disease.
Although the major role for EUS is in staging the primary tumor and regional lymph nodes, occasionally this modality reveals abnormal appearing distant lymph nodes in the celiac axis region; in this situation, EUS-guided biopsy should be performed for confirmation of metastatic disease.
Perhaps the most important modality in the diagnostic armamentarium for distant metastatic disease detection in esophageal cancer is PET scanning. In 5–40% of patients, PET may reveal previously occult distant metastases in nodal and non-nodal sites27–29, with moderate sensitivity (∼67%) and high specificity (∼97%)[23] (Figs. 8 and 9). It may also reveal osseous metastases that are not detected using conventional bone scintigraphy[30]. Using a logistic regression model in patients examined preoperatively with CT, EUS and/or PET, one investigation found that PET was the only modality that predicted intended curative resection, due to the exclusion of distant metastases; furthermore, these authors concluded that PET may be used to prevent unnecessary surgical explorations in patients who did have M1 disease[31]. In another study, the use of FDG-PET changed the clinical management of 27/68 patients (40%): in 12 therapy was changed from curative to palliative (due to detection of previously unsuspected distant metastases), in three from palliative to curative, while in 12 other patients there was a change in the treatment modality or delivery but not in the treatment intent[32].
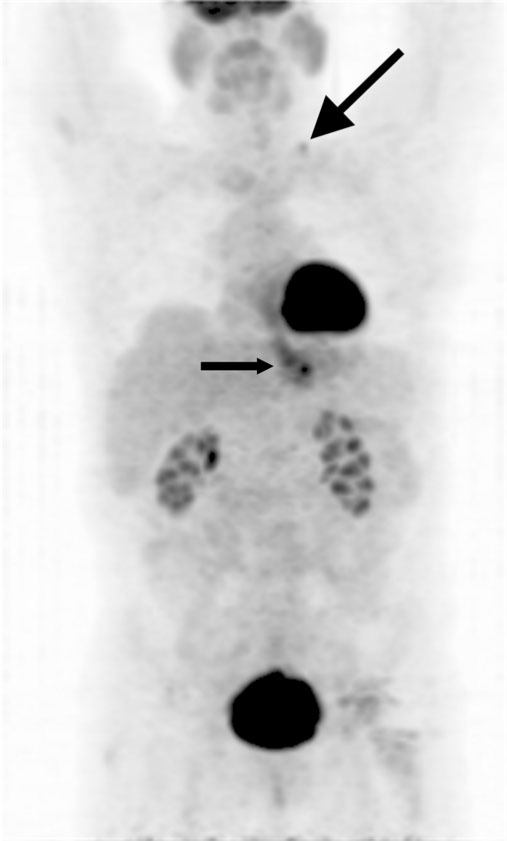
Figure 8.
Projection PET image shows an FDG-avid distal esophageal adenocarcinoma (small arrow) and a previously occult distant metastasis within a left supraclavicular lymph node (large arrow), constituting M1b disease.
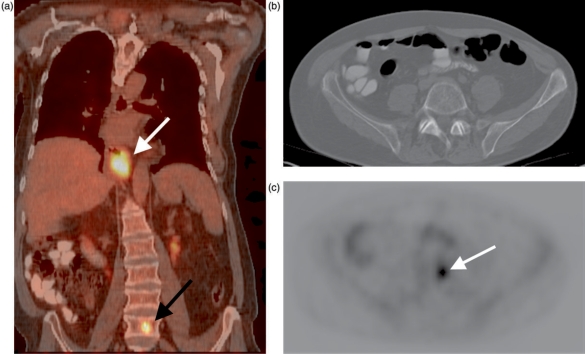
Figure 9.
Fused PET-CT image reveals an FDG-avid distal esophageal carcinoma (white arrow, A) and a previously occult distant vertebral body metastasis (black arrow, A). The spine appears normal on an axial CT image at the level of the metastasis (B); axial PET image at the same level shows the lesion (arrow, C).
Magnetic resonance imaging (MRI)
Several authors have reported that there is no real difference in staging accuracy between MRI and CT for esophageal cancer[13,20]. MRI is more costly than CT and less widely available; in addition, it is more difficult to perform, as respiratory and cardiac gating and control of swallowing are needed, in order to optimize images and avoid motion artifacts. Therefore, MRI is rarely used at most institutions for routine staging[15,33]. Experimental work using external surface coils has shown that T2-weighted high resolution MRI is capable of producing detailed images of the esophageal wall; such techniques may be useful for staging in the future[34].
Workup guidelines
Multiple staging modalities are available for evaluating patients with newly diagnosed esophageal cancer. The following guidelines are followed by multiple authors, yet may be varied according to local preferences and availability of imaging technology. Patients should initially undergo a history and physical exam, in order to detect gross evidence of metastatic disease. In addition, complete upper gastrointestinal (GI) endoscopy or barium upper GI series is indicated to assess for mucosal extent of disease. A CT of the chest and abdomen with bolus administration of intravenous contrast should then be performed to evaluate the primary tumor for T4 disease and to look for lymph node, visceral, and other distant metastatic disease. If the CT shows no distant metastases, and if the tumor is in the cervical, upper thoracic or mid-thoracic esophagus, bronchoscopy is generally performed to assess for airway invasion. PET has been shown to be more accurate than CT in diagnosing distant metastases[35]. Therefore, if the disease appears to be resectable at CT, patients may then undergo PET scanning for detection of occult distant metastases; suspicious lesions should be biopsied for confirmation of disease. Assuming the PET study shows no evidence of distant metastatic disease, EUS will then be performed for better T and N stage evaluation; suspicious lymph nodes detected by EUS (regional or celiac axis) should undergo EUS-guided fine needle aspiration biopsy.
At some institutions, patients with T1N0 disease on EUS and M0 disease on CT and PET will undergo endoscopic therapy or surgery, whereas those with deeper extent of tumor and/or tumor involved regional lymph nodes will undergo neoadjuvant chemoradiation therapy followed by surgery. M1 disease is usually treated non-surgically, with chemoradiotherapy. Some centers perform laparoscopy to look for occult abdominal metastases, particularly for tumors arising at the gastroesophageal junction36–38.
A cost effectiveness study comparing CT, EUS with fine needle aspiration biopsy (EUS-FNA), PET and thoracoscopy/laparoscopy found that CT plus EUS-FNA was the most inexpensive strategy and offered more quality adjusted life-years, on average, than all other strategies except for PET plus EUS-FNA[39]. The latter strategy, although slightly more effective, was also more expensive. The authors recommended use of PET plus EUS-FNA unless resources are scarce or PET is unavailable. At many institutions, PET is now being replaced by PET-CT, thereby potentially eliminating the need to choose between CT and PET in an individual patient.
Conclusion
Most patients with esophageal carcinoma already have tumor spread beyond the esophageal wall at the time of diagnosis, resulting in a dismal prognosis. Only those few patients with limited disease are suitable for potentially curative therapy. Staging information gleaned from imaging is helpful in triaging patients to the appropriate method of therapy; in particular, imaging detection of distant metastases prevents unnecessary surgery.
References
- [1].Bogot N, Quint L. CT in esophageal cancer. In: Rankin SC, editor. Carcinoma of the esophagus. Cambridge: Cambridge University Press; 2008. pp. p. 62–84. [Google Scholar]
- [2].New York: Springer-Verlag; 2002. AJCC cancer staging handbook. [Google Scholar]
- [3].Patti MG, Gantert W, Way LW. Surgery of the esophagus. Anatomy and physiology. Surg Clin North Am. 1997;77:959–70. doi: 10.1016/s0039-6109(05)70600-9. [DOI] [PubMed] [Google Scholar]
- [4].Riquet M, Saab M, Le Pimpec Barthes F, Hidden G. Lymphatic drainage of the esophagus in the adult. Surg Radiol Anat. 1993;15:209–11. doi: 10.1007/BF01627708. [DOI] [PubMed] [Google Scholar]
- [5].SEER NCI. 2008. Cancer of the esophagus. http://seer.cancer.gov/statfacts/html/esoph.html. [Google Scholar]
- [6].Quint LE, Hepburn LM, Francis IR, Whyte RI, Orringer MB. Incidence and distribution of distant metastases from newly diagnosed esophageal carcinoma. Cancer. 1995;76:1120–5. doi: 10.1002/1097-0142(19951001)76:7<1120::aid-cncr2820760704>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- [7].Cen P, Hofstetter WL, Lee JH, et al. Value of endoscopic ultrasound staging in conjunction with the evaluation of lymphovascular invasion in identifying low-risk esophageal carcinoma. Cancer. 2008;112:503–10. doi: 10.1002/cncr.23217. [DOI] [PubMed] [Google Scholar]
- [8].Rice TW. Clinical staging of esophageal carcinoma. CT, EUS, and PET. Chest Surg Clin N Am. 2000;10:471–85. [PubMed] [Google Scholar]
- [9].Panebianco V, Grazhdani H, Iafrate F, et al. 3D CT protocol in the assessment of the esophageal neoplastic lesions: can it improve TNM staging? Eur Radiol. 2006;16:414–421. doi: 10.1007/s00330-005-2851-5. [DOI] [PubMed] [Google Scholar]
- [10].Wayman J, Chakraverty S, Griffin SM, Doyle GJ, Keir MJ, Simpson W. Evaluation of local invasion by oesophageal carcinoma – a prospective study of prone computed tomography scanning. Postgrad Med J. 2001;77:181–4. doi: 10.1136/pmj.77.905.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Picus D, Balfe DM, Koehler RE, Roper CL, Owen JW. Computed tomography in the staging of esophageal carcinoma. Radiology. 1983;146:433–8. doi: 10.1148/radiology.146.2.6849089. [DOI] [PubMed] [Google Scholar]
- [12].Takashima S, Takeuchi N, Shiozaki H, et al. Carcinoma of the esophagus: CT vs MR imaging in determining resectability. AJR Am J Roentgenol. 1991;156:297–302. doi: 10.2214/ajr.156.2.1898802. [DOI] [PubMed] [Google Scholar]
- [13].Lehr L, Rupp N, Siewert JR. Assessment of resectability of esophageal cancer by computed tomography and magnetic resonance imaging. Surgery. 1988;103:344–50. [PubMed] [Google Scholar]
- [14].Rankin S. The role of computerized tomography in the staging of oesophageal cancer. Clin Radiol. 1990;42:152–3. doi: 10.1016/s0009-9260(05)81921-x. [DOI] [PubMed] [Google Scholar]
- [15].Kumbasar B. Carcinoma of esophagus: radiologic diagnosis and staging. Eur J Radiol. 2002;42:170–80. doi: 10.1016/s0720-048x(02)00030-x. [DOI] [PubMed] [Google Scholar]
- [16].Cerfolio RJ, Bryant AS. Maximum standardized uptake values on positron emission tomography of esophageal cancer predicts stage, tumor biology, and survival. Ann Thorac Surg. 2006;82:391–4. doi: 10.1016/j.athoracsur.2006.03.045. (discussion 394-5) [DOI] [PubMed] [Google Scholar]
- [17].van Vliet EP, Heijenbrok-Kal MH, Hunink MG, Kuipers EJ, Siersema PD. Staging investigations for oesophageal cancer: a meta-analysis. Br J Cancer. 2008;98:547–57. doi: 10.1038/sj.bjc.6604200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Balfe DM, Mauro MA, Koehler RE, et al. Gastrohepatic ligament: normal and pathologic CT anatomy. Radiology. 1984;150:485–90. doi: 10.1148/radiology.150.2.6691106. [DOI] [PubMed] [Google Scholar]
- [19].Schroder W, Baldus SE, Monig SP, Beckurts TK, Dienes HP, Holscher AH. Lymph node staging of esophageal squamous cell carcinoma in patients with and without neoadjuvant radiochemotherapy: histomorphologic analysis. World J Surg. 2002;26:584–7. doi: 10.1007/s00268-001-0271-5. [DOI] [PubMed] [Google Scholar]
- [20].Wu LF, Wang BZ, Feng JL, et al. Preoperative TN staging of esophageal cancer: comparison of miniprobe ultrasonography, spiral CT and MRI. World J Gastroenterol. 2003;9:219–24. doi: 10.3748/wjg.v9.i2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yoon YC, Lee KS, Shim YM, Kim BT, Kim K, Kim TS. Metastasis to regional lymph nodes in patients with esophageal squamous cell carcinoma: CT versus FDG PET for presurgical detection – prospective study. Radiology. 2003;227:764–70. doi: 10.1148/radiol.2281020423. [DOI] [PubMed] [Google Scholar]
- [22].Kato H, Miyazaki T, Nakajima M, et al. The incremental effect of positron emission tomography on diagnostic accuracy in the initial staging of esophageal carcinoma. Cancer. 2005;103:148–56. doi: 10.1002/cncr.20724. [DOI] [PubMed] [Google Scholar]
- [23].van Westreenen HL, Westerterp M, Bossuyt PM, et al. Systematic review of the staging performance of 18F-fluorodeoxyglucose positron emission tomography in esophageal cancer. J Clin Oncol. 2004;22:3805–12. doi: 10.1200/JCO.2004.01.083. [DOI] [PubMed] [Google Scholar]
- [24].Bar-Shalom R, Guralnik L, Tsalic M, et al. The additional value of PET/CT over PET in FDG imaging of oesophageal cancer. Eur J Nucl Med Mol Imaging. 2005;32:918–24. doi: 10.1007/s00259-005-1795-y. [DOI] [PubMed] [Google Scholar]
- [25].Yuan S, Yu Y, Chao KSC, et al. Additional value of PET/CT over PET in assessment of locoregional lymph nodes in thoracic esophageal squamous cell cancer. J Nucl Med. 2006;47:1255–9. [PubMed] [Google Scholar]
- [26].Gollub MJ, Lefkowitz R, Moskowitz CS, Ilson D, Kelsen D, Felderman H. Pelvic CT in patients with esophageal cancer. AJR Am J Roentgenol. 2005;184:487–90. doi: 10.2214/ajr.184.2.01840487. [DOI] [PubMed] [Google Scholar]
- [27].Meyers BF, Downey RJ, Decker PA, et al. The utility of positron emission tomography in staging of potentially operable carcinoma of the thoracic esophagus: results of the American College of Surgeons Oncology Group Z0060 trial. J Thorac Cardiovasc Surg. 2007;133:738–45. doi: 10.1016/j.jtcvs.2006.09.079. [DOI] [PubMed] [Google Scholar]
- [28].Blackstock AW, Farmer MR, Lovato J, et al. A prospective evaluation of the impact of 18-F-fluoro-deoxy-D-glucose positron emission tomography staging on survival for patients with locally advanced esophageal cancer. Int J Radiat Oncol Biol Phys. 2006;64:455–60. doi: 10.1016/j.ijrobp.2005.07.959. [DOI] [PubMed] [Google Scholar]
- [29].Luketich JD, Friedman DM, Weigel TL, et al. Evaluation of distant metastases in esophageal cancer: 100 consecutive positron emission tomography scans. Ann Thorac Surg. 1999;68:1133–6. doi: 10.1016/s0003-4975(99)00974-1. [DOI] [PubMed] [Google Scholar]
- [30].Kato H, Miyazaki T, Nakajima M, et al. Comparison between whole-body positron emission tomography and bone scintigraphy in evaluating bony metastases of esophageal carcinomas. Anticancer Res. 2005;25:4439–44. [PubMed] [Google Scholar]
- [31].van Westreenen HL, Heeren PA, van Dullemen HM, et al. Positron emission tomography with F-18-fluorodeoxyglucose in a combined staging strategy of esophageal cancer prevents unnecessary surgical explorations. J Gastrointest Surg. 2005;9:54–61. doi: 10.1016/j.gassur.2004.09.055. [DOI] [PubMed] [Google Scholar]
- [32].Duong CP, Demitriou H, Weih L, et al. Significant clinical impact and prognostic stratification provided by FDG-PET in the staging of oesophageal cancer. Eur J Nucl Med Mol Imaging. 2006;33:759–69. doi: 10.1007/s00259-005-0028-8. [DOI] [PubMed] [Google Scholar]
- [33].Thompson WM. Esophageal carcinoma. Abdom Imaging. 1997;22:138–42. doi: 10.1007/s002619900158. [DOI] [PubMed] [Google Scholar]
- [34].Riddell AM, Allum WH, Thompson JN, Wotherspoon AC, Richardson C, Brown G. The appearances of oesophageal carcinoma demonstrated on high-resolution, T2-weighted MRI, with histopathological correlation. Eur Radiol. 2007;17:391–9. doi: 10.1007/s00330-006-0363-6. [DOI] [PubMed] [Google Scholar]
- [35].Kinkel K, Lu Y, Both M, Warren RS, Thoeni RF. Detection of hepatic metastases from cancers of the gastrointestinal tract by using noninvasive imaging methods (US, CT, MR imaging, PET): a meta-analysis. Radiology. 2002;224:748–56. doi: 10.1148/radiol.2243011362. [DOI] [PubMed] [Google Scholar]
- [36].Buenaventura P, Luketich JD. Surgical staging of esophageal cancer. Chest Surg Clin N Am. 2000;10:487–97. [PubMed] [Google Scholar]
- [37].de Graaf GW, Ayantunde AA, Parsons SL, Duffy JP, Welch NT. The role of staging laparoscopy in oesophagogastric cancers. Eur J Surg Oncol. 2007;33:988–92. doi: 10.1016/j.ejso.2007.01.007. [DOI] [PubMed] [Google Scholar]
- [38].NCCN clinical practice guidelines in oncology. 2008. http://www.nccn.org. [Google Scholar]
- [39].Wallace MB, Nietert PJ, Earle C, et al. An analysis of multiple staging management strategies for carcinoma of the esophagus: computed tomography, endoscopic ultrasound, positron emission tomography, and thoracoscopy/laparoscopy. Ann Thorac Surg. 2002;74:1026–32. doi: 10.1016/s0003-4975(02)03875-4. [DOI] [PubMed] [Google Scholar]