Abstract
Please include a few sentences as an abstract of the paper so that it will have a presence in the online databases.
Keywords: CT screening, Lung cancer
Epidemiology and clinical presentation
Lung cancer is the leading cause of death from malignancy with an estimated 1.3 million deaths per year worldwide. The main risk factor is cigarette smoking which is believed to cause at least 85% of all lung cancers. Occupational exposure to asbestos as well as other substances (chromium, arsenic, etc.) has also been identified as a risk factor.
Lung cancer usually does not cause early symptoms. On the contrary, symptoms are usually due to locally advanced disease (infiltration or compression of adjacent organs such as the oesophagus (dysphagia), superior vena cava (SVC obstruction), spine (paraplegia), etc.) or distant metastases to the brain (seizures) or bone (pathological fractures). Rarely, haemoptysis may be found in early stage lung cancer.
Furthermore, clinical examination is not helpful for the detection of early stages of lung cancer. Therefore, most (>2/3) cancers are detected at advanced tumour stages. Therapy of advanced lung cancer is usually not curative, therefore the prognosis is poor with less than 15% overall 5-year survival. If, however, lung cancer is detected at earlier stages, either incidentally (e.g. chest radiograph or CT obtained for other reasons such as pulmonary embolism) prognosis is better. In non-small-cell lung cancer (NSCLC), 5-year survival is > 65% at stage IA (pT1, pN0, M0) and even > 80% at small (<1 cm) stage IA.
Motivation for lung cancer screening
Thus, it is hoped that diagnosis of lung cancer at early stages can result in higher cure rates. As symptoms or clinical examination are not able to diagnose early lung cancer, screening with regular chest radiography and/or sputum cytology was studied in the 1970s. Unfortunately, no reduction in lung cancer mortality was shown, probably because these tests are not sensitive enough for early tumour stages. Therefore, lung cancer screening with these methods was not recommended.
Recently, more sophisticated diagnostic tests with potentially higher sensitivity (e.g. sputum cytometry, analysis of molecular markers in exhaled air, sputum or blood) were suggested; of these low-dose radiation unenhanced CT (low-dose CT) is most likely to be feasible in clinical routine.
Current data on CT screening for lung cancer
During recent years several studies have been performed in Japan, North America and Europe testing low-dose CT as a screening tool in risk populations, mostly current or former cigarette smokers. The aim was to analyse the sensitivity for early lung cancer and test diagnostic algorithms for the work-up of detected abnormalities. This is necessary because most early lung cancers present as pulmonary nodules for which low-dose CT is highly sensitive, however, many pulmonary nodules even in smokers are due to benign lesions such as granulomas, hamartomas, etc. (Fig. 1).
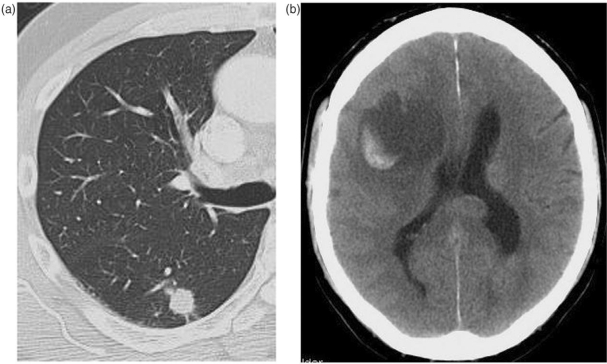
Figure 2.
Smoker with first seizure. (a) Ill-defined lobulated non-calcified nodule: biopsy confirmed adenocarcinoma; (b) CT brain demonstrates cerebral metastasis.
Figure 1.
Asymptomatic smoker, lobulated non-calcified nodule: biopsy confirmed hamartoma.
These feasibility studies all provided very similar results. Annual low-dose CT and management of detected abnormalities applying simple diagnostic algorithms based on size and density of nodules allowed detection of early stage lung cancer in much higher percentages of individuals than chest radiography and sputum cytology in previous studies. The management of nodules was mainly performed with follow-up low-dose CT, whereas other and more complex procedures (positron emission tomography (PET), fibre-optic bronchoscopy (FOB), percutaneous or surgical biopsy) were rarely required and the proportion of invasive procedures for benign lesions was acceptable (approx. 30%). Prevalence (i.e. cancers detected at the first examination) was higher than incidence (cancers detected at annual follow-up examinations). Resectability of cancers and cure rates were high and the percentage of cancers diagnosed between two examinations (interval cancers) was low.
Limitations of the current data
As all feasibility studies were designed as non-randomized one-arm trials they are not appropriate to assess the key question: “Does regular low-dose CT screening of subjects at risk for lung cancer actually prevent death from lung cancer?”
All the favourable results discussed above could be due to different biases of the non-comparative study designs. High 5-year survival can be solely due to the so-called lead-time bias, which describes the fact that the diagnosis (using a test in an asymptomatic subject) is automatically made earlier during the course of the disease, therefore the starting point of a 5-year follow-up is not comparable to the starting point in an individual with symptoms. Instead of 5-year survival, total disease-free survival should be measured long enough to compensate for the lead-time.
Screening with a diagnostic test at regular intervals will automatically cause a shift in the detection rates of tumours with different biological aggressiveness. More aggressive fast growing tumours are more likely to be diagnosed because of symptoms between two examinations than less aggressive slow growing tumours. Therefore, screening-detected tumours will automatically be less aggressive than interval tumours. This fact is described as the length-time bias.
In addition, patients at high risk for lung cancer (heavy smokers) are also at risk for other fatal diseases (e.g. coronary artery disease, stroke, etc.), therefore, detection of lung cancer at early stages may not prolong survival because of death from other causes. For a patient who dies from another cause before clinical symptoms of lung cancer develop the diagnosis of lung cancer is unnecessary. This fact, which automatically occurs in every screening setting is described as “overdiagnosis”.
In other words, the favourable results of the feasibility studies (high proportion of early stage NSCLC, prevalence higher than incidence, good resectability, good 5-year survival, low proportion of invasive procedures for benign lesions, etc.) are all prerequisites for CT screening to be effective but cannot serve as a proof that CT screening can actually save lives[1,2].
Prospective randomized controlled trials (RCT)
The only study design that is appropriate to answer the key question of lung cancer screening, (i.e. is there a reduction of mortality from lung cancer) requires a prospective controlled randomized trial (RCT) in which subjects at risk for lung cancer are randomized to a screening arm (with regular CT) and a control arm (without regular CT). Only if mortality from lung cancer in the screening arm is significantly lower than in the control arm, can CT screening be accepted as beneficial. Currently, one large RCT on CT screening for lung cancer in active and former smokers is under way in the US, the “National Lung Screening Trial (NLST)”. More than 53,000 subjects were randomized in 2002 and are currently being followed. The first results are not expected before 2009[3]. Other smaller trials with similar designs are under way in Europe, the largest of which is a multinational trial in the Netherlands and Belgium (NELSON trials). Data from the different studies may be pooled to increase the statistical significance of the results.
Recommendations
As long as mortality reduction through CT screening has not been demonstrated, all scientific societies and regulatory boards suggest applying CT screening only in studies but not in clinical routine.
References
- [1].Patz EF, Jr, Goodman PC, Bepler G. Screening for lung cancer. N Engl J Med. 2000;343:1627–33. doi: 10.1056/NEJM200011303432208. [DOI] [PubMed] [Google Scholar]
- [2].Bach PB, Niewoehner DE, Black WC. American College of Chest Physicians. Screening for lung cancer: the guidelines. Chest. 2003;123(Suppl):S83–8. doi: 10.1378/chest.123.1_suppl.83s. [DOI] [PubMed] [Google Scholar]
- [3].Church TR. National Lung Screening Trial Executive Committee. Chest radiography as the comparison for spiral CT in the National Lung Screening Trial. Acad Radiol. 2003;10:713–5. doi: 10.1016/s1076-6332(03)80095-8. [DOI] [PubMed] [Google Scholar]