Abstract
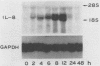
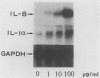
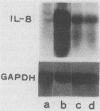
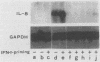
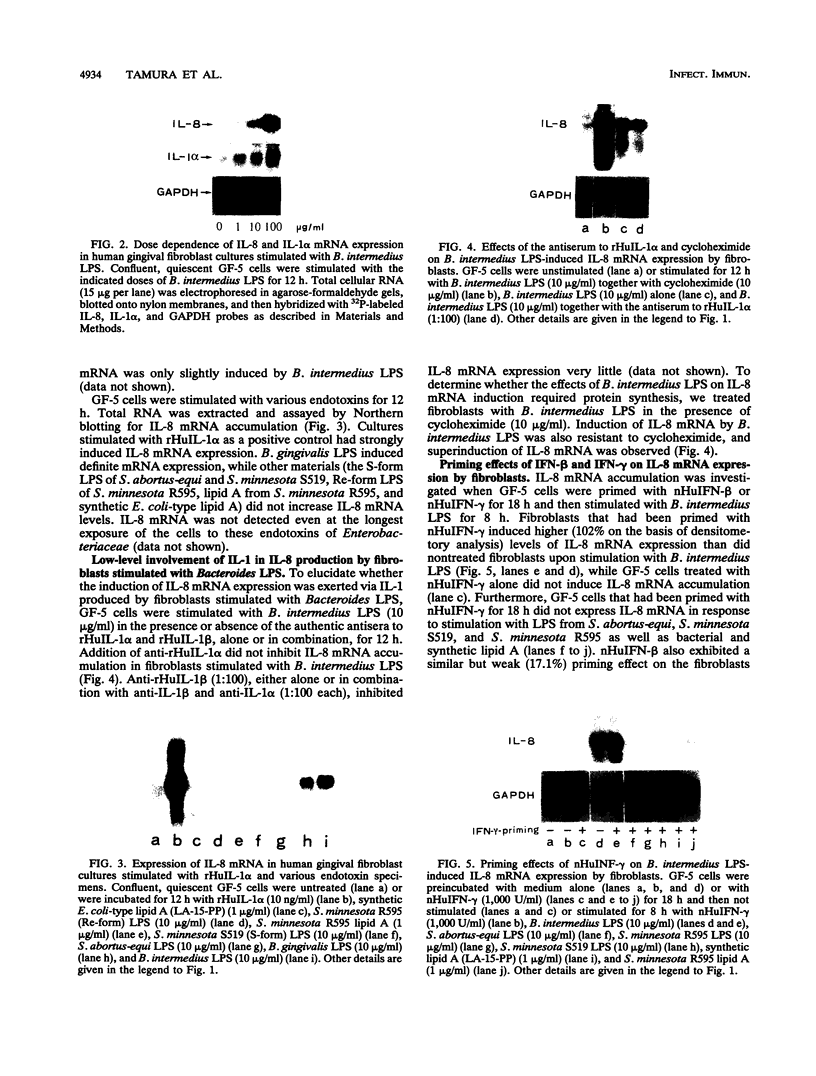
Lipopolysaccharides (LPS) prepared from Bacteroides intermedius (Prevotella intermedia) and Bacteroides (Porphyromonas) gingivalis by hot phenol-water extraction induced interleukin-8 (IL-8) mRNA in normal human gingival fibroblast cultures, as demonstrated by Northern (RNA) blot analysis. IL-8 mRNA levels began to increase after a 2-h exposure, reached a maximum after 12 h, and then dropped to the unstimulated level at 48 h. IL-8 mRNA levels were also enhanced in a dose-dependent manner. By contrast, LPS specimens from various Salmonella species with S and R chemotypes and bacterial [corrected] and synthetic lipid A preparations did not increase IL-8 mRNA levels in fibroblasts. Although recombinant human IL-1 alpha induced IL-8 mRNA expression in fibroblast cultures, an antiserum to recombinant human IL-1 alpha did not decrease the IL-8 mRNA accumulation induced by B. intermedius LPS. Fibroblasts primed with natural human gamma interferon (IFN-gamma) expressed higher IL-8 mRNA levels upon stimulation with B. intermedius LPS, but not with Salmonella LPS, compared with nontreated cells. Natural human IFN-beta exhibited a similar priming effect on the fibroblasts, and antiserum to IFN-beta added to the cultures together with B. intermedius LPS decreased the IL-8 mRNA levels. Therefore, endogenous IFN-beta enhanced IL-8 mRNA production in response to B. intermedius LPS in fibroblasts.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartold P. M., Haynes D. R. Interleukin-6 production by human gingival fibroblasts. J Periodontal Res. 1991 Jul;26(4):339–345. doi: 10.1111/j.1600-0765.1991.tb02072.x. [DOI] [PubMed] [Google Scholar]
- Bazzoni F., Cassatella M. A., Rossi F., Ceska M., Dewald B., Baggiolini M. Phagocytosing neutrophils produce and release high amounts of the neutrophil-activating peptide 1/interleukin 8. J Exp Med. 1991 Mar 1;173(3):771–774. doi: 10.1084/jem.173.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carveth H. J., Bohnsack J. F., McIntyre T. M., Baggiolini M., Prescott S. M., Zimmerman G. A. Neutrophil activating factor (NAF) induces polymorphonuclear leukocyte adherence to endothelial cells and to subendothelial matrix proteins. Biochem Biophys Res Commun. 1989 Jul 14;162(1):387–393. doi: 10.1016/0006-291x(89)92009-3. [DOI] [PubMed] [Google Scholar]
- Charon J. A., Luger T. A., Mergenhagen S. E., Oppenheim J. J. Increased thymocyte-activating factor in human gingival fluid during gingival inflammation. Infect Immun. 1982 Dec;38(3):1190–1195. doi: 10.1128/iai.38.3.1190-1195.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dahinden C. A., Kurimoto Y., De Weck A. L., Lindley I., Dewald B., Baggiolini M. The neutrophil-activating peptide NAF/NAP-1 induces histamine and leukotriene release by interleukin 3-primed basophils. J Exp Med. 1989 Nov 1;170(5):1787–1792. doi: 10.1084/jem.170.5.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzink J. L., Socransky S. S., Haffajee A. D. The predominant cultivable microbiota of active and inactive lesions of destructive periodontal diseases. J Clin Periodontol. 1988 May;15(5):316–323. doi: 10.1111/j.1600-051x.1988.tb01590.x. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. Preparation and properties of a standardized lipopolysaccharide from salmonella abortus equi (Novo-Pyrexal). Zentralbl Bakteriol Orig A. 1979 Apr;243(2-3):226–244. [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Obin M. S., Brock A. F., Luis E. A., Hass P. E., Hébert C. A., Yip Y. K., Leung D. W., Lowe D. G., Kohr W. J. Endothelial interleukin-8: a novel inhibitor of leukocyte-endothelial interactions. Science. 1989 Dec 22;246(4937):1601–1603. doi: 10.1126/science.2688092. [DOI] [PubMed] [Google Scholar]
- Hamada S., Koga T., Nishihara T., Fujiwara T., Okahashi N. Characterization and immunobiologic activities of lipopolysaccharides from periodontal bacteria. Adv Dent Res. 1988 Nov;2(2):284–291. doi: 10.1177/08959374880020021301. [DOI] [PubMed] [Google Scholar]
- Hamada S., Takada H., Ogawa T., Fujiwara T., Mihara J. Lipopolysaccharides of oral anaerobes associated with chronic inflammation: chemical and immunomodulating properties. Int Rev Immunol. 1990;6(4):247–261. doi: 10.3109/08830189009056635. [DOI] [PubMed] [Google Scholar]
- Jandinski J. J., Stashenko P., Feder L. S., Leung C. C., Peros W. J., Rynar J. E., Deasy M. J. Localization of interleukin-1 beta in human periodontal tissue. J Periodontol. 1991 Jan;62(1):36–43. doi: 10.1902/jop.1991.62.1.36. [DOI] [PubMed] [Google Scholar]
- Kabashima H., Maeda K., Iwamoto Y., Hirofuji T., Yoneda M., Yamashita K., Aono M. Partial characterization of an interleukin-1-like factor in human gingival crevicular fluid from patients with chronic inflammatory periodontal disease. Infect Immun. 1990 Aug;58(8):2621–2627. doi: 10.1128/iai.58.8.2621-2627.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamagata Y., Miyasaka N., Inoue H., Hashimoto J., Iida M. [Cytokine production in inflamed human gingival tissues--interleukin-6]. Nihon Shishubyo Gakkai Kaishi. 1989 Dec;31(4):1081–1087. doi: 10.2329/perio.31.1081. [DOI] [PubMed] [Google Scholar]
- Komuro T., Galanos C. Analysis of Salmonella lipopolysaccharides by sodium deoxycholate-polyacrylamide gel electrophoresis. J Chromatogr. 1988 Oct 26;450(3):381–387. doi: 10.1016/s0021-9673(01)83593-7. [DOI] [PubMed] [Google Scholar]
- Kristensen M. S., Paludan K., Larsen C. G., Zachariae C. O., Deleuran B. W., Jensen P. K., Jørgensen P., Thestrup-Pedersen K. Quantitative determination of IL-1 alpha-induced IL-8 mRNA levels in cultured human keratinocytes, dermal fibroblasts, endothelial cells, and monocytes. J Invest Dermatol. 1991 Sep;97(3):506–510. doi: 10.1111/1523-1747.ep12481543. [DOI] [PubMed] [Google Scholar]
- Larsen C. G., Anderson A. O., Appella E., Oppenheim J. J., Matsushima K. The neutrophil-activating protein (NAP-1) is also chemotactic for T lymphocytes. Science. 1989 Mar 17;243(4897):1464–1466. doi: 10.1126/science.2648569. [DOI] [PubMed] [Google Scholar]
- Lee T. H., Lee G. W., Ziff E. B., Vilcek J. Isolation and characterization of eight tumor necrosis factor-induced gene sequences from human fibroblasts. Mol Cell Biol. 1990 May;10(5):1982–1988. doi: 10.1128/mcb.10.5.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard E. J., Skeel A., Yoshimura T., Noer K., Kutvirt S., Van Epps D. Leukocyte specificity and binding of human neutrophil attractant/activation protein-1. J Immunol. 1990 Feb 15;144(4):1323–1330. [PubMed] [Google Scholar]
- March C. J., Mosley B., Larsen A., Cerretti D. P., Braedt G., Price V., Gillis S., Henney C. S., Kronheim S. R., Grabstein K. Cloning, sequence and expression of two distinct human interleukin-1 complementary DNAs. Nature. 1985 Jun 20;315(6021):641–647. doi: 10.1038/315641a0. [DOI] [PubMed] [Google Scholar]
- Masada M. P., Persson R., Kenney J. S., Lee S. W., Page R. C., Allison A. C. Measurement of interleukin-1 alpha and -1 beta in gingival crevicular fluid: implications for the pathogenesis of periodontal disease. J Periodontal Res. 1990 May;25(3):156–163. doi: 10.1111/j.1600-0765.1990.tb01038.x. [DOI] [PubMed] [Google Scholar]
- Matsushima K., Morishita K., Yoshimura T., Lavu S., Kobayashi Y., Lew W., Appella E., Kung H. F., Leonard E. J., Oppenheim J. J. Molecular cloning of a human monocyte-derived neutrophil chemotactic factor (MDNCF) and the induction of MDNCF mRNA by interleukin 1 and tumor necrosis factor. J Exp Med. 1988 Jun 1;167(6):1883–1893. doi: 10.1084/jem.167.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayrand D., Holt S. C. Biology of asaccharolytic black-pigmented Bacteroides species. Microbiol Rev. 1988 Mar;52(1):134–152. doi: 10.1128/mr.52.1.134-152.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim J. J., Zachariae C. O., Mukaida N., Matsushima K. Properties of the novel proinflammatory supergene "intercrine" cytokine family. Annu Rev Immunol. 1991;9:617–648. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- Schröder J. M., Christophers E. Secretion of novel and homologous neutrophil-activating peptides by LPS-stimulated human endothelial cells. J Immunol. 1989 Jan 1;142(1):244–251. [PubMed] [Google Scholar]
- Schröder J. M., Mrowietz U., Morita E., Christophers E. Purification and partial biochemical characterization of a human monocyte-derived, neutrophil-activating peptide that lacks interleukin 1 activity. J Immunol. 1987 Nov 15;139(10):3474–3483. [PubMed] [Google Scholar]
- Schröder J. M., Sticherling M., Henneicke H. H., Preissner W. C., Christophers E. IL-1 alpha or tumor necrosis factor-alpha stimulate release of three NAP-1/IL-8-related neutrophil chemotactic proteins in human dermal fibroblasts. J Immunol. 1990 Mar 15;144(6):2223–2232. [PubMed] [Google Scholar]
- Sismey-Durrant H. J., Hopps R. M. Effect of lipopolysaccharide from Porphyromonas gingivalis on prostaglandin E2 and interleukin-1-beta release from rat periosteal and human gingival fibroblasts in vitro. Oral Microbiol Immunol. 1991 Dec;6(6):378–380. doi: 10.1111/j.1399-302x.1991.tb00510.x. [DOI] [PubMed] [Google Scholar]
- Strieter R. M., Kasahara K., Allen R., Showell H. J., Standiford T. J., Kunkel S. L. Human neutrophils exhibit disparate chemotactic factor gene expression. Biochem Biophys Res Commun. 1990 Dec 14;173(2):725–730. doi: 10.1016/s0006-291x(05)80095-6. [DOI] [PubMed] [Google Scholar]
- Strieter R. M., Kunkel S. L., Showell H. J., Remick D. G., Phan S. H., Ward P. A., Marks R. M. Endothelial cell gene expression of a neutrophil chemotactic factor by TNF-alpha, LPS, and IL-1 beta. Science. 1989 Mar 17;243(4897):1467–1469. doi: 10.1126/science.2648570. [DOI] [PubMed] [Google Scholar]
- Strieter R. M., Phan S. H., Showell H. J., Remick D. G., Lynch J. P., Genord M., Raiford C., Eskandari M., Marks R. M., Kunkel S. L. Monokine-induced neutrophil chemotactic factor gene expression in human fibroblasts. J Biol Chem. 1989 Jun 25;264(18):10621–10626. [PubMed] [Google Scholar]
- Takada H., Hirai H., Fujiwara T., Koga T., Ogawa T., Hamada S. Bacteroides lipopolysaccharides (LPS) induce anaphylactoid and lethal reactions in LPS-responsive and -nonresponsive mice primed with muramyl dipeptide. J Infect Dis. 1990 Aug;162(2):428–434. doi: 10.1093/infdis/162.2.428. [DOI] [PubMed] [Google Scholar]
- Takada H., Mihara J., Morisaki I., Hamada S. Induction of interleukin-1 and -6 in human gingival fibroblast cultures stimulated with Bacteroides lipopolysaccharides. Infect Immun. 1991 Jan;59(1):295–301. doi: 10.1128/iai.59.1.295-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga K., Nakamura Y., Sakata K., Fujimori K., Ohkubo M., Sawada K., Sakiyama S. Enhanced expression of a glyceraldehyde-3-phosphate dehydrogenase gene in human lung cancers. Cancer Res. 1987 Nov 1;47(21):5616–5619. [PubMed] [Google Scholar]
- Walz A., Peveri P., Aschauer H., Baggiolini M. Purification and amino acid sequencing of NAF, a novel neutrophil-activating factor produced by monocytes. Biochem Biophys Res Commun. 1987 Dec 16;149(2):755–761. doi: 10.1016/0006-291x(87)90432-3. [DOI] [PubMed] [Google Scholar]
- Yoshimura T., Matsushima K., Tanaka S., Robinson E. A., Appella E., Oppenheim J. J., Leonard E. J. Purification of a human monocyte-derived neutrophil chemotactic factor that has peptide sequence similarity to other host defense cytokines. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9233–9237. doi: 10.1073/pnas.84.24.9233. [DOI] [PMC free article] [PubMed] [Google Scholar]