Abstract
Our eventual aim is to predict, using non-invasive imaging techniques, the biological behaviour of individual cirrhotic nodules. We are some distance away from this, so our current objective is to define imaging features which predict the histologic findings. This short review summarises the current capabilities and limitations of non-invasive imaging in detecting small hepatocellular carcinomas (HCCs) in cirrhosis. Extracellular contrast media used with ultrasound (US), computed tomography (CT) or magnetic resonance imaging (MRI) can characterise nodules according to the predominance of arterial or portal inflow, and most HCCs will be recognised by their arterial hypervascularity. Adding intracellular (liver-specific) MRI contrast agents provides a significant improvement in early detection and in specificity for HCC. Nodules can be classified on dual contrast MRI as clearly malignant, clearly benign, or borderline (needing careful surveillance). Future imaging research needs to establish the histology of small hypervascular nodules, the evolution of hypervascular nodules and of dysplastic nodules, and to seek imaging features which predict microvascular invasion. Currently, cirrhotic patients with either suspicious nodules on screening US or rising AFP should have cross-sectional imaging with multi-phase CT or preferably MRI. Dual-contrast MRI with liver-specific agents should be used to improve diagnostic specificity for small lesions. Borderline nodules should be followed at agreed intervals using the same imaging technique each time. Pre-operative staging in surgical candidates should include CT of thorax, abdomen and pelvis and bone scintigraphy.
Keywords: Hepatocellular carcinoma, diagnosis, liver, cirrhosis, MRI
Development of HCC in cirrhosis
The sequential progression from regenerative nodules via low-grade then high-grade dysplastic nodules to well-differentiated then moderately and poorly differentiated hepatocellular carcinoma (HCC) is now firmly established. It is also clear that some HCCs arise directly from regenerative nodules without the intervening stage of dysplasia.
Screening with sonography
Although MRI is the best imaging technique for demonstrating nodular architecture in cirrhosis, it is unsuitable for screening[1–9]. Sonography is superior to computed tomography (CT) in detecting nodular change, even though CT is better for finding small HCCs. Patients in whom suspicious nodules are found on sonography, and those with rising alpha-fetoprotein (AFP), should be investigated further with CT or preferably magnetic resonance imaging (MRI). Demonstration of arterial hypervascularity using ultrasound contrast agents is a valid technique for differentiation of established HCC from benign or dysplastic nodules, but cannot be relied on for examining the whole of the liver since the contrast enhancement is transitory and the field of view with ultrasound probes is limited. Even if HCC is detected by contrast-enhanced ultrasound (US), CT or MRI is still needed for staging.
Blood flow to cirrhotic nodules and HCC
Studies using CT with contrast injection into the hepatic artery (CTHA) and CT with portal contrast enhancement following injection into splenic and superior mesenteric arteries (CTAP) have shown how the changes in arterial and portal inflow are correlated with nodule vasculature and histology. The main conclusion is that stepwise de-differentiation from regenerative via dysplastic to frankly malignant nodules is accompanied by falling portal inflow and increasing arterial flow. However, recent studies have also shown that the early stages of carcinogenesis are associated with loss of portal tracts, so both arterial and portal inflow are reduced, and even some well-differentiated HCCs have diminished arterial inflow. It is only at the later stage of overt malignant change that the number of unpaired arteries typically becomes sufficient to produce arterial hypervascularity on US, CT or MRI. High-grade dysplastic nodules and early HCCs may be hypovascular.
Imaging appearances of HCC
HCCs are typically larger than surrounding regenerative nodules, but show no other reliably distinctive features on unenhanced US or CT. On unenhanced MRI, HCCs can be hyper-, hypo- or iso-intense on T1 images. Hyperintensity on T2 images is a fairly reliable but insensitive indicator of malignancy in a cirrhotic nodule.
The mainstay of diagnosis is the demonstration of arterial hypervascularity using conventional (extracellular) contrast agents with either US, CT or MRI. Rapid washout of the contrast from the lesion is specific for malignancy, but is often absent, particularly with small early HCCs.
Using liver-specific (intracellular) MRI contrast agents, HCCs show decreasing uptake with increasing de-differentiation. Advanced HCC shows no uptake of these agents whilst well-differentiated and early HCCs show uptake which is variably diminished or occasionally similar to that of benign nodules. The combination of increased arterialisation with decreased or absent uptake of intracellular contrast agents is highly specific for HCC.
American Association for the Study of Liver Diseases (AASLD)/European Association for the Study of the Liver (EASL) criteria for HCC
Criteria for the imaging diagnosis of HCC established by both EASL and AASLD are that nodules larger than 2 cm which are hypervascular on any imaging method may be regarded as HCC, and nodules 1–2 cm in size which are hypervascular on any two imaging techniques are also regarded as HCC. Although these criteria provide a useful guideline, they are not comprehensive. Some nodules larger than 2 cm are not hypervascular, a minority (about 10%) of HCCs are iso- or hypovascular, some hypervascular nodules larger than 1 cm are not HCCs, and some hypervascular nodules are smaller than 1 cm. Recent studies which have used sequential imaging and biopsy of indeterminate small nodules have shown that a substantial proportion of HCCs of under 2 cm are missed when EASL criteria are applied.
Additional diagnostic features on MRI
The presence of fat is indicative of HCCs (only rarely do dysplastic nodules contain sufficient fat to be visible on imaging), but only a minority of HCCs show this feature. Iron deposition is readily recognised on MRI but is non-specific and unhelpful in diagnosis of HCC. Cellular function may be assessed using iron oxide particles of colloidal size which are taken up by Kupffer cells in normally functioning liver parenchyma. Specific chelates of gadolinium and manganese are extracted by normally functioning hepatocytes. Combining extracellular and intracellular contrast agents allows assessment of both nodule vascularity and cellular function at the same examination. The combination of increased arterialisation with diminished cellular function is highly specific for HCC whilst regenerative nodules show both normal vascularity and normal cellular function. Hypervascular nodules which show normal cellular function, and nodules with abnormal cellular function which are not hypervascular, should both be regarded as borderline.
Choice of technique for HCC diagnosis
US is less sensitive than CT or MRI, although there is some evidence that combining US and CT increases sensitivity for detecting HCC. However, we are now approaching consensus that MRI is superior to either CT or US. All three methods rely on the single parameter of arterial hypervascularity and when we add the additional indicator of functional assessment using intracellular contrast agents in MRI, the results are improved further. Dual contrast MRI (DCMRI), has been shown to be superior to multidetector computed tomography (MDCT) and to unenhanced and single contrast MRI, and is approximately equivalent to the more invasive combination of CTHA with CTAP. In the only explant-correlated series so far published using this technique, DCMRI detected 92% of HCCs in the size range 1–2 cm.
Dysplastic nodules
Dysplastic nodules cannot be characterised on US or CT. On MRI, dysplastic nodules are typically hyperintense on T1 images and hypointense on unenhanced T2 images. Some dysplastic nodules are distinctly larger than regenerative nodules in the adjacent liver, but still show normal vascularity and cellular function. Increased arterial vascularity has been described in a few cases. Most dysplastic nodules show normal uptake of intracellular contrast agents. It is well recognised that HCC may develop as a small focus within a dysplastic nodule, giving the typical imaging appearance of ‘nodule-in-nodule’. More commonly, dysplastic nodules remain stable on consecutive imaging studies.
Proposal for imaging classification of cirrhotic nodules
Traditionally we try to correlate imaging features with pathology, so as to predict the histologic findings at surgery or explantation. However, what we are really trying to do is to predict the future behaviour of nodules rather than their pathologic appearances, so it may be helpful to use a classification based on the imaging appearances:
Type 1: nodules of similar size to those in the surrounding liver, showing the same vascularity and cellular function.
Type 2a: nodules showing normal vascularity and cellular function, but of distinctly larger size than surrounding nodules.
Type 2b: nodules of any size which show increased arterial hypervascularity, but normal cellular function.
Type 2c: nodules of any size which show normal vascularity, but decreased or absent cellular function.
Type 3: nodules of any size which show increased arterial hypervascularity and reduced or absent cellular function.
By this system, type 1 represents benign regenerative nodules, type 3 represents established HCC and all the type 2 categories represent borderline nodules with malignant potential.
Small hypervascular nodules
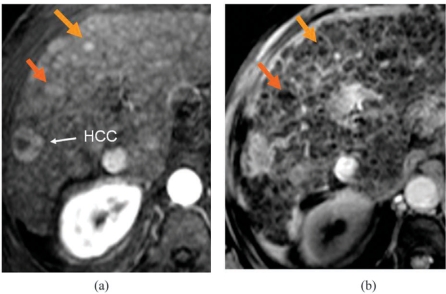
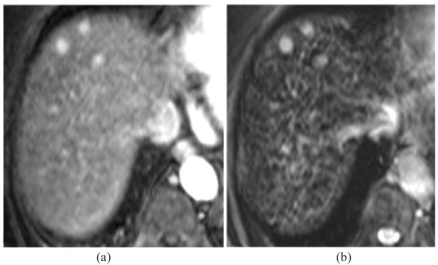
Focal areas of increased arterial hypervascularity may arise in the cirrhotic liver as a result of haemangiomas, local arterial-to-portal shunts, and small branch portal vein thromboses. Most of these vascular anomalies can be distinguished from genuine nodules by their irregular shape, peripheral location, and different signal intensity on MRI. However, some are indistinguishable from round or oval nodules in the parenchyma. In Budd–Chiari syndrome – as distinct from cirrhosis – small hypervascular foci are known to be arterialised hyperplastic nodules with no cellular features of malignancy. In the late-stage cirrhotic liver, small arterialised nodules are difficult to identify at explantation unless they larger or look different from surrounding nodules to the naked eye, so histologic correlation is difficult. Surveillance studies have shown that some nodules persist unchanged, some disappear, and some grow to become overt HCCs. The proportion of round or oval hypervascular nodules of under 2 cm which are later found to be HCCs has been variously reported as 5%, 13%, 25%, and 28%. When cirrhotic patients with hypervascular foci on CT were investigated with superparamagnetic iron oxide (SPIO)-enhanced MRI, nodules which showed no SPIO uptake were confirmed histologically as HCC in 100% of cases, whilst those which showed SPIO uptake were confirmed as dysplastic nodules in 78%, confirming the experience of the Leeds group with DCMRI (Figs. 1 and 2).
Figure 1.
Small hypervascular nodules with SPIO uptake: benign. (a) Post-Gd arterial T1; (b) post-SPIO T2*.
Figure 2.
Small hypervascular lesions with no SPIO uptake: HCCs. (a) Post-Gd arterial T1; (b) post-SPIO T2*.
Staging of HCC
Whichever staging system is used (Okuda, TNM, Japan Integrated Staging (JIS), Cancer of the Liver Italian Program (CLIP), Barcelona Clinic Liver Cancer (BCLC), the input required from imaging is to demonstrate the number and size of HCCs, to look for vascular invasion, and to find extrahepatic spread. All imaging methods – properly used – will demonstrate large HCCs, so the only issue with number and size is whether we should include lesions of 1–2 cm and those under 1 cm if they have definitive characteristics of HCC, and whether to include those lesions which have suggestive characteristics.
Gross vascular invasion is readily detectable by all imaging techniques and our challenge is now to develop techniques for demonstrating microvascular invasion. So far only very limited pointers can be suggested. In patients with mature HCCs larger than 2 cm, a well-defined capsule is often demonstrable by imaging. Where this correlates with a fibrotic capsule at histology, it is a good indicator that there is no microvascular invasion. Some lesions without a capsule show quite marked early arterial enhancement in the peri-lesional liver parenchyma (‘corona’ enhancement) and there is some experimental evidence that this can be correlated with microscopic invasion of local portal vein branches. However, at present this feature cannot be relied on.
Summary and recommendations
Patients with either suspicious nodules on screening US or rising AFP should have cross-sectional imaging with multi-phase CT or preferably MRI.
Dual-contrast MRI with liver-specific agents should be used to improve diagnostic specificity for small lesions.
Borderline nodules should be followed at agreed intervals using the same imaging technique each time.
Pre-operative staging in surgical candidates should include CT of thorax, abdomen and pelvis and a bone scan.
References
- [1].Ward J, Guthrie JA, Scott DJ, et al. Hepatocellular carcinoma in the cirrhotic liver: double-contrast MR imaging for diagnosis. Radiology. 2000;216:154–62. doi: 10.1148/radiology.216.1.r00jl24154. [DOI] [PubMed] [Google Scholar]
- [2].Jeong YY, Mitchell DG, Kamishima T. Small (<20 mm) enhancing hepatic nodules seen on arterial phase MR imaging of the cirrhotic liver: clinical implications. Am J Roentgenol. 2002;178:1327–34. doi: 10.2214/ajr.178.6.1781327. [DOI] [PubMed] [Google Scholar]
- [3].Burrel M, Llovet JM, Avuso C, et al. MRI angiography is superior to helical CT for detection of HCC prior to liver transplantation: an explant correlation. Hepatology. 2003;38:1034–42. doi: 10.1053/jhep.2003.50409. [DOI] [PubMed] [Google Scholar]
- [4].Bhartia B, Ward J, Guthrie JA, Robinson PJ. Hepatocellular carcinoma in cirrhotic livers: double-contrast thin-section MR imaging with pathologic correlation of explanted tissue. Am J Roentgenol. 2003;180:577–84. doi: 10.2214/ajr.180.3.1800577. [DOI] [PubMed] [Google Scholar]
- [5].Kwak HS, Lee JM, Kim CS. Preoperative detection of hepatocellular carcinoma: comparison of combined contrast-enhanced MR imaging and combined CT during arterial portography and CT hepatic arteriography. Eur Radiol. 2004;14:447–57. doi: 10.1007/s00330-003-2070-x. [DOI] [PubMed] [Google Scholar]
- [6].O’Malley ME, Takayama Y, Sherman M. Outcome of small (10–20 mm) arterial phase-enhancing nodules seen on triphasic liver CT in patients with cirrhosis or chronic liver disease. Am J Gastroenterol. 2005;100:1523–8. doi: 10.1111/j.1572-0241.2005.41814.x. [DOI] [PubMed] [Google Scholar]
- [7].Efremidis SC, Hytiroglou P, Matsui O. Enhancement patterns and signal-intensity characteristics of small hepatocellular carcinoma in cirrhosis: pathologic basis and diagnostic challenges. Eur Radiol. 2007;17:2969–82. doi: 10.1007/s00330-007-0705-z. [DOI] [PubMed] [Google Scholar]
- [8].Forner A, Vilana R, Ayuso C, et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: Prospective validation of the non-invasive diagnostic criteria for hepatocellular carcinoma. Hepatology. 2008;47:97–104. doi: 10.1002/hep.21966. [DOI] [PubMed] [Google Scholar]
- [9].Willatt JM, Hussain HK, Adusumilli S, et al. MR imaging of hepatocellular carcinoma in the cirrhotic liver: challenges and controversies. Radiology. 2008;247:311–30. doi: 10.1148/radiol.2472061331. [DOI] [PubMed] [Google Scholar]