Abstract
Periarcuate frontal cortex is involved in the control of smooth pursuit eye movements, but its role remains unclear. To better understand the control of pursuit by the “frontal pursuit area” (FPA), we applied electrical microstimulation when the monkeys were performing a variety of oculomotor tasks. In agreement with previous studies, electrical stimulation consisting of a train of 50-μA pulses at 333 Hz during fixation of a stationary target elicited smooth eye movements with a short latency (∼26 ms). The size of the elicited smooth eye movements was enhanced when the stimulation pulses were delivered during the maintenance of pursuit. The enhancement increased as a function of ongoing pursuit speed and was greater during pursuit in the same versus opposite direction of the eye movements evoked at a site. If stimulation was delivered during pursuit in eight different directions, the elicited eye velocity was fit best by a model incorporating two stimulation effects: a directional signal that drives eye velocity and an increase in the gain of ongoing pursuit eye speed in all directions. Separate experiments tested the effect of stimulation on the response to specific image motions. Stimulation consisted of a train of pulses at 100 or 200 Hz delivered during fixation so that only small smooth eye movements were elicited. If the stationary target was perturbed briefly during microstimulation, normally weak eye movement responses showed strong enhancement. If delivered at the initiation of pursuit, the same microstimulation caused enhancement of the presaccadic initiation of pursuit for steps of target velocity that moved the target either away from the position of fixation or in the direction of the eye movement caused by stimulation at the site. Stimulation in the FPA increased the latency of saccades to stationary or moving targets. Our results show that the FPA has two kinds of effects on the pursuit system. One drives smooth eye velocity in a fixed direction and is subject to on-line gain control by ongoing pursuit. The other causes enhancement of both the speed of ongoing pursuit and the responses to visual motion in a way that is not strongly selective for the direction of pursuit. Enhancement may operate either at a single site or at multiple sites. We conclude that the FPA plays an important role in on-line gain control for pursuit as well as possibly delivering commands for the direction and speed of smooth eye motion.
INTRODUCTION
There is now substantial evidence implicating an area that we call the “frontal pursuit area” (FPA) in smooth pursuit eye movements. The FPA has also been called the “smooth eye movement part of the frontal eye fields” and lies just caudal to and below the part of the frontal eye fields that is involved in saccadic eye movements (e.g., Gottlieb et al. 1993, 1994). Physiological evidence of its importance for pursuit comes from lesions (Keating 1991, 1993; Lynch 1987; MacAvoy et al. 1991; Shi et al. 1998), electrical stimulation (Gottlieb et al. 1993, 1994; Tian and Lynch 1996a), imaging (Berman et al. 1999; Petit and Haxby 1999; Petit et al. 1997), and unit recording studies (Fukushima et al. 2000; Gottlieb et al. 1994; MacAvoy et al. 1991; Tanaka and Fukushima 1998).
The anatomical connections of the FPA are also appropriate for a role in pursuit eye movements. The FPA and adjacent saccadic frontal eye fields have reciprocal connections with extrastriate visual motion areas such as the middle temporal area (MT) and the medial superior temporal area (MST) (e.g., Huerta et al. 1987; Leichnetz 1989; Stanton et al. 1988a, 1995; Tian and Lynch 1996b), both of which are thought to be important for pursuit. The FPA also sends strong outputs to the caudate nucleus (Cui et al. 2000; Yan et al. 1999) where a recent imaging study found a strong increase in the regional blood flow during pursuit (O’Driscoll et al. 2000). The descending output from the FPA and adjacent frontal eye fields projects to the cerebellum via the dorsomedial pontine nuclei and the nucleus reticularis tegmenti pontis (Huerta et al. 1986; Leichnetz 1989; Leichnetz et al. 1984; Stanton et al. 1988b; Yan et al. 1999), and there is a strong projection from the inferior frontal eye fields to the dorsolateral pontine nuclei (DLPN) in macaque monkeys (Leichnetz 1989). Despite the accumulation of the evidence that the FPA is involved in pursuit, it is not known whether the FPA has a unique role or if it simply provides a cortical representation of the eye-velocity command for pursuit. In the present study and an earlier report (Tanaka and Lisberger 2001), we have begun to search for possible unique functions of the FPA by analyzing the interaction among visual stimuli, pursuit eye movement, and electrical microstimulation in the FPA.
Our analysis of possible functions for the FPA has stemmed from behavioral studies showing that there are at least two different operations performed within the pursuit system (Goldreich et al. 1992; Grasse and Lisberger 1992; Keating and Pierre 1996; Krauzlis and Lisberger 1994; Krauzlis and Miles 1996a; Tanaka and Lisberger 2000). One operation converts visual motion into eye velocity, while the other provides an on-line “gain control” that regulates either or both of the strength of the visual-motor transmission for pursuit and the gain of the eye-velocity commands for pursuit. The most direct evidence for the on-line gain control was provided by the experiments of Schwartz and Lisberger (1994), who showed that eye movement responses were tiny if a brief target perturbation was presented during fixation but were large if the same perturbation was presented during the maintenance of pursuit. In addition, recent analyses have suggested that post-saccadic enhancement of pursuit is mediated by the same on-line gain control (Lisberger 1998) and that the gain control may be spatially selective in a way that causes target choice (Gardner and Lisberger 2001).
The neural loci of on-line gain control are not known. Because lesions made in MST or FPA cause deficits in the initiation and the maintenance of pursuit (references given in the preceding text) and because the pursuit-related neurons recorded from these areas carry both retinal and extra-retinal signals, it seems plausible that MST, FPA, or both participate in gain control (Grasse and Lisberger 1992; Keating and Pierre 1996; Schwartz and Lisberger 1994). We have now tested this hypothesis for the FPA with the use of electrical microstimulation. Our data show that activation of the FPA modulates the on-line gain of pursuit during both the initiation and the maintenance of pursuit.
Some of these results have been presented in a brief report (Tanaka and Lisberger 2001).
METHODS
Animal preparation
Data were collected from two male rhesus monkeys (Macaca mulatta, monkeys PCK and OLV), weighing 8–10 kg. All experimental protocols described in the following text were approved in advance by the Institutional Animal Care and Use Committee of the University of California, San Francisco. The procedures for animal preparation were similar to those described previously (Lisberger and Westbrook 1985). Monkeys were trained to sit in a primate chair and to fixate a spot of light to obtain liquid reward. Under isoflurane anesthesia and using sterile procedure, a head holder was installed over the skull using orthopedic stainless steel plates and dental acrylic. Within a few weeks after the first surgery, a coil of wire was implanted between the sclera and Tenon’s capsule to record eye movements (Judge et al. 1980) under the same surgical condition. For all subsequent training and experiments, the implanted head holder was used to secure the animal’s head to the ceiling of the primate chair so that horizontal and vertical eye position could be recorded.
After training in oculomotor tasks was complete, the monkeys participated in behavioral experiments that lasted for several months. A third surgery then was performed to implant a stainless steel cylinder over the right arcuate sulcus. We used a trephine to create holes in the skull centered at A26.5, L20 for monkey PCK and A26, L13 for monkey OLV. The cylinder was tilted 30–31° from the parasaggital plane to allow electrode penetrations through the dura roughly perpendicular to the cortical surface. The animals received analgesia with intramuscular injections of buprenorphine (Buprenex, 0.01 mg/kg) for 2–3 days following each surgery. Topical antibiotics were administered around the implants as necessary. The water intake was controlled daily, and the weight of the animal was checked before each experimental or training session.
Visual stimulus and behavioral tasks
The animals faced an analog oscilloscope (Hewlett Packard 1304A) that was located 28 cm from the eyes and subtended 42 × 36° of visual angle. Visual stimuli were presented on the oscilloscope at a refresh rate of 250 Hz under the control of a Pentium PC computer. A 0.2° diam, white spot with luminance of 3.8 cd/m2 served as a visual stimulus. All experiments were carried out in a dark room. The horizontal and vertical eye-position signals were calibrated by having the animals fixate a stationary target at known visual angles. Thereafter targets and electrical stimulation were presented in individual trials. In each trial, eye position was compared with target position, and the animal was reinforced by drops of water or juice for maintaining eye position within a window that surrounded target position throughout the trial. The trial was aborted and followed by a newly selected trial if the monkey failed to maintain eye position within the specified window. Different window sizes were used at different times during different types of target motions (see following text).
Three behavioral paradigms were used: fixation task, saccade task, and pursuit task. In all tasks, only a single target was presented at any given time. Target motion could be in any of eight directions including the four cardinal directions and the four 45° oblique directions. Trials that presented target motion in different directions and those with or without electrical stimulation were interleaved randomly within a block.
FIXATION TASK
A stationary target appeared at the center of the screen for 2,500–3,000 ms. In some trials, the target remained stationary throughout the trial. In others, the stationary target underwent a brief perturbation 1,000–1,500 ms after its appearance. The perturbation velocity consisted of a single cycle of a 10-Hz sine wave with peak-to-peak velocity of 20°/s. This yielded a maximum position excursion of 0.32°. The monkeys were required to maintain eye position within 1° of target position in fixation trials and normally did not make any saccades.
SACCADE TASK
A target appeared at the center of the screen for a random duration of 1,000–1,500 ms and then jumped 4–16° and remained stationary for an additional 1,500 ms. The monkey was required to fixate within 1° while the target was stationary. The fixation window was suspended for 300–800 ms at the time of the target step, and the monkey was required to fixate within 4° when the requirements were reinstated.
PURSUIT TASK
A target appeared and remained stationary for a random duration of 1,000–1,500 ms. The target then executed a “step-ramp” of position (Rashbass 1961) consisting of 1–6° steps and ramps at 5–30°/s. Except for the experiments summarized in Figs. 13 and 14, the direction of target motion was opposite to the direction of target step, and the size of the step was adjusted so that the target crossed the position of fixation 200 ms after the onset of motion. As a consequence, monkeys initiated pursuit without catch-up saccades in most trials. When we examined the effects of electrical stimulation during the maintenance of pursuit, the fixation target appeared at an eccentric location on the screen before executing step-ramp motion. The initial position was adjusted so that the target would cross the center of the screen 600 ms after the onset of step-ramp motion. When we examined the effects of electrical stimulation on the initiation of pursuit or searched for the pursuit-related regions, the fixation target appeared at the center of the screen before executing step-ramp motion.
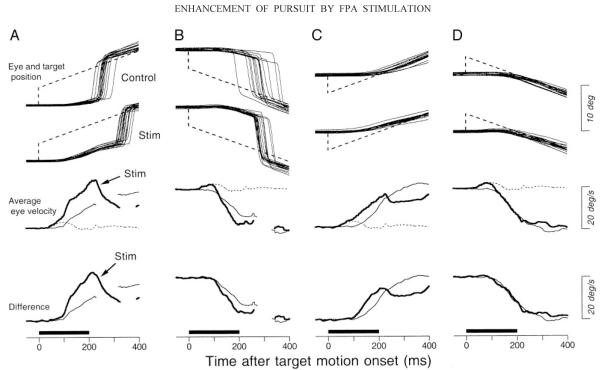
FIG. 13.
Effects of electrical stimulation on the initiation of pursuit. Top 2 rows: horizontal eye and target position aligned on the target motion onset for trials with (Stim) or without (Control) electrical stimulation. Third row: average eye velocity in 3 different task conditions. The dashed traces show responses elicited by stimulation without target motion and are the same for A through D. The fine, continuous traces show responses to target motion in the absence of stimulation. The bold traces show responses to target motion during microstimulation. Interruptions in traces indicate intervals when catch-up saccades were so frequent that data from fewer than 5 trials were available for averaging. Bottom row: comparison of the responses to target motion with or without stimulation. The bold trace shows the time course of the difference between eye velocity for target motion during stimulation and that during stimulation alone. The bold horizontal bars below each column indicate the times of stimulation. A: the target stepped and ramped rightward. B: the target stepped and ramped leftward. C: the target stepped leftward and ramped rightward. D: the target rightward and ramped leftward. Step amplitude was always 4° and ramp speed was always 20°/s.
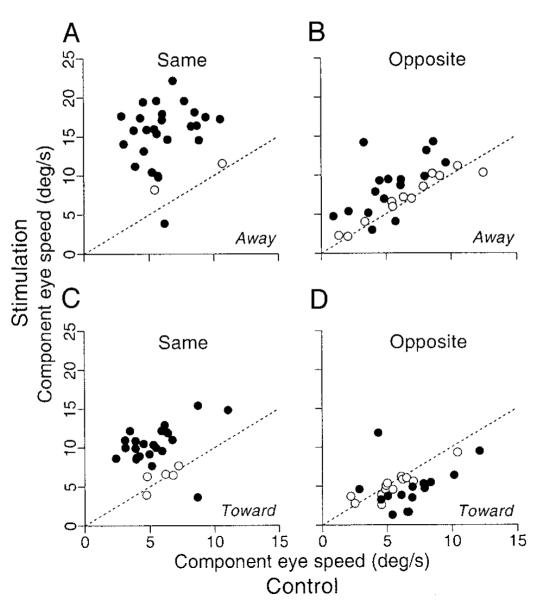
FIG. 14.
Summary of the effects of stimulation on the pursuit initiation. Each graph plots eye speed measured 180 ms after the onset of target motion for trials that included stimulation of the FPA as a function of that during nonstimulation controls. Data for the stimulation trials were measured after subtracting the response to electrical stimulation alone and projecting the remaining response onto the same axis as the eye velocity evoked during the initiation of pursuit without stimulation. The 4 graphs show data sorted by the direction of target motion relative to the target step (“away” or “toward” the position of fixation) and by the direction of pursuit relative to the direction of smooth eye movements elicited by electrical stimulation at given sites (“same” or “opposite”). - - -, a unity slope and would obtain if stimulation did not affect eye velocity. ●, data from a site with statistically significant effects of electrical stimulation (t-test, P < 0.05); ○, data from sites without significant effects.
Physiological procedures
Tungsten microelectrodes (Frederick Haer and Company) were lowered through the intact dura with a manual hydraulic micromanipulator (Narishige MO-95) while the monkeys performed either the pursuit task or the saccade task. We searched for pursuit-related regions in the periarcuate cortex by recording unit activity and by testing the effects of electrical microstimulation on eye movements every ∼500 μm along each penetration. A train of cathodal pulses (width, 0.2 ms) was delivered through the electrode as electrical stimulation. The current intensity was monitored by measuring the voltage across a serially connected 1-kΩ resister and was maintained at 50 μA. When we examined the electrically elicited smooth eye movements or searched for the sites likely to be related to pursuit, the train of stimulation pulses was 75 or 375 ms in duration at 333 Hz. When we examined the effects of stimulation on the responses to brief perturbations of the target, the train of stimulation pulses was either 100 or 200 ms in duration at 100 or 200 Hz. All stimulation experiments were performed at sites believed to be located in the gray matter on the basis of the neuronal activity recorded at the site.
Data acquisition and analysis
Horizontal and vertical eye-velocity signals were obtained by passing the eye-position voltages through analog differentiators (−6 dB/octave above 25 Hz). Data were digitized, sampled each channel at 1 kHz, and stored on disk for later analysis on a UNIX workstation. We initially reviewed the eye-position and -velocity traces for each trial on a video monitor, detected saccades visually, and marked them with a mouse-controlled cursor. Portions of the eye-velocity traces during saccades were not used for the subsequent analyses, which were done with Matlab (The MathWorks). Most analyses consisted of aligning the eye movement responses to identical stimuli on the onset of target motion and computing averages of horizontal and vertical eye velocity as a function of time. Points from individual eye-velocity traces were included in the average only if they did not occur during a saccade, so that there was a different number of samples in the average computed for each time point. Time points were drawn in our figures and used for further analysis only if the average had been constructed from at least five trials. In some cases, further analysis consisted of measuring the magnitude of the response from the average eye-velocity trace. In other instances, measurements were made directly from individual traces. Details are provided at the relevant places in RESULTS.
We assessed the effect of electrical stimulation by comparing the eye velocities from interleaved trials that had delivered identical target motions with and without electrical stimulation. First, we computed the “difference eye velocity,” defined as the millisecond-by-millisecond difference between the average eye velocities obtained from trials that provided identical target motions with and without electrical stimulation. This yielded estimates of the horizontal and vertical eye velocity evoked by the electrical stimulation, which we define as and . Then, for a 100-ms interval after the onset of electrical stimulation, we computed the electrically evoked eye speed as . The maximum electrically evoked eye speed over the analysis interval was taken as an estimate of the response size. Finally, the direction of the electrically elicited eye movements was computed as tan-1(Ėv[t]/Ėh[t]) at the time of the maximum eye speed and expressed as a polar angle: rightward, upward, leftward and downward movements corresponded to 0, 90, 180, and −90°, respectively.
The latency of the electrically-elicited smooth eye movements was measured by applying a technique modified from Carl and Gellman (1987). We obtained baseline eye speed by calculating eye speed in individual trials and obtaining the mean and SD of eye speed in the stimulation trials for the 100-ms interval before the stimulation onset. Then a regression line was fitted to the average eye speed for the period from 5 ms before to 10 ms after the average eye speed exceeded 2 SDs of the mean of the baseline. The point where the regression line crossed the baseline mean was taken as the movement onset. The latency of saccades was measured from individual eye-position traces by determining the time when eye speed exceeded 100°/s. For this analysis only, horizontal and vertical eye velocity were estimated by computing regression slopes of every seven eye-position samples.
We have not yet obtained histological verification of stimulation sites because both animals are currently in use for other projects.
RESULTS
Selection of stimulation sites
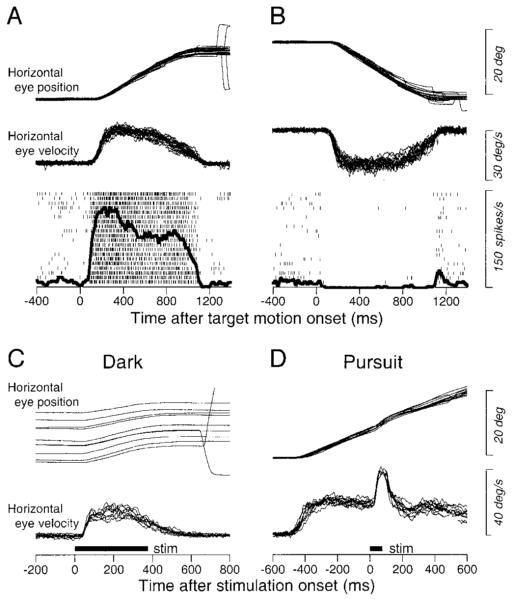
We have analyzed the smooth eye movement responses elicited from 73 stimulation sites in two monkeys. Sites were identified by the presence of pursuit-related neurons, the positive effect of electrical stimulation, or both as illustrated in Fig. 1. The top panels show the activity of a pursuit-related neuron that discharged vigorously during rightward (ipsiversive) pursuit at 20°/s (Fig. 1A). It began to discharge ∼90 ms after the onset of rightward target motion and showed strong sustained activity for the duration of rightward pursuit (Fig. 1A); its slight background discharge was suppressed during leftward pursuit (Fig. 1B). At the same site, a train of stimulation pulses (50 μA, 375 ms at 333 Hz) in the dark consistently elicited rightward smooth eye movements without eliciting saccades (Fig. 1C). The direction of the stimulation-evoked smooth eye movements was just upward from pure rightward (+2.8°). A shorter train of pulses (75 ms) with the same parameters evoked a brisk eye movement response when presented during sustained pursuit of rightward step-ramp target motion at 20°/s (Fig. 1D).
FIG. 1.
Criteria for selection of stimulation sites. A and B: activity of a pursuit-related neuron with ipsiversive (rightward) directional preference during tracking of step-ramp target motion to the right (A) or left (B) at 20°/s. From top to bottom, traces are superimposed trials of horizontal eye position and horizontal eye velocity and superimposed rasters and spike density, aligned on the onset of target motion. Rapid deflections associated with small corrective saccades were removed from eye-velocity traces during the maintenance of pursuit. C and D: superimposed trials of the smooth eye movements evoked by electrical stimulation (50 μA, 333 Hz) in the dark (C) and during ongoing pursuit (D). The bold horizontal lines labeled “stim” show the duration of microstimulation.
In general, we found a coincidence of pursuit-related activity and electrically evoked smooth eye movements. In agreement with previous studies (Gottlieb et al. 1993, 1994), the smooth eye movement sites were located posterior to the sites where contraversive saccades were elicited by stimulation (Fig. 16).
FIG. 16.
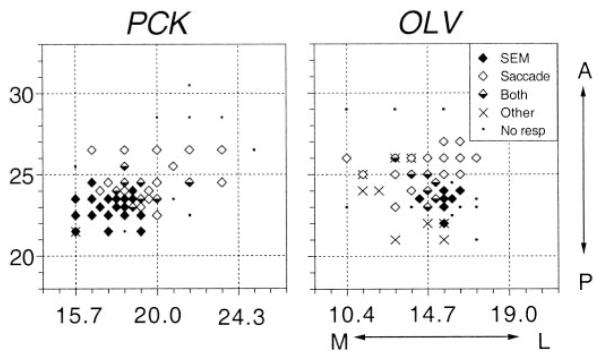
Location of the electrode penetrations in 2 monkeys (PCK and OLV). The stereotaxic coordinates of the entering points of electrodes are plotted for 2 right hemispheres. Symbols indicate the type of movement elicited by stimulation, according to the key in the top right of the map for monkey OLV. SEM, smooth eye movements; A, anterior; P, posterior; M, medial; L, lateral.
Latency of smooth eye movements evoked by electrical stimulation
Figure 2 shows the distributions of the latency of the electrically evoked smooth eye movements when the monkeys fixated a central stationary target (A) or tracked a target moving at 20°/s (B). Latency averaged 25.6 ± 6.1 ms during fixation and 21.9 ± 3.3 ms during pursuit. Although the means were similar, a paired t-test revealed significant differences in latency between fixation and pursuit (P < 0.0001, df = 72). At individual sites, the latency of the evoked smooth eye movement during fixation minus that during pursuit ranged from −12.9 to 18.9 ms (mean, 3.7 ± 5.5 ms; median, 2.3 ms). For data obtained during pursuit, electrical stimulation was delivered during each of the eight pursuit directions (4 cardinal and 4 oblique directions), and latency was estimated from the direction that produced the largest electrically evoked eye speed. In 70 of 73 sites (96%), the smooth eye movements evoked during pursuit were largest during pursuit in the direction same as the eye movements evoked from that site during fixation. For one site, the elicited eye movements were too small to use the automated procedure to measure latency, so the latency was estimated visually.
FIG. 2.
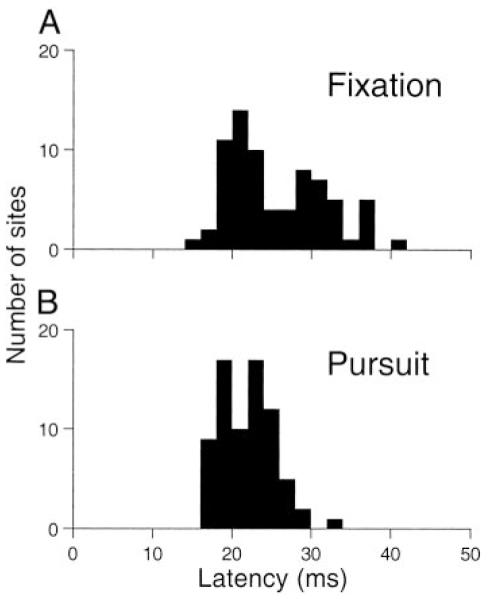
Distribution of latency of smooth eye movements elicited by electrical stimulation of the frontal pursuit area (FPA) during fixation (A) and during the maintenance of pursuit at 20°/s (B). Each histogram shows data from 73 stimulation sites.
Enhancement of smooth eye movements evoked by microstimulation during ongoing pursuit
We reported previously that the magnitude of the electrically elicited smooth eye movements was larger if stimulation was delivered during pursuit than during fixation (Tanaka and Lisberger 2001). We have now stimulated at more sites than were included in our earlier report and analyzed the data more extensively. The means of the peak electrically evoked eye speed were 7.1 ± 5.3°/s during fixation and 18.6 ± 8.1°/s during pursuit. These were statistically different (paired t-test, P < 0.0001, df = 72). The present section will provide new information about the enhancement of the response to electrical stimulation during ongoing pursuit.
EFFECTS OF PURSUIT SPEED AT THE TIME OF MICROSTIMULATION
The size of the electrically evoked smooth eye movement depended on the speed of pursuit at the time of stimulation. A typical effect is illustrated in Fig. 3A, which shows the time courses of the electrically evoked eye movements for stimulation during pursuit at different target velocities (solid traces) superimposed on averages of eye velocity for the equivalent time in the control trials without electrical stimulation (dashed traces). Stimulation of this site in the right FPA increased the speed of rightward pursuit and decreased the speed of leftward pursuit transiently. The magnitude of electrically evoked responses changed systematically as a function of ongoing eye velocity: larger responses for faster speeds of rightward target motion, indicated by positive values of target velocity. Since a previous study had shown that the direction and magnitude of elicited eye movements sometimes depended on eye position (Gottlieb et al. 1993), we contrived the target motions in this experiments so that microstimulation was delivered as the tracking target crossed the center of the screen: the target appeared 2, 4, 8, or 12° from the center of the screen and executed step-ramp motion with 1, 2, 4, or 6° steps away from this initial fixation position for target velocities of 5, 10, 20, or 30°/s, respectively.
FIG. 3.
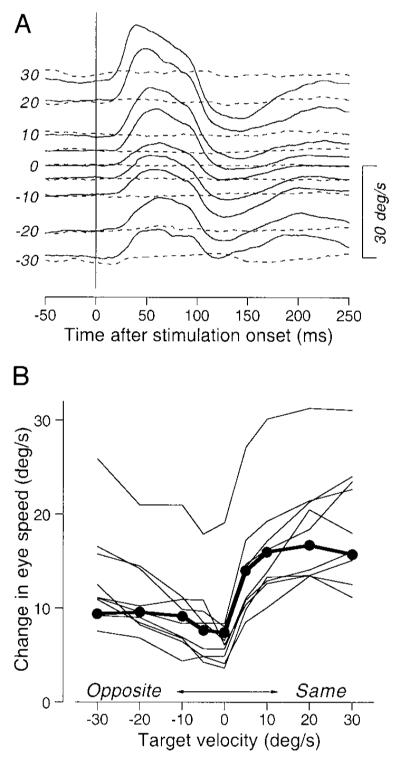
Effects of target velocity on the magnitude of electrically-elicited smooth eye movements. A: examples of the time courses of average horizontal eye velocity for stimulation with a 75-ms train of pulses at 333 Hz, 50 μA during tracking of different target velocities. Solid and dashed lines, data obtained with and without stimulation. Numbers at the beginning of traces indicate target velocity. The positive numbers indicate rightward target motion. Upward deflections of average traces indicate rightward eye movements. B: amplitudes of the responses to stimulation as a function of target velocity. The positive and negative values of target velocity indicate target motion in the direction of electrically elicited smooth eye movements (Same) or the opposite direction (Opposite). Each connected line indicates data from single stimulation site. The filled symbols connected by thick lines plot measurements made from the data in A.
The results of varying target velocity were very consistent for the 10 sites at which we performed this experiment. For each site, we attempted to choose the axis of target motion so that microstimulation would be delivered during pursuit in approximately the same or opposite direction as the response to stimulation delivered during fixation (6 horizontal, 3 oblique, and 1 vertical). Analysis after the experiment revealed that we were fairly successful: the difference between the directions of the axis of target motion used for these experiments and the electrically evoked smooth eye movements during fixation averaged 17.4 ± 17.1° (n = 10). At all 10 sites, the peak speed of electrically evoked smooth eye movements depended on both the speed and direction of target motion along the chosen axis (Fig. 3B). The speed of the electrically evoked smooth eye movements generally increased as a function of target speed, and the eye movements were considerably larger when the monkey performed pursuit in the direction of electrically evoked movements (positive values on the abscissa) than in the opposite direction.
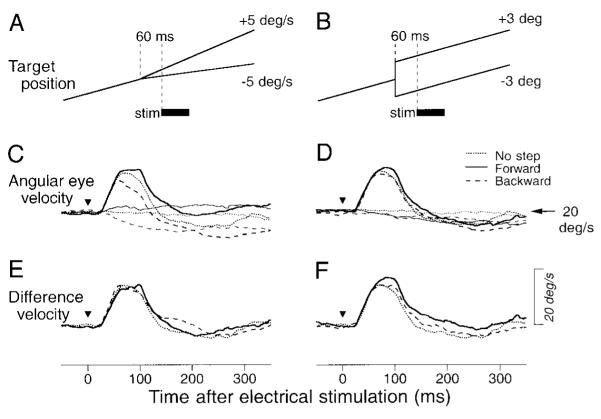
Is the effect illustrated in Fig. 3B related to the ongoing pursuit eye velocity or to the image speed present at the time of electrical stimulation, which averaged 0.68, 1.18, 1.97, and 2.14°/s for target motion 5, 10, 20, and 30°/s, respectively? Figure 4, A and B, illustrates the experimental paradigm we used to test and reject the latter possibility. Just before the electrical stimulation was delivered, the ongoing ramp target motion was interrupted with either a 5°/s step of target velocity (A) or a 3° step of target position (B) along the axis of target motion. Electrical stimulation (75 ms, 333 Hz) was applied 60 ms later in half of the randomized trials. To isolate the responses to electrical stimulation, we performed a millisecond-by-millisecond subtraction of the average eye speeds in the trials without electrical stimulation (Fig. 4, C and D, thin traces) from those in identical trials with electrical stimulation (Fig. 4, C and D, thick traces). Neither the direction of image velocity (Fig. 4E) nor image position (Fig. 4F) at the time of electrical stimulation altered the response: the difference eye velocities representing the responses to electrical stimulation were essentially identical for conditions that provided onward image positions or velocities (solid traces), backward image positions or velocities (long dashed traces), or no additional visual stimulus beyond that already present during target motion at constant speed (short dashed traces).
FIG. 4.
Absence of the effect of retinal events at the time of electrical stimulation on the magnitude of response. A, C, and E: responses to retinal velocity errors. B, D, and F: responses to retinal position errors. A and B: schematic drawings of experiments. A target moving at 20°/s changed velocity by 5°/s (A) or position by 3° (B) when it crossed the center of the screen. The thick bar labeled “stim” indicates the time of microstimulation. C and D: averages of angular eye velocity for stimulation (thick lines) or control (thin lines) trials. Traces are aligned on the onset of stimulation or equivalent time in the nonstimulation controls (triangles). Different line types indicate different directions of image velocity (C) or position (D) relative to ongoing target motion: dotted traces, control trials; solid traces, retinal stimulus in direction of target motion; long dashed traces: retinal stimulus in direction opposite to target motion. E and F: difference eye velocity, documenting the time courses of the difference in eye speed between the stimulation trial and nonstimulation control.
We repeated these experiments at 11 other stimulation sites, and the effects of target steps on the magnitudes of evoked movements were evaluated by using three-way repeated-measures ANOVA. The factors were the direction of pursuit (same as vs. opposite to elicited movements), the direction of target step (forward vs. backward), and the kind of target step (position vs. velocity). There were significant effects of pursuit direction (P < 0.0001), but no significant effects of the direction of target step (P = 0.57) and the kind of target step (P = 0.97). No interaction effects were found between each pair of factors. Thus the sizes of electrically elicited smooth eye movements during the maintenance of pursuit were determined mostly by ongoing eye (or target) velocity rather than by the image position or motion coded in the retinal inputs from the tracking target.
EFFECTS OF PURSUIT DIRECTION AT THE TIME OF MICROSTIMULATION
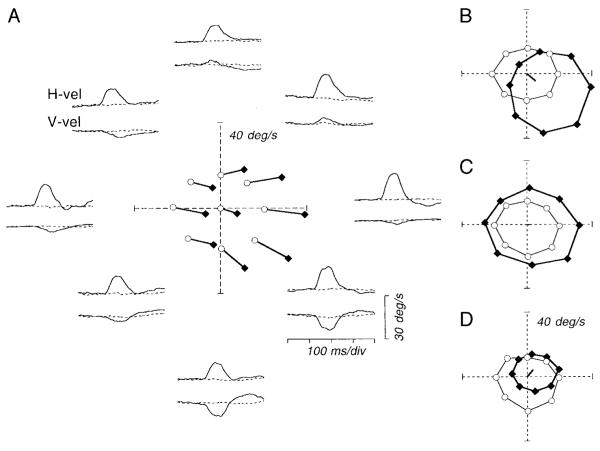
We applied electrical stimulation (75 ms, 333 Hz) in the FPA during fixation and during pursuit at a fixed speed of 20°/s in eight different directions, yielding traces like those shown around the perimeter of Fig. 5A, where the position of the traces indicates the direction of pursuit at the time of stimulation. To quantify the responses to microstimulation of the FPA during fixation and pursuit, we determined the time of the largest peak in the electrically evoked eye speed across all directions of target motion and measured the eye velocity in each trace at that time. In practice, the time of the peak response amplitude was nearly the same for all directions of pursuit, so this analysis yielded the effectively same results as would one based on measuring the peak response. In the polar plot at the center of Fig. 5A, we have plotted these estimates of eye velocity for both control trials and stimulation trials. Each vector starting at a control value (open symbols) and ending at a stimulation value (filled symbols) represents the evoked eye velocity for stimulation delivered during each direction of pursuit. The pair of connected symbols with the control value at the origin shows the responses during fixation with and without electrical stimulation. Inspection of Fig. 5A shows that the direction of the electrically evoked movement was largely independent of whether the monkey was fixating or pursuing at the time of stimulation and of the direction of pursuit. In contrast, for this site, the amplitude of the evoked eye velocity depended on the conditions at the time of stimulation. The response was larger during pursuit than during fixation, and was largest for stimulation during rightward (ipsiversive) pursuit.
FIG. 5.
Effects of electrical stimulation of the FPA during pursuit in different directions. A: pairs of traces around the polar plot show horizontal eye velocity (H-vel) or vertical eye velocity (V-vel) during the maintenance of pursuit, positioned to coincide with the direction of pursuit. In each pair of traces, the solid and dashed traces show data for the stimulation trial and the nonstimulation control, respectively. The traces start 500 ms after the onset of target motion. For the stimulation trials, the train of stimulation pulses (duration, 75 ms) was delivered 600 ms after the onset of target motion. The polar plot at the center of A shows eye velocity 90 ms after stimulation onset (filled symbols) and at the equivalent time for the controls (open symbols). Data for the same target motion are connected by lines. B—D: examples of the stimulation-evoked responses for 3 other sites. Each set of 8 data points for the stimulation trials (filled symbols) or controls (open symbols) is connected by lines. The thick line starting at the origin of each polar plot is a vector that indicates the direction and amplitude of eye velocity elicited by stimulation during fixation.
The other way to show the results of these experiments is to connect the points for eye velocity in the presence or absence of electrical stimulation for pursuit in the eight different directions, providing separate polar plots for eye velocity in the presence or absence of microstimulation. For example, Fig. 5B shows such a plot for the data obtained from another stimulation site, documenting the most common response pattern. In the absence of electrical stimulation (open symbols), the sustained eye speed was similar during pursuit in all eight directions, resulting in a nearly circular plot. When electrical stimulation was applied during pursuit, the resulting plot (filled symbols) was shifted to the right and down, and the size of the circle was larger than in the absence of electrical stimulation. The eye movement evoked by electrical stimulation during fixation (solid line starting from the center of the graph) was also to the right and down but of a smaller amplitude than the change in the position of the center of the polar plot obtained for electrical stimulation during pursuit. Of 44 sites, 29 were like the ones shown in Fig. 5, A and B: microstimulation increased eye speed during pursuit in some directions and decreased eye speed during pursuit in other directions. At 12 sites, the stimulation increased eye speed in all or some directions of pursuit without changing eye velocity in the other directions (Fig. 5C). Finally, at three sites, microstimulation decreased eye speed during pursuit in some directions without causing much response during pursuit in the other directions (Fig. 5D).
Polar plots like those in Fig. 5 suggest a model in which stimulation of the FPA has two separate effects. One effect is based on a “directional signal” and causes eye velocity in a direction that is independent of the direction of ongoing pursuit. The other effect can be thought of as a gain applied to ongoing pursuit eye velocity. The two effects can be summarized as
where is the eye movement recorded during stimulation, is the eye velocity present during companion target motions without stimulation, r is the effect of stimulation on the gain of ongoing eye velocity, df is the size of the directional signal if stimulation is delivered during fixation, and q is the effect of ongoing pursuit on the gain of the directional signal. We also define
so that dp indicates the direction and size of the directional signal if FPA stimulation is delivered during pursuit. We next use this model to quantify the interaction between ongoing pursuit velocity and the smooth eye movements evoked by electrical stimulation in the FPA. Within the possible parameter space of r, dp, and df, there are three possible interactions that are widely recognized in thinking about how signals are combined in the nervous system. In Fig. 6, the arrow emanating from the origin of the axes shows the directional signals injected by electrical stimulation of FPA during fixation (df), the dashed polygon connects the values of eye velocity during sustained pursuit in eight directions in the absence of electrical stimulation, and the solid polygon connects the values of eye velocity for sustained pursuit during electrical stimulation of the FPA. Vector averaging of pursuit velocity and the signals injected by electrical stimulation (e.g., Groh et al. 1997) would occur if r = 0.5. Vector averaging predicts that electrical stimulation should make eye speed smaller during pursuit in all directions: the solid polygon should be smaller than the dashed polygon (Fig. 6A). Summation of pursuit velocity and the response to electrical stimulation would occur if r = 1.0. Summation predicts that electrical stimulation should shift the eye velocity by the same amount for each direction of pursuit: the solid and dashed polygons should remain the same size but the solid polygon should be shifted relative to the dashed polygon (Fig. 6B). Finally, if r > 1.0 and the stimulation enhances the gain of ongoing pursuit for all directions as well as injects directional eye-velocity signals, then the response should be like that illustrated in Fig. 6C, where the solid polygon is larger than the dashed polygon and is shifted as well. In each of these situations df and dp could be equal or different: if ongoing pursuit enhances the directional signals injected by the FPA stimulation, then dp > df.
FIG. 6.
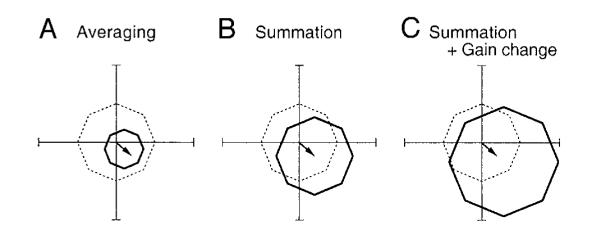
Patterns of eye velocity predicted from 3 different computations that might account for the interaction of ongoing pursuit and electrical stimulation. The dashed lines in each polar plot indicate control eye velocity during pursuit in 8 directions, and the solid lines connect eye velocity predicted in stimulation trials. The arrows starting from the origin of each polar plot illustrate the eye velocity evoked by electrical stimulation during fixation. Predictions for the interaction of ongoing pursuit and electrical stimulation are: vector average (A), vector summation (B), and vector summation with omnidirectional enhancement of pursuit eye velocity (C).
We analyzed our data in the framework of Fig. 6 by calculating a least-squares fit to the data from each stimulation site with three different equations
where dp and r are defined in the preceding text, and S and C are the sets of eight eye velocities obtained from stimulation trials and controls, respectively. We evaluated the validity of these three models by comparing the difference between the actual data and the prediction of the models. For all 44 stimulation sites at which this experiment was done, the data were fitted best by the third model, which incorporated the gain change of pursuit and summation with a directional signal injected by microstimulation. The mean (±SD) error of the fits for each pursuit direction across 44 stimulation sites were 13.8 ± 3.7, 5.7 ± 2.4, and 2.9 ± 1.1°/s for the predictions of averaging, summation, or summation plus gain change, respectively. The coefficient of determination averaged 0.68 ± 0.08, 0.94 ± 0.04, and 0.98 ± 0.01 for the three models, respectively.
To document the effect of electrical stimulation in the FPA during the maintenance of pursuit, we summarize the values of r, dp, and df derived from the third model for the 44 sites we studied. The mean value of r was 1.24 ± 0.19 and r was greater than unity for all but five sites (Fig. 7A), implying that electrical stimulation in the FPA almost always had one effect that increased pursuit speed in the direction of ongoing pursuit. The fixed smooth eye velocity injected during pursuit (dp) was biased toward ipsiversive with vertical components, although there were some sites where electrical stimulation during pursuit injected a small contraversive eye-velocity signal (Fig. 7B). The direction of the eye velocity caused by microstimulation during pursuit (dp) was similar to that during fixation (df). The difference in direction for a given site averaged 11.2 ± 11.9°, n = 44). Comparison of the magnitudes of df and dp (Fig. 7D) shows that these values were well correlated each other (r = 0.90, n = 44, P < 0.0001) with a regression coefficient of 1.58. This indicates that the directional component of smooth eye velocity injected by electrical stimulation was ∼1.58 times larger during pursuit at 20°/s than during fixation with remarkable consistency. In contrast, the size of directional signal injected by stimulation during pursuit was poorly correlated with the amount of gain enhancement of ongoing pursuit caused by stimulation (Fig. 7C, r = 0.41, n = 44, P < 0.01).
FIG. 7.
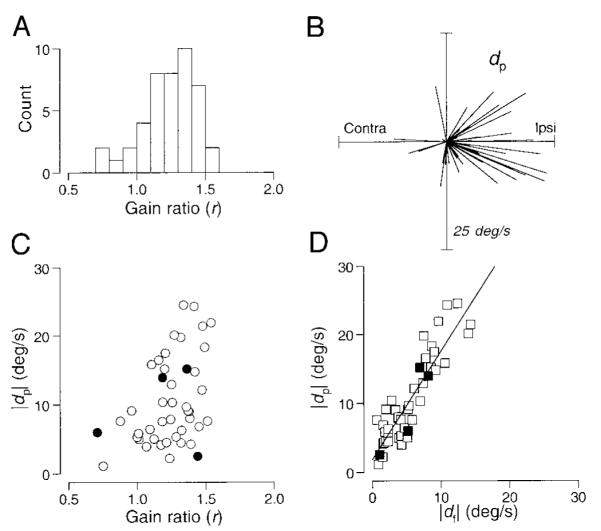
Quantitative analysis of the interaction between ongoing pursuit and the eye movements evoked by electrical stimulation for 44 sites. Data were fitted by the equation, S = r*C + dp, where r represents the ratio of pursuit gain and dp represents the directional component injected during pursuit. A: distribution of the gain term (r). B: polar plot where each line is a vector showing the direction and amplitude of the eye velocity caused by stimulation during pursuit (dp). C: relationship between the size of the eye movement evoked by stimulation during pursuit (dp) and the gain enhancement of ongoing pursuit (r). The correlation coefficient was 0.41. D: amplitude of the eye velocity caused by stimulation during the maintenance of pursuit (dp) compared with that during fixation (df). The regression line has a slope of 1.58, a y intercept of 1.84, and the correlation coefficient was 0.90. In C and D, each point shows data from a single stimulation site; filled symbols indicate measurements from the data shown in Fig. 5.
Further evidence that the directional signals and gain control are independent effects
The data in Figs. 5 and 7 are consistent with the idea that there are two different effects of microstimulation in the FPA. One is the injection of a signal that drives pursuit in a fixed direction and that is subject to ∼1.58-fold enhancement during pursuit at 20°/s versus during fixation. The other is an enhancement in the speed of ongoing pursuit for all directions. These two effects would cause different directions of eye movements if stimulation was delivered during pursuit opposite to the direction of evoked eye movements during fixation. If they had different time courses, then they might yield a bidirectional modulation of eye velocity around the control data. This prediction was borne out at 12 of 73 stimulation sites. For example, Fig. 8 shows data for a site at which microstimulation during fixation elicited smooth eye movements to the right and upward (+41.4°). Stimulation during pursuit to the right and up (same as the eye movement evoked from fixation) increased the speed of ongoing pursuit transiently (Fig. 8A). The evoked eye velocity had a latency of ∼25 ms, and its time course was monophasic. In contrast, the time course of the eye velocity evoked during pursuit in the opposite direction was biphasic (Fig. 8B). Initially, stimulation increased the speed of ongoing left and downward pursuit with a latency of ∼20 ms. Later with a latency of 40 ms from the onset of stimulation the direction of eye acceleration reversed, and 80 ms after the onset of stimulation the speed of left and downward pursuit became less than control. The initial component is in the direction expected from an omni-directional increase in the speed of ongoing pursuit, while the later component is in the same direction as the eye movement evoked by microstimulation during fixation. The reversal seen in Fig. 8B and in 11 other sites would be expected if the latency of the directional component was longer than that of the gain enhancement, and the two components were comparable in size. At 49 other sites, we found more subtle evidence for oppositely directed directional component and gain enhancement: stimulation during pursuit in the opposite direction from the elicited eye movements evoked no change in eye velocity (n = 7) or a smaller response (n = 42) than did stimulation during pursuit in the direction of the directional signals. This would occur if the directional component was equal to or larger than the eye movement caused by the effects of stimulation on gain control, and these two components had similar latencies. We did not find any sign of conflict of the two components in the remaining 12 sites where stimulation decreased (n = 3) or increased (n = 5) the speed of pursuit for both directions, or injected fixed eye-velocity signals irrespective of ongoing pursuit directions (n = 4).
FIG. 8.
Evidence for the presence of 2 components with different time courses resulting from FPA stimulation. Averages of eye velocity (—) are aligned on the onset of stimulation applied during the maintenance of pursuit. . . . , the mean plus 1 SD. - - -, average eye velocity in the nonstimulation control trials. Stimulation of this site during fixation elicited smooth eye movements in right-up direction. The → at the left of each panel indicate the direction of pursuit during stimulation. A: right and up. B: left and down. → in A also indicates the direction of the eye movement evoked by stimulation at this site during fixation. Stimulation was applied for 75 ms starting 600 ms after target motion onset. Target speed was 20°/s.
Enhancement of visual-motor gain during fixation by electrical stimulation in the FPA
We now revisit a phenomenon published in an earlier brief communication (Tanaka and Lisberger 2001) to provide additional quantitative evaluation of the phenomenon and to demonstrate temporal contingencies in the interaction of eye movements evoked by visual motion and electrical stimulation of the FPA. In the earlier publication, we used the experimental design outlined in Fig. 9 to show that electrical stimulation in the FPA enhanced the smooth eye velocity evoked by a brief target perturbation during fixation. Although Fig. 9 is very similar to Fig. 2 of our prior publication (Tanaka and Lisberger 2001), it shows data from a different stimulation site. Briefly, a fixation target appeared at the center of the screen for 1,000–1,500 ms and then underwent a brief oscillation consisting of a single cycle of a 10-Hz sine wave (±10°/s) with initial motion to the right, left, up (Fig. 9A), down (Fig. 9B), or in one of the four 45° oblique directions. The responses to the perturbation of target velocity were small in the absence of electrical stimulation (Fig. 9, C and D) and larger if microstimulation was applied in the FPA (200 ms, 200 Hz) at the onset of target motion (continuous traces in Fig. 9, E and F). Electrical stimulation alone at the same time in the fixation trials without target perturbations elicited small eye movements (2.2°/s) in the up-right direction (63°; dashed traces in Fig. 9, E and F). To estimate the response to the perturbations of target motion in conjunction with microstimulation, we computed the difference: eye velocity evoked by perturbations during microstimulation minus that evoked by stimulation alone. To reveal enhancement, the difference eye velocity (thick continuous traces in Fig. 9, G and H) was compared with the responses to perturbation in the absence of electrical stimulation (thin dotted traces in Fig. 9, G and H).
FIG. 9.
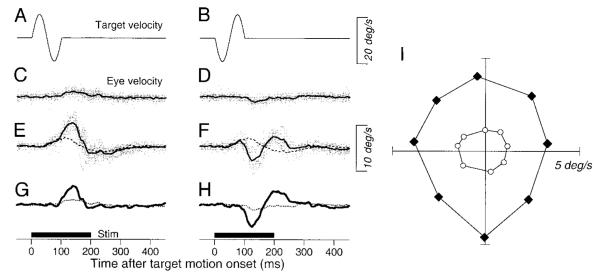
Example data from a stimulation site showing the enhancement of the responses to perturbation of a stationary target. A and B: the central fixation target underwent a perturbation that caused velocity to undergo a single cycle of a 10 Hz, ±10°/s sine wave with the initial component upward (A) or downward (B). C and D: vertical eye velocity evoked by the perturbation without electrical stimulation. E and F: responses to the target perturbation in the presence of electrical stimulation. In E and F, the broken line shows the average of the responses to electrical stimulation during fixation of a stationary target. In C—F, the gray traces show the responses in all individual trials, and the black bold traces show the average eye velocity. G and H: comparison of the average vertical eye velocity to the perturbation in the presence (bold solid traces) and absence (fine dotted traces) of electrical stimulation. The bold horizontal lines labeled “stim” below G and H show the time of stimulation. I: polar plot showing the size and direction of responses to target motion in 8 different directions. The filled symbols indicate responses with electrical stimulation, whereas the open symbols indicate those without stimulation. Data were obtained from a different site than that shown in a similar figure in Tanaka and Lisberger (2001).
Microstimulation in the FPA caused enhancement of the response in the direction of the perturbations. In Fig. 9, G and H, for example, the enhanced response to the perturbation is in the direction of the original perturbation (Fig. 9, A and B) rather than in the direction of the smooth eye velocity evoked by microstimulation. To quantify the directionality of the enhancement, we measured the response to the initial half cycle of target perturbation by measuring the peak average eye velocity in the interval from 100 to 150 ms after the onset of the perturbation in each of eight directions. Figure 9I contains a polar plot of the peak responses for all eight directions of target perturbation. The responses to the perturbations were three times as large during microstimulation trials (filled symbols) as during the control trials (open symbols), while the directions of the responses were changed by an average of only 9.0 ± 5.5° (n = 8) at this site.
The enhancement of the responses to target perturbation was omni-directional for this and most of our stimulation sites. The directional preference of the enhancement for each given site was evaluated statistically in the previous report (Tanaka and Lisberger 2001). Briefly, the direction of the change in eye movements caused by stimulation was distributed uniformly for 30 of 33 sites (Rayleigh’s test, P > 0.05). Furthermore, Mardia’s circular-linear analysis showed that the direction and amplitude of the enhancement were correlated each other for only 6 of 33 stimulation sites (P < 0.05). In the present paper, we quantify the directionality of the change in perturbation responses in a different way using the same set of stimulation data published previously. Because a previous study in our laboratory has shown that the enhanced responses to a brief perturbation during the performance of pursuit is largest if the perturbation is along the axis of ongoing pursuit direction (Schwartz and Lisberger 1994), we also asked whether the enhancement of the responses to a brief perturbation during stimulation of the FPA is stronger along a certain axis.
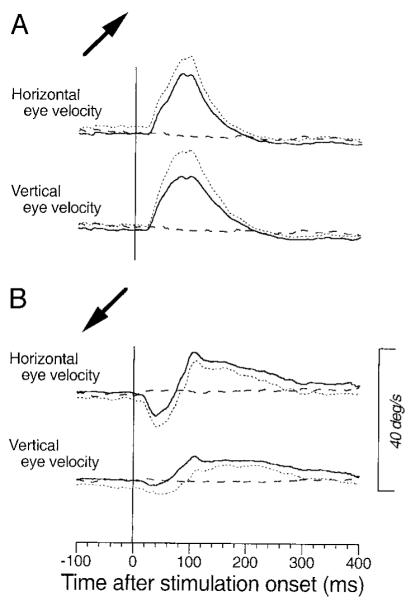
For each stimulation site and direction of target perturbation, we obtained the enhanced eye velocity by subtracting the responses in control trials from those in stimulation trials. Figure 10A plots data from another stimulation site in which the responses to perturbation were enhanced for all motion directions with a strong directional preference. We then fitted an ellipse to the set of eight data points. We computed the axial bias index (Iax) as
where a and b were the lengths of the major and the minor axes of the fitted ellipse, respectively. The directional bias (Idr) was computed in a similar way as
where c and d were the lengths of vectors from the origin to the ends of the major axis of the fitted ellipse (c > d, → in Fig. 10A). We performed this analysis for 25 of 33 stimulation sites in which stimulation changed the gain of the responses to target perturbation by 0.1. For smaller changes in gain, the data were sufficiently noisy that it was difficult to have confidence in the parameters of the best-fit ellipses.
FIG. 10.
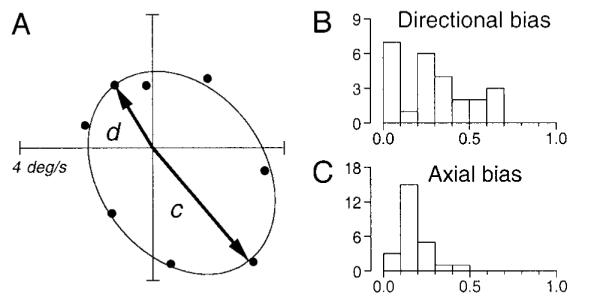
Quantitative analyses showing the lack of selectivity of enhancement for the direction or axis of target perturbation during fixation. A: symbols plot the difference in responses to target motion between stimulation trials and controls for 8 directions of perturbation at a single stimulation site. —, the best-fitting ellipse. →, vectors from the origin to the 2 ends of the major axis of the ellipse. B: distribution of the directional bias defined as 1– d/c. C: distribution of the axial bias defined by 1– b/a, where a and b are the lengths of major and minor axes of the ellipse, respectively. The ordinates of B and C indicate number of stimulation sites.
Figure 10, B and C, shows distributions of these indexes. For both indexes, the value closer to unity indicates stronger preferences to a certain direction (Idr) or axis (Iax) of target motion. In general, both indices were <0.5 at the overwhelming majority of sites, indicating that the enhancement was not strongly specific to a certain direction or axis. The site summarized in Fig. 10A had one of the strongest biases in our sample: the values of Idr and Iax were 0.51 and 0.13, respectively. The site summarized in Fig. 9I had much less directional bias: the values of Idr and Iax were 0.05 and 0.17, respectively. The means for all 25 sites we analyzed were 0.29 ± 0.21 (Idr) and 0.17 ± 0.09 (Iax).
Comparison of effects of FPA stimulation on eye velocity during pursuit and on responses to perturbations during fixation
We have now documented three effects of microstimulation in the FPA on different components of pursuit: injection of a directional signal that is enhanced 1.58-fold during ongoing pursuit at 20°/s; enhancement of the gain of steady-state eye velocity in a nondirectional way; and enhancement of the smooth eye velocity evoked by a brief perturbation of target velocity during fixation. We documented the latter two effects in separate blocks at 33 stimulation sites, allowing direct comparison of the size of the two different forms of gain enhancement.
For each of these 33 sites, Fig. 11 plots the effect of microstimulation in the FPA on the gain of the responses to perturbations during fixation as a function of the effect on eye velocity during the maintenance of pursuit. For the responses to target perturbation during fixation, we measured the gain by using the same method as the previous study (Tanaka and Lisberger 2001). For both the control responses and the responses during stimulation, the gain was computed as where AE is the area within the polygon defined by each set of eight data points for different target directions (e.g., Fig. 9I) and AT is the area within the polygon defined by the peaks of target velocity (always 282.84). We then estimated the effect of microstimulation as the gain during stimulation minus that during fixation without stimulation. For stimulation during the maintenance of pursuit, we used the same approach. For the control and stimulation responses, the gain was computed as where AEis the area within the polygon defined by each set of eight data points for different target directions (e.g., Fig. 5, B–D) and AT is the area within the polygon defined by target velocity (always 1131.4). We then estimated the effect of microstimulation as the gain during stimulation minus that during pursuit without stimulation. The gain changes were smaller for the responses to perturbation than those observed during pursuit in most sites, and the correlation coefficient was 0.55 (n = 33, P < 0.001). The analysis in Fig. 11 uses a difference between the gains with and without stimulation. A similar analysis using the ratio of the gains with and without stimulation yielded the same basic result. The gain ratio for perturbations was now much larger than that for pursuit maintenance because the gains of the responses to perturbations in the absence of stimulation were very small, but the correlation coefficient was about the same (r = 0.48, P < 0.01).
FIG. 11.
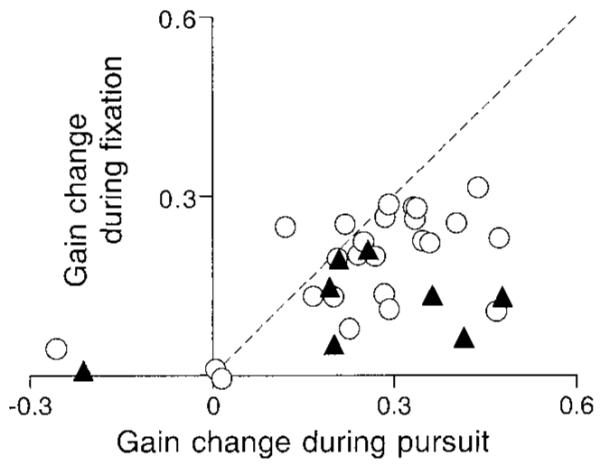
Relationship between the amount of stimulation-induced enhancement of eye velocity during pursuit (x axis) and during stimulation-induced enhancement of the response to a brief perturbation of target motion during fixation (y axis). ○ and ▲, data from different monkeys. - - -, unity slope. Correlation coefficient was 0.55.
Time window for enhancement of visual-motor gain during fixation by electrical stimulation in the FPA
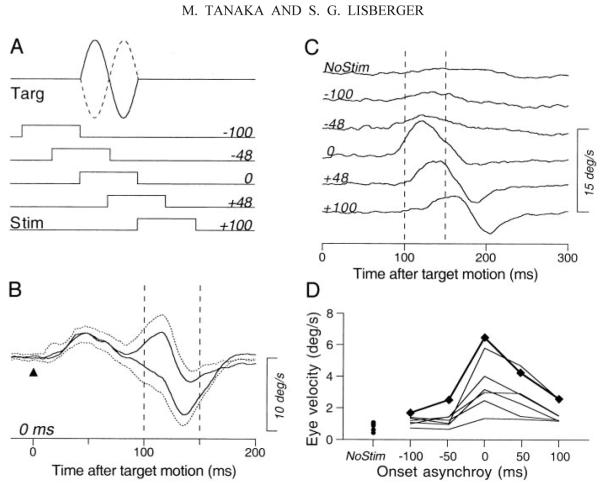
In the experiments reported so far, we used a 200-ms train of stimulation pulses to ensure the conjunction of the electrical stimulation and the 100-ms target perturbation. To determine the most effective time for stimulation, we next used a 100-ms train of stimulation pulses and varied the time between the onset of microstimulation and the 100-ms duration perturbations of target motion. Figure 12A illustrates the experimental design. As before, a single cycle of a 10 Hz, ±10°/s sine wave perturbed a central fixation target 1,000–1,500 ms after it appeared. Perturbations had a phase of 0° (continuous trace) or 180° (dashed trace) and a 100-ms train of 21 pulses was applied in the FPA before, after, or at the same time as the target motion onset. Figure 12B shows an example of the average eye velocities evoked by the two perturbations when the stimulation was applied at the onset of target motion, where the solid traces show the average responses for perturbations with a phase of 0 or 180° and the dotted traces indicate the SDs. The responses to the two perturbations diverged during the interval from 85 to 165 ms after the onset of the perturbation. To assess the response to the perturbations and reject the smooth eye velocity caused by the microstimulation, we computed the difference: eye velocity evoked by the 0° phase perturbation minus that evoked by the 180° phase perturbation.
FIG. 12.
Effect of timing of stimulation in the FPA on the enhancement of the responses to target perturbations. A: schematic diagram showing the timing of the stimuli. Traces labeled “targ” show the 2 polarities of perturbation velocity along the horizontal meridian. Rectangles labeled “stim” show the timing of 100-ms-duration trains of 200-Hz stimulation pulses at different delays. The numbers to the right of each stimulus marker indicate the time of electrical stimulation relative to the onset of target perturbation in milliseconds. B: example of responses for synchronous perturbations and electrical stimulation. The solid traces show averages of horizontal eye velocity for 2 different polarities of perturbation aligned on the onset of target motion (triangle). The dotted traces indicate 1 SD. The 2 vertical dashed lines indicate the interval used to measure the magnitudes of responses. C: time courses of the responses to perturbation for different times between the onset of microstimulation and the perturbation. The traces are the differences of average eye velocity for the 2 perturbation polarities. Two vertical dashed lines indicate the 50-ms interval during which peaks of the responses were measured. The numbers to the left of the traces indicate the time between the onset of microstimulation and the perturbation. Trace labeled “NoStim” shows the response in the nonstimulation control. D: peak response amplitude as a function of stimulation timing. Each set of connected data points indicates single experiment. Data points connected by bold lines were obtained from the experiment shown in B and C.
The responses to the perturbation were largest when microstimulation was delivered either at the same time as or shortly after the onset of the perturbation. Figure 12C shows the difference eye velocity as a function of the time since the onset of the perturbation for different intervals between stimulation and target perturbation (numbers at the beginning of the traces). When the electrical stimulation was applied before the onset of target motion (negative values of interval between stimulation and target perturbation), responses to the perturbation were small and comparable to those in trials that presented perturbations in the absence of stimulation (trace labeled NoStim). When the stimulation was applied 0, 48, or 100 ms after the onset of target motion, however, the eye movement responses to the target perturbation were clearly enhanced.
To summarize the effect of the timing of electrical stimulation, we measured the peak of the difference eye velocity in the interval from 100 to 150 ms after target motion onset (delimited by vertical dashed lines in Fig. 12C). The effect of timing was similar in all seven sites we studied. As shown in Fig. 12D, the responses to the perturbations in this interval were maximal when the electrical stimulation was applied at the same time as the onset of target motion (0 on the x axis in Fig. 12D). The enhancement decreased as the electrical stimulation was applied later (positive values on the x axis in Fig. 12D) and was nearly absent when electrical stimulation was applied before the target motion (negative values on the x axis in Fig. 12D).
Superposition of the enhanced response and the control response revealed that the enhanced responses in Fig. 12C started 85, 100, and 130 ms after the target motion onset or 85, 52, and 30 ms after stimulation onset for trials with intervals of 0, 48, and 100 ms, respectively. The enhanced responses lasted 80, 120, and 120 ms, meaning that they terminated 65, 72, and 150 ms after the end of the perturbation for trials with intervals of 0, 48, and 100 ms, respectively. The time courses of the responses were similar at all seven sites we studied. It was curious that electrical stimulation would cause an enhancement that outlasted the normal response. With this paradox in mind, we attempted to fit the time course of the responses with a model that placed filters both upstream and downstream from the site of gain control. We were not successful.
Enhancement of the initiation of pursuit by electrical stimulation in the FPA
We have shown thus far that stimulation of the FPA enhances the visual-motor processing for pursuit, at least for the slightly contrived situation where we have probed the state of the pursuit system by presenting a brief perturbation of a stationary target. We now use the more-natural situation that is commonly used to analyze the gain of the pursuit system: the initiation of pursuit for a step-ramp of target position, providing a step of target velocity starting at different locations in the visual field and moving in different directions with respect to the position of fixation.
In the trials illustrated in Fig. 13, a target appeared 4° to the right (A and D) or left (B and C) and moved horizontally at 20°/s either toward (C and D) or away from (A and B) the position of fixation. For each site, we customized the trials used to study the initiation of pursuit so that target motion was along the axis of the smooth eye movements elicited by a train of high-frequency stimulation pulses (333 Hz), either in or opposite the direction of the evoked eye movements. As before, a 200-ms duration train of stimulation pulses, of either 100 Hz (11 sites) or 200 Hz (17 sites), was delivered for 200 ms at the onset of target motion (horizontal bars below the traces) in half of the randomly interleaved trials. The stimulus configuration and the general sequence of pursuit and saccades can be seen best in the position traces in the top two rows of Fig. 13. However, the smooth eye movements themselves can be appreciated better in the average eye-velocity traces shown in the bottom two rows.
Consider first the configuration in Fig. 13A, where the target stepped in the direction of the smooth eye movement evoked by electrical stimulation during fixation and then ramped away from the position of fixation. The third row of traces in Fig. 13 shows the eye velocities elicited in the presence and absence of electrical stimulation: electrical stimulation itself caused little smooth eye movement (dotted trace), while strongly enhancing the eye velocity at the initiation of pursuit (bold trace) relative to the eye velocity at the initiation of pursuit in the absence of stimulation (fine continuous trace). To view the eye velocity evoked by target motion without contamination from the direct effects of stimulation, we again computed the “difference eye velocity,” defined as the point-by-point difference of the eye velocity caused by target motion in the presence of micro-stimulation and that evoked by stimulation alone (bold traces in 4th row of Fig. 13). Inspection of the four columns of Fig. 13 reveals that microstimulation in the FPA enhanced the initiation of pursuit for targets that moved either rightward (A) or leftward (B) away from the position of fixation. When the target moved toward the position of fixation, however, the FPA stimulation enhanced pursuit initiation only for target motion in the direction of the stimulation-evoked smooth eye movements (C).
We measured horizontal and vertical eye velocity 180 ms after target motion onset for the four combinations of target motion, and subtracted the mean of the responses to electrical stimulation alone. We then computed the component of the enhanced eye velocity that was along the axis of eye velocity during the initiation of pursuit without stimulation. The results for each combination of target step and ramp are summarized in Fig. 14, which plots component eye speed in stimulation trials versus that in nonstimulation controls. For target motion in the direction of the electrically evoked smooth eye movement, microstimulation enhanced the initiation of pursuit for target motion toward and away from the position of fixation (Fig. 14, A and C), and the effect was statistically significant in almost all sites (filled symbols, unpaired t-test, P < 0.05). For target motion in the opposite direction, the enhancement was much smaller and was statistically significant only for target motion away from the position of fixation (Fig. 14B) and only in a fraction of sites (14/28, 50%). When the target moved toward the position of fixation and in the direction opposite to elicited eye movements, electrical stimulation either did not alter the initiation of pursuit statistically (13/28, 46%) or caused statistically significant but small depression of eye velocity at the initiation of pursuit (13/28, 46%). Statistically significant enhancement was rare (2/28, 7%).
Effects of electrical stimulation in the FPA on saccade latency
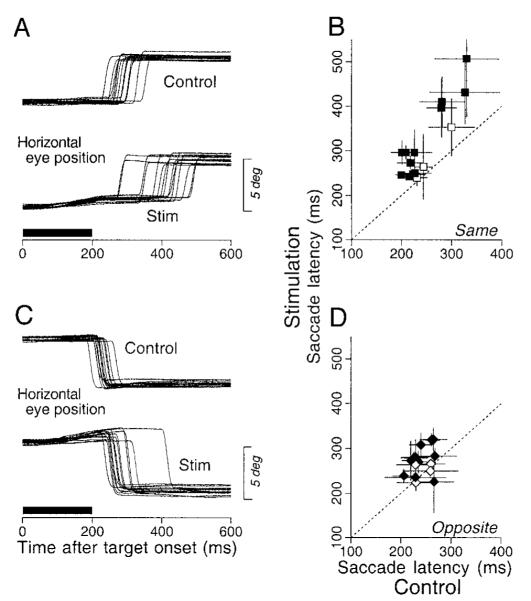
One possible explanation for the enhancement of the responses to perturbations during fixation and the enhancement of the initiation of pursuit is that microstimulation in the FPA facilitates the release of fixation, a process that is necessary to initiate both pursuit and saccades (Krauzlis and Miles 1996b). If the enhancement of pursuit was due to facilitation of the release of fixation, then the FPA stimulation should shorten the latency of saccades. Figure 15, A and C, shows the effect of microstimulation in the FPA on saccades to stationary targets for the same site used in Fig. 1. The central fixation target stepped 4° either to the right (A) or to the left (C) 1,000–1,500 ms after its onset and stimulation was applied at 100 Hz for 200 ms starting at the time the target was displaced. Compared to control trials without stimulation (Fig. 15, A and C, top traces), the latency was prolonged considerably for saccades in the direction of the smooth eye movement evoked by stimulation (Fig. 15A, bottom traces) and slightly for saccades in the opposite direction (Fig. 15C, bottom traces). The mean latencies for control and stimulation trials were 280 and 396 ms for ipsiversive saccades and 220 and 273 ms for contraversive saccades, and both differences were statistically significant (Kormogorov-Smirnov test, P < 0.0001).
FIG. 15.
Effects of low-frequency stimulation in the FPA on the latency of saccades. A and C: horizontal eye position aligned on the onset of 4° target step. The top set of superimposed traces shows control responses and the bottom set shows responses during stimulation. The bold horizontal bars indicate the time of stimulation. Data are from the same site as in Fig. 1. B and D: summary of the stimulation effects on saccade latency. Means of saccade latencies in the stimulation trials are plotted as a function of those in controls. Data are presented in separate panels according to the direction of saccades relative to the direction of the stimulation-evoked smooth eye movements (“same” vs. “opposite”). The dashed line in each graph has unity slope, and would obtain if there were no changes in latency. Error bars show ±1 SD. The filled symbols and open symbols plot data from sites showing effects that were or were not statistically significant differences (Kormogorov-Smirnov test, P < 0.05).
We examined the effects of microstimulation in the FPA on the latency of saccades for 15 sites using low-frequency stimulation to minimize the size of elicited smooth eye movements (100 Hz for 9 sites, 200 Hz for 6 sites). As before, the saccade trials were customized for each site so that we studied saccades along the axis of the smooth eye movement evoked by high-frequency microstimulation. The results from all 15 sites are summarized in Fig. 15, B and D, which plots the mean and SD of saccadic latency in stimulation trials as a function of those in controls. For saccades to targets in the direction of smooth eye movements evoked by electrical stimulation (Fig. 15B), the stimulation caused saccade latency to be significantly longer at 12 of 15 sites (80%, shown as filled symbols, Kormogorov-Smirnov test, P < 0.05). The same stimulation caused smaller but significant increases in the latency of saccades in the direction opposite to the evoked smooth movements for 10 of 15 sites (67%, Fig. 15D). Microstimulation cause the latencies to be lengthened by averages of 68.9 and 27.7 ms for saccades in the same and opposite direction as the electrically evoked smooth eye movements, respectively. These data do not support the hypothesis that FPA stimulation facilitates release from fixation in a way that is common to saccades and pursuit.
Location of stimulation sites
Figure 16 illustrates the locations of electrode penetrations made in this study. Because both monkeys are still being used for other unit recording and stimulation experiments, we can provide only the stereotaxic coordinates of the stimulation sites. The location of each electrode penetration was determined by the X-Y stage attached on the top of the recording cylinder that was tilted 30–31° from the parasaggital plane (see METHODS). Under the assumption that the path of the electrode was perpendicular to the cortical surface, the medial-lateral coordinates of the entrances of electrode penetrations were calculated by projecting the X-Y stage coordinates onto the axial plane. As shown in Fig. 16, most of the smooth eye movement sites (filled symbols) were located posterior to the sites where electrical stimulation elicited contraversive saccades (open symbols). Along penetrations in which both saccades and smooth eye movements were elicited, the smooth eye movement sites were usually located deeper than the saccadic sites. Stimulation of more dorsomedial sites sometimes elicited shoulder, neck, jaw, ear, or eyelid movements (crosses). Thus the smooth eye movement sites were located between the saccadic frontal eye fields and the premotor cortex, in a location that was consistent with the previous studies (Gottlieb et al. 1993, 1994).
DISCUSSION
We have found five effects of stimulation in the smooth eye movement region of the arcuate sulcus in the frontal cortex on smooth eye movements. One effect is a smooth eye movement that is evoked during fixation of a stationary target: in agreement with prior studies, the eye movement is biased toward but not exclusively ipsiversive (Gottlieb et al. 1993, 1994; Mac-Avoy et al. 1991). In our experiments, the smooth eye movement was somewhat slower than reported previously (7.1 vs. 11.1°/s) (Gottlieb et al. 1993) probably because the intensity of stimulation current used in the present study was smaller than those used in the previous studies (50 vs. ≤100 μA). A second and third effect appeared if the same stimulation was presented during pursuit and could be modeled as a combination of enhancement (by pursuit) of the eye movement evoked during fixation and enhancement (by microstimulation) of the sustained eye velocity during pursuit. The fourth effect was enhancement of the smooth eye movement evoked by brief perturbation of a stationary target, reported first in a short paper (Tanaka and Lisberger 2001). The fifth and final effect was enhancement of the presaccadic initiation of pursuit for some but not all target motion conditions. We will discuss each of the latter four enhancement effects separately, placing them in the context of other data, before considering the relation among the different effects and the neural mechanisms that might mediate them.
Enhancement of electrically induced smooth eye movement by pursuit
The enhancement of smooth eye movements due to the performance of pursuit is a ubiquitous finding in the pursuit pathways. For natural stimulation, the response to a brief perturbation of target motion is larger during pursuit than during fixation (Schwartz and Lisberger 1994). For electrical stimulation, the evoked smooth eye movement is larger during pursuit for stimulation in MT (Groh et al. 1997; Komatsu and Wurtz 1989), MST (Komatsu and Wurtz 1989), DLPN (May et al. 1985), and the vermis of the cerebellum (Krauzlis and Miles 1998). In our experiments, we were not able to demonstrate directly that pursuit enhanced the response to stimulation of the FPA because stimulation during pursuit also altered the gain of ongoing pursuit. However, our results were fitted well by a model that described the results as the sum of an evoked, directional eye movement and a gain change in ongoing smooth eye velocity that will be considered in the next section. The size of the directional component of smooth eye movement deduced during pursuit averaged 1.58 times that measured during fixation with relatively little variation from site to site, suggesting that pursuit enhanced the response to stimulation of the FPA. Thus it appears that stimulation of the FPA activates pathways through the site that modulates the gain of signals that drive pursuit. Further, either there are multiple sites of gain modulation or there is a single site that is shared by outputs from MT, MST, the FPA, the pontine nucleus, and the vermis.
Enhancement of eye velocity during the maintenance of pursuit by electrical stimulation
Prior experiments have noted that the direction of ongoing smooth eye movement modulates the size of the smooth eye movements evoked by electrical stimulation in the pursuit pathways. For example, in MT and MST (Komatsu and Wurtz 1989) and the cerebellar vermis (Krauzlis and Miles 1998), the size of elicited eye velocity was larger during pursuit in the opposite versus same direction as the elicited movements. We observed the opposite: the eye velocity elicited from most FPA sites was largest when the monkeys pursued in the same direction as the smooth eye movements elicited by stimulation during fixation. By stimulating during maintained pursuit in eight directions, we were able to show that the effect of stimulation could be attributed to the single enhanced directional effect discussed above plus an omni-directional increase in the gain of ongoing eye velocity. The gain increase is particularly striking because it appeared to result from enhancement of the ongoing eye velocity and was largely independent of the image motion and position present at the time of stimulation. Further, if our two-component model of this experiment is accurate, it indicates that the gain enhancement usually causes eye velocity to be substantially different from target velocity. Because we used brief trains of stimulus pulses for these experiments, we cannot say whether the effect of stimulation obviated visual inputs or if they would have reined eye velocity back to target velocity over several hundred milliseconds.
Enhancement of responses to perturbations by electrical stimulation during fixation
Perturbations provide a way to probe the state of the pursuit system under different initial conditions, without altering that state if the perturbations are chosen correctly. For example, Schwartz and Lisberger (1994) showed that the responses to a single cycle of a 10-Hz sine wave were enhanced during pursuit relative to those during fixation. The magnitude of the enhancement was related to ongoing pursuit velocity and was stronger for perturbations along the axis of pursuit than for perturbations along the orthogonal axis, at least for target velocities of ≤20°/s. In this paper and our short report (Tanaka and Lisberger 2001), we have shown that the effect of ongoing pursuit on the response to a perturbation can be mimicked by concurrent stimulation of the FPA during fixation. For FPA stimulation, the enhancement was always in the direction of the response to the perturbation rather than in the direction of the smooth eye movements also evoked by stimulation of the FPA. Thus the enhancement was typically omni-directional without any strong bias toward the axis of smooth eye movements elicited from the same stimulation site. This subtle difference between the electrically induced and naturally elicited enhancement may result because the electrical stimulation corresponded to target motion at speeds of 30°/s (Tanaka and Lisberger 2001), a target speed that did not cause strong axial selectivity of the enhancement (Schwartz and Lisberger 1994). Alternatively, the omni-directional enhancement caused by electrical stimulation of the FPA may reflect the spatially imprecise nature of electrical stimulation, which could have been activating nearby neurons with different directional preferences.
Two controls implied that the stimulation-induced enhancement of the response to a perturbation of target motion during fixation was due to direct effects of FPA output at some level of sensory-motor processing for pursuit. First, we could not explain the enhancement as a secondary consequence of the smooth eye velocity evoked by electrical stimulation. The use of low-frequency stimulation to demonstrate enhancement rendered the evoked eye movements quite small, and quantitative analysis verified that the evoked eye movements were too small to account for the enhancement at almost all of the stimulation sites (Tanaka and Lisberger 2001). Second, we could exclude the possibility that enhancement resulted from the release of fixation because the same low-frequency stimulation prolonged latency of saccades, as previously shown by Burman and Bruce (1997).
Enhancement of the initiation of pursuit by electrical stimulation
FPA stimulation also enhanced the presaccadic initiation of pursuit. The enhancement was most compelling for target motion in the direction of the smooth eye movement evoked at each site and was stronger for target motion away from versus toward the position of fixation. We recently reported enhancement of the initiation of pursuit when a stationary, eccentric cue was presented just before the onset of pursuit, and the enhancement was again more compelling for target motion away from versus toward the position of fixation (Tanaka and Lisberger 2000). Because of their similarity, we postulate that the two effects are mediated by the same mechanism.
Consideration of a number of prior studies provides a potential explanation for the stimulus selectivity of the enhancement of presaccadic pursuit by FPA stimulation. First, given that stimulation of the FPA evokes a smooth eye movement in one direction, it is plausible that responses to target motion in that direction might be more subject to enhancement, although it is curious that we did not see this bias in the enhancement of the response to perturbations during fixation. Second, it is well known that the initiation of pursuit for a step of target velocity depends on the initial position of moving images and on whether the target is moving toward or away from the position of fixation (Lisberger and Westbrook 1985). Presaccadic eye velocity is greater for target motion toward the position of fixation from near the fovea and becomes smaller as a function of the initial eccentricity of the moving images. Further, there is an asymmetry of initial eye acceleration for target motion toward versus away from the position of fixation across the same retinal eccentricity. Lisberger (1998) recently showed that both of these effects are abolished by strong enhancement of the immediate postsaccadic pursuit. He suggested that the throttle on presaccadic pursuit resulted from a low setting of pursuit gain and not from any weakness of the visual inputs. To explain the effects of eccentricity and target direction on the presaccadic pursuit, he proposed that the gain of pursuit is set to a high value for targets moving toward the position of fixation from a small eccentricity: if the gain is already high, then FPA stimulation might cause a smaller enhancement for these stimulus configurations due to a ceiling effect. If the gain of pursuit is normally low for targets moving away from the position of fixation, then stimulation of the FPA could cause a strong enhancement.
Possible roles of FPA in the pursuit system
Prior reports have provided a strong basis for the conclusion that the FPA is important for smooth pursuit eye movements. Based on the results of recording, stimulation, and ablation studies, it seemed reasonable to assume that the output of the FPA provides an important signal for controlling the direction and possibly the speed of pursuit. Our data add to this picture by showing that stimulation of the FPA modulates the gain of pursuit under a variety of circumstances for both brief visual perturbations and during pursuit maintenance in the absence of large image motion. Further, modulation of the gain of pursuit has been suggested to account for a variety of pursuit behaviors (Krauzlis and Lisberger 1994; Luebke and Robinson 1988; Robinson 1965) and naturally occurring pursuit deficits (e.g., M. Churchland and Lisberger 2000; Grasse and Lisberger 1992; Kiorpes et al. 1996) and has been demonstrated directly in both monkeys (Goldreich et al. 1992; Schwartz and Lisberger 1994) and humans (A. Churchland and Lisberger 2000).
We suggest that the FPA plays a critical role in regulating the gain of the sensory-motor processing for pursuit. The FPA seems suited for this function because it contains pursuit-related neurons that carry both retinal and extraretinal signals (Fukushima et al. 2000; Gottlieb et al. 1994; Tanaka and Fukushima 1998), because lesions made in this area reduce the gain of pursuit (Keating 1991; Lynch 1987; MacAvoy et al. 1991; Shi et al. 1998), and because stimulation of this area changes the gain of pursuit under a variety of conditions. The activity of pursuit-related neurons in the FPA is related to pursuit velocity (Fukushima et al. 2000; Gottlieb et al. 1994), so the output of the FPA also could account for the fact that the enhancement of the response to target perturbations is proportional to the speed of tracking (Schwartz and Lisberger 1994; Tanaka and Lisberger 2001).
Possible neural sites of enhancement and gain control
Current models of pursuit suggest that visual signals are represented as a population code in the visual motion pathways, namely area MT (e.g., Groh et al. 1997; Priebe et al. 2001). They are then decoded to create a command for smooth eye acceleration that is integrated and turned into commands for eye velocity by a positive feedback circuit that may be instantiated in the cerebellum, the cerebral cortex, or both (Lisberger et al. 1987). As we summarized in the preceding text, there is now a considerable sum of data implying that pursuit is subject to one or more forms of gain control that mediate the various enhancement effects reported here and elsewhere. Some models of pursuit have suggested that the gain control is within the internal positive feedback circuit in the pursuit system (Keating and Pierre 1996; Krauzlis and Lisberger 1994; Krauzlis and Miles 1996a; Robinson et al. 1986), while we have implied inadvertently that gain control is exercised while the signals form a command for eye acceleration, before the positive feedback circuit.
These possibilities are summarized by the diagram in Fig. 17. The diagram shows that the FPA may have access to the pursuit system through as many as three conceptually different pathways. One provides directional signals to drive pursuit, and these enter the pursuit pathways at a site upstream from gain control. It injects fixed signals independent of the state of the pursuit system and is expected from previous stimulation studies in the FPA. The poor correlation between the size of the directional signals and the amount of enhancement of ongoing pursuit caused by microstimulation at a given site, as well as the occasional observation of two response components with different latencies and directions during pursuit in the direction opposite to the directional component, argues that they are mediated by separate mechanisms. The second and third output pathways from the FPA regulate the gain of pursuit in an omni-directional manner at different stages of processing. One (GACC) is placed before the positive feedback pathway (PF) and regulates the gain of visual-motor conversion for pursuit. The other (GVEL) is placed within the positive feedback pathway and regulates how well this pathway integrates eye acceleration commands to create signals that drive eye velocity.
FIG. 17.
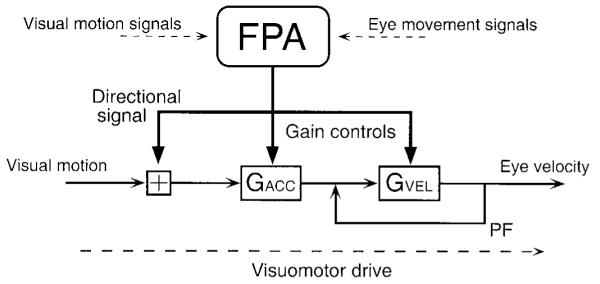
Summary diagram showing the postulated effects of FPA stimulation on pursuit. Signals that drive pursuit flow from left to right, starting from “visual motion” and ending with “eye velocity.” The square box with plus sign indicates summing junctions. The boxes labeled GACC and GVEL are variable gain elements that may be under control of the FPA. The FPA injects a directional signal upstream from the 2 variable gain elements and may be able to influence the values of the 2 gain elements. The positive feedback pathway (PF) surrounding GVEL is thought to act as an integrator, and converts visual motion commands for eye acceleration into motor commands for eye velocity.
Our data do not resolve the number and localization of the sites of gain control, but they do provide some constraints. The correlation of the enhancement of the responses to target perturbation with the size of the gain changes of ongoing pursuit accounted for only 30% of the variance (r = 0.55 in Fig. 11). The poor correlation might be attributed to the fact that different stimulation parameters were used (high- vs. low-frequency pulses), but it is also plausible that these two effects are mediated by separate mechanisms, at least to a large degree. Because it was independent of the image motion present at the time of stimulation, the effect of stimulation on the gain of ongoing pursuit seems to map most easily onto a stage of processing where signals are represented as eye-velocity commands, presumably GVEL within the positive feedback circuit. We have assumed that the enhancement of the responses to brief perturbations of target velocity would occur before the positive feedback pathway at GACC, but this assumption could be wrong. Our data do not exclude the possibility that the former site may play only a minor role. However, Missal and Heinen (2001) have recently demonstrated that stimulation of the supplementary eye field causes effects that seem to require a gain control upstream from the positive feedback circuit. Therefore, it seems most likely that the two sites of gain control are both important during pursuit and that they are activated in poorly correlated amounts from different sites in the FPA.
Acknowledgments
We thank all members of our laboratory staff: S. Tokiyama and G. Musacchia for technical assistance, K. MacLeod and L. Montgomery for surgical assistance, M. Meneses for animal care, S. Ruffner for software support, D. Kleinhesselink for network management, K. McGary for electronics, L. Bocskai for construction of mechanical devices, and N. Molyneaux for administrative assistance.
This study was supported by the Howard Hughes Medical Institute and by National Institute of Neurological Disorders and Stroke Grant P01-NS-34835.
REFERENCES
- Berman RA, Colby CL, Genovese CR, Voyvodic JT, Luna B, Thulborn KR, Sweeney JA. Cortical networks subserving pursuit and saccadic eye movements in humans: an FMRI study. Hum Brain Mapp. 1999;8:209–225. doi: 10.1002/(SICI)1097-0193(1999)8:4<209::AID-HBM5>3.0.CO;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burman DD, Bruce CJ. Suppression of task-related saccades by electrical stimulation in the primate’s frontal eye field. J Neurophysiol. 1997;77:2252–2267. doi: 10.1152/jn.1997.77.5.2252. [DOI] [PubMed] [Google Scholar]
- Carl JR, Gellman RS. Human smooth pursuit: stimulus-dependent responses. J Neurophysiol. 1987;57:1446–1463. doi: 10.1152/jn.1987.57.5.1446. [DOI] [PubMed] [Google Scholar]
- Churchland AK, Lisberger SG. Human smooth pursuit is subject to online gain control. Soc Neurosci Abstr. 2000;26(13):641. [Google Scholar]
- Churchland MM, Lisberger SG. Apparent motion produces multiple deficits in visually guided smooth pursuit eye movements of monkeys. J Neurophysiol. 2000;84:216–235. doi: 10.1152/jn.2000.84.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui DM, Yan YJ, Lynch JC. Basal ganglia circuits related to visual pursuit in monkey. Soc Neurosci Abstr. 2000;26(11):641. [Google Scholar]
- Fuchs AF, Robinson DA. A method for measuring horizontal and vertical eye movement chronically in the monkey. J Appl Physiol. 1966;21:1068–1070. doi: 10.1152/jappl.1966.21.3.1068. [DOI] [PubMed] [Google Scholar]
- Fukushima K, Sato T, Fukushima J, Shinmei Y, Kaneko CR. Activity of smooth pursuit-related neurons in the monkey periarcuate cortex during pursuit and passive whole-body rotation. J Neurophysiol. 2000;83:563–587. doi: 10.1152/jn.2000.83.1.563. [DOI] [PubMed] [Google Scholar]
- Gardner J, Lisberger SG. Linked target selection for saccadic and smooth pursuit eye movements. J Neurosci. 2001;21:2075–2084. doi: 10.1523/JNEUROSCI.21-06-02075.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldreich D, Krauzlis RJ, Lisberger SG. Effect of changing feedback delay on spontaneous oscillations in smooth pursuit eye movements of monkeys. J Neurophysiol. 1992;67:625–638. doi: 10.1152/jn.1992.67.3.625. [DOI] [PubMed] [Google Scholar]
- Gottlieb JP, Bruce CJ, Macavoy MG. Smooth eye movements elicited by microstimulation in the primate frontal eye field. J Neurophysiol. 1993;69:786–799. doi: 10.1152/jn.1993.69.3.786. [DOI] [PubMed] [Google Scholar]
- Gottlieb JP, Macavoy MG, Bruce CJ. Neural responses related to smooth-pursuit eye movements and their correspondence with electrically elicited smooth eye movements in the primate frontal eye field. J Neurophysiol. 1994;72:1634–1653. doi: 10.1152/jn.1994.72.4.1634. [DOI] [PubMed] [Google Scholar]
- Grasse KL, Lisberger SG. Analysis of a naturally occurring asymmetry in vertical smooth pursuit eye movements in a monkey. J Neurophysiol. 1992;67:164–179. doi: 10.1152/jn.1992.67.1.164. [DOI] [PubMed] [Google Scholar]
- Groh JM, Born RT, Newsome WT. How is a sensory map read Out? Effects of microstimulation in visual area MT on saccades and smooth pursuit eye movements. J Neurosci. 1997;17:4312–4330. doi: 10.1523/JNEUROSCI.17-11-04312.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta MF, Krubitzer LA, Kaas JH. Frontal eye field as defined by intracortical microstimulation in squirrel monkeys, owl monkeys, and macaque monkey. I. Subcortical connections. J Comp Neurol. 1986;253:415–439. doi: 10.1002/cne.902530402. [DOI] [PubMed] [Google Scholar]
- Huerta MF, Krubitzer LA, Kaas JH. Frontal eye field as defined by intracortical microstimulation in squirrel monkeys, owl monkeys, and macaque monkeys. II. Cortical connections. J Comp Neurol. 1987;265:332–361. doi: 10.1002/cne.902650304. [DOI] [PubMed] [Google Scholar]
- Judge SJ, Richmond BJ, Chu FC. Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res. 1980;20:535–538. doi: 10.1016/0042-6989(80)90128-5. [DOI] [PubMed] [Google Scholar]
- Keating EG. Frontal eye field lesions impair predictive and visually-guided pursuit eye movements. Exp Brain Res. 1991;86:311–323. doi: 10.1007/BF00228954. [DOI] [PubMed] [Google Scholar]
- Keating EG. Lesions of the frontal eye field impair pursuit eye movements, but preserve the predictions driving them. Behav Brain Res. 1993;53:91–104. doi: 10.1016/s0166-4328(05)80268-2. [DOI] [PubMed] [Google Scholar]
- Keating EG, Pierre A. Architecture of a gain controller in the pursuit system. Behav Brain Res. 1996;81:173–181. doi: 10.1016/s0166-4328(96)89078-4. [DOI] [PubMed] [Google Scholar]
- Kiorpes L, Walton PJ, O’keefe LP, Movshon JA, Lisberger SG. Effects of early-onset artificial strabismus on pursuit eye movements and on neuronal responses in area MT of macaque monkeys. J Neurosci. 1996;16:6537–6553. doi: 10.1523/JNEUROSCI.16-20-06537.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu H, Wurtz RH. Modulation of pursuit eye movements by stimulation of cortical areas MT and MST. J Neurophysiol. 1989;62:31–47. doi: 10.1152/jn.1989.62.1.31. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Lisberger SG. A model of visually-guided smooth pursuit eye movements based on behavioral observations. J Comp Neurosci. 1994;1:265–283. doi: 10.1007/BF00961876. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Miles FA. Transitions between pursuit eye movements and fixation in the monkey: dependence on context. J Neurophysiol. 1996a;76:1622–1638. doi: 10.1152/jn.1996.76.3.1622. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Miles FA. Decreases in the latency of smooth pursuit and saccadic eye movements produced by the “gap paradigm” in the monkey. Vision Res. 1996b;36:1973–1985. doi: 10.1016/0042-6989(95)00307-x. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Miles FA. Role of the oculomotor vermis in generating pursuit and saccades: effects of microstimulation. J Neurophysiol. 1998;80:2046–2062. doi: 10.1152/jn.1998.80.4.2046. [DOI] [PubMed] [Google Scholar]
- Leichnetz GR. Inferior frontal eye field projections to the pursuit-related dorsolateral pontine nucleus and middle temporal area (MT) in the monkey. Vis Neurosci. 1989;3:171–180. doi: 10.1017/s0952523800004478. [DOI] [PubMed] [Google Scholar]
- Leichnetz GR, Smith DJ, Spencer RF. Cortical projections to the paramedian tegmental and basilar pons in the monkey. J Comp Neurol. 1984;228:388–408. doi: 10.1002/cne.902280307. [DOI] [PubMed] [Google Scholar]
- Lisberger SG. Postsaccadic enhancement of initiation of smooth pursuit eye movements in monkeys. J Neurophysiol. 1998;79:1918–1930. doi: 10.1152/jn.1998.79.4.1918. [DOI] [PubMed] [Google Scholar]
- Lisberger SG, Morris EJ, Tychsen L. Visual motion processing and sensory-motor integration for smooth pursuit eye movements. Annu Rev Neurosci. 1987;10:97–129. doi: 10.1146/annurev.ne.10.030187.000525. [DOI] [PubMed] [Google Scholar]
- Lisberger SG, Westbrook LE. Properties of visual inputs that initiate horizontal smooth pursuit eye movements in monkeys. J Neurosci. 1985;5:1662–1273. doi: 10.1523/JNEUROSCI.05-06-01662.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luebke AE, Robinson DA. Transition dynamics between pursuit and fixation suggest different systems. Vision Res. 1988;28:941–946. doi: 10.1016/0042-6989(88)90103-4. [DOI] [PubMed] [Google Scholar]
- Lynch JC. Frontal eye field lesions in monkeys disrupt visual pursuit. Exp Brain Res. 1987;68:437–441. doi: 10.1007/BF00248811. [DOI] [PubMed] [Google Scholar]
- Macavoy MG, Gottlieb JP, Bruce CJ. Smooth-pursuit eye movement representation in the primate frontal eye field. Cereb Cortex. 1991;1:95–102. doi: 10.1093/cercor/1.1.95. [DOI] [PubMed] [Google Scholar]
- May JG, Keller EL, Crandall WF. Changes in eye velocity during smooth pursuit tracking induced by microstimulation in the dorsolateral pontine nucleus of the macaque. Soc Neurosci Abstr. 1985;11:79. [Google Scholar]
- Missal M, Heinen SJ. Facilitation of smooth pursuit initiation by electrical stimulation in the supplementary eye fields. J Neurophysiol. 2001;86:2413–2425. doi: 10.1152/jn.2001.86.5.2413. [DOI] [PubMed] [Google Scholar]
- Morrow MJ, Sharpe JA. Deficits of smooth-pursuit eye movement after unilateral frontal lobe lesions. Ann Neurol. 1995;37:443–451. doi: 10.1002/ana.410370406. [DOI] [PubMed] [Google Scholar]
- O’driscoll GA, Wolff AV, Benkelfat C, Florencio PS, Lal S, Evans AC. Functional neuroanatomy of smooth pursuit and predictive saccades. Neuroreport. 2000;11:1335–1340. doi: 10.1097/00001756-200004270-00037. [DOI] [PubMed] [Google Scholar]
- Petit L, Clark VP, Ingeholm J, Haxby JV. Dissociation of saccade-related and pursuit-related activation in human frontal eye fields as revealed by fMRI. J Neurophysiol. 1997;77:3386–3390. doi: 10.1152/jn.1997.77.6.3386. [DOI] [PubMed] [Google Scholar]
- Petit L, Haxby JV. Functional anatomy of pursuit eye movements in humans as revealed by fMRI. J Neurophysiol. 1999;82:463–471. doi: 10.1152/jn.1999.82.1.463. [DOI] [PubMed] [Google Scholar]
- Priebe NJ, Churchland MM, Lisberger SG. Reconstruction of target speed for the guidance of pursuit eye movements. J Neurosci. 2001;21:3196–3206. doi: 10.1523/JNEUROSCI.21-09-03196.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashbass C. The relationships between saccadic and smooth tracking eye movements. J Physiol (Lond) 1961;159:326–338. doi: 10.1113/jphysiol.1961.sp006811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DA. The mechanics of human smooth pursuit eye movement. J Physiol (Lond) 1965;180:569–591. doi: 10.1113/jphysiol.1965.sp007718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DA, Gordon JL, Gordon SE. A model of the smooth pursuit eye movement system. Biol Cybern. 1986;55:43–57. doi: 10.1007/BF00363977. [DOI] [PubMed] [Google Scholar]
- Schwartz JD, Lisberger SG. Initial tracking conditions modulate the gain of visuo motor transmission for smooth pursuit eye movements in monkeys. Vis Neurosci. 1994;11:411–424. doi: 10.1017/s0952523800002352. [DOI] [PubMed] [Google Scholar]
- Shi D, Friedman HR, Bruce CJ. Deficits in smooth-pursuit eye movements after muscimol inactivation within the primate’s frontal eye field. J Neurophysiol. 1998;80:458–464. doi: 10.1152/jn.1998.80.1.458. [DOI] [PubMed] [Google Scholar]
- Stanton GB, Bruce CJ, Goldberg ME. Topography of projections to posterior cortical areas from the macaque frontal eye fields. J Comp Neurol. 1995;353:291–305. doi: 10.1002/cne.903530210. [DOI] [PubMed] [Google Scholar]
- Stanton GB, Goldberg ME, Bruce CJ. Frontal eye field efferents in the macaque monkey. I. Subcortical pathways and topography of striatal and thalamic terminal fields. J Comp Neurol. 1988a;271:473–492. doi: 10.1002/cne.902710402. [DOI] [PubMed] [Google Scholar]
- Stanton GB, Goldberg ME, Bruce CJ. Frontal eye field efferents in the macaque monkey. II. Topography of terminal fields in midbrain and pons. J Comp Neurol. 1988b;271:493–506. doi: 10.1002/cne.902710403. [DOI] [PubMed] [Google Scholar]
- Tian JR, Lynch JC. Functionally defined smooth and saccadic eye movement subregions in the frontal eye field of Cebus monkeys. J Neurophysiol. 1996a;76:2740–2753. doi: 10.1152/jn.1996.76.4.2740. [DOI] [PubMed] [Google Scholar]
- Tian JR, Lynch JC. Corticocortical input to the smooth and saccadic eye movement subregions of the frontal eye field in Cebus monkeys. J Neurophysiol. 1996b;76:2754–2771. doi: 10.1152/jn.1996.76.4.2754. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Fukushima K. Neuronal responses related to smooth pursuit eye movements in the periarcuate cortical area of monkeys. J Neurophysiol. 1998;80:28–47. doi: 10.1152/jn.1998.80.1.28. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Lisberger SG. Context-dependent smooth eye movements evoked by stationary visual stimuli in trained monkeys. J Neurophysiol. 2000;84:1748–1762. doi: 10.1152/jn.2000.84.4.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Lisberger SG. Regulation of the gain of visually-guided smooth pursuit eye movements by frontal cortex. Nature. 2001;409:191–194. doi: 10.1038/35051582. [DOI] [PubMed] [Google Scholar]
- Yan YJ, Cui DM, Lynch JC. Efferent targets of the pursuit subregion of the frontal eye field in Cebus monkey include the superior colliculus, pontine nuclei, and caudate nucleus. Soc Neurosci Abstr. 1999;25:1397. [Google Scholar]