Abstract
Colorectal cancer is a common malignancy that afflicts many in the western world. Imaging studies are frequently used to evaluate patients in the screening, staging and surveillance of colorectal cancer. Cross sectional imaging studies such as ultrasound, computed tomography and magnetic resonance imaging provide anatomic and morphologic information about tumor and patterns of spread. Positron emission tomography (PET) differs in that it provides information about tumor metabolism.[18F]Fluorodeoxyglucose PET has been clinically used for the evaluation of patients with a wide variety of cancers since most malignancies, including colorectal cancer, typically show increased glucose metabolism. This review present the positron emission tomography/computed tomography imaging findings that may be encountered in the diagnosis, staging and follow-up of patients with colorectal cancer.
Keywords: Colorectal cancer, positron emission TOMOGRAPHY, computed tomography
Introduction
Colorectal cancer is a common malignancy that afflicts many in the western world. Approximately 150,000 new cases will be diagnosed in the United States in 2008 and approximately 50,000 deaths will be attributed to the disease this year[1]. Most patients are diagnosed with colorectal cancer in the sixth and seventh decade of life[1]. Approximately 30% of colorectal cancers occur in the sigmoid, 25% occur in the rectum and 25% occur in the cecum and ascending colon. Histologically, colon cancers are adenocarcinomas that form moderately to well-differentiated glands that secrete varying amounts of mucin[2].
Imaging studies are frequently used to evaluate patients in the screening, staging and surveillance of colorectal cancer. This review presents the positron emission tomography (PET)/computed tomography (CT) imaging findings that may be encountered in the diagnosis, staging and follow-up of patients with colorectal cancer.
Screening
The adenoma–carcinoma sequence theory is well established and suggests that many colon cancers develop directly from adenomatous polyps. The malignant potential of a polyp is largely determined by its size. Polyps greater than 2 cm in size have a greater than 40% risk of being cancerous, while those less than 0.5 cm are essentially at no risk for harboring malignancy. Other features of a polyp that predispose to malignancy are villous architecture and degree of cellular atypia and dysplasia[3]. The cumulative risk for developing invasive carcinoma in unresected polyps has been reported to be 2.5% at 5 years, 8% at 10 years and 24% at 20 years[4].
With the knowledge that colon cancers develop slowly over time, most often from preexisting adenomas, screening is of great importance for prevention of colon cancer. The ideal screening test should be safe, accurate and inexpensive. While there are several screening methods currently in use, such as fecal occult blood testing, optical colonoscopy and imaging studies such as barium enema or CT colonography, none of them meets all of these criteria at the present time. While imaging studies generally provide an anatomic or structural snapshot of abnormalities, PET imaging differs in that it provides information about metabolic activity and function.
[18F]Fluorodeoxyglucose (18F-FDG) PET has been clinically used for the evaluation of patients with a wide variety of cancers since most malignancies, including colorectal cancer typically show increased glucose metabolism. The greatest difficulty in using PET for colonic abnormalities is the presence of physiologic uptake in the gastrointestinal tract. The exact etiology of FDG uptake in the colon is not entirely clear. Some suggest that increased FDG uptake is due to uptake into mucosal structures. Differences in the histology of the intestinal glands in the ascending colon, descending colon, rectum, and small intestine can cause regional differences in FDG uptake and standard uptake value (SUV) readings. The presence of lymphoid tissue in the colon may contribute to FDG uptake. Increased activation of glandular structures in the ascending colon compared with other regions could induce increased FDG uptake or even excretion into the lumen[5]. Muscular activity with peristalsis may be another minor contributor to physiologic colon uptake.
Aside from normal colonic uptake of FDG, both benign and malignant colonic lesions can be detected by FDG-PET. A study by Yasuda et al. looked at 110 patients and found that precancerous adenomatous polyps can be detected incidentally on whole body images performed for other indications with a sensitivity of 24%[6]. The investigators in this study showed that benign colonic adenomas with FDG uptake could not be distinguished from FDG avid carcinomas of the colon. Lesion size was an important consideration in this study. The positivity rate for PET rises with increasing polyp size, with 90% positivity in lesions greater than 13 mm[6]. Another study by van Kouwen et al. showed similar findings with higher detection rates with increasing size (72% sensitivity with size >11 mm) and grade of dysplasia of the adenomatous polyps[7].
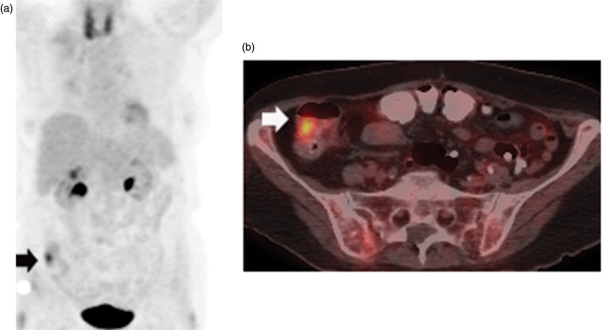
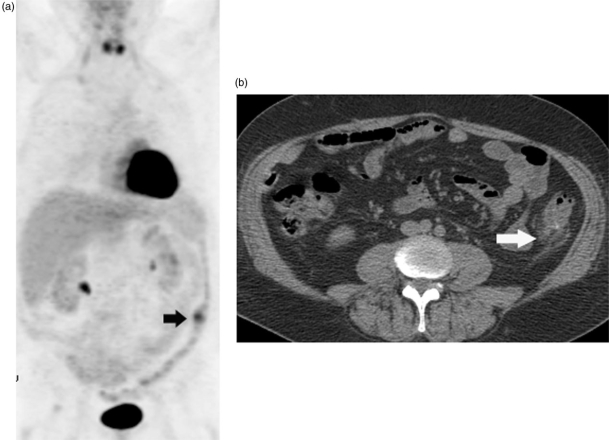
Since colonic uptake is frequently seen on FDG-PET imaging, it is important to determine whether the process is focal or diffuse to help distinguish physiologic from pathologic activity. While the measurement of SUV does not allow the differentiation of benign from malignant processes of the colon, the presence of focal colonic FDG uptake as an incidental finding on PET/CT justifies a colon screening examination and PET/CT fusion can be particularly helpful for localization of lesions (Fig. 1)[8]. FDG-PET may also identify inflammatory diseases of the colon such as inflammatory bowel disease or diverticulitis (Fig. 2).
Figure 1.
Coronal MIP image (a) of an FDG-PET scan in a patient with a history of lymphoma who presented for routine surveillance shows focal uptake in the right lower quadrant (arrow) corresponding to a lesion in the cecum (arrow) on axial fused PET/CT (b) which proved to be a 3-cm adenomatous polyp at colonoscopy.
Figure 2.
Coronal MIP PET image (a) of an FDG-PET scan shows a focal area of uptake in the descending colon (arrow). Corresponding axial CT (b) image shows diverticulosis and surrounding stranding (arrow) compatible with diverticulitis.
A few studies have investigated the feasibility of combining FDG-PET with CT colonography (CTC), a relatively new technique that provides an endoluminal perspective of the colon. A prospective study by Gollub et al. evaluated 17 patients with a combined PET/CT examination after colonic cleansing and insufflation of the colon with carbon dioxide. These investigators found that PET/CTC was a feasible technique allowing excellent image correlation in polyps measuring greater than 10 mm and showing promise in accurate anatomic correlation of both malignant and premalignant lesions of the colon[9]. Cost, availability and relative non-specificity make this technique less than feasible at the present time for widespread colorectal screening.
Staging
Once a diagnosis of colorectal cancer is established, staging becomes important for prognostication and to determine appropriate therapy. Complete surgical removal of tumor, along with regional lymphatics affords the best prognosis for patients with colorectal cancer. Recurrence rates are largely dependent upon the initial stage at diagnosis. Neoadjuvant or adjuvant chemotherapy and radiation therapy are therefore increasingly administered to decrease the incidence of recurrence[10]. In patients with rectal cancer, pre-operative therapy can help to downstage more advanced tumors, which allows sphincter preservation[10]. Once a tumor is invasive, it may extend through the layers of the colonic wall and invade adjacent structures[2,11]. Lymphatic, hematogenous and peritoneal spread may also occur.
PET can be a useful for pre-operative staging of colorectal cancer. The greatest value of PET lies in the fact that total body coverage allows detection of distant sites of disease. PET and PET/CT are clearly limited for T staging of the primary tumor due to limited spatial resolution and inability to distinguish the layers of the colonic wall. Transrectal ultrasound (US) and magnetic resonance imaging (MRI) provide much better anatomic resolution and are of greater value for T staging[11].
Nodal staging can be difficult with cross sectional imaging techniques such as US, CT and MRI. On cross-sectional imaging, size (greater than 1 cm) remains the primary criterion for predicting nodal metastasis, although it is well known that size is not an ideal indicator of disease. The advantage of PET lies in the ability to use metabolic activity to help distinguish benign from malignant adenopathy at sites away from the immediate vicinity of the primary tumor. Nodes in the immediate vicinity of the primary tumor are very difficult to detect with PET due to FDG activity of the primary which may obscure small lymph nodes. Small nodes are also not easily detected with PET. The overall sensitivity for nodal staging is therefore reported to be quite low, only 29%[12,13]. It is important not to confuse physiologic activity in the urinary system with tumor spread in the retroperitoneum or pelvis and fused PET/CT has an advantage in anatomic localization over PET alone.
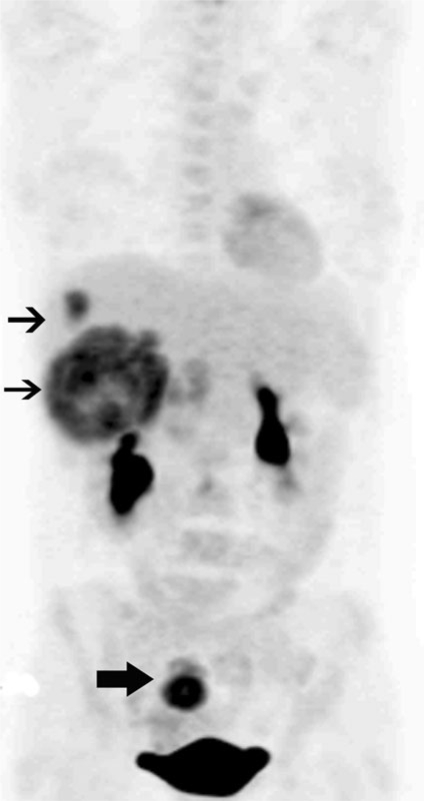
Accurate staging also requires the detection of distant sites of metastatic disease (Fig. 3). This is important because limited disease spread such as to the liver may be resected for cure. Resection of colorectal cancer metastases with or without hepatic arterial perfusion therapy can lead to up to 60% 10-year survival in selected patients[14]. Therefore, pre-operative knowledge of tumor extent is very important to determine if curative resection is feasible. It has been suggested that FDG-PET is more sensitive than CT in the detection of hepatic and pulmonary metastases and in identifying other sites of intra-abdominal disease[13]. FDG-PET showed greatest accuracy in the detection of liver metastases with reported accuracy up to 99%, sensitivity up to 100% and specificity up to 98%[15]. It is important to keep in mind that lesion size is an important criterion for detection and small hepatic lesions are still not easily detected due to relatively high background liver activity. Also the limited spatial resolution of PET alone makes surgical planning difficult.
Figure 3.
Coronal MIP PET image shows a primary FDG avid tumor in the rectosigmoid (thick arrow) with FDG avid metastases to the liver (thin arrows) in a patient with newly diagnosed colorectal cancer.
PET may also identify sites of disease that may preclude surgery or change the surgical approach. Several studies have shown that findings on PET and PET/CT results in change in stage and thereby alters management in up to 1/3 of patients16–18. In a study of patients with low rectal cancers, FDG-PET/CT altered treatment plans in 38% of patients largely through the detection of unsuspected inguinal adenopathy[19].
FDG-PET has also been used to predict response to pre-operative therapy and thereby predict outcome in several different malignancies including rectal cancer20–24. In a study by Guillem et al., 15 patients with locally advanced rectal cancer underwent FDG-PET imaging before and after completion of chemoradiation. All patients showed some degree of response to pre-operative therapy based on pathologic examination. The mean percentage decrease in SUVmax was 69% for patients that remained free of disease at a median follow-up of 42 months; the SUVmax decreased by only 37% in patients who eventually developed recurrence[20].
Surveillance
Although most patients with colorectal carcinoma undergo surgery with the intent of cure, nearly 4 out of 10 patients experience relapse of disease[25,26]. Over the past decade, aggressive surgical approaches to metastatic disease are being practiced and nearly 30% of patients undergo resection of recurrent disease with increased long term survival[27]. With the use of newer chemotherapeutic agents, many lesions which are deemed unresectable can be downsized thereby allowing potentially curative surgery[28]. Even in patients who have surgically unresectable disease, increasing use of newer chemotherapeutic agents, when given early show improved survival[26,29].
Nearly 85% of recurrences occur within the first 3 years after surgery and nearly none occur after 5 years[27]. Hence most surveillance strategies focus resources on the first 3 years following surgery. Various approaches to surveillance are used by clinicians from the strategy of ‘call me if you have symptoms’ to the use of aggressive monitoring with regular clinic visits, periodic tumor marker assays, cross sectional imaging, ultrasound and endoscopy. Recognizing the benefit of early treatment in resectable metastases despite acknowledging the lack of sufficient data to determine the optimal frequency of tests, the American Society of Clinical Oncology (ASCO) in 2005 recommended that assay by carcinoembryonic antigen (CEA) be performed every 3 months for the first 3 years, CT scan of the chest, abdomen and pelvis be performed every year for the first 3 years and an endoscopy at 3 years in patients with stage 2 and stage 3 colorectal carcinoma[27].
In patients with a history of colorectal cancer, PET is commonly used as a problem solving tool when there is a high index of suspicion for recurrence as evidenced by a rising CEA but when the routine diagnostic work up is equivocal. Flanagan et al. reported that FDG-PET found disease in 15 of 22 patients with elevated CEA but negative diagnostic work up. They showed a positive predictive value of 89% and a negative predictive value of 100%[30]. In a similar study, Flamen et al. report a sensitivity of 75% and a positive predictive value of 79% in a retrospective study of 50 patients[31]. However, these studies were done with stand alone PET instruments between 1993 and 1996 and between 1996 and 1999 respectively. Significant technical improvements providing hybrid images and images of a higher resolution and quality have taken place since that time.
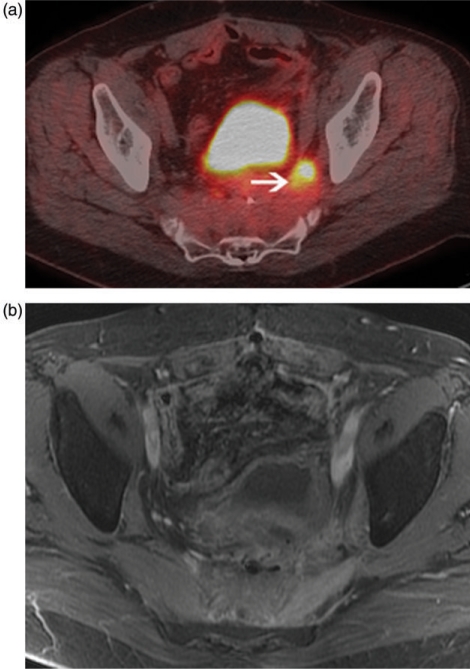
Another common application of PET in patients with recurrent disease is in surgical planning, particularly in patients who develop resectable metastases in the liver or lungs. Identification of occult metastases in such patients would avoid unnecessary surgery in many and alter management significantly. In a meta analysis, Wiering and colleagues report that FDG-PET changed clinical management in 31.6% of patients[32]. PET in pre-surgical planning decreases the number of futile surgeries and, may also lead to increased survival by allowing for better patient selection[33,34]. FDG-PET also affords some benefit in rectal cancer patients who have been treated with surgery and chemoradiotherapy with subsequent development recurrence in the pelvis. Early identification of pelvic recurrence is necessary for surgery to be of any benefit and distinguishing tumor from post-treatment fibrosis can be a challenge for conventional cross sectional imaging studies; FDG-PET can be useful in this regard (Fig. 4)[35]. In patients who are candidates for curative resection of local recurrence, FDG-PET can show other sites of disease that would avoid unnecessary surgery.
Figure 4.
Fused PET/CT image (a) shows an FDG avid area along the left pelvic sidewall (arrow) with diffuse pre-sacral thickening without a distinct mass on contrast enhanced MRI (b) in a patient with colorectal cancer treated with chemoradiation and surgery, now with rising tumor markers. Biopsy of the FDG avid area proved recurrence.
It is important to be aware of some limitations of PET. FDG-PET has less spatial resolution when compared to other cross sectional imaging modalities such as CT and MRI. Currently, the resolution of most commercially available PET scanners is in the range of 1.3 to 1.5 cm and lesions smaller than this size may not be detected because of volume averaging[36]. Overall high background hepatic activity also makes PET for assessment of small hepatic metastases difficult. Despite a few reports of relative superiority of PET in detecting hepatic metastases compared to cross sectional imaging, the lack of clear anatomic landmarks and inability to detect small lesions are major limitations with PET alone. Another limitation of FDG-PET that must be kept in mind when imaging patients with colorectal cancer is the relative insensitivity for detection of mucinous tumors likely due to the paucicellularity of these tumors[37]. The use of neoadjuvant chemotherapy before surgery can also decrease the sensitivity of PET in lesion detection[38]. Serosal metastases on the surface of the large and small bowel may still be missed, due to physiological bowel activity. Pulmonary metastases, particularly small ones, may be missed because of partial volume artifacts amplified by breathing. In addition to all of the above mentioned false negatives on FDG-PET, it is important to keep in mind that inflammation can result in false positive FDG uptake.
Conclusion
In summary, FDG-PET has been used for detection, staging and surveillance of disease in colorectal cancer patients. Physiologic activity in the gastrointestinal tract can be problematic and careful correlation with fused CT images should be performed to improve specificity. FDG-PET also provides information for staging particularly with regard to the presence of distant metastatic disease. There is insufficient data to justify the routine use of FDG-PET in detecting recurrence in patients with colorectal cancer, mainly due to the lack of large randomized trials. PET is still considered a modality with emerging applications. In areas such as post-operative surveillance of colorectal carcinoma where the surgical treatment options and chemotherapy strategies are being constantly redefined, PET CT may find additional future applications, particularly with the development of new, more specific radiotracers.
References
- [1].Jemal A, Siegel R, Ward E, et al. Cancer statistics 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- [2].Gore RM. Colorectal cancer: clinical and pathologic features. Radiol Clin North Am. 1997;35:404–29. [PubMed] [Google Scholar]
- [3].Morson BC. Evolution of cancer of the colon and rectum. Cancer. 1974;34:845–9. doi: 10.1002/1097-0142(197409)34:3+<845::aid-cncr2820340710>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- [4].Stryker SJ, Wolff BG, Culp CE, Libbe SD, Ilstrup DM, MacCarty RL. Natural history of untreated colonic polyps. Gastroenterology. 1987;93:1009–13. doi: 10.1016/0016-5085(87)90563-4. [DOI] [PubMed] [Google Scholar]
- [5].Nakada K, Brown RS, Fisher SJ, et al. Histological localization of tritiated FDG in the GI tract: an autoradiographic study. J Nucl Med. 2001;42(suppl):25. [Google Scholar]
- [6].Yasuda S, Fujii H, Nakahara T, et al. 18F-FDG-PET detection of colonic adenomas. J Nucl Med. 2001;42:989–92. [PubMed] [Google Scholar]
- [7].van Kouwen M, Nagengast F, Jansen J, Oyen W, Drenth J. 2-(18F)-Fluoro-2-deoxy-D-glucose positron emission tomography detects clinical relevant adenomas of the colon: a prospective study. J Clin Oncol. 2005;23:3713–17. doi: 10.1200/JCO.2005.02.401. [DOI] [PubMed] [Google Scholar]
- [8].Gutman F, Alberini J-L, Wartski M, et al. Incidental colonic focal lesions detected by FDG-PET/CT. AJR. 2005;185:495–500. doi: 10.2214/ajr.185.2.01850495. [DOI] [PubMed] [Google Scholar]
- [9].Gollub MJ, Akhurst T, Markowitz AJ, et al. Combined CT colonography and 18F-FDG-PET of colon polyps: potential technique for selective detection of cancer and precancerous lesions. AJR. 2007;188:130–8. doi: 10.2214/AJR.05.1458. [DOI] [PubMed] [Google Scholar]
- [10].Sauer R, Becker H, Hohenberger W, et al. Pre-operative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–40. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- [11].Balfe D, Semin M. In: Husband JES, Reznek RH, editors. Imaging in oncology. Oxford: Isis Medical Media; 1998. Colorectal cancer; pp. 129–150. [Google Scholar]
- [12].Kantorva I, Lipska L, Belohlavek O, et al. PET preoperative staging of colorectal cancer comparison with conventional staging and its impact on treatment decision making. J Nucl Med. 2003;44:1784–8. [PubMed] [Google Scholar]
- [13].Abel NH, Doerr RJ, Lamonica DM, et al. Staging of primary colorectal carcinomas with fluorine-18 fluorodexoyglucose whole-body PET: correlation with histopathologic and CT findings. Radiology. 1998;206:755–60. doi: 10.1148/radiology.206.3.9494497. [DOI] [PubMed] [Google Scholar]
- [14].Kemeny NE, Gonen M. Hepatic arterial infusion after liver resection. N Engl J Med. 2005;352:734–5. doi: 10.1056/NEJM200502173520723. [DOI] [PubMed] [Google Scholar]
- [15].Nahas CSR, Akhurst T, Yeung H, et al. Positron emission tomography detection of distant metastatic or synchronous disease in patients with locally advanced rectal cancer receiving preoperative chemoradiation. Ann Surg Oncol. 2007;15:704–11. doi: 10.1245/s10434-007-9626-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fong Y, Saldinger PF Akhurst T, et al. Utility of 18F-FDG positron emission tomography scanning on selection of patients for resection of hepatic colorectal metastases. Am J Surg. 1999;178:282–7. doi: 10.1016/s0002-9610(99)00187-7. [DOI] [PubMed] [Google Scholar]
- [17].Selzner M, Hany TF, Wildbrett P, et al. Does the novel PET/CT imaging modality impact on the treatment of patients with metastatic colorectal cancer of the liver? Ann Surg. 2004;240:1027–34. doi: 10.1097/01.sla.0000146145.69835.c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Davey K, Heriot AG, Mackay J, et al. The impact of 18-fluorodexoyglucose positron emission tomography-computed tomography on the staging and management of primary rectal cancer. Dis Colon Rectum. 2008;51:997–1003. doi: 10.1007/s10350-008-9244-1. [DOI] [PubMed] [Google Scholar]
- [19].Gearhart SL, Frassica D, Rosen R, et al. Improved staging with pretreatment positron emission tomography/computed tomography in low rectal cancer. Ann Surg Oncol. 2006;13:397–404. doi: 10.1245/ASO.2006.04.042. [DOI] [PubMed] [Google Scholar]
- [20].Guillem JG, Moore HG, Akhuurst T, et al. Sequential preoperative fluorodeoxyglucose-positron emission tomography assessment of response to preoperative chemoradiation: a means for determining long term outcomes of rectal cancer. J Am Coll Surg. 2004;199:1–7. doi: 10.1016/j.jamcollsurg.2004.02.024. [DOI] [PubMed] [Google Scholar]
- [21].Kalff V, Duong C, Drummond E, Matthews JP, Hicks RJ. Findings on 18F-FDG-PET scans after neoadjuvant chemoradiation provides prognostic stratification in patients with locally advanced rectal carcinoma subsequently treated by radical surgery. J Nucl Med. 2006;47:14–22. [PubMed] [Google Scholar]
- [22].Denecke T, Rau B, Hoffman KT, et al. Comparison of CT, MRI and FDG-PET in response prediction of patients with locally advanced rectal cancer after multimodal preoperative therapy: is there a benefit in using functional imaging? Eur Radiol. 2005;15:1648–97. doi: 10.1007/s00330-005-2658-4. [DOI] [PubMed] [Google Scholar]
- [23].Oku S, Nakagawa K, Momose T, et al. FDG-PET after radiotherapy is a good prognostic indication of rectal cancer. Ann Nucl Med. 2002;16:409–16. doi: 10.1007/BF02990079. [DOI] [PubMed] [Google Scholar]
- [24].Calvo FA, Competr M, Matute R, et al. 18F-FDG positron emission tomography staging and restaging in rectal cancer treated with preoperative chemoradiation. Int J Radiat Oncol Biol Phys. 2004;58:528–35. doi: 10.1016/j.ijrobp.2003.09.058. [DOI] [PubMed] [Google Scholar]
- [25].Kievit J. Follow-up of patients with colorectal cancer: numbers needed to test and treat. Eur J Cancer. 2002;38:986–99. doi: 10.1016/s0959-8049(02)00061-8. [DOI] [PubMed] [Google Scholar]
- [26].Chau I, Allen MJ, Cunningham D, et al. The value of routine serum carcino-embryonic antigen measurement and computed tomography in the surveillance of patients after adjuvant chemotherapy for colorectal cancer. J Clin Oncol. 2004;22:1420–9. doi: 10.1200/JCO.2004.05.041. [DOI] [PubMed] [Google Scholar]
- [27].Desch CE, Benson 3rd AB, Somerfield MR, et al. Colorectal cancer surveillance: 2005 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2005;23:8512–19. doi: 10.1200/JCO.2005.04.0063. [DOI] [PubMed] [Google Scholar]
- [28].Adam R, Avisar E, Ariche A, et al. Five-year survival following hepatic resection after neoadjuvant therapy for nonresectable colorectal. Ann Surg Oncol. 2001;8:347–53. doi: 10.1007/s10434-001-0347-3. [DOI] [PubMed] [Google Scholar]
- [29].Tournigand C, Andre T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229–37. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- [30].Flanagan FL, Dehdashti F, Ogunbiyi OA, Kodner IJ, Siegel BA. Utility of FDG-PET for investigating unexplained plasma CEA elevation in patients with colorectal cancer. Ann Surg. 1998;227:319–23. doi: 10.1097/00000658-199803000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Flamen P, Hoekstra OS, Homans F, et al. Unexplained rising carcinoembryonic antigen (CEA) in the postoperative surveillance of colorectal cancer: the utility of positron emission tomography (PET) Eur J Cancer. 2001;37:862–9. doi: 10.1016/s0959-8049(01)00049-1. [DOI] [PubMed] [Google Scholar]
- [32].Wiering B, Krabbe PF, Jager GJ, Oyen WJ, Ruers TJ. The impact of fluor-18-deoxyglucose-positron emission tomography in the management of colorectal liver metastases. Cancer. 2005;104:2658–70. doi: 10.1002/cncr.21569. [DOI] [PubMed] [Google Scholar]
- [33].Strasberg SM, Dehdashti F, Siegel BA, Drebin JA, Linehan D. Survival of patients evaluated by FDG-PET before hepatic resection for metastatic colorectal carcinoma: a prospective database study. Ann Surg. 2001;233:293–9. doi: 10.1097/00000658-200103000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wiering B, Krabbe PF, Dekker HM, Oyen WJ, Ruers TJ. The role of FDG-PET in the selection of patients with colorectal liver metastases. Ann Surg Oncol. 2007;14:771–9. doi: 10.1245/s10434-006-9013-0. [DOI] [PubMed] [Google Scholar]
- [35].Ito K, Kato T, Tadokoro M, et al. Recurrent rectal cancer and scar: differentiation with PET and MR imaging. Radiology. 1992;182:549–52. doi: 10.1148/radiology.182.2.1732979. [DOI] [PubMed] [Google Scholar]
- [36].Mawlawi O, Podoloff DA, Kohlmyer S, et al. Performance characteristics of a newly developed PET/CT scanner using NEMA standards in 2D and 3D modes. J Nucl Med. 2004;45:1734–42. [PubMed] [Google Scholar]
- [37].Berger L, Nicholson SA, Dehadashti F, et al. FDG-PET evaluation of mucinous neoplasms: correlation of FDG uptake with histopathologic features. Am J. Roengenol. 2000;174:1005–8. doi: 10.2214/ajr.174.4.1741005. [DOI] [PubMed] [Google Scholar]
- [38].Lubezky N, Metser U, Geva R, et al. The role and limitations of 18-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) scan and computerized tomography (CT) in restaging patients with hepatic colorectal metastases following neoadjuvant chemotherapy: comparison with operative and pathological findings. J Gastrointest Surg. 2007;11:472–8. doi: 10.1007/s11605-006-0032-8. [DOI] [PMC free article] [PubMed] [Google Scholar]