Abstract
The iron chelate, ferric nitrilotriacetate (FeNTA), induces acute proximal tubular necrosis as a consequence of lipid peroxidation and oxidative tissue damage. Chronic exposure of FeNTA leads to a high incidence of renal adenocarcinomas in rodents. NF-e2-related factor 2 (Nrf2) is a transcription factor that is activated by oxidative stress and electrophiles, and regulates the basal and inducible expression of numerous detoxifying and antioxidant genes. To determine the roles of Nrf2 in regulating renal gene expression and protecting against oxidative stress-induced kidney damage, wild-type and Nrf2-null mice were administered FeNTA. Renal Nrf2 protein translocated to the nucleus at 6 h after FeNTA treatment. FeNTA increased mRNA levels of Nrf2 target genes, including NQO1, GCLC, GSTpi1/2, Mrp1, 2, and 4 in kidneys from wild-type mice, but not Nrf2-null mice. Protein expression of NQO1, a prototypical Nrf2 target gene, was increased in wild-type mice, with no change in Nrf2-null mice. FeNTA produced more nephrotoxicity in Nrf2-null mice than wild-type mice as indicated by higher serum urea nitrogen and creatinine levels, as more urinary NAG, stronger 4-hydroxynonenal protein adduct staining, and more extensive proximal tubule damage. Furthermore, pretreatment with CDDO-Im, a potent small molecule Nrf2 activator, protected mice against FeNTA-induced renal toxicity. Collectively, these results suggest that activation of Nrf2 protects mouse kidneys from FeNTA-induced oxidative stress damage by coordinately up-regulating the expression of cytoprotective genes.
Keywords: Nrf2, FeNTA, NQO1, oxidative stress, Mrp, kidney
INTRODUCTION
The iron chelate, ferric nitrilotriacetate (FeNTA), is a potent nephrotoxicant (Hamazaki et al., 1985; 1986). FeNTA induces a free radical-mediated oxidative damage that leads to renal proximal tubular necrosis as well as a high incidence of renal cell carcinomas in rodents (Li et al., 1987). Treatment of rats with a single dose of FeNTA transiently causes oxidative stress, as revealed by renal accumulation of the lipid peroxidation product, 4-hydroxynonenal (4-HNE) and the DNA base-modified product, 8-hydroxy-2′-deoxyguanosine (Toyokuni et al., 1994; 1997).
Nuclear factor erythroid 2 related factor 2 (Nrf2) belongs to the cap’n’ collar family of basic leucine zipper transcription factors. Nrf2 is responsible for the basal and inducible expression of a battery of antioxidative genes, cytoprotective enzymes, and export transporters, such as glutathione-S-transferases (GST), NAD(P)H:quinone oxidoreductase 1 (NQO1), heme oxygenase-1 (HO-1), and multidrug resistance-associated proteins (Mrps) that together constitute a defense system against oxidative stress (Ishii et al., 2000; Hayashi et al., 2003; Vollrath et al., 2006; Maher et al., 2007). The cytosolic regulatory protein Kelch-like ECH-associated protein 1 (Keap1) sequesters Nrf2 in the cytoplasm under basal conditions. Upon exposure to electrophilic or oxidative stresses, Nrf2 is released from Keap1 repression and translocates to the nucleus where it heterodimerizes with small Maf proteins to enhance transcription of target genes via cis-acting antioxidant response elements in the promoters (Ishii et al, 2002; Kobayashi et al., 2006).
Numerous studies show that Nrf2 protects cells from xenobiotic and oxidative stresses, and consequently from carcinogenesis in a variety of tissues, such as liver, lung, and brain (Enomoto et al., 2001; Cho et al., 2002; Burton et al., 2006). Nrf2 protects the kidney from renal injury after hepatic ischemia-reperfusion injury, and aged Nrf2-deficient female mice develop lupus-like autoimmune nephritis (Yoh et al., 2001; Hirayama et al, 2003; Tanaka et al., 2007). However, little is known about the ability of Nrf2 to regulate cytoprotective gene expression in the kidneys and protect against chemical-induced renal injury.
This study will investigate the susceptibility of Nrf2-null mice to chemical-induced renal oxidative stress and toxicity using ferric-nitrilotriacetate (FeNTA). Nrf2 translocation and activation were evaluated by western blot using nuclear extracts from kidneys of mice after FeNTA treatment. Immunohistochemical detection of 4-HNE protein adducts was used as a marker of renal oxidative stress and lipid peroxidation. Serum urea nitrogen and creatinine along with histopathology were quantified to evaluate FeNTA-induced renal toxicity. The mRNA expression of Nrf2 target genes were determined by branched DNA (bDNA) signal amplification. Furthermore, it was determined whether pretreatment with the synthetic triterpenoid, CDDO-Im (1-[2-cyano-3-,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole), a novel Nrf2 activator protects the kidneys from FeNTA-induced toxicity.
METHODS
Materials
CDDO-Im (1-[2-cyano-3-,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole) was kindly provided by Dr. Michael Sporn (Dartmouth Medical School and Dartmouth College, Hanover, New Hampshire). Ferric nitrate enneahydrate and sodium dicarbonate, nitrilotriacetic acid disodium salt, and all other chemicals were purchased from Sigma-Aldrich (St. Louis, MO). Serum urea nitrogen and creatinine kits were purchased from Stanbio (Boerne, TX). Urinary N-acetyl-beta-glucosaminidase (NAG) was quantified using a colorimetric assay from Diazyme Laboratories (San Diego, CA).
Animals
Male C57BL/6 mice (aged 8 weeks) were purchased from Jackson Laboratory (Bar Harbor, ME) and were used for dose-response, time-course, and the CDDO-Im protection study. Male wild-type and Nrf2-null mice on a mixed C57BL/6 and AKR background (aged 8–10 weeks) were obtained from Dr. Jefferson Chan (University of California, Irvine) and bred in our animal-care facility. Animals received humane care as outlined in the Guide for the Care and Use of Laboratory Animals (NIH publication 86–23, revised 1985). Studies were approved by the University of Kansas Medical Center Institutional Animal Care and Use Committee.
In the dose-response study, FeNTA was administered to mice at 4 different doses (saline as vehicle, 1, 2.5, and 5 mg Fe/kg). Blood samples and kidneys were removed 6 h after FeNTA treatment. In the time-course study, 5 mg Fe/kg of FeNTA was administered to mice. At various times (3, 6, 24, and 48 h) after FeNTA treatment, blood samples were obtained, and kidneys removed. For studies with wild-type and Nrf2-null mice, FeNTA was administered to mice at 5 mg Fe/kg, and blood samples and kidneys were obtained at 3, 6, and 24 h after FeNTA treatment. Urine samples were collected at 3 h after FeNTA treatment by bladder puncture.
In a separate experiment to characterize the effect of CDDO-Im on mRNA expression of Nrf2 target genes in kidney, CDDO-Im (0.3, 1, and 3 mg/kg i.p.) was administered to C57BL/6 mice, and kidneys were collected 6 h thereafter. Furthermore, pretreatment with CDDO-Im (1 mg/kg i.p. in DMSO, 24 and 48 h prior to FeNTA administration) or DMSO was administered in a volume of 5 ml/kg before FeNTA injection (5 mg Fe/kg). Blood was taken 24 h after FeNTA injection.
Preparation of FeNTA
The FeNTA solution was prepared immediately before use. Ferric nitrate enneahydrate and nitrilotriacetic acid disodium salt were each dissolved in deionized water to form 300 and 600 mmol/L solutions. They were mixed at the volume ratio of 1:2 (molar ratio, 1:4) and the pH was adjusted with dihydrocarbonate to 7.4.
Serum Urea Nitrogen and Creatinine
Blood samples were collected at 6, 24, and 48 h after FeNTA treatment. Serum samples were analyzed by standard enzymatic-colorimetric assays using urea nitrogen and creatinine assay kits in accordance with the manufacturer’s protocols. The absorption of each sample was assessed spectrophotometricly at wavelengths of 600 and 520 nm, respectively.
Urinary NAG and Creatinine
Urinary NAG and creatinine concentrations were determined using urinary NAG and creatinine assay kits in accordance with the manufacturer’s protocols. The absorption of each sample was assessed spectrophotometricly at 505 and 520 nm, respectively. Urinary NAG values were normalized to urinary creatinine levels.
RNA Isolation
Total RNA was isolated using RNAzol B reagent (Tel Test Inc., Friendswood, TX) according to the manufacturer’s protocol. The concentration of total RNA in each sample was quantified spectrophotometrically at 260 nm. The integrity of each RNA sample was evaluated by formaldehyde-agarose gel electrophoresis before analysis.
bDNA Assay
The mRNA expression of mouse HO-1, NQO1, glutamate cysteine ligase catalytic subunit (GCLC), GSTpi1/2, and Mrp1-4 were quantified using the bDNA assay as previously described (QuantiGene, High Volume bDNA Signal Amplification Kit; Panomics, Fremont, CA) (Onyenwoke and Wiegel, 2007). Multiple oligonucleotide probe sets (containing capture, label, and blocker probes) specific to mouse GCLC, and GSTpi1/2 mRNA transcripts were designed using ProbeDesigner software v1.0 (Bayer Corp., Diagnostics Div.) (Supplementary Table 1). The probe set for GSTpi1/2 cannot distinguish between isoforms 1 and 2 of GSTpi. Specific mouse HO-1, NQO1, and Mrp1-4 probe sets were described previously (Aleksunes et al., 2005; Maher et al., 2005).
Western Blot Analysis of Nrf2 and NQO1 Expression
Nuclear proteins were prepared from mouse kidneys by using the NE-PER kit (Pierce, Rockford, IL) as per the manufacturer’s recommendations. Cytosolic fractions were obtained as described previously (Aleksunes et al., 2006). Protein concentration was determined using Bio-Rad protein assay reagents (Bio-Rad Laboratories, Hercules, CA). Immunochemical detection of nuclear Nrf2 and cytosolic NQO1 proteins was performed using an anti-Nrf2 antibody (H-300, Santa Cruz Biotechnology, Santa Cruz, CA) and an anti-NQO1 antibody (ab2346, Novus Biological, Littleton, CO), respectively. Equal protein loading was confirmed using β-actin (ab8227, Abcam, Cambridge, MA) as a loading control. Nrf2 and NQO1 protein-antibody complexes were detected using an enhanced chemiluminescent kit (Amersham Life Science, Arlington Heights, IL) and exposed to Fuji Medical X-ray film (Fisher Scientific, Springfield, NJ). Intensity of protein bands was quantified using the Discovery Series Quantity One 1-D Analysis software (Bio-Rad Laboratories, Hercules, CA).
NQO1 Activity Assay
NQO1 activity (nmol/min/mg protein) was calculated by measuring the colorimetric reduction of 2,6-dichlorophenolindophenol (DCPIP). The disappearance of DCPIP was quantified at 600 nm over 1 min as described by Ernster with modifications by Benson (Ernster, 1967; Benson et al., 1980). NQO1 activity was quantified in 1-ml reactions (27°C) containing cytosol, 200 μmol/L NADPH, 40 μmol/L DCPIP and Tris-HCl buffer (25 mmol/L Tris-HCl, pH 7.4, 0.7 mg/ml bovine serum albumin). Parallel reactions were performed with 20 μmol/L dicumarol. The rate of dicumarol-sensitive NQO1 activity was determined as the difference between the uninhibited and dicumarol-inhibited rates and was normalized to total cytosolic protein as previously described (Benson et al., 1980).
Histopathology
Kidney samples were fixed in 10% formalin containing 1% zinc sulfate (Fisher Scientific, Springfield, NJ) prior to routine processing and paraffin embedding. Kidney sections (5 μm in thickness) were stained with hematoxylin and eosin. The pathological examination of sections by light microscopy was performed in a blinded fashion by a veterinary pathologist for the presence and severity of tubular degeneration, necrosis, protein casts, and dilated lumens. Histopathology scoring was as follows: no significant lesions = grade 0; minimal injury affecting less than 10% of renal tubule cells = grade 1; mild injury affecting 10–25% of renal tubule cells = grade 2; moderate injury affecting 25–40% of renal tubule cells = grade 3; marked injury affecting 40–50% of renal tubule cells = grade 4; or severe injury affecting more than 50% of renal tubule cells = grade 5.
Immunohistochemistry
Sections of formalin-fixed, paraffin-embedded kidneys were deparaffinized in xylene, then rehydrated through a graded alcohol series. Endogenous peroxidase activity was blocked with 3% H2O2 for 10 min and antigen retrieval was performed by incubating slides in citrate buffer (10 mM) at 95°C for 10 min. For immunohistochemical staining of 4-hydroxynonenal (4-HNE), sections were blocked with 5% goat serum in phosphate-buffered saline for 1 h. The anti-4HNE antibody (ALX-210-767, Axxora, San Diego, CA) was diluted 1:500 in 1% goat serum and applied to slides for 1 h. Protein-antibody complexes were visualized using the anti-rabbit Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA). 4-HNE immunostaining was visualized by developing with 3,3′-diaminobenzidine substrate (Vector Laboratories, Burlingame, CA). Tissues were counterstained with hematoxylin, followed by dehydration in ethanol and clearing with xylene. Kidney sections from 3 to 4 mice per group were stained and representative images are shown. Negative control staining was performed by incubating sections without primary antibody. Representative sections from the cortex were imaged using a Zeiss Axioskope2Plus microscope (Donsanto Corp., Natick, MA) equipped with a Optronics Camera and MagnaFire Software v2.0 (Optronics, Goleta, CA).
Statistical Analysis
In the time-course study, statistical differences between control and FeNTA treatment groups were determined using Student’s t test with significance set at p< 0.05. In the other studies, FeNTA-treated mice at the various doses and control groups, pretreatment with CDDO-Im to control and FeNTA-treated mice, and wild-type and Nrf2-null mice were analyzed by analysis of variance, followed by a Duncan’s multiple range post hoc test. Significance was set at p < 0.05. Bars represent mean ± S.E.M.
RESULTS
Effect of FeNTA on serum urea nitrogen and creatinine
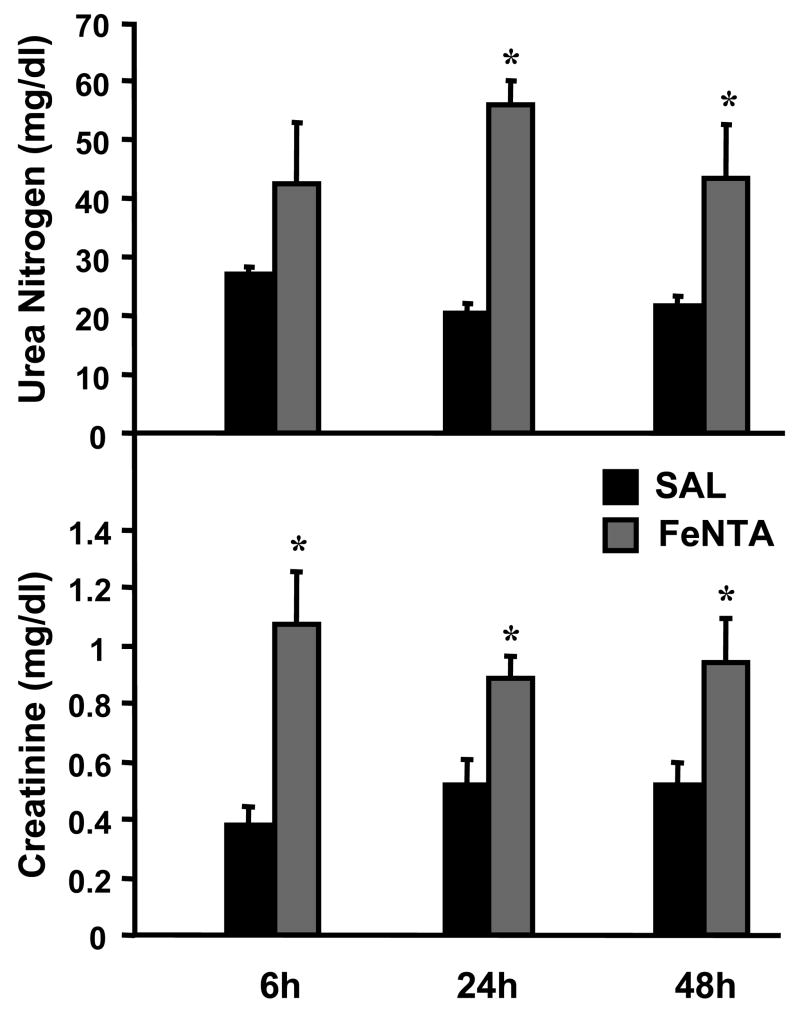
FeNTA is a known nephrotoxicant in rodents (Hamazaki et al., 1985; 1986). Thus, renal toxicity was evaluated by serum urea nitrogen and creatinine. Serum urea nitrogen was increased to 56 and 43 mg/dl at 24 and 48 h after FeNTA (5 mg/kg) treatment (compared to 20 mg/dl in vehicle-treated mice), respectively (Fig. 1). Serum creatinine was increased to 0.89–1.07 mg/dl at 6, 24, and 48 h after FeNTA (5 mg/kg) treatment (compared to 0.38–0.53 mg/dl in vehicle-treated mice) (Fig. 1). Because serum creatinine was increased at 6 h, this time point was used for subsequent dose-response experiments.
Fig. 1. Time course of renal toxicity after FeNTA (5 mg/kg) treatment.
At 6, 24, and 48 h after saline (SAL) or FeNTA treatment, urea nitrogen and creatinine levels were quantified in serum. Data are expressed as mean ± S.E.M. (each group, n = 5 animals). Asterisks (*) represent statistically significant differences (p < 0.05) between vehicle and FeNTA groups.
Dose-response of FeNTA on Nrf2 translocation in mouse kidneys
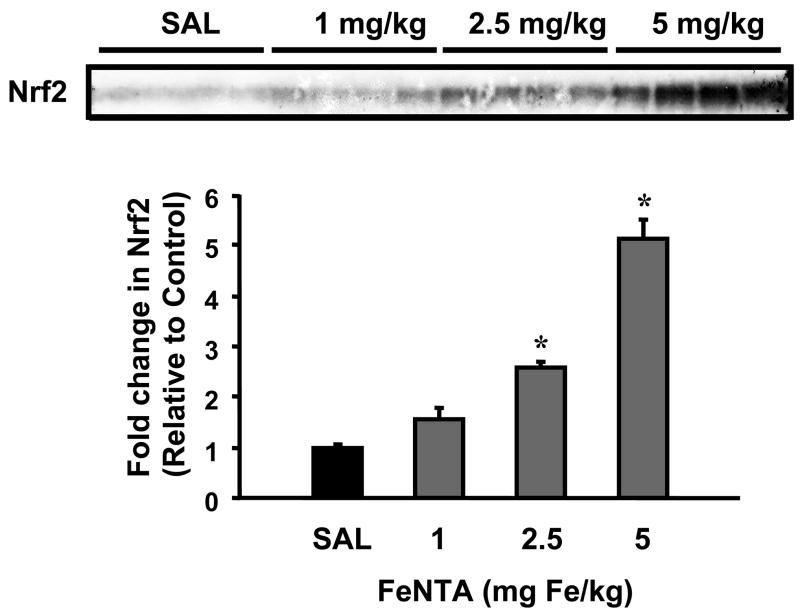
To determine whether Nrf2 is released from sequestration in kidneys after FeNTA treatment, nuclear accumulation of Nrf2 protein was determined by Western blot. Nrf2 protein translocated to the nucleus dose dependently 6 h after FeNTA treatment (Fig. 2).
Fig. 2. Dose response of nuclear Nrf2 protein expression in kidneys 6 h after FeNTA treatment.
Nuclear extracts was made from kidneys of control (saline, SAL) and FeNTA-treated mice at different doses (1, 2.5, and 5 mg Fe/kg body) at 6h. A western blot of nuclear extracts stained with an antibody that detects mouse Nrf2 (50 μg protein/lane) is shown. Immunoreactive bands were semiquantitated by densitometric analysis. Data were normalized to SAL control and expressed as mean ± S.E.M. (each group, n = 4 animals). Asterisks (*) represent statistically significant differences (p < 0.05) compared to vehicle-treated mice.
Dose-response of FeNTA on the mRNA expression of Nrf2 target genes
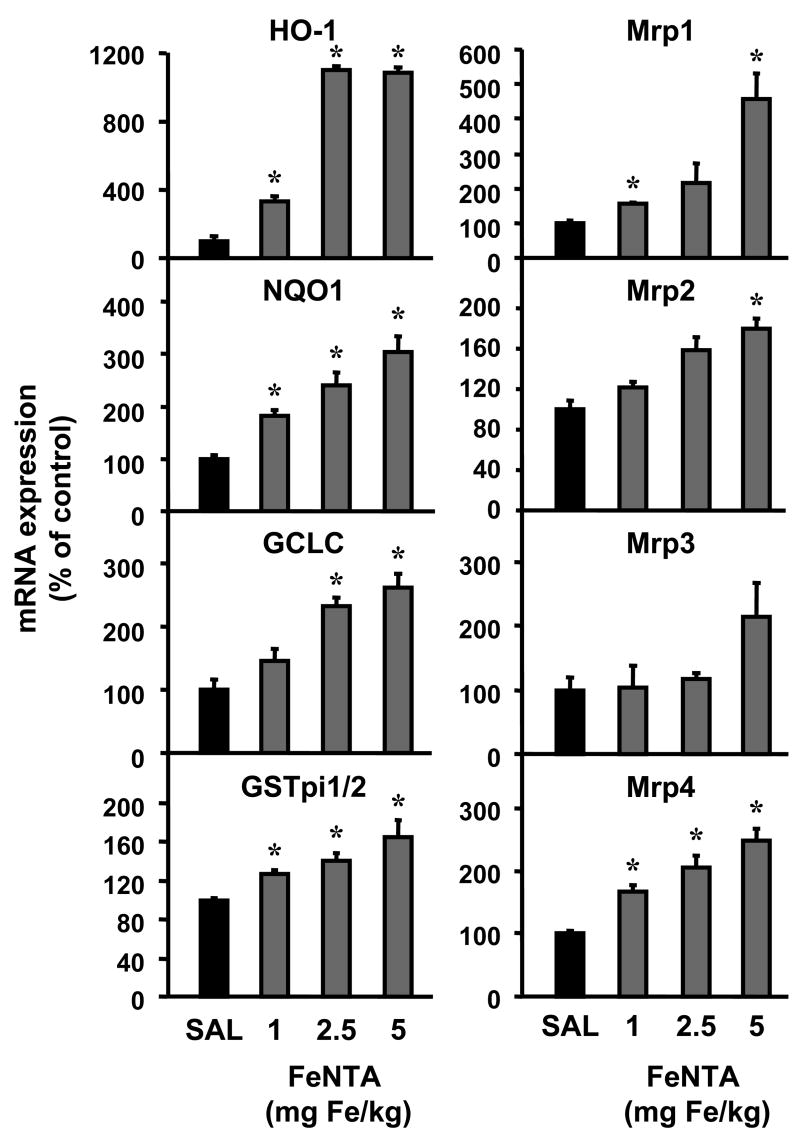
HO-1 mRNA was increased dose dependently up to 2.5 mg/kg FeNTA at 6 h, but was not further induced at 5 mg/kg FeNTA (Fig. 3). NQO1, GCLC, GSTpi1/2, and Mrp1, 2, and 4 mRNA were also increased dose dependently (Fig. 3). Mrp3 mRNA was unchanged by FeNTA.
Fig. 3. Dose response of kidney mRNA expression of Nrf2 targets 6 h after FeNTA treatment.
Total RNA was isolated from kidneys of control (saline, SAL) and FeNTA-treated mice at different doses (0, 1, 2.5, and 5 mg Fe/kg body) and analyzed by the bDNA assay as described in MATERIALS AND METHODS. Data are presented as mean (percent of control) ± S.E.M. (each group, n = 4 or 7 animals). Asterisks (*) represent statistically significant differences (p < 0.05) compared to vehicle-treated mice.
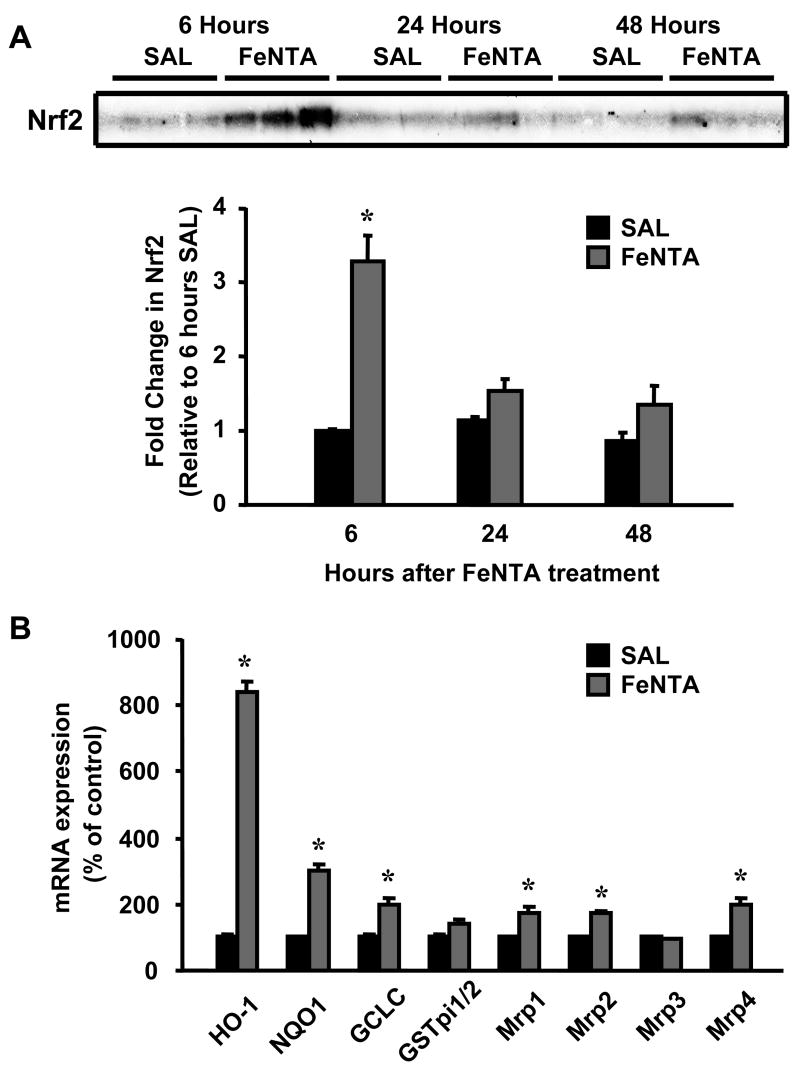
Effect of FeNTA treatment on Nrf2 translocation and mRNA expression of Nrf2 target genes
Levels of Nrf2 protein in nuclear extracts increased 230% at 6 h after FeNTA (5 mg/kg) treatment and returned to control levels by 24 h (Fig. 4A). The mRNA expression of Nrf2 target genes, including detoxification and efflux Mrp transporters was quantified by the bDNA assay. HO-1, NQO1, GCLC, and Mrp1, 2, and 4 mRNA were increased maximally 6 h after FeNTA (5 mg/kg) treatment by 740, 200, 100, 72, 74, and 99%, respectively (Fig. 4B), and with the exception of NQO1 returned to control values by 24 h (data not shown). GSTpi1/2 and Mrp3 were increased 56 and 45%, respectively, 24 h after FeNTA treatment (data not shown).
Fig. 4.
A: Time course of nuclear Nrf2 protein expression in kidneys after FeNTA (5 mg/kg) treatment. Nuclear extracts were made from kidneys of control (saline, SAL) and FeNTA-treated mice at different time points (6, 24, 48 h). A western blot of nuclear extracts stained with an antibody that detects mouse Nrf2 (50 μg protein/lane) is shown. Immunoreactive bands were semiquantified by densitometric analysis. Data were normalized to controls and expressed as mean ± S.E.M. (each group, n = 3 animals). Asterisks (*) represent statistically significant differences (p < 0.05) between control and FeNTA groups. B: mRNA expression of Nrf2 target genes in kidneys 6 h after FeNTA (5 mg/kg) treatment. Total RNA was isolated from control (saline, SAL) and FeNTA-treated mouse kidneys and analyzed by the bDNA assay as described in MATERIALS AND METHODS. Data are presented as mean (percent of control) ± S.E.M. (each group, n = 5 animals). Asterisks (*) represent statistically significant differences (p < 0.05) between control and FeNTA groups.
Protein expression and activity of NQO1 after FeNTA treatment
NQO1 is a prototypical Nrf2 target gene (Venugopal and Jaiswal, 1996). Western blots using an anti-NQO1 antibody confirmed that the changes in NQO1 mRNA expression observed in kidneys after FeNTA resulted in elevated protein levels. NQO1 protein expression was increased 24 h after FeNTA (5 mg/kg) treatment and returned to baseline levels at 48 h. NQO1 activity also increased 24 h after FeNTA (5 mg/kg) treatment (data not shown).
Renal toxicity of FeNTA in wild-type and Nrf2-null mice
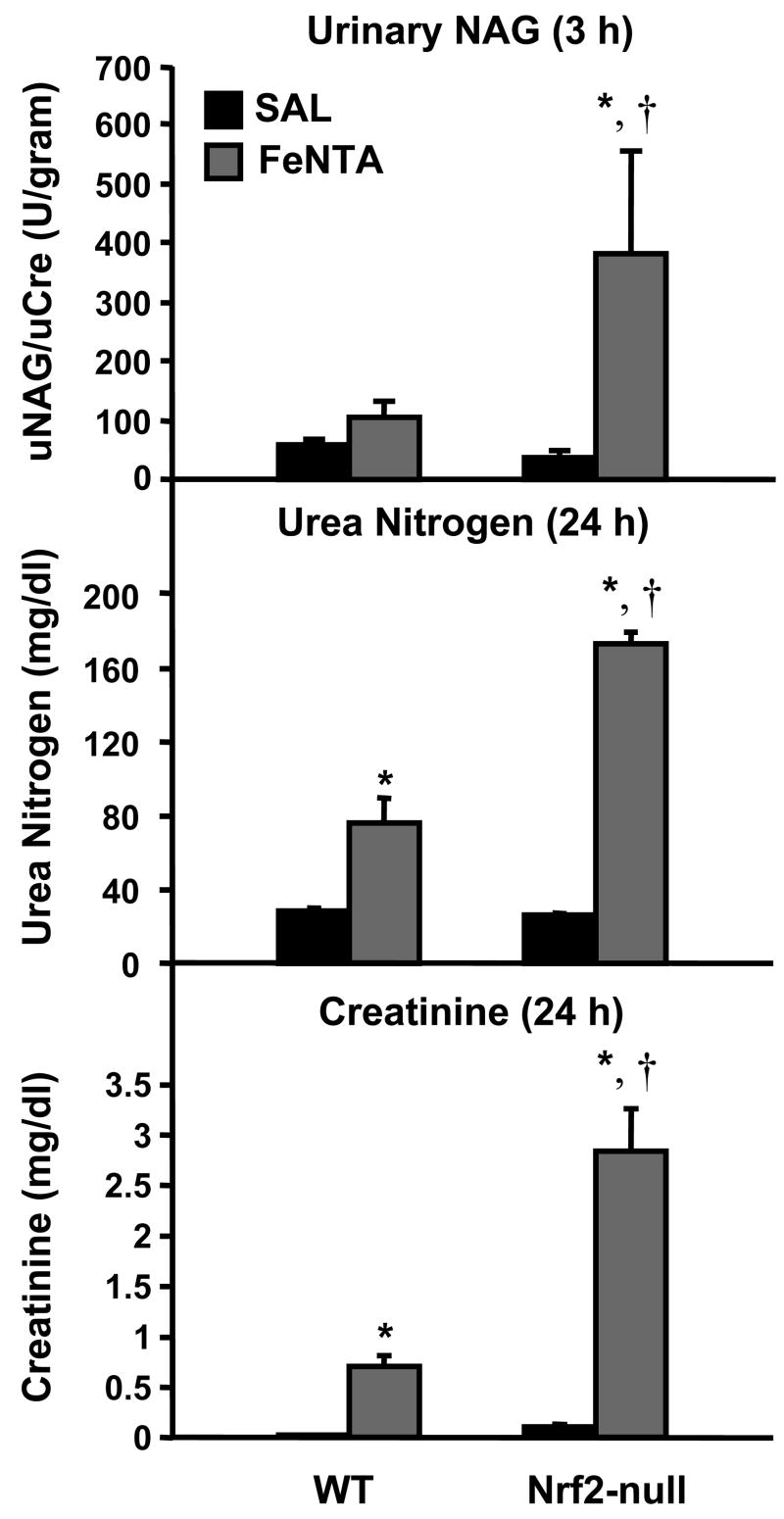
Urinary NAG is an enzyme in proximal tubular cells that enters the urine during proximal tubular injury (Le Hir et al., 1979). Because urinary NAG is used to detect early kidney injury, it was quantified 3 h after FeNTA (5 mg/kg) treatment. FeNTA tended to increase urinary NAG in wild-type mice, but was markedly increased in Nrf2-null mice (Fig. 5). FeNTA (5 mg/kg) increased serum urea nitrogen and creatinine 24 h after administration to both genotypes, with higher elevations in Nrf2-null mice (Fig. 5).
Fig. 5. Renal toxicity in wild-type and Nrf2-null mice after FeNTA (5 mg/kg) treatment.
At 3 h after FeNTA treatment, urinary NAG was quantified. At 24 h after saline (SAL) or FeNTA treatment, serum urea nitrogen and creatinine levels were quantified. Data are expressed as mean ± S.E.M. (each group, n = 4 animals). Asterisks (*) represent a statistically significant difference (p < 0.05) from vehicle-treated of the same genotype; daggers (†) represent a statistically significant difference (p < 0.05) from FeNTA-treated wild-type mice.
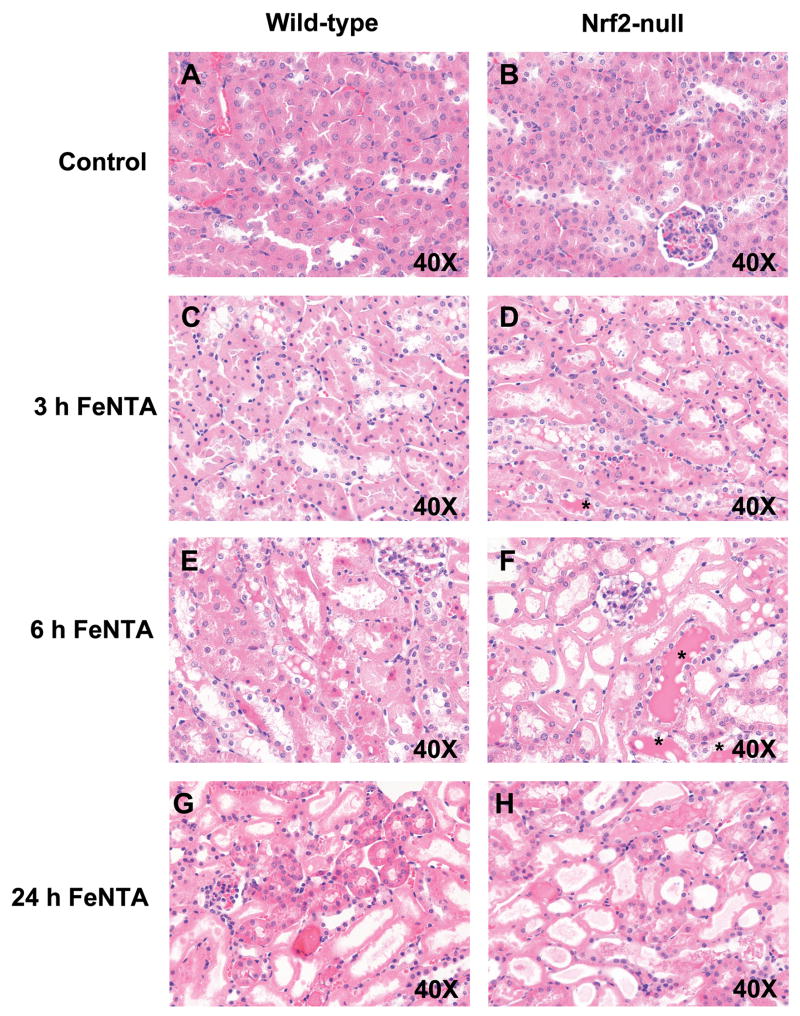
Histopathology of kidney sections from wild-type and Nrf2-null mice after FeNTA treatment
Histological examination of kidney sections from vehicle-treated wild-type and Nrf2-null mice did not show significant lesions (Fig. 6A and 6B). In general, the severity of renal tubular injury increased from 3 to 6 h in both genotypes with greater damage in the kidneys of FeNTA-treated Nrf2-null mice. At 3 h after FeNTA (5 mg/kg) treatment, proximal tubular degeneration and necrosis were observed in both genotypes, but were more extensive in Nrf2-null mice (75% of mice compared to 0% of wild-type mice) (Fig. 6C and 6D). Kidneys from FeNTA-treated Nrf2-null mice demonstrated more apoptotic cells compared to wild-types. By 6 h after FeNTA (5 mg/kg) treatment, tubular degeneration and necrosis were more prominent than 3 h (Fig. 6E and 6F). Necrotic tubules from both genotypes had complete loss of epithelial cell detail with intact basement membranes. In addition, necrotic tubules contained eosinophilic amorphous material and pyknotic and karyorhectic debris. Of note, tubular casts (asterisks) were more numerous and larger in kidney sections from FeNTA-treated Nrf2-null mice at 6 h (Fig. 6E and 6F). It is important to note that one Nrf2-null mouse died at this time point. By 24 h after FeNTA, wild-type mice exhibited a greater number of viable tubules compared to null mice (Fig. 6G wild-type; Fig. 6H Nrf2-null). Loss of tubular epithelium was extensive in the kidneys of Nrf2-null mice (100% of Nrf2-null mice; 50% of wild-type mice). Nrf2-null mice also exhibited marked cellular degeneration of remaining tubules, protein casts, and dilated lumens.
Fig. 6. Histological examination of kidney sections from wild-type and Nrf2-null mice after FeNTA treatment.
Images are presented as follows: (A) wild-type control; (B) Nrf2-null control; and FeNTA-treated (C) wild-type 3 h; (D) Nrf2-null 3 h; (E) wild-type 6 h; (F) Nrf2-null 6 h; (G) wild-type 24 h; (H) Nrf2-null 24 h. Asterisks depict protein casts. Magnifications: 40X.
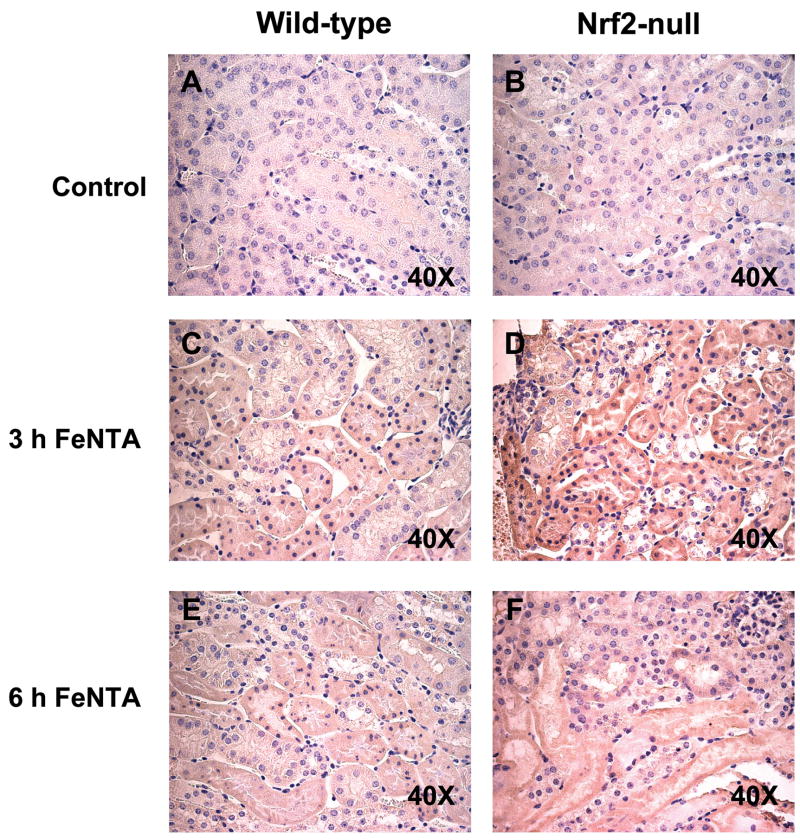
Immunohistochemical detection of 4-HNE protein adducts in wild-type and Nrf2-null mice after FeNTA treatment
Immunohistochemical staining was performed on paraffin kidney sections from control and FeNTA-treated mice to determine the extent of lipid peroxidation using 4-HNE protein adduct staining as a marker. 4-HNE adducts were minimally detected in kidney sections from control mice of both genotypes (Fig. 7A and 7B). In contrast, strong mostly cytoplasmic 4-HNE staining (brown) of proximal tubule epithelial cells was observed in cortex sections from FeNTA-treated mice at 3 h. Staining was stronger in Nrf2-null mice compared to wild-type mice (Fig. 7C and 7D). A decrease in the intensity of 4-HNE-adducted proteins was observed at 6 h after FeNTA (5 mg/kg) treatment, but more staining was still observed in Nrf2-null mice (Fig. 7E and 7F). At 24 h, there was no difference between genotypes due to resolution of staining (data not shown).
Fig. 7. Immunohistochemical staining of 4-HNE adducts in kidney sections from wild-type and Nrf2-null mice after FeNTA treatment.
The staining of 4-HNE appears brown in the photomicrographs (40X). (A) wild-type control; (B) Nrf2-null control; (C) wild-type 3 h after FeNTA; (D) Nrf2-null 3 h after FeNTA; (E) wild-type 6 h after FeNTA; (F) Nrf2-null 6 h after FeNTA.
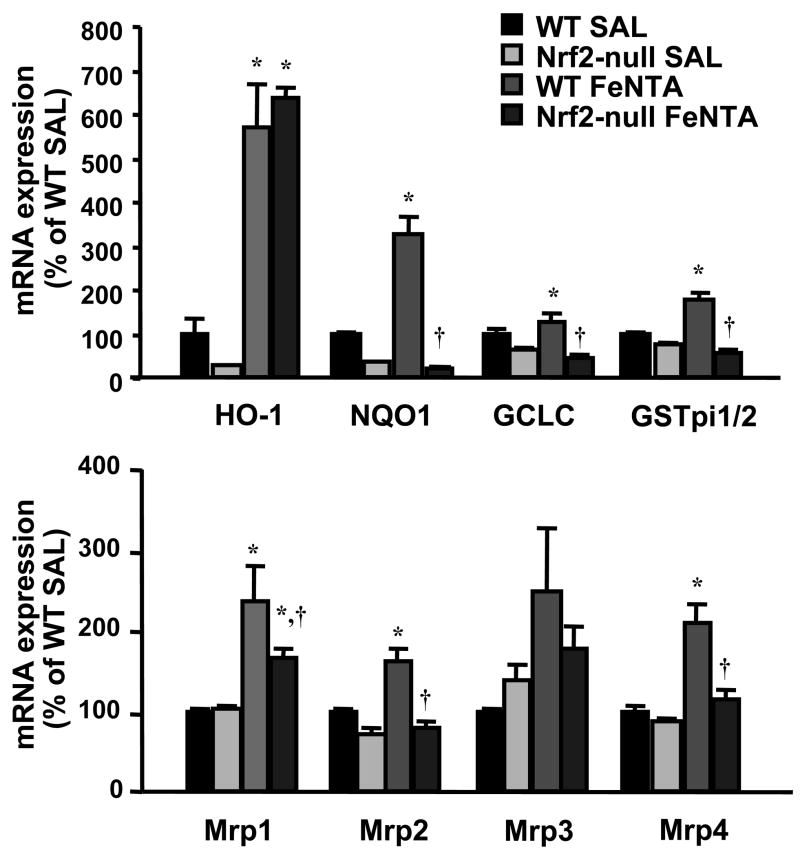
Effect of FeNTA on mRNA expression of Nrf2 target genes in wild-type and Nrf2-null mice
To determine whether induction of detoxification and transporter genes during kidney injury is via Nrf2, wild-type and Nrf2-null mice were subjected to FeNTA (5 mg/kg) treatment, and the renal mRNA expression of Nrf2 target genes was quantified at 6 h. The mRNA levels of HO-1, NQO1, GSTpi1/2, and Mrp2 were lower in vehicle-treated Nrf2-null mice compared to vehicle-treated wild-type mice, suggesting that Nrf2 partially regulates the constitutive expression of these genes (Fig. 8). HO-1 mRNA was increased 470% in wild-type mice 6 h after FeNTA treatment, and was also elevated in Nrf2-null mice, suggesting that Keap1-Nrf2 and additional regulatory pathways may induce HO-1 expression (Fig. 8). Renal NQO1, GCLC, GSTpi1/2, and Mrp1, 2, and 4 mRNA were increased in wild-type mice 6 h after FeNTA treatment by 230, 81, 78, 140, 62, and 110%, respectively, which was largely blocked in Nrf2-null mice (Fig. 8).
Fig. 8. mRNA expression of Nrf2 target genes in kidneys from wild-type and Nrf2-null mice 6 h after FeNTA (5 mg/kg) treatment.
Total RNA was isolated from control and FeNTA-treated mouse kidneys and analyzed by the bDNA assay as described in MATERIALS AND METHODS. Data are presented as mean (percent of WT control) ± S.E.M. (each group, n = 4 or 5 animals). WT-SAL, vehicle-treated wild-type mice; Nrf2-null SAL, vehicle-treated null mice; WT-FeNTA, FeNTA-treated wild-type mice; Nrf2-null FeNTA, FeNTA-treated null mice. Asterisks (*) represent a statistically significant difference (p < 0.05) from vehicle-treated mice of the same genotype; daggers (†) represent a statistically significant difference (p < 0.05) from WT FeNTA group.
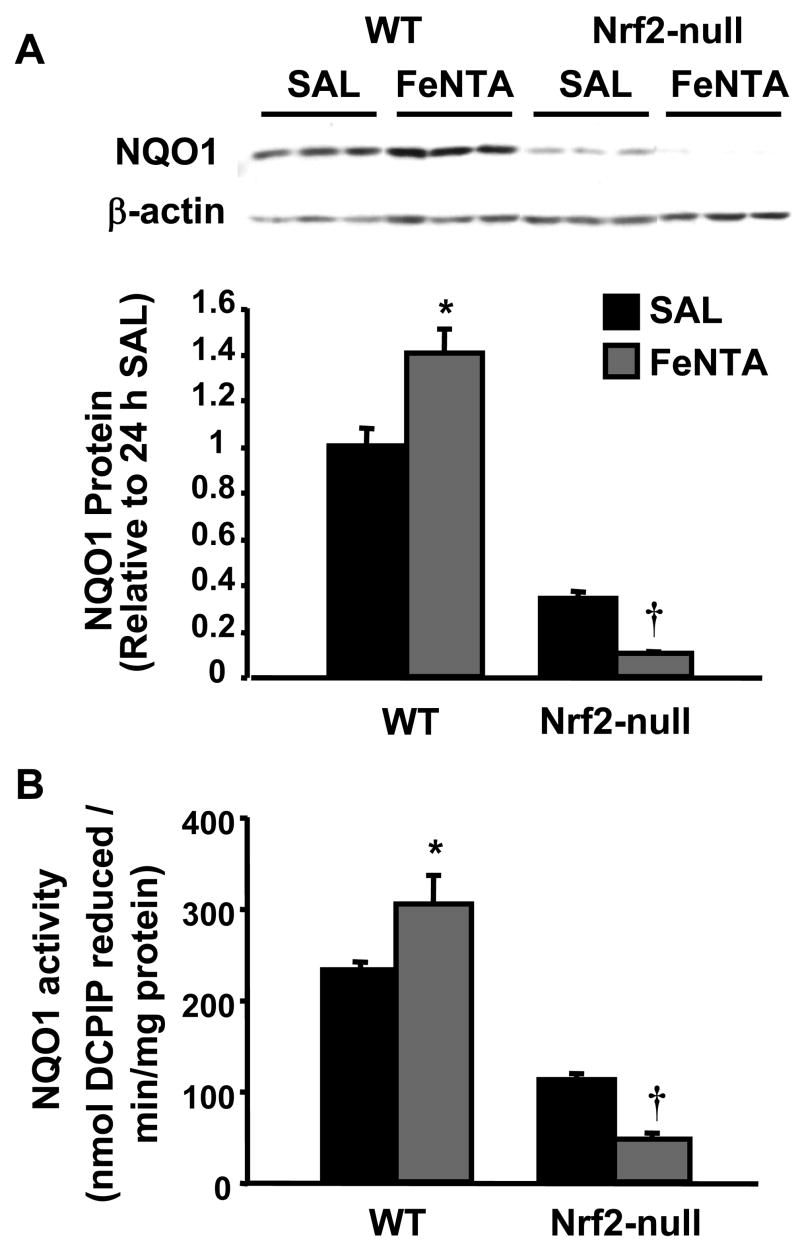
Protein expression and activity of NQO1 after FeNTA treatment in wild-type and Nrf2-null mice
Western blots were performed to confirm that elevated NQO1 mRNA expression observed in kidneys after FeNTA results in protein changes. FeNTA (5 mg/kg) increased NQO1 protein expression in wild-type mice, but not in Nrf2-null mice (Fig. 9A). FeNTA increased NQO1 activity 31% in wild-type mice, but not in Nrf2-null mice (Fig. 9B). Thus, FeNTA increases NQO1 mRNA, protein, and enzyme activity via Nrf2.
Fig. 9. NQO1 protein expression and activity in kidneys from wild-type and Nrf2-null mice 24 h after FeNTA (5 mg/kg) treatment.
A; A western blot of kidney cytosolic proteins stained with an antibody that detects mouse NQO1 (50 μg protein/lane) is shown. Immunoreactive bands were semiquantified by densitometric analysis. Data are expressed as mean (percent of WT control) ± S.E.M. (each group, n = 3 or 4 animals). β-actin staining is shown as a loading control. B; Data are expressed as mean nmol of DCPIP reduced/min/mg protein ± S.E.M. (each group, n = 3 or 4 animals). Asterisks (*) represent a statistically significant difference (p < 0.05) from vehicle-treated mice of the same genotype; daggers (†) represent a statistically significant difference (p < 0.05) from FeNTA-treated wild-type mice.
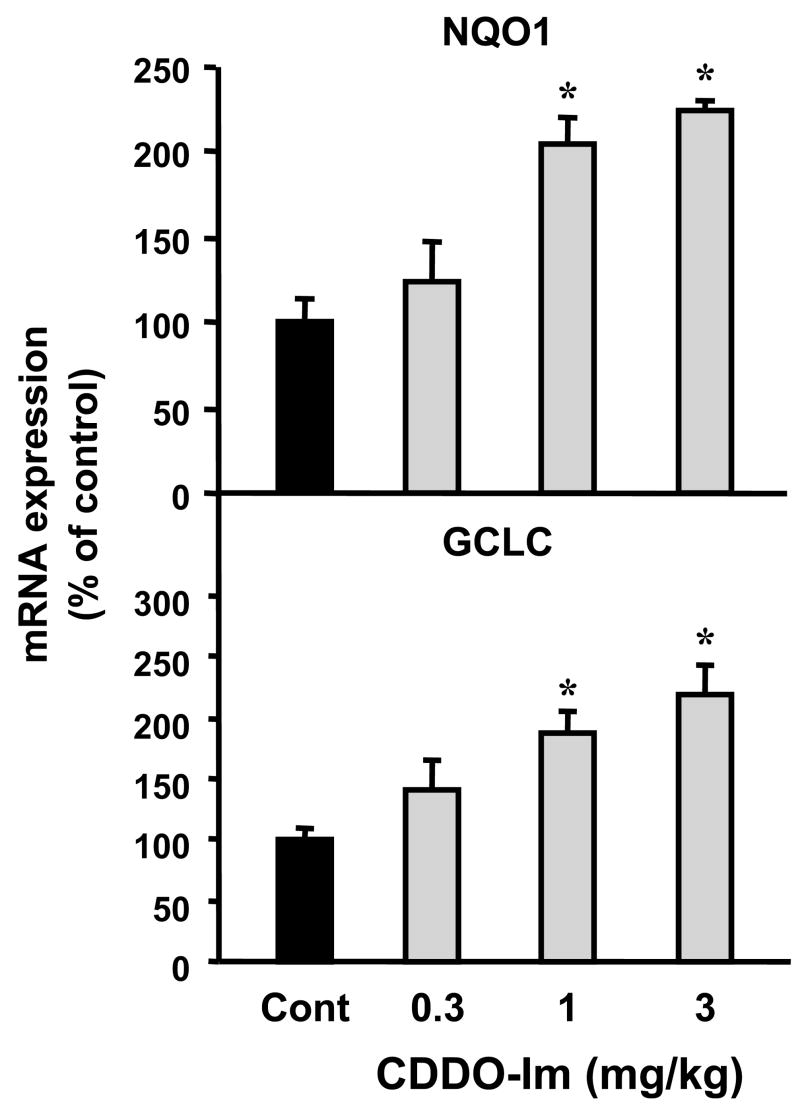
Effect of CDDO-Im on the mRNA expression of Nrf2 target genes
It is known that CDDO-Im can activate Nrf2 in various organs (Liby et al., 2005; Thimmulappa et al., 2006). This experiment was conducted to determine whether CDDO-Im similarly increases expression of Nrf2 target genes in mouse kidneys. Doses of 1 and 3 mg/kg CDDO-Im increased levels of NQO1 and GCLC mRNA by 87%–120% (Fig. 10). No change in mRNA expression of any Nrf2 target was observed at the lowest dose of CDDO-Im (0.3 mg/kg).
Fig. 10. Dose response of Nrf2 target gene expression after CDDO-Im administration.
Total RNA was isolated from CDDO-Im-treated mouse kidneys at different dose levels (0, 0.3, 1, and 3 mg/kg) and analyzed by the bDNA assay as described in MATERIALS AND METHODS. Data are presented as mean (percent of control) ± S.E.M. (each group, n = 3–5 animals). Asterisks (*) represent statistically significant differences (p < 0.05) between control and CDDO-Im groups.
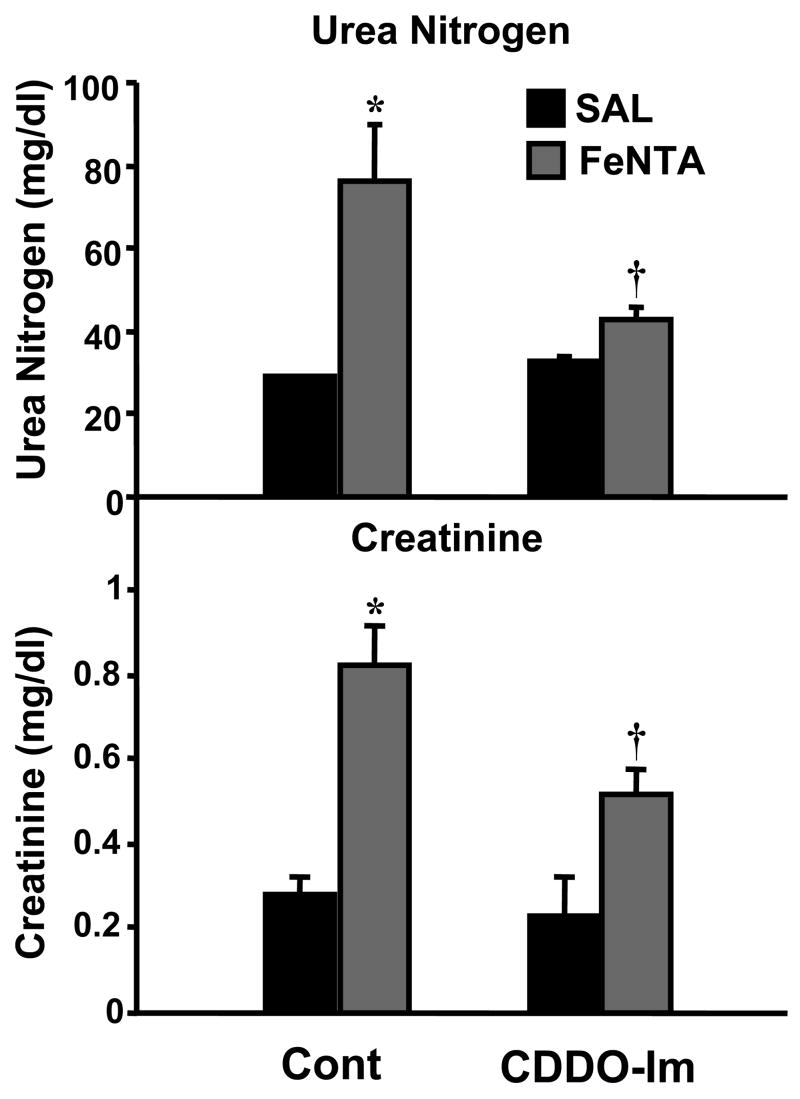
Effect of CDDO-Im on FeNTA-induced renal toxicity
The final experiment was to test whether CDDO-Im protects against FeNTA-induced renal toxicity. For this purpose, mice were pretreated with CDDO-Im (1 mg/kg) 24 and 48 h prior to FeNTA (5 mg/kg) administration and tissues removed 24 h later. Treatment with CDDO-Im alone did not alter serum urea nitrogen or creatinine levels. FeNTA treatment increased serum urea nitrogen and creatinine. Pretreatment with CDDO-Im prior to FeNTA injection (5 mg/kg) attenuated increases in serum urea nitrogen and creatinine, indicating that CDDO-Im protects against FeNTA-induced renal toxicity (Fig. 11).
Fig. 11. Effect of CDDO-Im pretreatment on FeNTA-induced renal toxicity.
CDDO-Im (1 mg/kg) was administered 24 and 48 h prior to FeNTA injection (5 mg Fe/kg), and blood was collected 24 h after FeNTA treatment. Serum urea nitrogen and creatinine were quantified. Data are presented as mean ± S.E.M. (each group, n = 4–12 animals). Control, vehicle group; CDDO-Im, two doses of CDDO-Im group; FeNTA, FeNTA (5mg Fe/kg) group; FeNTA CDDO-Im, two doses of CDDO-Im prior to FeNTA injection. Asterisks (*) represent a statistically significant difference (p < 0.05) from control group; daggers (†) represent a statistically significant difference (p < 0.05) from FeNTA group.
DISCUSSION
Numerous studies have shown that Nrf2 protects many cell types and organ systems from oxidative stress, inflammation, and carcinogenesis through antioxidant response element-mediated transcriptional activation of several detoxifying and antioxidant enzymes (Enomoto et al., 2001; Cho et al., 2002; Ishii et al., 2002; Burton et al., 2006). However, little is known about the defensive role of Nrf2 in the kidneys (Yoh et al., 2001; Hirayama et al., 2003; Tanaka et al., 2007). The purpose of the present study was to determine whether Nrf2 regulates detoxification and transporter genes in the kidneys during oxidative stress and whether Nrf2 protects against oxidative injury in kidney. For this purpose, FeNTA was administered to wild-type and Nrf2-null mice to induce renal oxidative stress and toxicity (Hamazaki et al., 1985; 1986; Kanki et al., 2008; Li et al., 1987). In addition, the ability of the novel Nrf2 activator, CDDO-Im, to protect against FeNTA nephrotoxicity was tested.
A previous study demonstrated that levels of oxidative stress markers, such as 2-thiobarbituric acid-reactive substances, 8-hydroxy-2′-deoxyguanosine, and 4-HNE increase and reach a peak between 1 and 6 h after FeNTA treatment (Okada et al., 1999). Notably, 2-thiobarbituric acid-reactive substances increased dose dependently. In the present study, lipid peroxidation as detected by 4-HNE protein adducts increased 3 h after FeNTA treatment in both genotypes, with greater staining in Nrf2-null mice (Fig. 7C and 7D). Similar to a recent report, Nrf2-null mice exhibit enhanced sensitivity to FeNTA-induced nephrotoxicity (Kanki et al., 2008). Histopathological evaluation revealed more extensive tubule necrosis, more numerous and larger protein casts and dilated tubules in Nrf2-null mice. These data are supported by higher increases in serum urea nitrogen, creatinine, and urinary NAG in Nrf2-null mice after FeNTA. In order to determine the mechanism for increased susceptibility of Nrf2-null mice to FeNTA, constitutive and inducible expression of detoxification and transporter genes were quantified.
Under basal conditions, Nrf2 is sequestered in the cytoplasm by Keap1. Upon exposure to oxidative stress or electrophiles, Nrf2 translocates to the nucleus. Therefore, to determine whether FeNTA might activate Nrf2, we examined the accumulation of Nrf2 protein in the nucleus after FeNTA treatment by western blot (Fig. 2 and 4A). Nuclear translocation of Nrf2 occurred early (6 h) and returned to control values 24 h after FeNTA treatment (Fig. 4A). FeNTA administration dose dependently increased Nrf2 translocation (Fig. 2). The data suggest that FeNTA treatment generated oxidative stress very rapidly, and in turn, stimulated Nrf2 nuclear translocation.
To determine whether FeNTA increased Nrf2-mediated transcription in the kidneys, various Nrf2 target genes, including Phase II enzymes, antioxidant enzymes, and transporters were quantified in FeNTA-treated mice. Most of these genes, except for Mrp3, were induced dose dependently at 6 h after FeNTA treatment, and returned to control levels 24 h after FeNTA treatment (Fig. 3 and 4B). Taken together, these data indicate that the nuclear accumulation of Nrf2 is functional and results in time-dependent gene induction.
To determine whether up-regulation of cytoprotective genes is dependent on Nrf2 expression, FeNTA was also administered to Nrf2–null mice. FeNTA induced most of the prototypical Nrf2 target genes, such as NQO1 and GCLC, in wild-type mice, but not in the Nrf2-null mice, indicating that FeNTA regulates these genes via Nrf2 (Fig. 8) (Venugopal et al., 1996; McWalter et al., 2004). The Pi-class of GSTs plays a pivotal role in the detoxification of xenobiotics, preventing carcinogenesis, and enabling drug resistance. Transient transfection studies have shown that Nrf2 directly activates the GSTpi1 gene (Ikeda et al., 2002). A previous study showed that class Pi GST isozyme mRNA and protein expression were induced after FeNTA treatment, and that the induction may be important in mediating cell repair and/or increasing resistance to subsequent injury (Fukuda et al., 1996). In the present study, GSTpi1/2 mRNA expression was elevated in wild-type mice, but not in Nrf2-null mice, indicating the induction of GSTpi1/2 by FeNTA is via Nrf2 (Fig. 8). HO-1 was induced in both wild-type and Nrf2-null mice, suggesting that the induction of this gene may involve Nrf2 and additional signaling pathways (Fig. 8). The promoter region of the human HO-1 gene contains a number of putative binding sites for transcription factors, including nuclear factor-κB, activator proteins 1 and 2 and CCAAT/enhancer-binding proteins (Alam and Den, 1992; Lavrovsky et al., 1994; Alam et al., 2003). Thus, HO-1 could be induced through alternate pathways in Nrf2-null mice treated with FeNTA.
Mrps play a major role in hepatobiliary and renal elimination of many structurally diverse xenobiotics, including organic anions and drug conjugates (Bodo et al., 2003). Mrps also transport endogenous molecules, such as leukotriene C4, prostaglandin, bilirubin glucuronide, and bile acids (Homolya et al., 2003). Recent studies have demonstrated that mouse Mrp1-4 are regulated by Nrf2 (Hayashi et al., 2003; Vollrath et al., 2006; Maher et al., 2007). In this study, FeNTA increased the mRNA expression of Mrp1, 2, and 4 in wild-type mice, but not in Nrf2-null mice, indicating that the induction of these genes by FeNTA is Nrf2 dependent (Fig. 8). Previous studies have shown that Mrp2 excretes glutathione conjugates of 4-HNE into bile (Ji et al., 2002; Reichard et al., 2003). Mrp2 and 4 are localized to the brush border membrane in kidneys (Schaub et al., 1997; Chen et al., 2005). Taken together, increased Mrp2 and/or Mrp4 expression after FeNTA treatment may enhance the excretion of 4-HNE metabolites, including 4-HNE mercapturic acid and 1,4-dihydroxynonene mercapturic acid, into urine (Alary et al., 1995). In the present study, more 4-HNE staining was observed in Nrf2-null mice than wild-type mice after FeNTA treatment (Fig. 7). This may be a consequence of the inability of Nrf2-null mice to induce Mrp2 and Mrp4 mRNA and eliminate 4-HNE metabolites. Overall, greater retention of 4-HNE may contribute to enhanced toxicity in Nrf2-null mice.
Because NQO1 is known as a prototypical Nrf2 target gene, protein expression and activity of NQO1 were quantified after FeNTA treatment (Venugopal and Jaiswal, 1996). NQO1 protein expression and activity were increased 24 h after FeNTA treatment in an Nrf2-dependent fashion (Fig. 9). NQO1 plays a role in detoxifying superoxide, regenerating endogenous antioxidants, as well as metabolizing xenobiotics. A recent study demonstrated that human NQO1 is capable of reducing iron to a less reactive form (Alary et al., 1995). Thus, the induction of NQO1 after FeNTA treatment could limit oxidative stress and renal injury by these mechanisms. Similarly, the reduced constitutive and impaired induction of NQO1 in Nrf2-null mice likely contributes to their enhanced susceptibility to FeNTA.
It is known that metallothionein-1 plays a protective role against FeNTA-mediated renal oxidative damage (Iqbal et al., 2003). In the present study, metallothionein-1 mRNA was increased in kidneys from wild-type mice, but it was further increased in Nrf2-null mice (data not shown). These data suggest that metallothionein-1 may attempt to compensate for loss of the antioxidant defense system by the Keap1-Nrf2 signaling pathway.
Lastly, it was tested whether pretreatment with an Nrf2 activator before FeNTA, would protect the kidney from FeNTA-induced renal injury. The synthetic triterpenoid derivative, CDDO-Im is a potent inducer of cytoprotective enzymes and inhibitors of inflammation. Previous studies have shown that CDDO-Im activates Nrf2 and increases expression of a number of antioxidant and detoxification genes through Keap1-Nrf2-antioxidant response element signaling (Liby et al., 2005; Thimmulappa et al., 2006). In the present study, a single dose of CDDO-Im increased NQO1 and GCLC in a dose-dependent fashion, suggesting that CDDO-Im also activates Nrf2 in the kidney (Fig. 10). Administration of CDDO-Im 24 and 48 h prior to FeNTA injection protected mice from FeNTA-induced renal toxicity (Fig. 11). Although it is necessary to use Nrf2-null mice to confirm the specificity of CDDO-Im to Nrf2, using both genetic (Nrf2-null mice) and pharmacological (CDDO-Im) approaches, the present study demonstrates that Nrf2 protects against FeNTA-induced renal toxicity in mice.
In conclusion, FeNTA activates Nrf2 in kidneys and induces expression of Nrf2 target genes, including efflux transporters as well as antioxidant and Phase II enzymes. This coordinate induction of genes protects the kidneys from the progression of oxidative damage after FeNTA administration. From a toxicological standpoint, activation of Nrf2 target genes in preclinical studies may represent a biomarker for renal injury. Similarly, CDDO-Im-mediated protection against FeNTA-induced damage suggests that development of drugs which activate Nrf2-mediated transcription may be a novel approach for preventing and/or treating renal pathologies with an oxidative stress component.
Supplementary Material
Acknowledgments
This work was supported by NIH grants ES-09649, ES-09716, ES-07079, and RR021940.
ABBREVIATIONS
- bDNA
branched DNA signal amplification
- CDDO-Im
(1-[2-cyano-3-,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole)
- DCPIP
2,6-dichlorophenolindophenol
- FeNTA
ferric nitrilotriacetate
- 4-HNE
4-hydroxynonenal
- GCLC
glutamate cysteine ligase catalytic subunit
- GST
glutathione-S-transferase
- HO-1
heme oxygenase-1
- Keap1
Kelch-like ECH-associated protein 1
- Mrp
Multidrug resistance-associated protein
- NAG
N-acetyl-beta-glucosaminidase
- NQO1, NAD(P)H
quinone oxidoreductase 1
- Nrf2
NF-e2-related factor 2
- RLU
relative light units
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alam J, Den Z. Distal AP-1 binding sites mediate basal level enhancement and TPA induction of the mouse heme oxygenase-1 gene. J Biol Chem. 1992;267:21894–21900. [PubMed] [Google Scholar]
- Alam J, Killeen E, Gong P, Naquin R, Hu B, Stewart D, Ingelfinger JR, Nath KA. Heme activates the heme oxygenase-1 gene in renal epithelial cells by stabilizing Nrf2. Am J Physiol Renal Physiol. 2003;284:F743–752. doi: 10.1152/ajprenal.00376.2002. [DOI] [PubMed] [Google Scholar]
- Alary J, Bravais F, Cravedi JP, Debrauwer L, Rao D, Bories G. Mercapturic acid conjugates as urinary end metabolites of the lipid peroxidation product 4-hydroxy-2-nonenal in the rat. Chem Res Toxicol. 1995;8:34–39. doi: 10.1021/tx00043a004. [DOI] [PubMed] [Google Scholar]
- Aleksunes LM, Scheffer GL, Jakowski AB, Pruimboom-Brees IM, Manautou JE. Coordinated expression of multidrug resistance-associated proteins (Mrps) in mouse liver during toxicant-induced injury. Toxicol Sci. 2006;89:370–379. doi: 10.1093/toxsci/kfi332. [DOI] [PubMed] [Google Scholar]
- Aleksunes LM, Slitt AM, Cherrington NJ, Thibodeau MS, Klaassen CD, Manautou JE. Differential expression of mouse hepatic transporter genes in response to acetaminophen and carbon tetrachloride. Toxicol Sci. 2005;83:44–52. doi: 10.1093/toxsci/kfi013. [DOI] [PubMed] [Google Scholar]
- Benson AM, Hunkeler MJ, Talalay P. Increase of NAD(P)H:quinone reductase by dietary antioxidants: possible role in protection against carcinogenesis and toxicity. Proc Natl Acad Sci U S A. 1980;77:5216–5220. doi: 10.1073/pnas.77.9.5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodó A, Bakos E, Szeri F, Váradi A, Sarkadi B. The role of multidrug transporters in drug availability, metabolism and toxicity. Toxicol Lett. 2003;133:140–141. doi: 10.1016/s0378-4274(02)00497-6. [DOI] [PubMed] [Google Scholar]
- Burton NC, Kensler TW, Guilarte TR. In vivo modulation of the Parkinsonian phenotype by Nrf2. Neurotoxicology. 2006;27:1094–1100. doi: 10.1016/j.neuro.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Chen C, Slitt AL, Dieter MZ, Tanaka Y, Scheffer GL, Klaassen CD. Up-regulation of Mrp4 expression in kidney of Mrp2-deficient TR- rats. Biochem Pharmacol. 2005;70:1088–1095. doi: 10.1016/j.bcp.2005.06.019. [DOI] [PubMed] [Google Scholar]
- Cho HY, Jedlicka AE, Reddy SP, Kensler TW, Yamamoto M, Zhang LY, Kleeberger SR. Role of NRF2 in protection against hyperoxic lung injury in mice. Am J Respir Cell Mol Biol. 2002;26:175–182. doi: 10.1165/ajrcmb.26.2.4501. [DOI] [PubMed] [Google Scholar]
- Enomoto A, Itoh K, Nagayoshi E, Haruta J, Kimura T, O’Connor T, Harada T, Yamamoto M. High sensitivity of Nrf2 knockout mice to acetaminophen hepatotoxicity associated with decreased expression of ARE-regulated drug metabolizing enzymes and antioxidant genes. Toxicol Sci. 2001;59:169–177. doi: 10.1093/toxsci/59.1.169. [DOI] [PubMed] [Google Scholar]
- Ernster L. DT-diaphorase. Methods Enzymol. 1967;10:309–317. doi: 10.1016/0076-6879(90)86122-c. [DOI] [PubMed] [Google Scholar]
- Fukuda A, Osawa T, Oda H, Toyokuni S, Satoh K, Uchida K. Oxidative stress response in iron-induced renal carcinogenesis: acute nephrotoxicity mediates the enhanced expression of glutathione S-transferase Yp isozyme. Arch Biochem Biophys. 1996;329:39–46. doi: 10.1006/abbi.1996.0189. [DOI] [PubMed] [Google Scholar]
- Hamazaki S, Okada S, Ebina Y, Midorikawa O. Acute renal failure and glucosuria induced by ferric nitrilotriacetate in rats. Toxicol Appl Pharmacol. 1985;77:267–274. doi: 10.1016/0041-008x(85)90326-6. [DOI] [PubMed] [Google Scholar]
- Hamazaki S, Okada S, Ebina Y, Fujioka M, Midorikawa O. Nephrotoxicity of ferric nitrilotriacetate. An electron-microscopic and metabolic study. Am J Pathol. 1986;123:343–350. [PMC free article] [PubMed] [Google Scholar]
- Hayashi A, Suzuki H, Itoh K, Yamamoto M, Sugiyama Y. Transcription factor Nrf2 is required for the constitutive and inducible expression of multidrug resistance-associated protein 1 in mouse embryo fibroblasts. Biochem Biophys Res Commun. 2003;310:824–829. doi: 10.1016/j.bbrc.2003.09.086. [DOI] [PubMed] [Google Scholar]
- Hirayama A, Yoh K, Nagase S, Ueda A, Itoh K, Morito N, Hirayama K, Takahashi S, Yamamoto M, Koyama A. EPR imaging of reducing activity in Nrf2 transcriptional factor-deficient mice. Free Radic Biol Med. 2003;34:1236–1242. doi: 10.1016/s0891-5849(03)00073-x. [DOI] [PubMed] [Google Scholar]
- Homolya L, Varadi A, Sarkadi B. Multidrug resistance-associated proteins: Export pumps for conjugates with glutathione, glucuronate or sulfate. Biofactors. 2003;17:103–114. doi: 10.1002/biof.5520170111. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Serria MS, Kakizaki I, Hatayama I, Satoh K, Tsuchida S, Muramatsu M, Nishi S, Sakai M. Activation of mouse Pi-class glutathione S-transferase gene by Nrf2(NF-E2-related factor 2) and androgen. Biochem J. 2002;364:563–570. doi: 10.1042/BJ20011756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal M, Noor R, Mizuno R, Okada S. Protective role of zinc-metallothionein (Zn-MT) in iron nitrilotriacetate (Fe-NTA)-induced renal oxidative damage. Redox Rep. 2003;8:163–167. doi: 10.1179/135100003225001557. [DOI] [PubMed] [Google Scholar]
- Ishii T, Itoh K, Takahashi S, Sato H, Yanagawa T, Katoh Y, Bannai S, Yamamoto M. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J Biol Chem. 2000;275:16023–16029. doi: 10.1074/jbc.275.21.16023. [DOI] [PubMed] [Google Scholar]
- Ishii T, Itoh K, Yamamoto M. Roles of Nrf2 in activation of antioxidant enzyme genes via antioxidant responsive elements. Methods Enzymol. 2002;348:182–190. doi: 10.1016/s0076-6879(02)48637-5. [DOI] [PubMed] [Google Scholar]
- Ji B, Ito K, Suzuki H, Sugiyama Y, Horie T. Multidrug resistance-associated protein2 (MRP2) plays an important role in the biliary excretion of glutathione conjugates of 4-hydroxynonenal. Free Radic Biol Med. 2002;33:370–378. doi: 10.1016/s0891-5849(02)00906-1. [DOI] [PubMed] [Google Scholar]
- Kanki K, Umemura T, Kitamura Y, Ishii Y, Kuroiwa Y, Kodama Y, Itoh K, Yamamoto M, Nishikawa A, Hirose M. A possible role of Nrf2 in prevention of renal oxidative damage by ferric nitrilotriacetate. Tox Path. 2008 doi: 10.1177/0192623307311401. In Press. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Kang MI, Watai Y, Tong KI, Shibata T, Uchida K, Yamamoto M. Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1. Mol Cell Biol. 2006;26:221–229. doi: 10.1128/MCB.26.1.221-229.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavrovsky Y, Schwartzman ML, Levere RD, Kappas A, Abraham NG. Identification of binding sites for transcription factors NF-kappa B and AP-2 in the promoter region of the human heme oxygenase 1 gene. Proc Natl Acad Sci U S A. 1994;91:5987–5991. doi: 10.1073/pnas.91.13.5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Hir M, Dubach UC, Schmidt U. Quantitative distribution of lysosomal hydrolases in the rat nephron. Histochemistry. 1979;63:245–251. doi: 10.1007/BF00644546. [DOI] [PubMed] [Google Scholar]
- Li JL, Okada S, Hamazaki S, Ebina Y, Midorikawa O. Subacute nephrotoxicity and induction of renal cell carcinoma in mice treated with ferric nitrilotriacetate. Cancer Res. 1987;47:1867–1869. [PubMed] [Google Scholar]
- Liby K, Hock T, Yore MM, Suh N, Place AE, Risingsong R, Williams CR, Royce DB, Honda T, Honda Y, Gribble GW, Hill-Kapturczak N, Agarwal A, Sporn MB. The synthetic triterpenoids, CDDO and CDDO-imidazolide, are potent inducers of heme oxygenase-1 and Nrf2/ARE signaling. Cancer Res. 2005;65:4789–4798. doi: 10.1158/0008-5472.CAN-04-4539. [DOI] [PubMed] [Google Scholar]
- Maher JM, Dieter MZ, Aleksunes LM, Slitt AL, Guo G, Tanaka Y, Scheffer GL, Chan JY, Manautou JE, Chen Y, Dalton TP, Yamamoto M, Klaassen CD. Oxidative and electrophilic stress induces Mrp transporters via the Nrf2 transcriptional pathway. Hepatology. 2007;46:1597–1610. doi: 10.1002/hep.21831. [DOI] [PubMed] [Google Scholar]
- Maher JM, Slitt AL, Cherrington NJ, Cheng X, Klaassen CD. Tissue distribution and hepatic and renal ontogeny of the multidrug resistance-associated protein (Mrp) family in mice. Drug Metab Dispos. 2005;33:947–955. doi: 10.1124/dmd.105.003780. [DOI] [PubMed] [Google Scholar]
- McWalter GK, Higgins LG, McLellan LI, Henderson CJ, Song L, Thornalley PJ, Itoh K, Yamamoto M, Hayes JD. Transcription factor Nrf2 is essential for induction of NAD(P)H:quinone oxidoreductase 1, glutathione S-transferases, and glutamate cysteine ligase by broccoli seeds and isothiocyanates. J Nutr. 2004;134:3499S–3506S. doi: 10.1093/jn/134.12.3499S. [DOI] [PubMed] [Google Scholar]
- Okada K, Wangpoengtrakul C, Osawa T, Toyokuni S, Tanaka K, Uchida K. 4-Hydroxy-2-nonenal-mediated impairment of intracellular proteolysis during oxidative stress. Identification of proteasomes as target molecules. J Biol Chem. 1999;274:23787–23793. doi: 10.1074/jbc.274.34.23787. [DOI] [PubMed] [Google Scholar]
- Onyenwoke RU, Wiegel J. Iron (III) reduction: A novel activity of the human NAD(P)H:oxidoreductase. Biochem Biophys Res Commun. 2007;353:389–393. doi: 10.1016/j.bbrc.2006.12.022. [DOI] [PubMed] [Google Scholar]
- Reichard JF, Doorn JA, Simon F, Taylor MS, Petersen DR. Characterization of multidrug resistance-associated protein 2 in the hepatocellular disposition of 4-hydroxynonenal. Arch Biochem Biophys. 2003;411:243–250. doi: 10.1016/s0003-9861(03)00002-x. [DOI] [PubMed] [Google Scholar]
- Schaub TP, Kartenbeck J, König J, Vogel O, Witzgall R, Kriz W, Keppler D. Expression of the conjugate export pump encoded by the mrp2 gene in the apical membrane of kidney proximal tubules. J Am Soc Nephrol. 1997;8:1213–1221. doi: 10.1681/ASN.V881213. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Maher JM, Chen C, Klaassen CD. Hepatic ischemia-reperfusion induces renal heme oxygenase-1 via NF-E2-related factor 2 in rats and mice. Mol Pharmacol. 2007;71:817–825. doi: 10.1124/mol.106.029033. [DOI] [PubMed] [Google Scholar]
- Thimmulappa RK, Scollick C, Traore K, Yates M, Trush MA, Liby KT, Sporn MB, Yamamoto M, Kensler TW, Biswal S. Nrf2-dependent protection from LPS induced inflammatory response and mortality by CDDO-Imidazolide. Biochem Biophys Res Commun. 2006;351:883–889. doi: 10.1016/j.bbrc.2006.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyokuni S, Tanaka T, Hattori Y, Nishiyama Y, Yoshida A, Uchida K, Hiai H, Ochi H, Osawa T. Quantitative immunohistochemical determination of 8-hydroxy-2′-deoxyguanosine by a monoclonal antibody N45.1: its application to ferric nitrilotriacetate-induced renal carcinogenesis model. Lab Invest. 1997;76:365–374. [PubMed] [Google Scholar]
- Toyokuni S, Uchida K, Okamoto K, Hattori-Nakakuki Y, Hiai H, Stadtman ER. Formation of 4-hydroxy-2-nonenal-modified proteins in the renal proximal tubules of rats treated with a renal carcinogen, ferric nitrilotriacetate. Proc Natl Acad Sci U S A. 1994;91:2616–2620. doi: 10.1073/pnas.91.7.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopal R, Jaiswal AK. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc Natl Acad Sci U S A. 1996;93:14960–14965. doi: 10.1073/pnas.93.25.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollrath V, Wielandt AM, Iruretagoyena M, Chianale J. Role of Nrf2 in the regulation of the Mrp2 (ABCC2) gene. Biochem J. 2006;395:599–609. doi: 10.1042/BJ20051518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoh K, Itoh K, Enomoto A, Hirayama A, Yamaguchi N, Kobayashi M, Morito N, Koyama A, Yamamoto M, Takahashi S. Nrf2-deficient female mice develop lupus-like autoimmune nephritis. Kidney Int. 2001;60:1343–1353. doi: 10.1046/j.1523-1755.2001.00939.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.