Abstract
A correct histological diagnosis, careful staging and detection of tumour response to treatment are all crucial in the management of sarcomas. Imaging is important in all of these stages. Sarcomas have distinct biological and treatment-related features posing challenges for imaging. For example, size measurements may not adequately reflect response rates. Techniques which can measure tissue function rather than generate merely anatomical data such as positron emission tomography (PET) are rapidly gaining interest. We discuss the importance of imaging in different stages of patient management, emphasising the unique characteristics of sarcoma. Furthermore, we discuss the potential of PET for the various indications, focussing on therapy evaluation.
Keywords: Diagnostic imaging, radionuclide imaging, positron emission tomography, sarcoma, gastrointestinal stromal tumors, therapeutics
Introduction
Sarcomas are a rare and heterogeneous group of mesenchymal-derived tumours with distinct molecular features. They are subclassified into bone and soft-tissue sarcoma (STS). The latter group consists of over 50 subtypes including gastro-intestinal stromal tumour (GIST)[1]. In STS, treatment consists of surgery and in selected subtypes or stages of disease also of radiotherapy and/or chemotherapy. In bone sarcoma, such as Ewing's sarcoma and osteosarcoma, treatment always includes (neo-)adjuvant chemotherapy. Targeting underlying molecular events may provide spectacular benefits, as demonstrated in GIST and dermatofibrosarcoma protuberans (DFSP)2–5. Prognosis depends on the extent of the disease, requiring optimal staging, and the possibility for radical resection of the primary tumour. Prognosis drops dramatically once the sarcoma is metastasised or worsens, in case of Ewing's sarcoma and osteosarcoma, if the histological response to neoadjuvant chemotherapy is limited. Early, adequate therapy evaluation prevents prolonged exposure to toxic yet ultimately unsuccessful treatment, which in some cases may be substituted by an alternative, more effective one. An ideal evaluation method should provide information in an objective and reproducible fashion. At present, especially since the introduction of targeted therapies, the call for functional rather than mere anatomical imaging is increasing.
Diagnosis, grading and staging
Histological classification is a crucial first step in suspected sarcomas since tumour type and grade have major impact on prognosis and management. The heterogeneity of sarcomas poses the risk for sampling error from a single biopsy while repeated biopsy risks tumour spread. Currently, imaging of the primary tumour is mainly performed by magnetic resonance imaging (MRI) or computed tomography (CT), depending on the tumour localisation. Both modalities provide important anatomical information and have also been used to assess tissue composition6–8 to select the biopsy site most likely to have the highest grade present. Furthermore, local tissue reaction and invasion may give an impression of the malignancy grade, which helps avoiding false reassurance in non-representative biopsy. Staging is also crucial, as the mainstay of therapy is radical surgery. In peripheral sarcomas limb-sparing surgery is largely facilitated as MRI enables assessment of the anatomical extension of the tumour, as well as its relationship with the neurovascular bundle and other adjacent structures. Still, large and disabling surgical interventions are no exception. This is acceptable in the setting of localised sarcoma but not in metastasised disease. CT is the imaging technique of choice to detect pulmonary metastasis and [99mTc]polyphosphate bone scintigraphy is useful to stage sarcomas allowing whole-body screening for bone metastases[9] and – in bone-forming sarcomas – soft-tissue metastases[10]. More recently, whole-body MRI was found more sensitive than bone scanning in the detection of osseous metastases from Ewing's sarcoma[11]. The basis for these findings is the intramedullary accumulation of tumour cells replacing the normal marrow before reactive osteoblastic response occurs. MRI directly reveals neoplastic bone marrow infiltrates[11].
Response evaluation and restaging
Available imaging modalities for therapy evaluation are essentially the same as in staging and grading[12,13] and the choice is guided by tumour or metastasis localisation. Bone scintigraphy, however, is not sufficiently specific for assessment of response[14,15]. Much work has been done to standardise the interpretation of radiological evaluation methods on the basis of size. This resulted in the World Health Organization (WHO) criteria[16–18], later replaced by the simplified European Organisation for Research and Treatment of Cancer Response Evaluation Criteria in Solid Tumours (EORTC/RECIST)[19–21]. These criteria have imperfections, both in general and specifically for sarcoma. First, they were originally based on the change in tumour size which could reliably be detected by palpation[22]. Therefore marked size reduction is required before a tumour is considered responsive to therapy. Although much smaller changes can now be detected, these RECIST criteria are still adhered to because of a supposed relationship between tumour size reduction and clinical benefit. The inter- and intratumoural heterogeneity and the rareness of sarcomas have prevented a reliable scientific foundation of the relationship between size and effects, although there are several reasons to question whether such a relationship exists.
First, sarcomas differ from other tumours as they contain large volumes of non-malignant cells and other stromal materials, which maintain a certain size even if all malignant components disappear23–25. Also, with the disappearance of tumour cells, rather than shrinkage, replacement with fibrous materials or calcification can be seen[26]. In bone sarcomas, surrounding bone has limited capacity to return to its normal size (Fig. 2)[12]. In GIST, progression under treatment may present as a nodule within a cystic mass, instead of mass enlargement[27]. Finally, the development of targeted tyrosine kinase inhibitors and antibodies[28] needs consideration. These treatments are cytostatic rather than cytoreductive and hence cause consolidation rather than reduction of tumour size.
Figure 2.
(a) A patient with Ewing's sarcoma of the left femur. Top, PET; middle, low-dose CT; bottom, fused images. After two cycles of polychemotherapy PET showed (good) partial metabolic response while MRI showed stable disease according to RECIST (−16%). The resection sample showed only microscopic residue of viable tumour (> 90% necrosis) and therefore correlated well with PET. (b) Axial contrast-enhanced spin echo T1-weighted MRI image with fat-saturation acquired on the same day.
In summary, the large changes in size required by WHO and RECIST criteria may be too stringent for the detection of progression or response in sarcoma. Furthermore, size reduction takes time. Thus, identification of tumour response – or lack thereof – may require several weeks or months. This time delay causes unnecessary costs and toxicity and may prevent a timely switch to alternative treatments. Moreover, the absence of progression of disease is increasingly considered as a relevant endpoint for clinical trials, replacing response rate[5,29].
To overcome the possibility that size does not represent tumour viability, histological evaluation can be performed. Histological evaluation for therapy-related changes, including the percentage of necrosis, provides adequate insight into the response to previous therapy and correlates well with prognosis. Limitations are that standardised approaches are only available for osteosarcoma and Ewing's sarcoma[30]. Heterogeneity of the tumour might again hamper a representative and reliable histological assessment based on small biopsies.
New developments
Improvements in anatomical imaging and interpretation
The above-mentioned restrictions of anatomical imaging during therapy are not only challenging for existing criteria, they are also demanding for the development of new ones. The appearance of a nodule within a mass has been proposed as a sign of recurrent GIST[27]. Choi and colleagues have shown that a 10% tumour size decrease or 15% tumour density decrease as determined by measuring the attenuation coefficient are sensitive and specific methods for assessing targeted therapy response in patients with GIST (the so-called ‘Choi criteria’) (Fig. 1)[31,32]. Dynamic contrast-enhanced MRI (DCE-MRI) is a technique sensitive to alterations in vascular permeability and blood flow. It has been reported as a sensitive imaging method for the evaluation of response to chemotherapy and it might help in differentiating viable tumour from vascularised granulation tissue[13,33]. It is, however, not widely used in clinical practice, as it is labour intensive and technically challenging[34]. These approaches incorporate an increasingly popular concept of imaging; i.e. it is not (only) size that matters, but more so the underlying tissue function, cell biology, physiology and biochemistry.
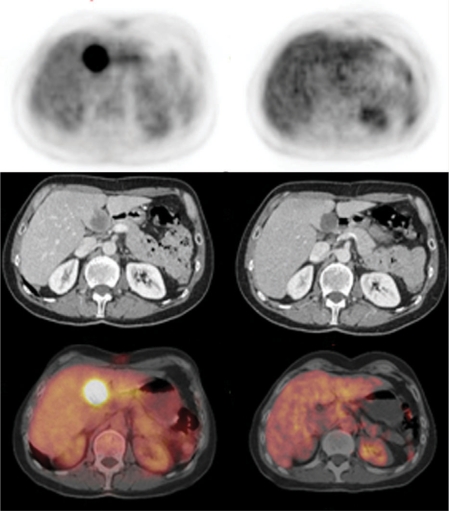
Figure 1.
Patient with liver metastasis from gastric GIST. Top, PET; middle, contrast-enhanced diagnostic CT; bottom, fused images. After 18 days of imatinib there was a complete metabolic response on PET, while CT showed stable disease according to RECIST (−15%) and partial response according to Choi et al.[32].
Positron emission tomography
Positron emission tomography (PET) is a technique with large potential because of its ability to image biological characteristics based on the differential utilisation of various substrates by cancer cells and normal tissue. Numerous PET radiopharmaceuticals are available, but the most widely used agent is [18F]fluorodeoxyglucose (FDG). This lead position is in part due to its approval as a tracer by the US Food and Drug Administration (FDA) for routine clinical use[35], its early development, and wide availability[36]. There is a clear rationale for its use. Mammalian cells depend on glucose as a major source of energy and of carbons. Glucose is transported into the cell via facilitative transporters (GLUT) present in all cell types. Many GLUT isoforms exist with tissue-specific expression, subject to environmental control (e.g. hypoxia)[37]. After membrane transport, glucose is phosphorylated by hexokinases to glucose 6-phosphate and is further metabolised in the glycolysis pathway. Increased glucose utilisation of malignant cells has been recognised for decades. Like glucose, FDG is transported into the cell cytoplasm where it is phosphorylated and becomes trapped inside the cell as dephosporylation hardly occurs.
Another well-characterised radiopharmaceutical is [18F]fluorodeoxythymidine (FLT)38–40. FLT is a pyrimidine analog that utilises the salvage pathway of DNA synthesis. Much like FDG, it is taken up through facilitated transport and diffusion and phosphorylated by a cell cycle-regulated enzyme, thymidine kinase 1 (TK1) and then becomes trapped in the cell. TK1 activity is higher in malignant cells than in normal cells; therefore, the uptake of FLT is a reflection of proliferative activity[41]. Recent data indicate that the sensitivity to detect most tumour types is lower than that of FDG PET. However, specificity could be higher since FLT does not accumulate in inflammatory cells[42], although the latter is not undisputed[43].
PET scans can be evaluated both qualitatively (visual assessment) or (semi)quantitatively[44]. Qualitative assessment is more practical for clinical use, but obviously less objective. Semiquantitative measurements of maximum standardised uptake value (SUV), average SUV and tumour-to-background ratio (TBR) have all been used. In recent years a gradual replacement of PET scans with hybrid PET-CT scanners has occurred.
PET in the various stages of imaging in sarcoma
Grading and staging
In theory, increased FDG uptake represents a metabolically active site45–47. Thus, PET scanning could aid biopsy guidance and should discriminate between sarcoma and benign conditions. However, non-malignant processes like inflammation and areas with variable physiologic FDG turnover such as brown fat and muscle may interfere with image analysis[48]. The inherently limited spatial resolution of PET compared to anatomical imaging has been largely solved by using hybrid PET-CT technology. Still, sensitivity for pulmonary and intrahepatic lesions may be relatively limited49–51.
To date, only one meta-analysis has been performed[51], indicating that FDG-PET can indeed discriminate between sarcomas and benign tumours and low and high grade sarcomas, although the methodological quality of the studies included was generally poor. Thus, there is an urgent need for further evidence to support the routine clinical use of FDG-PET for diagnosing and staging sarcomas[52].
Treatment evaluation
The same principles that make FDG PET an interesting new option in the diagnostic phase apply to evaluation during treatment. Particularly, PET allows quantification of tumour viability or proliferation. When listing and evaluating studies that have investigated the value of PET or PET/CT for therapy evaluation in sarcoma, it becomes clear that there is no generally accepted definition for a metabolic response in sarcoma on FDG-PET. Preliminary criteria have been published by the EORTC/RECIST group[53] and the National Cancer Institute[54], but the imaging protocols, measures of activity and definitions of response have varied and study size has been modest.
Bone sarcomas
Schulte et al.[55], Franzius et al.[56] and Nair et al.[57] used TBR to determine metabolic response to neoadjuvant chemotherapy in a total of 64 patients. In the first two studies, the decrease of FDG uptake after chemotherapy correlated well with histological response. Nair et al. found that tumour necrosis was accurately predicted on PET scan in 15/16 patients by visual assessment, 14/15 patients by TBR value on presurgery scans, and 7/15 patients using percent change of TBR on serial scans. Hawkins et al. measured changes in the tumour SUVmax in 69 patients and found a correlation with both post-chemotherapy SUVmax and post- to pre-treatment SUVmax ratio with histological response[58,59]. Iagaru implemented the RECIST criteria for PET analysis in a heterogeneous group of bone and soft tissue sarcoma patients in a protocol including both PET and PET/CT imaging. The pathological degree of necrosis after chemotherapy was concordant with PET in 57.1% of cases; the observed discrepancies were attributed to chemotherapy-induced inflammation. Inflammation can be induced by radiotherapy and certain cytotoxic agents60–62 sometimes presenting as the so-called fibrous pseudocapsule with inflammatory tissue that can form around the tumour[63]. Uptake in the latter has been attributed to passive accumulation of FDG as a result of altered cell membrane permeability in the initial phase of irreversible cell death[64].
For the detection of recurrent disease, Arush et al.[65] and Gerth et al.[66] recently studied a total of 72 patients including many Ewing's sarcoma cases. The first study confirms the high accuracy of FDG-PET/CT in the diagnosis of local relapse of sarcoma, while it failed in the detection of metastases in three patients. Gerth et al. compared PET and PET/CT and found that the sensitivity, specificity, and accuracy of single-modality PET were 71%, 95%, and 88%, respectively; the corresponding values for the hybrid PET/CT technique were 87%, 97%, and 94% (P < 0.0001). PET/CT thus was significantly more accurate than PET alone for the detection and localisation of lesions.
Soft-tissue sarcomas
In this group of tumours, PET has been used to evaluate not only chemotherapy effects, but also the effect of isolated limb perfusion (ILP), a technique with the possibility of local high-dosed therapy to facilitate limb-sparing surgery. For ILP, Nieweg and colleagues first reported a case of liposarcoma in which PET suggested complete response which was later confirmed by histological examination[67]. In larger groups of STS patients, it was shown by Van Ginkel et al. that based on the pre-treatment glucose consumption in soft-tissue sarcomas, one could predict the probability of a patient achieving complete response after ILP, although uptake in inflammatory tissue hampered the evaluation[68]. To overcome this, PET with l-[11C]tyrosine was used by the same group[69]. They were able to predict histological response by post-treatment uptake rates and inflammatory tissue did not interfere with viable tumour. More recently, a study from this group investigated the possibilities of FLT by PET/CT. Interestingly, uptake was correlated with the mitotic index of the tumours (r = 0.82 and P = 0.004 for SUVmax; r = 0.87 and P = 0.001 for SUVmean). After HILP, the uptake of FLT decreased significantly (P = 0.008 for SUVmax and P = 0.002 for SUVmean). Tumours with initially high FLT uptake showed a better response to HILP (r = 0.64, P < 0.05)[41].
In the evaluation of chemotherapy, Jones et al. described a homogeneously decreased FDG uptake throughout the tumour in responsive cases. Again, despite complete necrosis, persistent tumour FDG uptake was observed in fibrous pseudocapsules[63]. Shields describes two patients who underwent chemotherapy; in the responding patient a decrease of FDG uptake of 40% was seen while in the non-responding case uptake increased by 69%. Change in [11C]thymidine incorporation was more marked in the responder but remained stable in the non-responding case[70]. Changes in tumour SUVmax predicted outcomes in 46 patients with localised extremity STS by PET scanning in a study by Schuetze et al.[36]. Not only was a change in SUVmax >40% correlated with the amount of residual viable tumour, also multivariate analysis found a correlation between lack of response and increased risk of disease recurrence, metastasis and death. Peng et al. confirmed the association between permanent uptake and rapid relapse versus decreased uptake and favourable response in rhabdomyosarcoma[71], as did Kasper et al., by demonstrating a significant difference in the progression-free survival for patients with a decrease in the standardised uptake value in comparison with patients with an increased or stable SUV[72].
Hybrid PET/CT scanning has been performed in the reports by Evilevitch et al. and Park et al.[35,73]. In the Evilevitch study, FDG-PET was significantly more accurate than size-based criteria (RECIST) at assessing response to neoadjuvant therapy, correctly identifying all of the responders and 71% of the non-responders while only 25% of responding tumours were identified by size-based criteria. Moreover, threshold values ranging from 50% to 70% of baseline FDG uptake allowed assessment of response, thereby limiting the effect of remaining uptake in inflammatory lesions. Park et al. report that PET or PET/CT was highly effective in discriminating true recurrence in patients with suspected recurrence and was highly sensitive in detecting recurrence in asymptomatic patients.
Gastro-intestinal stromal tumours
The separate consideration of GIST from STS appears rather artificial, but there are some distinct features to FDG-PET in GIST. Comparability is better because the same tumour under the same treatment regimen is studied and adherence to standardised response criteria[53] has been rather strict. Also, directly from the first availability of imatinib for GIST treatment, it was shown that FDG-PET seems to predict response very early (Fig. 1)[23,24,74] and therefore it has been incorporated in a relatively large amount of study protocols, the larger of which we have listed in Table 1. Overall results of these studies have been that the main limitations are the occasional lack of pre-treatment FDG avidity[31,75] and the lower sensitivity for pulmonary and hepatic lesions[76,77]. Still, all agree that PET scanning is a sensitive and rapid indicator of response preceding size-based response by weeks or months[23,74,76–79]. Furthermore, response on PET scans is closely related to clinical symptom relief[74] and predicts clinical outcome[32,75,79].
Table 1.
Studies evaluating PET in GIST therapy evaluation
| First author | n | Histology | Daily imatinib dose (mg) | Modality for comparison | First follow-up PET | PET modality and interpretation method |
|---|---|---|---|---|---|---|
| Van Oosterom[74] | 17 | GIST | Various | CT | 8 days | PET; EORTC |
| Demetri[23] | 64 | GIST | 400/600 | CT or MRI | 24 hours | PET; NS |
| Stroobants[80] | 21 | GIST/STS | Various | CT | 8 days | PET; EORTC |
| Gayed[78] | 54 | GIST | NS | CT | 2 months | PET; EORTC |
| Antoch[76] | 20 | GIST | 400/600/800 | ‘All information after 6 months’ | 1 month | PET, PET/CT; EORTC |
| Jager[79] | 16 | GIST/STS | NS | CT, PFS | 1 week | PET; SUVmax decrease |
| Choi[31] | 29 | GIST | NS | CT; size and density | 2 months | PET; modified EORTC |
| Goldstein (abstract)[77] | 18 | GIST | 400/800 | CT, outcome | Unknown | PET; unknown |
| Goerres[75] | 28 | GIST | 400/800 | CT | Median 19 days | PET, PET/CT; EORTC |
| Choi[32] | 109 | GIST | 400/800 | PET | 2 months | CT, size and density; SUVmax decrease |
NS, not specified; PFS, progression-free survival; SUV, standardised uptake value, EORTC, according to the criteria published by Young et al.[53].
FDG-PET in GIST exceeds the role of a staging and re-staging modality. Different mutations with different therapeutic impact can exist synchronously in a patient[81]. Furthermore, a change in micro-environment can result in replacement of tumour cells with drug-resistant variant cells[27,82,83]. In this context, FDG-PET-evaluation can be used to select only progressive lesions for resection in patients with ongoing response of the remainder of the metastases[84], an approach that is still controversial versus the generally accepted surgical approaches in oncology.
Conclusion
Sarcomas have unique properties which not only increase demands on imaging but also pose specific problems. In the phase of diagnosis and staging, anatomical imaging techniques such as MRI for local tumour characterisation and CT for detection of pulmonary metastasis remain indispensable and reliable techniques. During treatment, the limitations of these techniques and their size-based evaluation in sarcoma become clearer. Although studies on the value of PET are of limited size and quality, PET is a promising modality especially for treatment evaluation in sarcoma, providing a rapid and reliable indication of response. The possibility to non-invasively detect tumour progression has already influenced clinical practice in GIST in a revolutionary way, with clear impact on patient management. PET scanning has inherent limitations which fortunately do not entirely overlap with those of anatomical imaging. Therefore, these techniques should be regarded as complementary. The studies comparing PET versus PET/CT underscore this statement. For future studies, the availability of objective criteria for response evaluation with PET would be highly instrumental to implement PET in a cost effective way for patient tailored sarcoma treatment.
References
- [1].de Alava E. Molecular pathology in sarcomas. Clin Transl Oncol. 2007;9:130–44. doi: 10.1007/s12094-007-0027-2. [DOI] [PubMed] [Google Scholar]
- [2].Wunder JS, Nielsen TO, Maki RG, O'Sullivan B, Alman BA. Opportunities for improving the therapeutic ratio for patients with sarcoma. Lancet Oncol. 2007;8:513–24. doi: 10.1016/S1470-2045(07)70169-9. [DOI] [PubMed] [Google Scholar]
- [3].Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–80. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- [4].Verweij J, van Oosterom A, Blay JY, et al. Imatinib mesylate (STI-571 Glivec, Gleevec) is an active agent for gastrointestinal stromal tumours, but does not yield responses in other soft-tissue sarcomas that are unselected for a molecular target. Results from an EORTC Soft Tissue and Bone Sarcoma Group phase II study. Eur J Cancer. 2003;39:2006–11. [PubMed] [Google Scholar]
- [5].Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet. 2004;364:1127–34. doi: 10.1016/S0140-6736(04)17098-0. [DOI] [PubMed] [Google Scholar]
- [6].Alyas F, James SL, Davies AM, Saifuddin A. The role of MR imaging in the diagnostic characterisation of appendicular bone tumours and tumour-like conditions. Eur Radiol. 2007;17:2675–86. doi: 10.1007/s00330-007-0597-y. [DOI] [PubMed] [Google Scholar]
- [7].Vilanova JC, Woertler K, Narvaez JA, et al. Soft-tissue tumors update: MR imaging features according to the WHO classification. Eur Radiol. 2007;17:125–38. doi: 10.1007/s00330-005-0130-0. [DOI] [PubMed] [Google Scholar]
- [8].Yu RS, Chen Y, Jiang B, Wang LH, Xu XF. Primary hepatic sarcomas: CT findings. Eur Radiol. 2008 doi: 10.1007/s00330-008-0997-7. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- [9].Hicks RJ. Functional imaging techniques for evaluation of sarcomas. Cancer Imaging. 2005;5:58–65. doi: 10.1102/1470-7330.2005.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ghaed N, Thrall JH, Pinsky SM, Johnson MC. Detection of extraosseous metastases from osteosarcoma with 99mTc-polyphosphate bone scanning. Radiology. 1974;112:373–5. doi: 10.1148/112.2.373. [DOI] [PubMed] [Google Scholar]
- [11].Daldrup-Link HE, Franzius C, Link TM, et al. Whole-body MR imaging for detection of bone metastases in children and young adults: comparison with skeletal scintigraphy and FDG PET. AJR Am J Roentgenol. 2001;177:229–36. doi: 10.2214/ajr.177.1.1770229. [DOI] [PubMed] [Google Scholar]
- [12].Suzuki C, Jacobsson H, Hatschek T, et al. Radiologic measurements of tumor response to treatment: practical approaches and limitations. Radiographics. 2008;28:329–44. doi: 10.1148/rg.282075068. [DOI] [PubMed] [Google Scholar]
- [13].van der Woude HJ, Bloem JL, Hogendoorn PC. Preoperative evaluation and monitoring chemotherapy in patients with high-grade osteogenic and Ewing's sarcoma: review of current imaging modalities. Skeletal Radiol. 1998;27:57–71. doi: 10.1007/s002560050339. [DOI] [PubMed] [Google Scholar]
- [14].Brisse H, Ollivier L, Edeline V, et al. Imaging of malignant tumours of the long bones in children: monitoring response to neoadjuvant chemotherapy and preoperative assessment. Pediatr Radiol. 2004;34:595–605. doi: 10.1007/s00247-004-1192-x. [DOI] [PubMed] [Google Scholar]
- [15].Bloem JL, Taminiau AH, Eulderink F, Hermans J, Pauwels EK. Radiologic staging of primary bone sarcoma: MR imaging, scintigraphy, angiography, and CT correlated with pathologic examination. Radiology. 1988;169:805–10. doi: 10.1148/radiology.169.3.3055041. [DOI] [PubMed] [Google Scholar]
- [16].Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207–14. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- [17].Van Glabbeke M, van Oosterom AT, Steward W, Verweij J, Mouridsen H. Selection of large and objectively measurable target lesions in EORTC phase II trials: impact on recruitment and response rate. EORTC Soft Tissue and Bone Sarcoma Group (STBSG) Eur J Cancer. 1993;29A:1943–7. doi: 10.1016/0959-8049(93)90449-p. [DOI] [PubMed] [Google Scholar]
- [18].Van Glabbeke M, van Oosterom AT, Oosterhuis JW, et al. Prognostic factors for the outcome of chemotherapy in advanced soft tissue sarcoma: an analysis of 2185 patients treated with anthracycline-containing first-line regimens – a European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J Clin Oncol. 1999;17:150–7. doi: 10.1200/JCO.1999.17.1.150. [DOI] [PubMed] [Google Scholar]
- [19].Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- [20].Therasse P, Le Cesne A, Van Glabbeke M, Verweij J, Judson I. RECIST vs. WHO: prospective comparison of response criteria in an EORTC phase II clinical trial investigating ET-743 in advanced soft tissue sarcoma. Eur J Cancer. 2005;41:1426–30. doi: 10.1016/j.ejca.2005.04.005. [DOI] [PubMed] [Google Scholar]
- [21].Therasse P, Eisenhauer EA, Verweij J. RECIST revisited: a review of validation studies on tumour assessment. Eur J Cancer. 2006;42:1031–9. doi: 10.1016/j.ejca.2006.01.026. [DOI] [PubMed] [Google Scholar]
- [22].Moertel CG, Hanley JA. The effect of measuring error on the results of therapeutic trials in advanced cancer. Cancer. 1976;38:388–94. doi: 10.1002/1097-0142(197607)38:1<388::aid-cncr2820380156>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- [23].Demetri GD, Von Mehren M., Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–80. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- [24].Joensuu H, Roberts PJ, Sarlomo-Rikala M, et al. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med. 2001;344:1052–6. doi: 10.1056/NEJM200104053441404. [DOI] [PubMed] [Google Scholar]
- [25].Einarsdottir H, Wejde J, Bauer HC. Pre-operative radiotherapy in soft tissue tumors. Assessment of response by static post-contrast MR imaging compared to histopathology. Acta Radiol. 2001;42:1–5. [PubMed] [Google Scholar]
- [26].van der Woude HJ, Bloem JL, Holscher HC, et al. Monitoring the effect of chemotherapy in Ewing's sarcoma of bone with MR imaging. Skeletal Radiol. 1994;23:493–500. doi: 10.1007/BF00223076. [DOI] [PubMed] [Google Scholar]
- [27].Shankar S, van Sonnenberg E, Desai J, Dipiro PJ, Van Den Abbeele A, Demetri GD. Gastrointestinal stromal tumor: new nodule-within-a-mass pattern of recurrence after partial response to imatinib mesylate. Radiology. 2005;235:892–8. doi: 10.1148/radiol.2353040332. [DOI] [PubMed] [Google Scholar]
- [28].Shor AC, Agresta SV, D'Amato GZ, Sondak VK. Therapeutic potential of directed tyrosine kinase inhibitor therapy in sarcomas. Cancer Control. 2008;15:47–54. doi: 10.1177/107327480801500106. [DOI] [PubMed] [Google Scholar]
- [29].Van Glabbeke M, Verweij J, Judson I, Nielsen OS. Progression-free rate as the principal end-point for phase II trials in soft-tissue sarcomas. Eur J Cancer. 2002;38:543–9. doi: 10.1016/s0959-8049(01)00398-7. [DOI] [PubMed] [Google Scholar]
- [30].Coffin CM, Lowichik A, Zhou H. Treatment effects in pediatric soft tissue and bone tumors: practical considerations for the pathologist. Am J Clin Pathol. 2005;123:75–90. doi: 10.1309/h0d4vd760nh6n1r6. [DOI] [PubMed] [Google Scholar]
- [31].Choi H, Charnsangavej C, de Castro FS, et al. CT evaluation of the response of gastrointestinal stromal tumors after imatinib mesylate treatment: a quantitative analysis correlated with FDG PET findings. AJR Am J Roentgenol. 2004;183:1619–28. doi: 10.2214/ajr.183.6.01831619. [DOI] [PubMed] [Google Scholar]
- [32].Choi H, Charnsangavej C, Faria SC, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007;25:1753–9. doi: 10.1200/JCO.2006.07.3049. [DOI] [PubMed] [Google Scholar]
- [33].Dyke JP, Panicek DM, Healey JH, et al. Osteogenic and Ewing sarcomas: estimation of necrotic fraction during induction chemotherapy with dynamic contrast-enhanced MR imaging. Radiology. 2003;228:271–8. doi: 10.1148/radiol.2281011651. [DOI] [PubMed] [Google Scholar]
- [34].Mar WA, Taljanovic MS, Bagatell R, et al. Update on imaging and treatment of Ewing sarcoma family tumors: what the radiologist needs to know. J Comput Assist Tomogr. 2008;32:108–18. doi: 10.1097/RCT.0b013e31805c030f. [DOI] [PubMed] [Google Scholar]
- [35].Park JY, Kim EN, Kim DY, et al. Role of PET or PET/CT in the post-therapy surveillance of uterine sarcoma. Gynecol Oncol. 2008;109:255–62. doi: 10.1016/j.ygyno.2008.01.030. [DOI] [PubMed] [Google Scholar]
- [36].Schuetze SM, Rubin BP, Vernon C, et al. Use of positron emission tomography in localized extremity soft tissue sarcoma treated with neoadjuvant chemotherapy. Cancer. 2005;103:339–48. doi: 10.1002/cncr.20769. [DOI] [PubMed] [Google Scholar]
- [37].Medina RA, Owen GI. Glucose transporters: expression, regulation and cancer. Biol Res. 2002;35:9–26. doi: 10.4067/s0716-97602002000100004. [DOI] [PubMed] [Google Scholar]
- [38].Been LB, Suurmeijer AJ, Cobben DC, Jager PL, Hoekstra HJ, Elsinga PH. [18F]FLT-PET in oncology: current status and opportunities. Eur J Nucl Med Mol Imaging. 2004;31:1659–72. doi: 10.1007/s00259-004-1687-6. [DOI] [PubMed] [Google Scholar]
- [39].Cobben DC, Elsinga PH, Hoekstra HJ, et al. Is 18F-3′-fluoro-3′-deoxy-l-thymidine useful for the staging and restaging of non-small cell lung cancer? J Nucl Med. 2004;45:1677–82. [PubMed] [Google Scholar]
- [40].Cobben DC, van der Laan BF, Maas B, et al. 18F-FLT PET for visualization of laryngeal cancer: comparison with 18F-FDG PET. J Nucl Med. 2004;45:226–31. [PubMed] [Google Scholar]
- [41].Been LB, Suurmeijer AJ, Elsinga PH, Jager PL, van Ginkel RJ, Hoekstra HJ. 18F-fluorodeoxythymidine PET for evaluating the response to hyperthermic isolated limb perfusion for locally advanced soft-tissue sarcomas. J Nucl Med. 2007;48:367–72. [PubMed] [Google Scholar]
- [42].van Waarde A, Cobben DC, Suurmeijer AJ, et al. Selectivity of 18F-FLT and 18F-FDG for differentiating tumor from inflammation in a rodent model. J Nucl Med. 2004;45:695–700. [PubMed] [Google Scholar]
- [43].Dimitrakopoulou-Strauss A, Strauss LG. The role of 18F-FLT in cancer imaging: does it really reflect proliferation? Eur J Nucl Med Mol Imaging. 2008;35:523–6. doi: 10.1007/s00259-007-0679-8. [DOI] [PubMed] [Google Scholar]
- [44].Aoki J, Endo K, Watanabe H, et al. FDG-PET for evaluating musculoskeletal tumors: a review. J Orthop Sci. 2003;8:435–41. doi: 10.1007/s10776-001-0539-6. [DOI] [PubMed] [Google Scholar]
- [45].Folpe AL, Lyles RH, Sprouse JT, Conrad EU, III, Eary JF. (F-18) fluorodeoxyglucose positron emission tomography as a predictor of pathologic grade and other prognostic variables in bone and soft tissue sarcoma. Clin Cancer Res. 2000;6:1279–87. [PubMed] [Google Scholar]
- [46].Ioannidis JP, Lau J. 18F-FDG PET for the diagnosis and grading of soft-tissue sarcoma: a meta-analysis. J Nucl Med. 2003;44:717–24. [PubMed] [Google Scholar]
- [47].Tateishi U, Yamaguchi U, Seki K, Terauchi T, Arai Y, Hasegawa T. Glut-1 expression and enhanced glucose metabolism are associated with tumour grade in bone and soft tissue sarcomas: a prospective evaluation by [18F]fluorodeoxyglucose positron emission tomography. Eur J Nucl Med Mol Imaging. 2006;33:683–91. doi: 10.1007/s00259-005-0044-8. [DOI] [PubMed] [Google Scholar]
- [48].Rosenbaum SJ, Stergar H, Antoch G, Veit P, Bockisch A, Kuhl H. Staging and follow-up of gastrointestinal tumors with PET/CT. Abdom Imaging. 2006;31:25–35. doi: 10.1007/s00261-005-0031-3. [DOI] [PubMed] [Google Scholar]
- [49].Franzius C, Daldrup-Link HE, Wagner-Bohn A, et al. FDG-PET for detection of recurrences from malignant primary bone tumors: comparison with conventional imaging. Ann Oncol. 2002;13:157–60. doi: 10.1093/annonc/mdf012. [DOI] [PubMed] [Google Scholar]
- [50].Gould MK, Maclean CC, Kuschner WG, Rydzak CE, Owens DK. Accuracy of positron emission tomography for diagnosis of pulmonary nodules and mass lesions: a meta-analysis. JAMA. 2001;285:914–24. doi: 10.1001/jama.285.7.914. [DOI] [PubMed] [Google Scholar]
- [51].Bastiaannet E, Groen H, Jager PL, et al. The value of FDG-PET in the detection, grading and response to therapy of soft tissue and bone sarcomas; a systematic review and meta-analysis. Cancer Treat Rev. 2004;30:83–101. doi: 10.1016/j.ctrv.2003.07.004. [DOI] [PubMed] [Google Scholar]
- [52].Fletcher JW, Djulbegovic B, Soares HP, et al. Recommendations on the use of 18F-FDG PET in oncology. J Nucl Med. 2008;49:480–508. doi: 10.2967/jnumed.107.047787. [DOI] [PubMed] [Google Scholar]
- [53].Young H, Baum R, Cremerius U, et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer. 1999;35:1773–82. doi: 10.1016/s0959-8049(99)00229-4. [DOI] [PubMed] [Google Scholar]
- [54].Shankar LK, Hoffman JM, Bacharach S, et al. Consensus recommendations for the use of 18F-FDG PET as an indicator of therapeutic response in patients in National Cancer Institute Trials. J Nucl Med. 2006;47:1059–66. [PubMed] [Google Scholar]
- [55].Schulte M, Brecht-Krauss D, Werner M, et al. Evaluation of neoadjuvant therapy response of osteogenic sarcoma using FDG PET. J Nucl Med. 1999;40:1637–43. [PubMed] [Google Scholar]
- [56].Franzius C, Sciuk J, Brinkschmidt C, Jurgens H, Schober O. Evaluation of chemotherapy response in primary bone tumors with F-18 FDG positron emission tomography compared with histologically assessed tumor necrosis. Clin Nucl Med. 2000;25:874–81. doi: 10.1097/00003072-200011000-00004. [DOI] [PubMed] [Google Scholar]
- [57].Nair N, Ali A, Green AA, et al. Response of osteosarcoma to chemotherapy. Evaluation with F-18 FDG-PET scans. Clin Positron Imaging. 2000;3:79–83. doi: 10.1016/s1095-0397(00)00037-6. [DOI] [PubMed] [Google Scholar]
- [58].Hawkins DS, Rajendran JG, Conrad EU, III, Bruckner JD, Eary JF. Evaluation of chemotherapy response in pediatric bone sarcomas by [F-18]-fluorodeoxy-d-glucose positron emission tomography. Cancer. 2002;94:3277–84. doi: 10.1002/cncr.10599. [DOI] [PubMed] [Google Scholar]
- [59].Hawkins DS, Schuetze SM, Butrynski JE, et al. [18F]Fluorodeoxyglucose positron emission tomography predicts outcome for Ewing sarcoma family of tumors. J Clin Oncol. 2005;23:8828–34. doi: 10.1200/JCO.2005.01.7079. [DOI] [PubMed] [Google Scholar]
- [60].Iagaru A, Masamed R, Chawla SP, Menendez LR, Fedenko A, Conti PS. F-18 FDG PET and PET/CT evaluation of response to chemotherapy in bone and soft tissue sarcomas. Clin Nucl Med. 2008;33:8–13. doi: 10.1097/RLU.0b013e31815c4fd4. [DOI] [PubMed] [Google Scholar]
- [61].Issels RD, Meier TH, Muller E, Multhoff G, Wilmanns W. Ifosfamide induced stress response in human lymphocytes. Mol Aspects Med. 1993;14:281–6. doi: 10.1016/0098-2997(93)90016-7. [DOI] [PubMed] [Google Scholar]
- [62].Huang TL, Liu RS, Chen TH, Chen WY, Hsu HC, Hsu YC. Comparison between F-18-FDG positron emission tomography and histology for the assessment of tumor necrosis rates in primary osteosarcoma. J Chin Med Assoc. 2006;69:372–6. doi: 10.1016/S1726-4901(09)70275-8. [DOI] [PubMed] [Google Scholar]
- [63].Jones DN, McCowage GB, Sostman HD, et al. Monitoring of neoadjuvant therapy response of soft-tissue and musculoskeletal sarcoma using fluorine-18-FDG PET. J Nucl Med. 1996;37:1438–44. [PubMed] [Google Scholar]
- [64].Kubota R, Kubota K, Yamada S, Tada M, Ido T, Tamahashi N. Active and passive mechanisms of [fluorine-18] fluorodeoxyglucose uptake by proliferating and prenecrotic cancer cells in vivo: a microautoradiographic study. J Nucl Med. 1994;35:1067–75. [PubMed] [Google Scholar]
- [65].Arush MW, Israel O, Postovsky S, et al. Positron emission tomography/computed tomography with 18fluoro-deoxyglucose in the detection of local recurrence and distant metastases of pediatric sarcoma. Pediatr Blood Cancer. 2007;49:901–5. doi: 10.1002/pbc.21150. [DOI] [PubMed] [Google Scholar]
- [66].Gerth HU, Juergens KU, Dirksen U, Gerss J, Schober O, Franzius C. Significant benefit of multimodal imaging: PET/CT compared with PET alone in staging and follow-up of patients with Ewing tumors. J Nucl Med. 2007;48:1932–9. doi: 10.2967/jnumed.107.045286. [DOI] [PubMed] [Google Scholar]
- [67].Nieweg OE, Pruim J, Hoekstra HJ, et al. Positron emission tomography with fluorine-18-fluorodeoxyglucose for the evaluation of therapeutic isolated regional limb perfusion in a patient with soft-tissue sarcoma. J Nucl Med. 1994;35:90–2. [PubMed] [Google Scholar]
- [68].van Ginkel RJ, Hoekstra HJ, Pruim J, et al. FDG-PET to evaluate response to hyperthermic isolated limb perfusion for locally advanced soft-tissue sarcoma. J Nucl Med. 1996;37:984–90. [PubMed] [Google Scholar]
- [69].van Ginkel RJ, Kole AC, Nieweg OE, et al. l-[1-11C]-tyrosine PET to evaluate response to hyperthermic isolated limb perfusion for locally advanced soft-tissue sarcoma and skin cancer. J Nucl Med. 1999;40:262–7. [PubMed] [Google Scholar]
- [70].Shields AF, Mankoff DA, Link JM, et al. Carbon-11-thymidine and FDG to measure therapy response. J Nucl Med. 1998;39:1757–62. [PubMed] [Google Scholar]
- [71].Peng F, Rabkin G, Muzik O. Use of 2-deoxy-2-[F-18]-fluoro-d-glucose positron emission tomography to monitor therapeutic response by rhabdomyosarcoma in children: report of a retrospective case study. Clin Nucl Med. 2006;31:394–7. doi: 10.1097/01.rlu.0000222954.38724.be. [DOI] [PubMed] [Google Scholar]
- [72].Kasper B, Dietrich S, Dimitrakopoulou-Strauss A, et al. Early prediction of therapy outcome in patients with high-risk soft tissue sarcoma using positron emission tomography. Onkologie. 2008;31:107–12. doi: 10.1159/000113795. [DOI] [PubMed] [Google Scholar]
- [73].Evilevitch V, Weber WA, Tap WD, et al. Reduction of glucose metabolic activity is more accurate than change in size at predicting histopathologic response to neoadjuvant therapy in high-grade soft-tissue sarcomas. Clin Cancer Res. 2008;14:715–20. doi: 10.1158/1078-0432.CCR-07-1762. [DOI] [PubMed] [Google Scholar]
- [74].van Oosterom AT, Judson I, Verweij J, et al. Safety and efficacy of imatinib (STI571) in metastatic gastrointestinal stromal tumours: a phase I study. Lancet. 2001;358:1421–3. doi: 10.1016/s0140-6736(01)06535-7. [DOI] [PubMed] [Google Scholar]
- [75].Goerres GW, Stupp R, Barghouth G, et al. The value of PET, CT and in-line PET/CT in patients with gastrointestinal stromal tumours: long-term outcome of treatment with imatinib mesylate. Eur J Nucl Med Mol Imaging. 2005;32:153–62. doi: 10.1007/s00259-004-1633-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Antoch G, Kanja J, Bauer S, et al. Comparison of PET, CT, and dual-modality PET/CT imaging for monitoring of imatinib (STI571) therapy in patients with gastrointestinal stromal tumors. J Nucl Med. 2004;45:357–65. [PubMed] [Google Scholar]
- [77].Goldstein D, Tan BS, Rossleigh M, Haindl W, Walker B, Dixon J. Gastrointestinal stromal tumours: correlation of F-FDG gamma camera-based coincidence positron emission tomography with CT for the assessment of treatment response – an AGITG study. Oncology. 2005;69:326–32. doi: 10.1159/000089765. [DOI] [PubMed] [Google Scholar]
- [78].Gayed I, Vu T, Iyer R, et al. The role of 18F-FDG PET in staging and early prediction of response to therapy of recurrent gastrointestinal stromal tumors. J Nucl Med. 2004;45:17–21. [PubMed] [Google Scholar]
- [79].Jager PL, Gietema JA, van der Graaf WT. Imatinib mesylate for the treatment of gastrointestinal stromal tumours: best monitored with FDG PET. Nucl Med Commun. 2004;25:433–8. doi: 10.1097/00006231-200405000-00002. [DOI] [PubMed] [Google Scholar]
- [80].Stroobants S, Goeminne J, Seegers M, et al. 18FDG-positron emission tomography for the early prediction of response in advanced soft tissue sarcoma treated with imatinib mesylate (Glivec) Eur J Cancer. 2003;39:2012–20. doi: 10.1016/s0959-8049(03)00073-x. [DOI] [PubMed] [Google Scholar]
- [81].Wardelmann E, Thomas N, Merkelbach-Bruse S, et al. Acquired resistance to imatinib in gastrointestinal stromal tumours caused by multiple KIT mutations. Lancet Oncol. 2005;6:249–51. doi: 10.1016/S1470-2045(05)70097-8. [DOI] [PubMed] [Google Scholar]
- [82].Rajagopalan H, Lengauer C. Aneuploidy and cancer. Nature. 2004;432:338–41. doi: 10.1038/nature03099. [DOI] [PubMed] [Google Scholar]
- [83].Desai J, Shankar S, Heinrich MC, et al. Clonal evolution of resistance to imatinib in patients with metastatic gastrointestinal stromal tumors. Clin Cancer Res. 2007;13:5398–405. doi: 10.1158/1078-0432.CCR-06-0858. [DOI] [PubMed] [Google Scholar]
- [84].Al-Batran SE, Hartmann JT, Heidel F, et al. Focal progression in patients with gastrointestinal stromal tumors after initial response to imatinib mesylate: a three-centre-based study of 38 patients. Gastric Cancer. 2007;10:145–52. doi: 10.1007/s10120-007-0425-8. [DOI] [PubMed] [Google Scholar]