Abstract
Correct staging of non small cell lung cancer (NSCLC) is vital for appropriate management. Initial staging is usually performed with computerised tomography (CT), but increasingly functional imaging using integrated positron emission tomography and CT (PET/CT) is being used to provide more accurate staging, guide biopsies, assess response to therapy and identify recurrent disease.
Keywords: Positron emission tomography, PET/CT, staging lung cancer, response to therapy, recurrent disease
Introduction
Lung cancer is the third commonest cancer in the European Union with 386,300 new cases diagnosed in 2006. It is the most common cause of cancer death with an estimated 334,800 deaths in 2006. Computed tomography (CT) is usually used for the initial staging and functional imaging using 2-[18F]fluorodeoxyglucose positron emission tomography (FDG-PET) is used to identify both mediastinal nodal involvement and distant metastases and also to assess response to therapy. The integration of PET and CT in PET/CT provides accurate anatomic localisation and improved staging over PET alone.
Staging
The majority of lung cancer (80%) is non-small cell lung cancer (NSCLC) and the treatment and prognosis depend on the anatomic extent of the disease at presentation and are defined using the TNM system. The International Association for the Study of Lung Cancer (IASLC) has recommended changes to the TNM staging, which will be incorporated into the 2009 edition[1]. In the revised classification T1 tumours will be divided into T1a (<2 cm) and T1b (2–3 cm). T2 tumours less than 5 cm will be T2a and those between 5 and 7 cm, T2b. T2 tumours greater than 7 cm will be reclassified as T3 tumours. Tumour nodules in the same lobe (previously T4) will now be T3 and malignant pleural effusions and nodules and pericardial effusions will become M1 (previously T4). Malignant parenchymal nodules in an ipsilateral, but separate lobe will be classified as T4 (previously M1), with nodules in the contralateral lung being M1a and distant metastases M1b. The nodal classification is unchanged.
Primary tumour (T status)
Twenty to thirty percent of patients present with a solitary pulmonary nodule (T1) and differentiation of benign from malignant disease may be difficult. If a standardised uptake value (SUV) of greater than 2.5 is used to indicate malignancy, the sensitivity, specificity and accuracy of FDG-PET for differentiating between benign and malignant nodules are 94%, 71% and 86% with positive predictive value (PPV) of 90% and negative predictive value (NPV) of 85%[2]. False positives will occur in tuberculosis, aspergillomas, rheumatoid nodules, Wegener's granulomatosis and amyloidosis. False negatives occur in small early stage adenocarcinoma and squamous cell carcinomas, bronchoalveolar cell carcinoma and some carcinoid tumours.
The exact extent of tumours is difficult to assess using CT for both chest wall and mediastinal invasion. Gross invasion can readily be identified but CT is inaccurate in differentiating contiguity from subtle invasion.
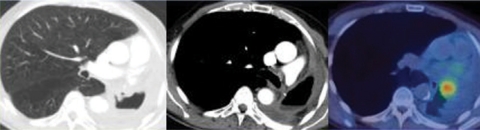
PET/CT has no real advantage over CT for chest wall or mediastinal invasion but is excellent at differentiating tumour from adjacent collapsed lung (Fig. 1) and is helpful in planning radiotherapy portals and reducing toxicity by the sparing of normal tissue[3]. Overall PET/CT is more accurate than contrast enhanced CT for the T staging of tumours (86% versus 79%) but not significantly so[4].
Figure 1.
Patient with NSCLC. PET/CT shows central tumour separate from distal consolidation.
Nodal status (N)
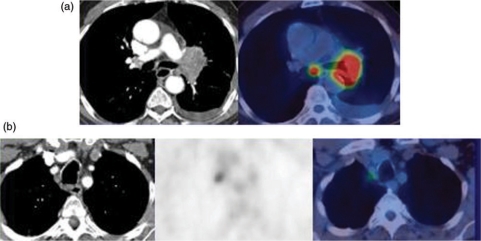
The presence of regional node metastases significantly alters prognosis in NSCLC. When disease has progressed outside the ipsilateral hemithorax the outcome is poor with less than 3% of patients with N3 disease surviving 5 years. In patients with N2 disease, the size, number and nodal levels involved influence survival. CT provides good anatomic definition but with significant over and under staging with a reported accuracy of 62–88% (Fig. 2).
Figure 2.
(a) NSCLC on the left with involved subcarinal (N2) nodes. (b) Same patient with involved right superior mediastinal nodes (N3), which are normal in size on CT.
FDG-PET has been shown to be more accurate than CT for staging mediastinal nodes in multiple studies and is cost effective. Detection is dependent not on size but on metabolic activity and an SUVmax of greater than 2.5 is often used as the cut off value, although increasing the SUVmax to 5.3 gives an accuracy for malignancy of greater than 92%[5]. In a meta-analysis by Gould et al.[6] PET was more sensitive and specific than CT for large nodes (85% and 90% versus 61% and 79% respectively) but with small nodes the specificity of PET decreased. PET is better than CT for N0, N2 and N3 disease but not N1disease and the accuracy for staging nodal uptake appears to be less for smokers compared to non-smokers particularly for N2 disease (72% versus 96%)[7]. The results comparing CT and PET from recent studies are shown in Table 1[4,8–12].
Table 1.
Accuracy of PET or PET/CT in nodal staging compared to CT
| Author, year | Number of subjects | CT (%) | PET (%) | PET/CT (%) |
|---|---|---|---|---|
| Yen[8], 2008a | 96 | 65.5 | 82.3 | |
| Melek[9], 2008 | 170 | 78 | 74 | |
| Shimm[4], 2005 | 106 | 69 | 84 | |
| Cerfolio[12], 2004 | 129 | 56 | 78 | |
| Yang[10], 2008 | 122 | 70 | 85 | |
| De Wever[11], 2007 | 50 | 66 | 70 | 80 |
aResults from an area with a high incidence of granulomatous disease.
False positive uptake in nodes occurs in sarcoidosis, amyloidosis, Wegener's granulomatosis, anthracosis tuberculosis, histoplasmosis and organising pneumonias. False negatives are also a problem. Lee et al.[13] in a study of patients who were clinically stage 1 found the incidence of unsuspected N2 disease was 6.5% in T1 tumours and 8.7% in T2 tumours. The risk factors for unsuspected N2 disease included a high SUV in the primary tumour, adenocarcinoma histology and central rather than peripheral tumours.
Meyers et al.[14] performed a cost analysis study in patients with stage 1 disease, and found 5.6% had unsuspected N2 disease, and that mediastinoscopy was not cost effective in this group. Many groups would therefore suggest that mediastinoscopy is not required in patients who are N0 on PET/CT. In patients who are N1 on PET/CT the incidence of unsuspected N2 disease in much higher (23.5%) and mediastinoscopy may be appropriate for this group.
Mediastinoscopy has been considered the gold standard for pre-operative staging but provides limited access to posterior mediastinal node groups. Endoscopic techniques either via the oesophagus for posterior mediastinal groups (levels 7–9) or via the bronchus for access to superior mediastinal, paratracheal, subcarinal and hilar nodes (levels 1, 2, 4, 7, 10 and 11) is increasingly being used. Eloubeidi et al.[15] found endoscopic ultrasound (EUS)-fine needle aspiration (FNA) positive N2 and N3 nodes in 37% of patients with negative mediastinoscopy and it was more accurate than CT or PET (98% vs. 41.5% vs. 40%). Similarly endobronchial ultrasound (EBUS) with guided biopsy is more accurate than CT or PET (98% vs. 60.8% vs. 72.5%)[16].
Metastatic disease (M status)
The commonest sites for metastatic disease in NSCLC are the brain, bone, liver and adrenals (in decreasing order) at presentation. FDG-PET is a whole body imaging system that will identify unsuspected metastases and PET/CT is significantly better than CT or PET alone for extra thoracic metastases[17], although it is limited in assessing brain metastases.
Metastases to the adrenals are not uncommon and FDG-PET can differentiate incidental adrenal adenomas, which have low uptake from adrenal metastases that exhibit increased uptake with a reported accuracy of 99%. PET/CT is more specific than PET alone for adrenal masses[18].
Metastases to the central nervous system are common and detected in 18% of patients with M1 disease at presentation. FDG-PET may not be very useful as the brain always shows increased metabolic activity and other isotopes such as [11C]methionine may be more sensitive.
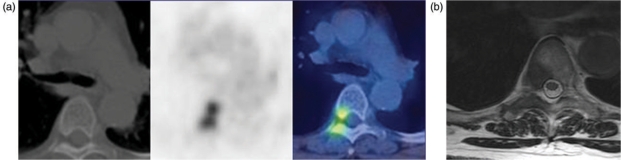
FDG-PET is more sensitive and accurate than isotope bone scans for bone metastases (91% and 94% versus 75% and 85%), with a very high PPV of 98% if the findings are concordant on PET/CT although this decreases to 61% if the CT is negative[19] (Fig. 3).
Figure 3.
(a) PET/CT shows uptake in the thoracic vertebra which is not identified on the diagnostic CT. (b) Same patient. Metastasis is seen on the MRI.
Prognosis
There are numerous reports on the prognostic value of FDG-PET using the SUV of the primary tumour although the values used are very variable. Cerfolio et al.[20] found tumours with an SUVmax of greater than 10 were more likely to be poorly differentiated and of advanced stage at diagnosis and the SUV was the best predictor of disease free survival. Goodgame et al.[21] using a median SUV of 5.5, found a recurrence rate of 14% in those with an SUV less than 5.5 compared to a 37% recurrence rate if the SUV was greater than 5.5.
Response to treatment
Patients with stage 1 disease are the most suitable candidates for surgery with survival rates of 57–85%. Patients with stage 111A NSCLC who have bulky N2 disease have a very poor prognosis, however in patients with ‘unexpected’ N2 disease the prognosis is much better with 20–30% 5-year survival if complete surgical resection can be performed. This is the rationale for attempted down staging of bulky N2 disease with induction chemotherapy prior to resection. Lorent et al.[22] found that patients who either had a partial response or stable disease in mediastinal nodes after induction chemotherapy had a 5-year survival of 35% following surgery compared to 9.4% in the non-responders.
FDG-PET has been shown to be better than CT in identifying responders in other tumours and re-staging with PET/CT appears to offer some advantages over CT or mediastinoscopy.
De Leyn[23] compared PET/CT with CT and mediastinoscopy for pre-surgical re-staging and found PET/CT was more accurate than CT (83% versus 60%) and mediastinoscopy (83% versus 60%) with a low sensitivity for mediastinoscopy (29%) as 60% of patients had incomplete mediastinoscopy due to fibrosis and adhesions. Although FDG-PET appears to be good at assessing the response in both the primary and metastases, it is less accurate for the response in the mediastinal nodes with a 20% false negative and 25% false positive rate and FDG positive nodes should undergo biopsy prior to definitive surgery.
FDG-PET can be used to stratify patients into prognostic groups following therapy. Eschmann et al.[24] found the change in SUV in the primary tumour following chemotherapy was predictive for long-term survival with a decrease of more than 60% predicting a much longer survival compared to those whose SUV decreased by less than 60%.
Recurrent disease
Approximately 30–50% of patients who undergo surgery will develop recurrent disease, with most (90%) occurring in the first 2 years. The recurrence rate is highest for T4 or N2 tumours (70%). Recurrences may be loco-regional (20–40%), distant (66–74%) or both (9–14%) (Fig. 4). FDG-PET is both sensitive and specific for recurrent disease with a high NPV and will alter treatment plans in up to 63% of patients.
Figure 4.
Recurrent disease shown on PET/CT in a patient who developed a broncho-pleural fistula 6 months after pneumonectomy.
Conclusion
FDG-PET/CT is now an established method for staging lung cancer and provides additional information compared to conventional imaging. Positive nodes may require biopsy either via mediastinoscopy or increasingly, endoscopic guided biopsy. FDG-PET also provides prognostic information on both initial and recurrent tumours.
References
- [1].Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2:706–14. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- [2].Yang SN, Liang JA, Lin FJ, Kwan AS, Kao CH, Shen YY. Differentiating benign and malignant pulmonary lesions with FDG-PET. Anticancer Res. 2001;21:4153–7. [PubMed] [Google Scholar]
- [3].van Baardwijk A, Baumert BG, Bosmans G, et al. The current status of FDG-PET in tumour volume definition in radiotherapy treatment planning. Cancer Treat Rev. 2006;32:245–60. doi: 10.1016/j.ctrv.2006.02.002. [DOI] [PubMed] [Google Scholar]
- [4].Shim SS, Lee KS, Kim BT. Non-small cell lung cancer: prospective comparison of integrated FDG PET/CT and CT alone for preoperative staging. Radiology. 2005;236:1011–9. doi: 10.1148/radiol.2363041310. [DOI] [PubMed] [Google Scholar]
- [5].Bryant AS, Cerfolio RJ, Klemm JM, Ohja B. Maximum standard uptake value of mediastinal lymph nodes on integrated FDG-PET-CT predicts pathology in patients with non-small cell lung cancer. Ann Thorac Surg. 2006;82:417–22. doi: 10.1016/j.athoracsur.2005.12.047. [DOI] [PubMed] [Google Scholar]
- [6].Gould MK, Sanders GD, Barnett PG, et al. Cost-effectiveness of alternative management strategies for patients with solitary pulmonary nodules. Ann Intern Med. 2003;138:724–35. doi: 10.7326/0003-4819-138-9-200305060-00009. [DOI] [PubMed] [Google Scholar]
- [7].Bryant AS, Cerfolio RJ. The clinical stage of non-small cell lung cancer as assessed by means of fluorodeoxyglucose-positron emission tomographic/computed tomographic scanning is less accurate in cigarette smokers. J Thorac Cardiovasc Surg. 2006;132:1363–8. doi: 10.1016/j.jtcvs.2006.07.032. [DOI] [PubMed] [Google Scholar]
- [8].Yen RF, Chen KC, Lee JM, et al. 18F-FDG PET for the lymph node staging of non-small cell lung cancer in a tuberculosis-endemic country: is dual time point imaging worth the effort? Eur J Nucl Med Mol Imaging. 2008;35:1305–15. doi: 10.1007/s00259-008-0733-1. [DOI] [PubMed] [Google Scholar]
- [9].Melek H, Gunluoglu MZ, Demir A, Akin H, Olcmen A, Dincer SI. Role of positron emission tomography in mediastinal lymphatic staging of non-small cell lung cancer. Eur J Cardiothorac Surg. 2008;33:294–9. doi: 10.1016/j.ejcts.2007.11.019. [DOI] [PubMed] [Google Scholar]
- [10].Yang W, Fu Z, Yu J. Value of PET/CT versus enhanced CT for locoregional lymph nodes in non-small cell lung cancer. Lung Cancer. 2008;61:35–43. doi: 10.1016/j.lungcan.2007.11.007. [DOI] [PubMed] [Google Scholar]
- [11].de Wever W, Ceyssens S, Mortelmans L, et al. Additional value of PET-CT in the staging of lung cancer: comparison with CT alone, PET alone and visual correlation of PET and CT. Eur Radiol. 2007;17:23–32. doi: 10.1007/s00330-006-0284-4. [DOI] [PubMed] [Google Scholar]
- [12].Cerfolio RJ, Ohja B, Bryant AS, Raghuveer V, Mountz JM, Bartolucci AA. The accuracy of integrated PET-CT compared with dedicated PET alone for the staging of patients with nonsmall cell lung cancer. Ann Thorac Surg. 2004;78:1017–23. doi: 10.1016/j.athoracsur.2004.02.067. (discussion 1017–23) [DOI] [PubMed] [Google Scholar]
- [13].Lee PC, Port JL, Korst RJ, Liss Y, Meherally DN, Altorki NK. Risk factors for occult mediastinal metastases in clinical stage I non-small cell lung cancer. Ann Thorac Surg. 2007;84:177–81. doi: 10.1016/j.athoracsur.2007.03.081. [DOI] [PubMed] [Google Scholar]
- [14].Meyers BF, Haddad F, Siegel BA, et al. Cost-effectiveness of routine mediastinoscopy in computed tomography- and positron emission tomography-screened patients with stage I lung cancer. J Thorac Cardiovasc Surg. 2006;131:822–9. doi: 10.1016/j.jtcvs.2005.10.045. [DOI] [PubMed] [Google Scholar]
- [15].Eloubeidi MA, Cerfolio RJ, Chen VK, Desmond R, Syed S, Ohja B. Endoscopic ultrasound-guided fine needle aspiration of mediastinal lymph node in patients with suspected lung cancer after positron emission tomography and computed tomography scans. Ann Thorac Surg. 2005;79:263–8. doi: 10.1016/j.athoracsur.2004.06.089. [DOI] [PubMed] [Google Scholar]
- [16].Yasufuku K, Nakajima T, Motoori K, et al. Comparison of endobronchial ultrasound, positron emission tomography, and CT for lymph node staging of lung cancer. Chest. 2006;130:710–8. doi: 10.1378/chest.130.3.710. [DOI] [PubMed] [Google Scholar]
- [17].de Wever W, Vankan Y, Stroobants S, Versdchakelen J. Detection of extrapulmonary lesions with integrated PET/CT in the staging of lung cancer. Eur Respir J. 2007;29:995–1002. doi: 10.1183/09031936.00119106. [DOI] [PubMed] [Google Scholar]
- [18].Blake MA, Slattery JM, Kalra MK, et al. Adrenal lesions: characterization with fused PET/CT image in patients with proved or suspected malignancy – initial experience. Radiology. 2006;238:970–7. doi: 10.1148/radiol.2383042164. [DOI] [PubMed] [Google Scholar]
- [19].Taira AV, Herfkens RJ, Gambhir SS, Quon A. Detection of bone metastases: assessment of integrated FDG PET/CT imaging. Radiology. 2007;243:204–11. doi: 10.1148/radiol.2431052104. [DOI] [PubMed] [Google Scholar]
- [20].Cerfolio RJ, Bryant AS, Ohja B, Bartolucci AA. The maximum standardized uptake values on positron emission tomography of a non-small cell lung cancer predict stage, recurrence, and survival. J Thorac Cardiovasc Surg. 2005;130:151–9. doi: 10.1016/j.jtcvs.2004.11.007. [DOI] [PubMed] [Google Scholar]
- [21].Goodgame B, Pillot GA, Yang Z, et al. Prognostic value of preoperative positron emission tomography in resected stage I non-small cell lung cancer. J Thorac Oncol. 2008;3:130–4. doi: 10.1097/JTO.0b013e318160c122. [DOI] [PubMed] [Google Scholar]
- [22].Lorent N, De Leyn P, Lievens Y, et al. Long-term survival of surgically staged IIIA-N2 non-small-cell lung cancer treated with surgical combined modality approach: analysis of a 7-year prospective experience. Ann Oncol. 2004;15:1645–53. doi: 10.1093/annonc/mdh435. [DOI] [PubMed] [Google Scholar]
- [23].De Leyn P, Stroobants S, De Wever W, et al. Prospective comparative study of integrated positron emission tomography-computed tomography scan compared with remediastinoscopy in the assessment of residual mediastinal lymph node disease after induction chemotherapy for mediastinoscopy-proven stage IIIA-N2 non-small-cell lung cancer: a Leuven Lung Cancer Group Study. J Clin Oncol. 2006;24:3333–9. doi: 10.1200/JCO.2006.05.6341. [DOI] [PubMed] [Google Scholar]
- [24].Eschmann SM, Friedel G, Paulsen F, et al. 18F-FDG PET for assessment of therapy response and preoperative re-evaluation after neoadjuvant radio-chemotherapy in stage 111 non-small cell lung cancer. Eur J Nucl Med Mol Imaging. 2007;34:463–71. doi: 10.1007/s00259-006-0273-5. [DOI] [PubMed] [Google Scholar]