Abstract
Dicer is an evolutionarily conserved ribonuclease III that is necessary for microRNA (miRNA) processing and the synthesis of small interfering RNAs from long double-stranded RNA. Although it has been shown that Dicer plays important roles in the mammalian germline and early embryogenesis, the functions of Dicer-dependent pathways in the somatic cells of the female reproductive tract are unknown. Using a transgenic line in which Cre recombinase is driven by the anti-Müllerian hormone receptor type 2 promoter, we conditionally inactivated Dicer1 in the mesenchyme of the developing Müllerian ducts and postnatally in ovarian granulosa cells and mesenchyme-derived cells of the oviducts and uterus. Deletion of Dicer in these cell types results in female sterility and multiple reproductive defects including decreased ovulation rates, compromised oocyte and embryo integrity, prominent bilateral paratubal (oviductal) cysts, and shorter uterine horns. The paratubal cysts act as a reservoir for spermatozoa and oocytes and prevent embryos from transiting the oviductal isthmus and passing the uterotubal junction to enter the uterus for implantation. Deep sequencing of small RNAs in oviduct revealed down-regulation of specific miRNAs in Dicer conditional knockout females compared with wild type. The majority of these differentially expressed miRNAs are predicted to regulate genes important for Müllerian duct differentiation and mesenchyme-derived structures, and several of these putative target genes were significantly up-regulated upon conditional deletion of Dicer1. Thus, our findings reveal diverse and critical roles for Dicer and its miRNA products in the development and function of the female reproductive tract.
MICRORNAS (miRNAS) ARE approximately 22-nucleotide, single-stranded, noncoding RNAs that are critical elements of gene regulatory networks in plants and animals (1). These small RNAs direct the translational repression or degradation of complementary target mRNAs, potentially functioning as molecular rheostats to fine tune cellular protein production (2). miRNAs are essential for mammalian development through their regulation of fundamental cellular processes, namely growth, differentiation, and apoptosis. They are synthesized as long primary transcripts that are cleaved by a ribonuclease (RNase) III, Drosha, to yield an approximately 70-nucleotide stem loop precursor miRNA (pre-miRNA). The pre-miRNA is exported to the cytoplasm and subsequently cleaved by a second RNase III, Dicer (encoded by Dicer1), to excise the approximately 22-nucleotide miRNA:miRNAa duplex, one strand of which is preferentially incorporated into the RNA-induced silencing complex. Unlike miRNAs, small interfering (siRNAs) are derived from larger double-stranded RNAs that are processed by Dicer. Whereas siRNAs function in mRNA degradation by forming siRNA:mRNA duplexes, miRNAs often bind to the 3′-untranslated region of mRNAs in a perfect or imperfect match to regulate mRNA cleavage or translational repression, respectively (reviewed in Refs. 3 and 4).
The role of miRNAs, siRNAs, and the processing enzyme Dicer in the regulation of gene expression has been shown to be conserved from yeast to mammals (5,6,7,8,9). For instance, recent reports have shown that miRNAs regulate the expression of a variety of steroid hormone receptors including human estrogen receptor α (10), the ecdysone receptor in fruit flies (11), and nuclear hormone receptors 23 and 25 in worms (12).
In mice, Dicer null and hypomorphic mutations result in postimplantation embryonic lethality [embryonic d 7.5 (E7.5) and E12.5-E14.5, respectively (9,13)]. Mutations in both Dicer and Dgcr8, an essential cofactor for Drosha, indicate that miRNAs are essential for embryonic stem cell differentiation (14,15). Loss of embryonic stem cells has also been shown to occur in the absence of Dicer (9), likely due to the inability of Dicer null stem cells to generate miRNAs, as demonstrated by high-throughput small RNA sequencing (16). Due to the embryonic lethality demonstrated by Dicer null and hypomorphic mice, the study of mammalian Dicer and its miRNA/siRNA products in late embryogenesis and postnatal development requires a conditional knockout (cKO) approach. Using tissue-specific promoters to drive the expression of Cre recombinase, cKO studies have shown that RNA silencing is required for the normal function of diverse cell and tissue types, including T cells (17,18), B cells (19), chondrocytes (20), skin and hair follicles (21,22), brain (23,24), heart (25,26), skeletal muscle (27), lung epithelium (28), pancreatic islets (29), limb mesoderm (30), and retina (31).
With regard to reproduction, Dicer is required for normal fertility. In male mice, Dicer1-deficient primordial germ cells and spermatogonia show a reduction in proliferation, resulting in a block in spermatogenesis and infertility after 8 months of age (32). In females, conditional deletion of Dicer1 has so far only been reported in germ cells. cKO of Dicer1 in mouse oocytes results in sterility due to meiotic defects secondary to disorganized spindle formation (33,34). More recently, characterization of mice homozygous for a hypomorphic allelic of Dicer1 has suggested that Dicer is involved in corpus luteum angiogenesis, leading to fertility defects (35). From this hypomorph model, however, it is unclear whether the observed phenotype arises secondary to deficiency of Dicer in endothelial cells or whether luteal cell function is directly compromised.
Given the fact that 1) steroid hormone receptors are essential for female reproduction, are expressed in multiple tissues of the reproductive tract, and have been shown to be regulated by miRNAs in human cell lines (10); and 2) granulosa cells actively proliferate during folliculogenesis, and cell division is also regulated by miRNAs, we hypothesized that depletion of Dicer in the somatic cells of the female reproductive tract would result in reproductive defects. To this end, we examined the consequences of deletion of Dicer using a cKO approach directed by a knockin of the Cre recombinase gene into the anti-Müllerian hormone receptor type 2 (Amhr2) locus. Cre-mediated recombination in Amhr2cre/+ mice has been observed in the developing Müllerian ducts, ovarian granulosa cells, oviducts, uterus, and somatic cells of the testis (36,37). Dicer1 cKO female mice are sterile and demonstrate decreased ovulation rates, compromised oocyte and embryo integrity, and defects in the oviducts and uterus, therefore revealing novel and essential roles for Dicer-dependent pathways in the development and function of the female reproductive tract.
RESULTS
Dicer1 cKO Female Mice Are Sterile
To study the functions of Dicer in the female reproductive tract, we generated a cKO mouse model using Cre-mediated recombination under the control of the Amhr2 promoter. Absence of Amhr2 or its ligand, anti-Müllerian hormone, results in persistent Müllerian duct syndrome in males and leads to fertility defects mainly in male mice (38,39). Amhr2-Cre is expressed embryonically in the mesenchyme of the developing Müllerian ducts and postnatally in ovarian granulosa cells and the smooth muscle and stromal cells of the oviducts and uterus (36,37,40,41). Temporal expression of Amhr2-Cre in the Müllerian duct mesenchyme has been previously characterized using two mouse reporter lines, ROSA (36) and EGFP (40). Results from these studies are conflicting; when ROSA reporter mice were used, expression was detected at E12.5 (36), whereas when an EGFP reporter line was used, expression was detected at E15.5, and it was not detected in all mesenchymal cells (40). Amhr2-Cre transgenic mice have been used to characterize the anti-Müllerian hormone signaling cascade in the mesenchyme surrounding the developing Müllerian ducts (36), TGFβ superfamily signaling in postnatal ovarian granulosa cells (42,43,44,45), and depletion of the orphan nuclear receptor, chicken ovalbumin upstream promoter transcription factor II (COUP-TFII), in the stromal cells and smooth muscle layers of the postnatal uterus (46).
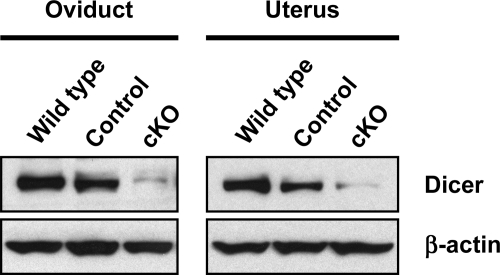
We crossed Dicer1+/− Amhr2cre/+ mice to Dicer1flox/flox mice to generate Dicer1flox/− Amhr2cre/+ experimental mice (designated throughout as Dicer1 cKO), Dicer1flox/− mice (designated as control), and Dicer1flox/+ mice (designated as wild type). Dicer1 cKO mice were born at the expected Mendelian frequency and appeared healthy. Tissue-specific recombination of the Dicer1 floxed allele was assessed by Western blot analysis in oviducts and uterus from wild-type, control, and Dicer1 cKO mice (Fig. 1). Tissues from control mice showed a decrease in Dicer levels compared with wild-type mice, and a further decrease in Dicer levels was observed in tissues from Dicer1 cKO mice. Complete absence of Dicer in these tissues was not expected because Amhr2-Cre is known to direct recombination only in mesenchyme-derived structures (i.e. smooth muscle and stromal cells) of the oviducts and uterus.
Figure 1.
Dicer1 cKO Oviducts and Uteri Have Decreased Dicer Levels
Western blot analysis of oviducts and uteri from 3- to 4-wk-old wild-type (Dicer1flox/+), control (Dicer1flox/−), and Dicer1 cKO (Dicer1flox/− Amhr2cre/+) mice. Membranes were probed with anti-Dicer antibody, and then stripped and reprobed with anti-β-actin to control for loading. Note that control tissues show a decrease in Dicer levels, whereas a further decrease was observed in Dicer1 cKO tissues. Complete absence of Dicer was not expected because Dicer1 was deleted only in tissues derived from the Müllerian duct mesenchyme.
To test the fertility of Dicer1 cKO and control females, we mated 10 females of each genotype to wild-type males beginning at 6 wk of age. Whereas control females had normal fecundity and fertility (9.2 ± 0.4 pups per litter; 1.2 ± 0.04 litters/month; see Table 1), Dicer1 cKO females were sterile over the same 13- to 30-wk period. These findings indicate that Dicer-dependent pathways in the female reproductive tract are necessary for fertility.
Table 1.
Fertility Testing of Dicer1flox/− and Dicer1 cKO Females
| Genotype | N | Litters | Total Pups | Pups/Litter | Litters/Month |
|---|---|---|---|---|---|
| Dicer1flox/− | 10 | 62 | 575 | 9.2 ± 0.4 | 1.2 ± 0.04 |
| Dicer1flox/− | 10 | 0 | 0 | 0 | 0 |
| Amhr2cre/+ |
Six-week-old Dicer1flox/− and Dicer1flox/− Amhr2cre/+ females were mated to wild-type males for 13–30 wk. Data are shown as the mean ± sem.
Paratubal Cysts Are Prominent in Dicer1 cKO Mice
Because Amhr2 drives expression of Cre in multiple tissues of the female reproductive tract, several potential etiologies could be responsible for the sterility in Dicer1 cKO females, including aberrant mating behavior, defects in ovarian folliculogenesis or ovulation, poor fertilization, compromised oocyte/embryo integrity, oviductal defects, or an impaired uterine decidual response. To gain insight into these possibilities, we examined the reproductive tracts of Dicer1 cKO females at various time points postnatally. In 12-wk-old mutants, we observed bilateral paratubal cysts that contained clear, colorless fluid (Fig. 2, A and B). Cysts were absent at birth [postnatal d 0 (P0); Fig. 2, C and D); however, abnormalities in the oviductal wall were visible in 7-d-old mice (P7, Fig. 2, E–H). Paratubal cysts were evident as early as P16 (Fig. 2, I–K) and expanded further by 3 wk of age (Fig. 2L) and older (Fig. 2M). Cysts were also typically larger and more abundant near the uterotubal junction and often contained opaque, flocculated debris (Fig. 2, L and M).
Figure 2.
Dicer1 cKO Females Develop Bilateral Paratubal Cysts
A, Reproductive tracts from 12-wk-old control (left) and cKO (right) female mice. The uterine (ut) horns of cKO females are shorter, and the oviducts (ovid) display bilateral fluid-filled cysts that are larger near the uterotubal junction than near the ovary (ov). B, Higher magnification of uterotubal junction in panel A (box). Newborn oviducts (P0) of control (C) and cKO (D) mice show that paratubal cysts are absent at birth. Compared with control oviducts (E), defects in the oviduct wall (F, arrow; G, higher magnification) are evident in cKO mice at postnatal day 7 (P7). Normal oviduct wall morphology is also present (F, arrowhead; H, higher magnification). Paratubal cysts (I, J, arrows; K, higher magnification) are evident at postnatal day 16 (P16). The cysts grow larger with age (L, 3-wk-old; M, 7.5-wk-old), and contain opaque, flocculated material (M, arrowhead). N, Oviduct of a 7.5-wk-old cKO female after injection of dye into the uterine lumen near the uterotubal junction. The dye entered the cysts and was extruded from the ampulla, indicating that the cysts are continuous with the lumen of the oviduct. The oviducts in panels M and N are oriented with the ovarian side (ampulla) at the top of the figure and the uterine side at the bottom.
To determine the structural relationship between the oviduct and the paratubal cysts in adult Dicer1 cKO mice, we injected dye into the uterine lumen near the uterotubal junction and examined its dispersal in the oviducts. The dye highlighted the tortuous path of the oviducts and also entered the cysts (Fig. 2N), indicating that the cysts were directly connected and continuous with the lumen of the oviducts. These findings suggest that the cysts in adult Dicer1 cKO mice may physically prevent the interaction of oocytes and spermatozoa, or alternatively prevent embryos from reaching the uterus.
Histological analysis of oviducts from control females at all ages demonstrated relatively uniform periodic acid Schiff (PAS)-positive columnar epithelium throughout most of the structure (Fig. 3, A, B, and E). In contrast, oviducts from 16-d-old Dicer1 cKO females showed distended areas lined by either simple columnar, cuboidal, or squamous-like epithelium (Fig. 3, C and D). Cellular debris was occasionally found in the lumen of these structures. In adult Dicer1 cKO females, the paratubal cysts were typically lined by a flattened or simple columnar epithelium (Fig. 3, F and G). In most cases, the cysts contained a significant amount of exudative material as well as ring-shaped PAS-positive structures (Fig. 3H).
Figure 3.
Histological Examination of Oviducts from 16-d-old (A–D) and 12-wk-old (E–H) Control (A, B, E) and cKO (C, D, F–H) Females
Control oviducts at all ages examined are lined by simple columnar epithelium (B, arrowhead and inset). D, High magnification of the area in panel C (box). Cysts present in 16-d-old oviducts are small, lined by columnar (D, arrowhead and top inset) or cuboidal (D, arrow and bottom inset) epithelium and often contain cellular remnants and PAS-positive material. F, Paratubal cysts in older animals are often multilobular and contain exudative material. Higher magnification of the area in panel F (box) indicates that the cysts are lined by a flattened (G, arrow and top inset) or simple columnar (G, arrowhead and bottom inset) epithelium. In addition, higher magnification of the area in panel F (asterisk) demonstrates that the cysts often contain ring-shaped PAS-positive structures (H).
Because paratubal cysts were present in 3-wk-old cKO females and grew larger as the mice aged, we hypothesized that reproductive hormones might influence such growth. However, treatment with pregnant mare serum gonadotropin (PMSG) followed by human chorionic gonadotropin (hCG), a LH analog, did not influence the size of the cysts. Furthermore, treatment of 3-wk-old mice with 17β-estradiol for 3 d had no significant effect on the size of the cysts, despite the fact that estrogen receptor α was present in the nuclei of epithelial cells lining the cysts (data not shown).
Ovarian Defects Are Limited in Dicer1 cKO Mice
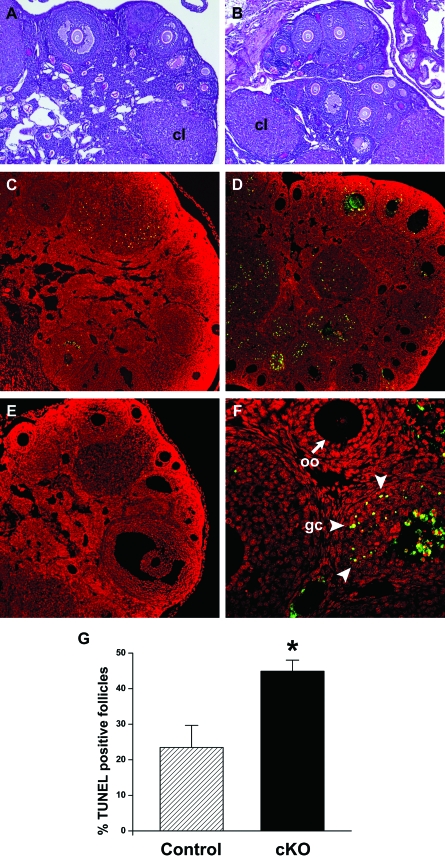
Because Amhr2-Cre transgenic mice have also been used to study the postnatal functions of genes expressed in developing follicles (42,43,44,45), we analyzed the consequences of Dicer1 deletion in ovarian granulosa cells. Histological analysis of ovaries from 3-wk-old control and Dicer1 cKO females showed no obvious differences. In addition, ovaries from 6-wk-old and 12-wk-old control and cKO females contained follicles at all stages of folliculogenesis as well as corpora lutea (Fig. 4, A and B). Despite these initial observations, follicle counts on 6-wk-old ovaries revealed a trend toward increased numbers of atretic follicles in Dicer1 cKO ovaries compared with controls (data not shown). To pursue this finding, we performed terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL) analysis on 6-wk-old ovaries from control and Dicer1 cKO females and found a significant increase in the proportion of TUNEL-positive follicles in mutant ovaries (Fig. 4, C–G). TUNEL-positive follicles contained labeled granulosa cells but not oocytes (Fig. 4F), and follicular fluid occasionally showed nonspecific staining. Thus, the ovaries of adult Dicer1 cKO females demonstrate essentially normal folliculogenesis with the exception of increased follicular atresia.
Figure 4.
Dicer1 cKO Ovaries Have Increased Apoptosis
Comparison of ovarian histology from 12-wk-old control (A) and cKO (B) females shows that Dicer1 mutant ovaries contain follicles at all stages of folliculogenesis as well as corpora lutea (cl). Despite these observations, we also noted a trend toward increased atretic follicles in cKO ovaries at 6 wk of age (data not shown). TUNEL staining of 6-wk-old ovaries from control (C) and cKO (D) mice revealed increased apoptotic granulosa cells (green/yellow) in the Dicer1 mutant. Nuclei are counterstained with propidium iodide (red). E, TUNEL-negative control. F, High magnification of TUNEL-positive follicles in the Dicer1 cKO ovary. Note that granulosa cells (gc, arrowheads) are TUNEL positive, whereas oocytes (oo, arrow) are negative. G, The proportion of TUNEL-positive follicles was quantified and found to be significantly higher in cKO ovaries (44.9 ± 5.4%) as compared with control ovaries (23.5 ± 10.8%). *, P < 0.05 (Student’s t test).
Ovulation and Early Embryonic Defects in Dicer1 cKO Females
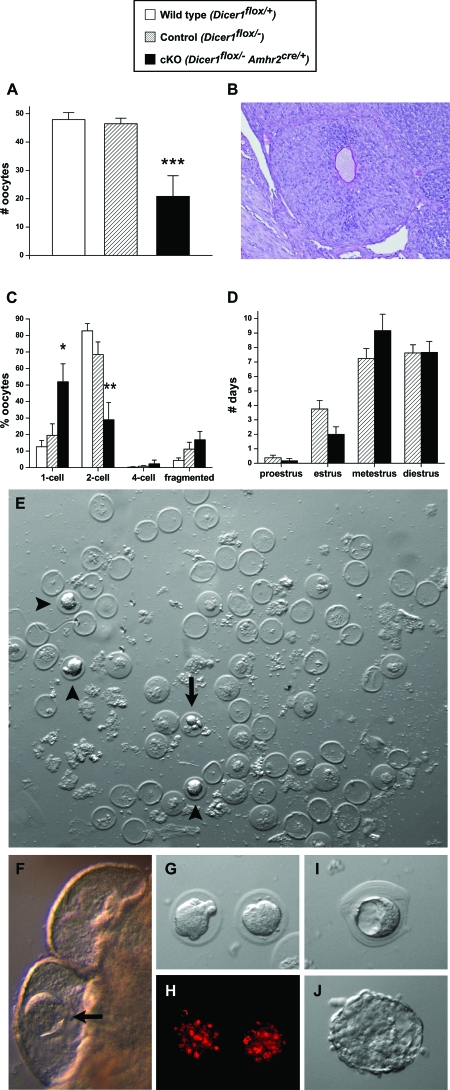
Because the ovaries of prepubertal Dicer1 cKO mice appeared normal, we next investigated the responsiveness of immature Dicer1 cKO and control females to gonadotropins. Three- to 4-wk-old mice were pharmacologically superovulated with PMSG followed by hCG and then mated with stud males of proven fertility to examine ovulation, fertilization, and early embryonic development. Dicer1 cKO females ovulated approximately half the number of oocytes compared with controls, whereas no significant differences in ovulation rates were found between controls and wild-type littermates (Fig. 5A). Histological analysis of Dicer1 cKO ovaries after superovulation showed the presence of trapped oocytes in luteinized follicles (Fig. 5B), further suggesting defects in ovulation. Although copulatory plugs were observed at similar frequencies for mutants and controls, a significantly lower proportion of oocytes from Dicer1 cKO mice progressed to the two-cell stage after overnight culture (Fig. 5C). This may be caused by decreased fertilization potential of oocytes from Dicer1 cKO females or by mitotic defects during the first division of the embryo. The results suggest that Dicer1 expression in granulosa cells is required for normal ovulation and indirectly for oocyte/embryo integrity.
Figure 5.
Dicer1 cKO Mice Demonstrate Abnormalities in Ovulation, Early Embryonic Development, and Estrous Cycles, and Blocked Transit of Embryos through the Oviduct
A, Average number of oocytes ovulated by 3- to 4-wk-old wild-type (n = 12; 47.9 ± 2.5 oocytes), control (n = 11; 46.5 ± 2.0), and Dicer1 cKO (n = 6; 20.8 ± 7.3) females after pharmacological superovulation. B, Oocyte trapped in a luteinized follicle of a Dicer1 cKO ovary after pharmacological superovulation. C, Average numbers of one-cell, two-cell, four-cell, and fragmented oocytes/embryos from wild-type (n = 11), control (n = 11), and cKO (n = 4) female mice after pharmacological superovulation, mating, collection, and culture. A significantly higher proportion of oocytes/embryos from Dicer1 mutants remained at the one-cell stage (wild type, 12.6 ± 3.8%; control, 19.6 ± 6.9%; cKO, 51.9 ± 10.9%), and a significantly lower proportion progressed to the two-cell stage (wild type, 82.8 ± 4.4%; control, 68.4 ± 7.7%; cKO, 29.0 ± 10.5%). D, Average number of days spent in each stage of the estrous cycle for adult control (n = 8) and cKO (n = 6) females over a 3-wk period. As compared with controls, Dicer1 cKO females trended toward spending less time in estrus (control, 3.8 ± 0.6 d; cKO, 2.0 ± 0.5 d) and more time in metestrus (control, 7.3 ± 0.7 d; cKO, 9.2 ± 1.1 d). E–J, Adult 10- to 12- wk-old control and Dicer1 cKO females were mated to stud males, and their uteri and paratubal cysts were flushed and examined microscopically 3.5 d after copulation. Whereas uteri of Dicer1 cKO females lacked embryos, the paratubal cysts of each mouse contained 15 to more than 100 zona pellucida remnants and several embryos. F, Blastocyst (arrow) inside of a paratubal cyst. A developmentally arrested embryo is shown in panel E (arrow). Morula stage embryos are shown in panels G, H (stained with propidium iodide), and E (arrowheads). We also observed blastocysts in an unusual onion skin-like matrix (I) and hatched embryos (J). *, P < 0.01; **, P < 0.001; ***, P < 0.0001 (one-way ANOVA, Tukey Honestly Significant Differences test).
Dicer1 cKO Females Have Normal Serum FSH and Estradiol Levels but Abnormal Estrous Cycles
The results described above indicate that Dicer1 cKO mice can mate with stud males and generate copulatory plugs when hormones are administered to override the normal hypothalamic-pituitary-gonadal axis. However, because the Dicer1 cKO females display ovarian defects, and the ovary plays a critical role in the regulation of the estrous cycle, we examined serum hormone levels and estrous cycles of adult control and Dicer1 cKO females. No significant differences were found in serum FSH levels of randomly cycling mice at 6 wk (control: 8.8 ± 2.3, n = 12; cKO: 8.0 ± 2.6, n = 9) and 12 wk (control: 3.3 ± 0.9, n = 4; cKO: 3.5 ± 0.5, n = 3). Likewise, serum estradiol levels were similar between control and cKO females at 6 wk (control: 12.1 ± 1.9, n = 12; cKO: 11.0 ± 2.2, n = 9) and 12 wk (control: 12.0 ± 3.2, n = 4; cKO: 13.0 ± 2.9, n = 4). However, by monitoring estrous cycles with daily vaginal smears over a 3-wk period, we observed that Dicer1 cKO females trended toward shorter estrus and longer metestrus phases as compared with control females (Fig. 5D). Thus, these findings confirm a defect in the ovaries that influences cyclicity of the females.
Oocytes, Spermatozoa, and Embryos Are Trapped in the Paratubal Cysts of Dicer1 cKO Oviducts
Because Dicer1 cKO female mice are sterile despite the fact that they ovulate oocytes that can be fertilized to produce embryos, we next addressed whether defects in the oviduct and/or uterus prevented implantation. As mentioned above and illustrated in Fig. 2M, a significant amount of debris accumulated in the paratubal cysts. We therefore examined Dicer1 cKO females that were mated with stud males; motile spermatozoa were observed in the paratubal cysts of Dicer1 cKO oviducts, indicating that in adult mice with prominent bilateral paratubal cysts, there may be an impediment for spermatozoa to reach the ampulla for fertilization. To investigate further, 10- to 12-wk-old Dicer1 cKO and control females were examined 3.5 d after natural mating with stud males. Whereas flushing of control uterine horns yielded blastocysts, none of the Dicer1 cKO females had embryos in their uteri. On closer examination, embryos were instead apparent in some of the paratubal cysts (see blastocyst in Fig. 5F). Flushing of the cysts of adult Dicer1 cKO mice at 3.5 d after coitus revealed a wide range of zona pellucida remnants (from 15 to >100), indicating that the oocytes were readily swept into the cysts (Fig. 5E). In addition, morula stage (Fig. 5, E, G, and H), blastocyst stage (Fig. 5I), and hatching stage embryos (Fig. 5J), as well as developmentally arrested embryos, could be collected from the cysts. Thus, there appeared to be a reduction in the potential of oocytes to be fertilized, but once fertilized, embryos that entered the oviductal isthmus were readily swept into the paratubal cysts, preventing entry into the uterus and the potential for implantation.
Dicer1 cKO Uteri Are Shorter but Demonstrate Normal Decidualization
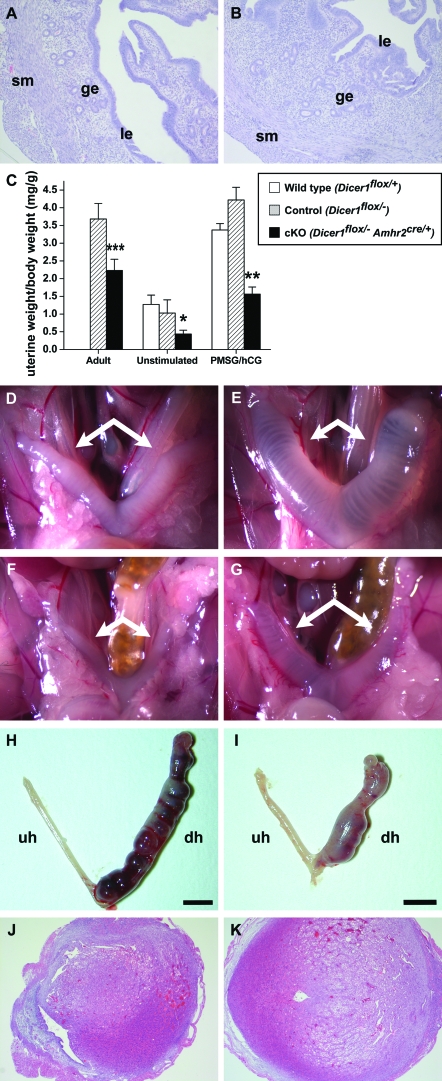
The uterus consists of two major compartments, the myometrium and the endometrium. The myometrium consists of two smooth muscle layers whereas the endometrial compartment consists of luminal and glandular epithelial cells as well as supporting stromal cells. Because the Amhr2 promoter directs the expression of Cre strongly to the smooth muscle cells of the myometrium and more weakly to the endometrial stromal cells (46), we next investigated the effect of Dicer1 loss in the uterus. At all ages examined, the uterine horns of Dicer1 cKO mice were shorter compared with those of control females and weighed less (Fig. 6C). A representative micrograph of uteri from 12-wk-old control and mutant females is shown in Fig. 2A. Despite these gross abnormalities, uteri from Dicer1 cKO females demonstrated histologically normal myometrium and endometrium (Fig. 6, A and B).
Figure 6.
Histological and Functional Analysis of Dicer1 cKO Uteri
Uteri from 12-wk-old control (A) and cKO (B) females display histologically normal myometrial (sm, smooth muscle) and endometrial (ge, glandular epithelium; le, luminal epithelium) compartments. C, Uterine weights were measured and normalized to body weights for adult 10- to 12-wk-old females 3.5 d after copulation with stud males and for 3- to 4-wk-old female mice. Uteri from adult Dicer1 cKO mice (n = 6) weighed significantly less than control uteri (n = 4). In addition, uteri from unstimulated (wild type, n = 6; control, n = 4; cKO, n = 5) and PMSG/hCG-treated (n = 3 for all groups) Dicer1 cKO mice weighed significantly less than wild-type uteri; however, the relative increase in uterine weight after hormonal stimulation was comparable between wild-type (2.7-fold) and cKO (3.6-fold) females. In addition, uteri from 3- to 4-wk-old wild-type (D and E), control (data not shown), and cKO (F and G) female mice were examined after 3-d treatment with 17β-estradiol (E and G) or vehicle alone (D and F). Although uteri from cKO females weighed less than wild-type and control uteri regardless of treatment group, the relative increase in uterine weight after estradiol stimulation was similar between cKO (2.6-fold) and wild-type females (3.2-fold). The uterine decidual response in control (H) and cKO (I) mice was also examined and found to be normal, despite the fact that cKO uterine horns are shorter than those of controls. In contrast to the unstimulated horns (uh), the decidualized horns (dh) from both control (J) and cKO (K) uteri display the expected transformation of stromal fibroblasts into epithelioid cells. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (Student’s t test).
To determine the functional response of Dicer1 cKO uteri to endocrine stimulation, mice were injected with PMSG followed by hCG to induce an increase in ovarian steroid hormone production. The mice were then killed 20 h later, and uterine weights were recorded and normalized to body weights. Because Dicer1 cKO uteri are smaller than those of controls, we also recorded uterine weights from unstimulated mice to evaluate the relative response to hormonal stimulation. Uteri from unstimulated and PMSG/hCG-treated Dicer1 cKO mice weighed significantly less than wild-type uteri; however, the relative increase in uterine weight after hormonal stimulation was comparable between wild-type (2.7-fold) and cKO (3.6-fold) females (Fig. 6C). These results suggest that the superovulatory regimen of gonadotropins induced ovarian estrogen production and that the uterus responded to estrogen in a normal fashion. To verify these findings, 3- to 4-wk-old Dicer1 cKO females were treated with 17β-estradiol or vehicle (sesame oil) for 3 d, and uterine weights were recorded (Fig. 6, D–G). Although uteri from cKO females weighed less than wild-type and control uteri regardless of treatment group, the relative increase in uterine weight after estradiol stimulation was similar between cKO (2.6-fold) and wild-type females (3.2-fold). Consistent with these observations, estrogen receptor α, the major form of estrogen receptor in the uterus, was present in Dicer1 cKO uteri at 12 wk of age (data not shown). Thus, although Dicer1 cKO uteri are shorter than those of control mice, they retain their ability to respond to gonadotropins and estrogen.
Embryo implantation depends on the uterine response to both estrogen and progesterone, and on the ability of stromal cells to decidualize. Although the estradiol response of Dicer1 cKO females appeared to be normal, progesterone responsiveness and stromal cell differentiation might be affected by loss of Dicer. Due to the ovulatory defect in Dicer1 cKO females, we chose to evaluate decidualization by inducing an artificial decidual reaction as previously described (47). Ten days after ovariectomy, control and Dicer1 cKO mice were subjected to a steroid hormone regimen (see Materials and Methods), and one uterine horn was given a decidual stimulus (injected with oil) while the contralateral horn served as an unstimulated control. The mice were then treated with estrogen and progesterone daily and killed on the fifth day after trauma. As shown in Fig. 6, H–K, induction of decidualization appeared to be normal in both control and Dicer1 cKO mice. Both showed a similar increase in uterine size compared with the unstimulated uterine horn (Fig. 6, H and I). In addition, histological analysis of the endometrial stroma of the decidual horn showed the expected transformation of fibroblasts to epithelioid cells in both control and Dicer1 cKO females (Fig. 6, J and K). Thus, absence of Dicer1 in the smooth muscle and stromal cells of the uterus appears to affect the growth but not the function of the uterus.
Conditional Deletion of Dicer1 in the Oviduct Leads to Specific Changes in miRNA Levels
Because Dicer processes miRNA precursors into mature miRNAs, we hypothesized that conditional deletion of Dicer1 would cause a global reduction in the levels of miRNAs in the affected tissues. To test this hypothesis, we performed Illumina/Solexa deep sequencing of small RNAs in oviducts of 3- to 4-wk-old wild-type and Dicer1 cKO mice, which already have visible paratubal cysts. Oviducts were the tissue of choice because they displayed the most striking phenotype. We generated 4,225,815 and 4,192,829 small RNA sequences for two independent wild-type samples, and 3,828,843 and 4,070,145 small RNA sequences for two independent Dicer1 cKO samples. Unexpectedly, the proportion of small RNA sequences that mapped to known mouse miRNAs was comparable between wild-type (79.3% and 79.6%) and Dicer1 cKO (78.4% and 79.3%) oviducts. Despite these global similarities, we detected 28 miRNAs that were down-regulated in cKO oviducts compared with wild type (Table 2).
Table 2.
Differentially Expressed Oviductal miRNAs and Putative Targets in Mesenchyme-Derived Compartments
| miRNA | Fold Change | Predicted Targets (Algorithm) |
|---|---|---|
| mmu-mir-449c | −1.66 | Cald1 (m); Wnt5a (m) |
| mmu-mir-449a | −1.60 | Wnt5a (m) |
| mmu-mir-143 | −1.59 | Mapk7 (t,m) |
| mmu-mir-375 | −1.59 | Wnt5a (t) |
| mmu-mir-27b | −1.51 | Cald1 (t,p); Hoxa10 (t,p); Hoxa13 (t,p); Mapk7 (t,p) |
| mmu-mir-99b | −1.48 | |
| mmu-mir-152 | −1.41 | Cnn1 (t); Ctnnb1 (m) |
| mmu-let-7i | −1.39 | Cald1 (t,p); Hoxa9 (t,p); Tagln (m) |
| mmu-mir-24 | −1.39 | Mapk7 (t,p) |
| mmu-mir-103 | −1.38 | Hoxa10 (p); Fzd1 (m); Mapk7 (m); Wnt4 (m) |
| mmu-mir-145 | −1.36 | Des (m) |
| mmu-mir-34b-3p | −1.34 | Cald1 (m) |
| mmu-mir-744 | −1.33 | Tagln (m); Mapk7 (m) |
| mmu-mir-29b | −1.33 | |
| mmu-mir-92b | −1.33 | Hoxa9 (t);Fzd1 (m) |
| mmu-mir-100 | −1.32 | |
| mmu-mir-30a | −1.32 | Hoxa10 (m); Vim (t); Hoxa11 (t,m) |
| mmu-mir-125b-5p | −1.30 | Wnt5a (m) |
| mmu-mir-146b | −1.28 | Wnt5a (m);Fzd1 (m); Mapk7 (m) |
| mmu-mir-340–5p | −1.28 | Cald1 (m); Hoxa10 (m) |
| mmu-mir-200c | −1.28 | |
| mmu-mir-23b | −1.26 | Hoxa11 (t,p); Wnt4 (m) |
| mmu-mir-30d | −1.25 | Vim (t); Hoxa11 (t,m) |
| mmu-mir-107 | −1.24 | Hoxa10 (p);Fzd1 (m); Wnt4 (m) |
| mmu-mir-185 | −1.22 | Wnt5a (m); Ctnnb1 (m) |
| mmu-mir-29a | −1.22 | Vim (p) |
| mmu-mir-676 | −1.21 | |
| mmu-mir-148a | −1.20 | Cnn1 (t) |
miRNAs from the oviducts of 3- to 4-wk-old Dicer1flox/+ and Dicer1 cKO females were profiled by deep sequencing. Fold changes are expressed relative to Dicer1flox/+. Genes in bold were significantly up-regulated in Dicer1 cKO oviducts by QPCR. Prediction algorithms: t, TargetScan; p, PicTar; m, miRanda.
To examine the potential functional consequences of these alterations, we used three different algorithms (TargetScan, PicTar, and miRanda) to predict the mRNA targets of these down-regulated miRNAs. Interestingly, 23 of the 28 miRNAs were predicted to target one to four genes known to be involved in Müllerian duct differentiation and mesenchyme-derived structures (Table 2). The Müllerian duct proliferates and differentiates in an anterior to posterior manner to form the oviduct, uterus, cervix, and upper part of the vagina in a process that is completed by postnatal day 15 (48). Müllerian duct differentiation and maturation involves members of the wingless family of secreted glycoproteins (Wnt4, Wnt5a, Wnt7a) and the homeobox transcription factors (Hoxa9, Hoxa10, Hoxa11, Hoxa13) (49,50). Hoxa9, Hoxa10, Hoxa11, and Hoxa13 are expressed along the axis of the Müllerian duct at birth, and their expression persists in the adult reproductive tract where they are proposed to define functional and tissue identities (51). Our analysis predicted Wnt4 and Wnt5a as targets for three and six miRNAs, respectively (Table 2). Similarly, all Hoxa genes mentioned above are predicted to be targets of one or more miRNAs. In addition, our analysis predicted changes in genes that are important for mesenchyme-derived structures, including smooth muscle differentiation markers such as transgelin (Tagln) and desmin (Des) (52,53,54,55).
Conditional Deletion of Dicer1 in the Oviduct Leads to Significant Changes in the Expression of Genes That Are Critical for Müllerian Duct Differentiation and Mesenchyme-Derived Structures
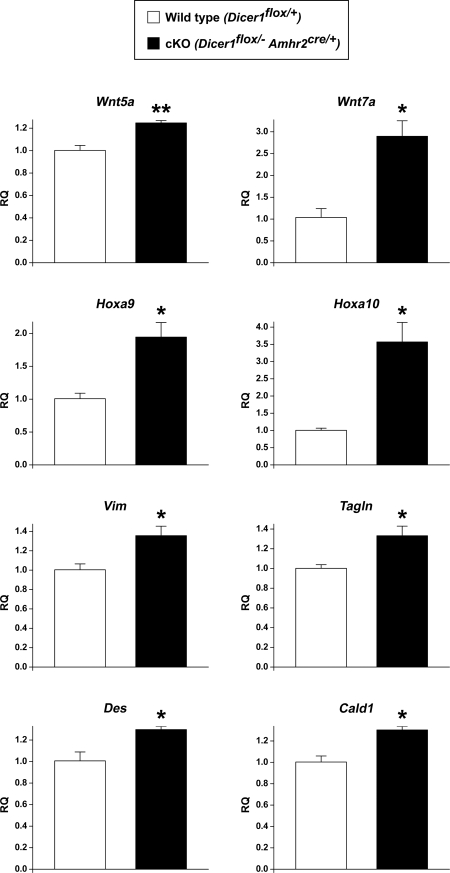
As indicated above, our target analysis of miRNAs that were down-regulated in oviducts from Dicer1 cKO mice predicted changes in various genes involved in Müllerian duct differentiation and genes relevant to mesenchyme-derived structures. One mechanism of miRNA function is through cleavage of mRNA targets. Therefore, we examined the expression of candidate target genes by performing quantitative real-time PCR (QPCR) analysis on oviducts from 3- to 4-wk-old wild-type and Dicer1 cKO females (Fig. 7 and Table 3). Hoxa9, which is expressed in the oviduct, was significantly up-regulated in Dicer1 cKO mice. Interestingly, Hoxa10, which is predominantly expressed in the uterus, was also significantly increased in Dicer1 cKO oviducts. In contrast, Hoxa13, which is normally expressed in the vagina, was below the level of detection in both control and cKO oviducts.
Figure 7.
Gene Expression Changes in Dicer1 cKO Oviducts
QPCR analysis of mRNA from 3- to 4-wk-old wild-type and Dicer1 cKO oviducts (mean ± sem; n = 3 independent pools of oviducts per genotype). Dicer1 cKO oviducts showed a significant increase in the relative quantity (RQ) of wingless family members (Wnt5a and Wnt7a), Hoxa genes (Hoxa9 and Hoxa10), the mesenchymal intermediate filament vimentin (Vim), the smooth muscle markers transgelin (Tagln) and desmin (Des), and the actin-binding protein caldesmon 1 (Cald1). These results and other gene expression changes are summarized in Table 3. *, P < 0.05; **, P < 0.01 (Student’s t test).
Table 3.
Summary of QPCR Results for Gene Expression in Oviducts of 3- to 4 wk-old Dicer1flox/+ and Dicer1 cKO Females
| Gene | Description | Fold Change |
|---|---|---|
| Wnt family and receptors | ||
| Wnt4 | Wingless-related MMTV integration site 4 | +1.26 |
| Wnt5a | Wingless-related MMTV integration site 5a | +1.24a |
| Wnt7a | Wingless-related MMTV integration site 7a | +2.79b |
| Fzd1 | Frizzled homolog 1 | +1.55 |
| Hoxa family | ||
| Hoxa9 | Homeo box A9 | +1.93b |
| Hoxa10 | Homeo box A10 | +3.55b |
| Hoxa13 | Homeo box A13 | Not detected |
| Smooth muscle markers | ||
| Acta2 | Actin, α 2, smooth muscle, aorta | +1.04 |
| Cnn1 | Calponin 1 | −1.24 |
| Des | Desmin | +1.29b |
| Myh11 | Myosin, heavy polypeptide 11, smooth muscle | −1.03 |
| Smtn | Smoothelin | +1.33 |
| Tagln | Transgelin | +1.33b |
| Oviductal epithelium markers | ||
| Foxj1 | Forkhead box J1 | −1.17 |
| Ovgp1 | Oviductal glycoprotein 1 | 1.00 |
| Other genes | ||
| Cald1 | Caldesmon 1 | +1.30b |
| Ctnnb1 | Catenin (cadherin-associated protein), β1 | +1.08 |
| Mapk7 | MAPK 7 | +1.23 |
| Vim | Vimentin | +1.35b |
Fold changes are expressed relative to Dicer1flox/+.
, P < 0.01;
, P < 0.05 (Student’s t test). MMTV, Mouse mammary tumor virus.
We also observed significant changes in the wingless family member Wnt5a, but not in Wnt4 levels. Embryonically, Wnt7a is expressed throughout the Müllerian duct epithelium, but it becomes restricted to the oviduct and uterine luminal epithelium soon after birth (50,56). Wnt7a plays a pivotal role in Müllerian duct patterning and in the maintenance of Hoxa10 and Hoxa11 gene expression in the uterus (56). Similar to Dicer1 cKO mice, Wnt7a knockout mice display uterine horns that are shorter and thinner than their control counterparts, as well as defects in oviduct coiling (56,57). Interestingly, we observed a significant increase in Wnt7a levels in Dicer1 cKO oviducts. Wnt proteins exert their actions through two signaling pathways, canonical and noncanonical, via interactions with members of the Frizzled (Fzd) family of receptors (58). The canonical pathway involves β-catenin (Ctnnb1) and its downstream targets (58). Fzd1 is expressed in the Müllerian duct mesenchyme and epithelium and has been proposed to mediate β-catenin activation by Wnt proteins in this tissue (40). In contrast to our prediction analysis, Ctnnb1 and Fzd1 were unchanged in Dicer1 cKO oviducts.
The mesenchyme of the Müllerian duct gives rise to the smooth muscle of the oviduct and uterus. Dicer1 cKO oviducts showed a significant increase in the smooth muscle markers transgelin (Tagln) and desmin (Des) (52,53,54,55), but not in other smooth muscle markers such as smooth muscle actin (Acta2) (59), the actin-linked regulatory protein calponin 1 (Cnn1) (60), myosin heavy chain 11 (Myh11) (59), and the cytoskeletal protein smoothelin (Smtn) (61). In addition, no significant changes were observed in MAPK 7 (Mapk7), which is required for vascular smooth muscle cell migration (62,63). Vimentin (Vim), the predominant filament in mesenchymal cells, was significantly up-regulated in Dicer1 cKO oviducts. Caldesmon 1 (Cald1), a protein that is present in both smooth muscle and epithelial cells of chicken and bovine oviducts (64,65), and that interacts with actin, myosin, tropomyosin, and calmodulin (60), was also significantly up-regulated in Dicer1 mutant oviducts. No significant changes were observed in forkhead box J1 (Foxj1), a marker for ciliated epithelial cells of the oviduct (66), or in oviductal glycoprotein 1 (Ovgp1), a marker for secretory epithelial cells of the oviduct (67,68).
DISCUSSION
Consistent with the important roles played by regulatory RNAs in mammalian development and postnatal physiology, deletion of Dicer1, the gene encoding the key enzyme involved in miRNA and siRNA biogenesis, in the female reproductive tract leads to multiple defects. In this study, we show that loss of Dicer1 in somatic cells of the ovary, oviduct, and uterus causes infertility due to defects in ovarian function and the presence of paratubal cysts. Our experiments demonstrate that ovaries from Dicer1 cKO females are without obvious defects at 3 wk of age, although superovulation of immature females yielded half the expected number of oocytes, suggesting an abnormal response to gonadotropin stimulation or defects in ovulation. Because the response to PMSG appears to be normal, at least in terms of granulosa cell proliferation, the decrease in ovulation rates might be attributed to altered LH responsiveness. Dicer1 cKO females also have abnormal estrous cycles, with shorter estrus and longer metestrous phases. In addition, histological analysis of adult ovaries showed increased follicle loss, which has been previously associated with alterations in estrous cycle length (69).
Experiments in which unstimulated adult female mice were mated to males of known fertility showed absence of blastocysts in the uteri of Dicer1 cKO females at 3.5 d after coitus, whereas control females showed normal blastocyst recovery. However, we were able to retrieve blastocysts, developmentally arrested embryos, and numerous zona pellucida remnants from the paratubal cysts of Dicer1 cKO females. These results suggest that although ovulation and fertilization occur in Dicer1 cKO females, fewer embryos reach the blastocyst stage, and all unfertilized oocytes and embryos become trapped in the oviductal cysts. Infertility caused by embryo entrapment in the oviduct has been previously reported to occur in cannabinoid receptor 1 null mice, which show defects in oviduct contraction, although in the absence of cysts (70). Therefore, both the physical and functional integrity of the oviduct is essential for normal reproduction.
Although we cannot rule out the idea that the oviductal environment may adversely affect embryonic development, the decreased ability of embryos from superovulated immature females to progress to the two-cell stage in vitro suggests that the quality of oocytes from Dicer1 cKO females is compromised. Because Dicer was deleted in the somatic cell compartment of the ovary, the observed defects in oocyte quality are likely due to altered communication between oocytes and granulosa cells (71). In this context, regulatory RNAs have been shown to be essential for follicular function in fruit flies, because combined loss of Dicer and its interacting protein R2D2, which is expressed in the somatic cells of the ovary, causes defective oogenesis (72).
To our knowledge, this is the first mouse model of paratubal cysts. These cysts were present in Dicer1 cKO females as early as postnatal day 16 and became larger with age, which suggested the possibility of hormonal regulation; however, we were unable to induce cyst growth in immature females by stimulation with either gonadotropins or estrogen. Despite this, we cannot discard the possibility that progesterone may have an impact on cyst growth. In addition, the presence of a large number of zona pellucida remnants in the cysts is intriguing, given the fact that the response of Dicer1 cKO females to gonadotropins is reduced compared with that of control mice, and the Dicer1 cKO estrous cycles are abnormal. A likely explanation is that the remnants are products of repeated estrous cycles in which oocytes became trapped in the cysts, with the zona pellucidae remaining long after oocyte degeneration.
Decreased levels of selected miRNAs have been demonstrated in other Dicer1 cKO mouse models (17,18,19,21,22,23,24,27,30,31) and in Dicer-deficient embryonic stem cells (14); however, global decreases in miRNA levels have rarely been reported in somatic cells of other Dicer1 cKO mouse models (20,26), although they have been shown to occur in Dicer1 cKO oocytes (34) and Dicer null embryonic stem cells (16). Our high-throughput sequencing of small RNAs showed comparable proportions of miRNA sequences in oviducts from 3- to 4-wk-old wild-type and Dicer1 cKO mice, which already have visible paratubal cysts. Further analysis showed that 28 miRNAs were down-regulated in Dicer1 cKO oviducts. Dicer is decreased only in mesenchyme-derived structures of the oviduct and uterus (i.e. smooth muscle and stromal cells), but not in the epithelium. Therefore, it is possible that normal levels of Dicer in the oviduct epithelium may mask a more dramatic decrease in miRNA levels. The fact that previous studies showed that not all mesenchymal cells in the Müllerian duct undergo recombination in the Amhr2-Cre mouse line (40) may also contribute. Additionally, because the spatiotemporal expression of miRNAs in the prenatal Müllerian duct and postnatal oviduct is unknown, it is possible that our findings accurately reflect the loss of miRNAs that are relevant to mesenchyme-derived structures of the oviduct after postnatal differentiation is completed.
Importantly, several genes involved in Müllerian duct differentiation were predicted to be targets of one or more miRNAs that were differentially expressed in Dicer1 cKO oviducts. Predicted targets included, but were not limited to, Wnt family genes, Hoxa genes, and smooth muscle markers. Abnormal expression of several of the putative targets was confirmed by QPCR analysis, whereas the levels of others were unchanged. For instance, Wnt4, Fzd1, Ctnnb1, and Cnn1 were predicted to be targeted by at least two miRNAs; however, we found no significant changes in their mRNA levels. MiRNA regulation of these genes may occur through translational repression instead of mRNA cleavage, in which case only protein levels would be altered.
Hoxa genes are expressed on the longitudinal axis of the Müllerian duct during embryogenesis, and their spatial distribution and expression persist in the adult reproductive tract. As predicted, our results show up-regulation of Hoxa9, which is normally expressed in the oviduct (51), and Hoxa10, which is important for development of the uterotubal junction and is expressed in the uterus (49,73). Similarly, Wnt5a, which is predominantly expressed in the uterine stroma and epithelium, was also up-regulated. The increase in Hoxa gene expression could be attributed to the loss of regulatory miRNAs; however, because none of the miRNAs that were differentially expressed in Dicer1 cKO oviducts have been confirmed to target Hoxa9, Hoxa10, or Wnt5a, we cannot rule out the possibility that abnormal expression of other factors may contribute to the observed increase. For example, Wnt7a, which was up-regulated in Dicer1 cKO oviducts, has been shown to activate and sustain expression of Hoxa10, possibly via regulation of Wnt5a (50). It is likely that the increase in Wnt7a mRNA is an indirect effect of Dicer1 deletion in the oviduct mesenchyme, because postnatal expression of Wnt7a is limited to the luminal epithelium of the uterus (50). This hypothesis is supported by studies showing that the molecular cues from the mesenchyme induce differentiation of the Müllerian duct epithelium 4–5 d after birth (74). We also found increased expression of the smooth muscle markers transgelin (Tagln) and desmin (Des), the intermediate filament vimentin (Vim), and caldesmon 1 (Cald1). The impact of such changes on the physiology of the oviduct and their contribution to the Dicer1 cKO phenotype remain to be elucidated.
Other models have been created to study the function of Dicer1 in the female reproductive tract. Whereas our studies were focused on the deletion of Dicer1 in the somatic cells surrounding the oocytes, as well as in the oviduct and uterus, two other groups demonstrated that loss of Dicer1 specifically in oocytes resulted in arrest at meiosis I and sterility (33,34). Likewise, studies in a hypomorphic Dicer1 mouse model showed that the vascular compartment of the corpus luteum was compromised, thereby affecting progesterone circulation and female reproduction (35). Surprisingly, this latter model did not show ovulation defects, embryo abnormalities, oviductal cysts, or altered uterine size, indicating that low levels of Dicer1 outside of the vasculature are sufficient to generate viable gametes and that only through ablation in specific cells can the roles of Dicer be observed.
Apart from decreased uterine size, Dicer1 cKO females do not show any defects in uterine function, as they respond to estrogen and decidual stimuli similar to controls. Because Amhr2-Cre does not ablate gene expression in the uterine epithelial cell compartment, and it is only weakly active in the uterine stroma, these results were expected. Recently, ablation of an endometrial stroma-expressed gene, chicken ovalbumin upstream promoter transcription factor II (COUP-TFII), showed two different phenotypes when conditionally ablated using Amhr2-Cre (46) or progesterone receptor (PR)-Cre transgenic mice (75). This was attributed to differences in the compartmental expression and relative efficiencies of Cre recombination (46). Because the PR-Cre mouse line efficiently ablates gene expression in all compartments of the uterus (76), it may be more suited to evaluate the role of Dicer in this tissue. Experiments are underway to understand how Dicer1 conditional deletion in epithelial cells of the uterus affects implantation and to define the changes in miRNAs/siRNAs that may be responsible for those effects.
MATERIALS AND METHODS
Generation and Genotyping of Dicer1 cKO Mice
The Dicer1 conditional allele (designated throughout as Dicer1flox) and Dicer1 null allele (designated throughout as Dicer1−) have been described (22) and were maintained on a C57BL/6J;129S5/SvEvBrd mixed hybrid background. Mice were genotyped from tail genomic DNA using PCR primers as described (32). Dicer1+/− mice were bred to Amhr2cre/+ mice (36) to generate Dicer1+/− Amhr2cre/+ mice, which were then bred to Dicer1flox/flox mice to generate Dicer1flox/− Amhr2cre/+ experimental mice (designated throughout as Dicer1 cKO), Dicer1flox/− mice (designated as control), and Dicer1flox/+ mice (designated as wild type). All mice were maintained in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Western Blot Analysis
Protein lysates from oviducts and uteri of 3- to 4-wk-old wild-type (Dicer1flox/+), control (Dicer1flox/−), and Dicer1 cKO (Dicer1flox/− Amhr2cre/+) female mice were electrophoresed through a NuPAGE 3–8% Tris-Acetate gel (Invitrogen, Carlsbad, CA) and then transferred to a nitrocellulose membrane. Immunoblotting was performed using a rabbit polyclonal antibody against Dicer1 at a 1:1000 dilution [ab1416, a kind gift from David Livingston (14)]. Blots were stripped and reprobed using a mouse monoclonal antibody against β-actin at a 1:10,000 dilution (Sigma A5441; Sigma Chemical Co., St. Louis, MO) to verify equal loading of samples. Bands were visualized using the SuperSignal West Pico Chemiluminescent Substrate Kit (Pierce Biotechnology, Rockford, IL). A total of three independent samples per genotype were analyzed in three independent experiments.
Fertility Analysis
To evaluate reproductive performance, 10 individually housed 6-wk-old Dicer1 cKO and Dicer1flox/− females were bred to wild-type C57BL/6J;129S5/SvEvBrd hybrid males with known fertility. The numbers of litters and pups were recorded over a 13- to 30-wk period.
Tissue Collection and Histological Analysis
Ovaries, oviducts, and uteri were dissected and fixed in 10% neutral buffered formalin for histology, snap frozen for protein or RNA isolation, or preserved in RNAlater (Applied Biosystems, ABI, Foster City, CA). Tissue processing and embedding were performed by the Department of Pathology Core Services laboratory (Baylor College of Medicine, Houston, TX). Histological sections were cut at 5 μm and stained with periodic acid-Schiff/hematoxylin or hematoxylin-eosin using standard techniques.
Oviductal Dye Injection
The oviducts and paratubal cysts of adult Dicer1 cKO females were dissected in M2 medium (Sigma) and dye (0.25% bromophenol blue, 0.25% xylene cyanol, and 15% Ficoll type 400 in deionized water) was injected into the uterine lumen near the uterotubal junction using a 30-gauge needle.
TUNEL Staining
Analysis of apoptosis in 6-wk-old ovaries was carried out by TUNEL assay using the ApopTag Plus Fluorescein In Situ Apoptosis Detection Kit (Chemicon International, Temecula, CA). Ovaries were fixed in 10% neutral buffered formalin, embedded, and sectioned at 5 μm. Three sections from each of three independent control and Dicer1 cKO ovaries were analyzed in parallel. TUNEL assay was performed according to the manufacturer’s instructions, and slides were mounted in Vectashield (Vector Laboratories, Inc., Burlingame, CA) containing propidium iodine to visualize chromatin. Slides were analyzed by confocal microscopy. The total number of TUNEL-positive follicles per section were counted and normalized to the total number of follicles. Data are presented as the average percent of TUNEL-positive follicles per section ± sem.
Superovulation and Isolation of Oocytes/Embryos
Superovulation experiments were carried out as described (77). Twenty-two to 25-d-old Dicer1flox/+, Dicer1flox/−, and Dicer1 cKO female mice were injected ip with 5 IU PMSG (Calbiochem, San Diego, CA) for 46 h, followed by ip injection with 5 IU Novarel (Ferring Pharmaceuticals, Parsippany, NJ). Mice were then bred to wild-type males with known fertility. Eggs and/or embryos were recovered from the ampulla of the oviduct 20 h after Novarel, collected in M2 medium (Sigma) containing 1 mg/ml hyaluronidase (Sigma) to dissociate cumulus cells, counted, and cultured overnight in M16 medium (Sigma). The numbers of one-cell, two-cell, four-cell, and fragmented oocytes/embryos were recorded.
Serum Analysis
Mice were anesthetized by isoflurane inhalation (Abbott Laboratories, North Chicago, IL), and blood was collected in Microtainer tubes (Becton Dickinson, Franklin Lakes, NJ) by closed cardiac puncture. Serum was separated by centrifugation and stored at −20 C until further use. FSH and estradiol measurements were performed by the University of Virginia Ligand Assay and Analysis Core (Specialized Cooperative Centers Program in Reproduction Research). Assay information is available at http://www.healthsystem.virginia.edu/internet/ crr/ligand.cfm.
Monitoring of Estrous Cycles
Over a 3-wk period, vaginal smears from 6- to 7-wk-old control and Dicer1 cKO female mice were collected each day between 1300 h and 1400 h. The smears were classified into one of four phases of the estrous cycle (proestrus, estrus, metestrus, diestrus) as described elsewhere (78). Data are presented as the average number of days spent in each phase of the estrous cycle ± sem.
Uterine Hormonal Stimulation
Three to 4-wk-old Dicer1flox/+, Dicer1flox/−, and Dicer1 cKO female mice were unstimulated or treated with one of the following regimens: 1) 5 IU PMSG ip for 46 h, followed by 5 IU Novarel ip for 20 h; 2) 2 μg 17β-estradiol in 100 μl sesame oil sc, once per day for 3 d (79); or 3) 100 μl sesame oil (vehicle only) sc, once per day for 3 d. Body weights and uterine weights were measured upon cervical dislocation, and the average uterine weight/body weight ± sem was calculated for each group. For each genotype, fold changes between the PMSG/hCG and unstimulated groups were calculated, and fold changes between the 17β-estradiol and vehicle-only groups were calculated.
Induction of Decidualization
The artificial decidual response has been previously described (47). Briefly, ovariectomized mice were treated with sc injections of 100 ng 17β-estradiol per mouse, once per day for 3 d. After 2 d rest, mice were then treated with sc injections of 1 mg progesterone and 6.7 ng 17β-estradiol per mouse, once per day for 3 d. One uterine horn was traumatized by an intraluminal injection of 50 μl of sesame oil 6 h after the third progesterone and 17β-estradiol injection. The contralateral horn was not traumatized and served as a control. Mice were then given sc injections of 1 mg progesterone and 6.7 ng 17β-estradiol per mouse, once per day. Mice were killed 5 d after the trauma by cervical dislocation while under anesthesia, Avertin (2,2-tribromoethyl alcohol; Sigma). At the time of dissection, uterine tissues were placed in 4% paraformaldehyde.
Deep Sequencing of Oviductal Small RNAs
Oviducts from 3- to 4-wk-old Dicer1flox/+ and Dicer1 cKO female mice were dissected, pooled by genotype (n = 2 pools per genotype; three to five mice per pool), and preserved in RNAlater (ABI). Total RNA was extracted using the mirVana miRNA Isolation Kit (ABI) and small RNA library construction was performed using the DGE-Small RNA Sample Prep Kit (Illumina, San Diego, CA) according to the manufacturer’s protocol. Purified cDNA was quantified using the Quant-iT PicoGreen dsDNA Kit (Invitrogen) and diluted to 10 nm for sequencing on the Illumina 1G Genome Analyzer at the University of Houston. All unique sequence reads with a minimum read count of 10 were aligned to mouse miRNA sequences in the miRNA database [miRBase version 11.0 (80)] as described elsewhere (81). For each sample, counts were normalized to the total number of small RNA sequences, and then for each miRNA, the average number of counts was compared between genotypes. miRNAs with fold change more than 1.2 and Dicer1flox/+ read count greater than 500 were considered to be differentially expressed.
Prediction of Putative miRNA:mRNA Functional Interactions
Three established miRNA target prediction algorithms, TargetScan [version 4.1 (82)], PicTar (83), and miRanda [January 2008 (84)], were used to identify potential mRNA targets for miRNAs that were differentially expressed between Dicer1flox/+ and Dicer1 cKO oviducts. Predictions were compiled from Microsoft Excel annotation worksheets consisting of all putative miRNA:mRNA interactions for each algorithm. Annotation worksheets are available at http://sigterms. sourceforge.net.
QPCR
Oviducts from 3- to 4-wk-old Dicer1flox/+ and Dicer1 cKO female mice were dissected, pooled by genotype (n = 3 pools per genotype; three to five mice per pool), and flash frozen. Total RNA was extracted using the RNeasy Mini Kit (QIAGEN, Valencia, CA) with on-column deoxyribonuclease digestion using the RNase-Free DNase Set (QIAGEN), all according to the manufacturer’s protocol. Total RNA (500 ng) was reverse transcribed in a 50-μl reaction using 250 U Superscript III reverse transcriptase (Invitrogen) and oligo(dT)12–18 primer (Invitrogen). Samples were diluted 50-fold, and 5 μl was used for each QPCR reaction. QPCR was performed on the ABI Prism 7500 Sequence Detection System using predesigned TaqMan Gene Expression Assays (ABI) or custom primers designed using Primer Express software (ABI), and mouse Gapdh as an endogenous control. The following TaqMan assays were used: Wnt4, Mm00437341_m1; Wnt5a, Mm00437347_m1; Wnt7a, Mm00437355_m1; Hoxa10, Mm00433966_m1; Hoxa13, Mm00433967_m1; Ctnnb1, Mm00483033_m1; Vim, Mm00449201_m1; and Gapdh, 4352339E. Custom primers were designed for the following genes and primer sequences are listed in Table 4: Acta2; Cald1; Cnn1; Des; Foxj1; Fzd1; Gapdh; Hoxa9; Mapk7; Myh11; Ovgp1; Smtn; and Tagln. TaqMan PCR was performed using TaqMan Universal PCR Master Mix (ABI), and PCR with custom primers was performed using SYBR Green PCR Master Mix (ABI) in 20 μl. The reaction conditions were as follows: 2 min at 50 C, 10 min at 95 C, followed by 40 cycles of 15 sec at 95 C (denaturation) and 1 min at 60 C (annealing/extension). Each sample was analyzed in duplicate or triplicate and a nontemplate control (nuclease-free water) sample was included on each plate for each primer-probe set. The relative quantity (RQ) of transcript was calculated using the 2-ΔΔCT method (85) and plotted as mean ± sem.
Table 4.
Primer Sequences for QPCR
| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| Acta2 | CCACCGCAAATGCTTCTAAGT | GGCAGGAATGATTTGGAAAGG |
| Cald1 | AGTCTCCAAGATTGACAGCCG | ATGCTCTTGATATTGCGGACG |
| Cnn1 | GGTGAAACCCCACGACATCTT | TTTGTCTTGGCCATGCTGG |
| Des | TACACCTGCGAGATTGATGCC | GCGCAATGTTGTCCTGATAGC |
| Foxj1 | CAAGATCACTCTGTCGGCCAT | CGAGGCACTTTGATGAAGCAC |
| Fzd1 | GTTTCGTGTCACTCTTCCGCA | CCCGAAAGGCCTGTTCATAGA |
| Gapdh | CAATGTGTCCGTCGTGGATCT | GCCTGCTTCACCACCTTCTT |
| Hoxa9 | CCTTCTCCGAAAACAATGCC | TCCTTCTCCAGTTCCAGCGT |
| Mapk7 | TGCCATCTCCGACAATACCA | TTCTCTTCTCGTTCTCGCTGG |
| Myh11 | CATCCTGACCCCACGTATCAA | ATCGGAAAAGGCGCTCATAGG |
| Ovgp1 | TGGCCAAGAATCTGCAGGATG | AACCGTGATGTGCCGAAGTTC |
| Smtn | TCACTACCTTCAGCCATGCCT | GCCATTAGCTGCTTCCACTGT |
| Tagln | CGATGGAAACTACCGTGGAGA | TGAAGGCCAATGACGTGCT |
Statistical Analysis
Data are presented as the mean ± sem. Statistical analysis was carried out using the JMP version 7.0.1 statistical package (SAS Software, Cary, NC). Statistical differences were tested using Student’s t test or one-way ANOVA followed by Tukey’s honestly significant differences test. P < 0.05 was considered statistically significant.
Acknowledgments
We thank Alexander Tarakhovsky for Dicer1flox/flox mice, Richard Behringer for Amhr2-Cre mice, David Livingston for Dicer1 antibodies, Chad Creighton for providing annotation worksheets for miRNA target prediction, and the University of Virginia Ligand Assay and Analysis Core (Specialized Cooperative Centers Program in Reproduction Research, Charlottesville, VA) for performing serum hormone assays. We thank the Cullen Foundation for their generous support of the University of Houston’s Institute for Molecular Design, and Zhenkang Xu and Karon Cassidy for sequencing. We also thank Mark Edson for help with uterine studies, Roopa Nalam for help with figures, and Angshumoy Roy and James Orengo for insightful discussions.
Footnotes
This work was supported by National Institutes of Health Grant CA60651 (to M.M.M.), the Specialized Cooperative Centers Program in Reproduction and Infertility (to F.J.D. and M.M.M.), a Program Project Development Grant from the Ovarian Cancer Research Fund (to P.H.G. and M.M.M.), T32GM008307 (to A.K.N.), the Joseph and Matilda Melnick Endowed Fund (to A.K.N.), and scholarships from Baylor Research Advocates for Student Scientists (to A.K.N. and H.L.F.). The University of Virginia Ligand Assay and Analysis Core (Specialized Cooperative Centers Program in Reproduction Research) was supported by Grant U54 HD28934 from National Institutes of Child Health and Human Development/National Institutes of Health.
Disclosure Statement: The authors have nothing to disclose.
First Published Online August 7, 2008
Abbreviations: cKO, Conditional knockout; E7.5, embryonic d 7.5; hCG, human chorionic gonadotropin; miRNA, microRNA; PAS, periodic acid Schiff; PMSG, pregnant mare serum gonadotropin; QPCR, quantitative real-time PCR; RNase, ribonuclease; TUNEL, terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling.
References
- Du T, Zamore PD 2005 microPrimer: the biogenesis and function of microRNA. Development 132:4645–4652 [DOI] [PubMed] [Google Scholar]
- Bartel DP, Chen CZ 2004 Micromanagers of gene expression: the potentially widespread influence of metazoan microRNAs. Nat Rev Genet 5:396–400 [DOI] [PubMed] [Google Scholar]
- Shyu AB, Wilkinson MF, van Hoof A 2008 Messenger RNA regulation: to translate or to degrade. EMBO J 27:471–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Belasco JG 2008 Let me count the ways: mechanisms of gene regulation by miRNAs and siRNAs. Mol Cell 29:1–7 [DOI] [PubMed] [Google Scholar]
- Reinhart BJ, Weinstein EG, Rhoades MW, Bartel B, Bartel DP 2002 MicroRNAs in plants. Genes Dev 16:1616–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE, Horvitz HR, Ruvkun G 2000 The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature 403:901–906 [DOI] [PubMed] [Google Scholar]
- Brennecke J, Cohen SM 2003 Towards a complete description of the microRNA complement of animal genomes. Genome Biol 4:228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wienholds E, Koudijs MJ, van Eeden FJ, Cuppen E, Plasterk RH 2003 The microRNA-producing enzyme Dicer1 is essential for zebrafish development. Nat Genet 35:217–218 [DOI] [PubMed] [Google Scholar]
- Bernstein E, Kim SY, Carmell MA, Murchison EP, Alcorn H, Li MZ, Mills AA, Elledge SJ, Anderson KV, Hannon GJ 2003 Dicer is essential for mouse development. Nat Genet 35:215–217 [DOI] [PubMed] [Google Scholar]
- Adams BD, Furneaux H, White BA 2007 The micro-ribonucleic acid (miRNA) miR-206 targets the human estrogen receptor-α (ERα) and represses ERα messenger RNA and protein expression in breast cancer cell lines. Mol Endocrinol 21:1132–1147 [DOI] [PubMed] [Google Scholar]
- Varghese J, Cohen SM 2007 microRNA miR-14 acts to modulate a positive autoregulatory loop controlling steroid hormone signaling in Drosophila. Genes Dev 21:2277–2282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes GD, Frand AR, Ruvkun G 2006 The mir-84 and let-7 paralogous microRNA genes of Caenorhabditis elegans direct the cessation of molting via the conserved nuclear hormone receptors NHR-23 and NHR-25. Development 133:4631–4641 [DOI] [PubMed] [Google Scholar]
- Yang WJ, Yang DD, Na S, Sandusky GE, Zhang Q, Zhao G 2005 Dicer is required for embryonic angiogenesis during mouse development. J Biol Chem 280:9330–9335 [DOI] [PubMed] [Google Scholar]
- Kanellopoulou C, Muljo SA, Kung AL, Ganesan S, Drapkin R, Jenuwein T, Livingston DM, Rajewsky K 2005 Dicer-deficient mouse embryonic stem cells are defective in differentiation and centromeric silencing. Genes Dev 19:489–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Medvid R, Melton C, Jaenisch R, Blelloch R 2007 DGCR8 is essential for microRNA biogenesis and silencing of embryonic stem cell self-renewal. Nat Genet 39:380–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese JM, Seila AC, Yeo GW, Sharp PA 2007 RNA sequence analysis defines Dicer’s role in mouse embryonic stem cells. Proc Natl Acad Sci USA 104:18097–18102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb BS, Nesterova TB, Thompson E, Hertweck A, O'Connor E, Godwin J, Wilson CB, Brockdorff N, Fisher AG, Smale ST, Merkenschlager M 2005 T cell lineage choice and differentiation in the absence of the RNase III enzyme Dicer. J Exp Med 201:1367–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muljo SA, Ansel KM, Kanellopoulou C, Livingston DM, Rao A, Rajewsky K 2005 Aberrant T cell differentiation in the absence of Dicer. J Exp Med 202:261–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koralov SB, Muljo SA, Galler GR, Krek A, Chakraborty T, Kanellopoulou C, Jensen K, Cobb BS, Merkenschlager M, Rajewsky N, Rajewsky K 2008 Dicer ablation affects antibody diversity and cell survival in the B lymphocyte lineage. Cell 132:860–874 [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Lu J, Cobb BS, Rodda SJ, McMahon AP, Schipani E, Merkenschlager M, Kronenberg HM 2008 Dicer-dependent pathways regulate chondrocyte proliferation and differentiation. Proc Natl Acad Sci USA 105:1949–1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andl T, Murchison EP, Liu F, Zhang Y, Yunta-Gonzalez M, Tobias JW, Andl CD, Seykora JT, Hannon GJ, Millar SE 2006 The miRNA-processing enzyme dicer is essential for the morphogenesis and maintenance of hair follicles. Curr Biol 16:1041–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi R, O'Carroll D, Pasolli HA, Zhang Z, Dietrich FS, Tarakhovsky A, Fuchs E 2006 Morphogenesis in skin is governed by discrete sets of differentially expressed microRNAs. Nat Genet 38:356–362 [DOI] [PubMed] [Google Scholar]
- Cuellar TL, Davis TH, Nelson PT, Loeb GB, Harfe BD, Ullian E, McManus MT 2008 Dicer loss in striatal neurons produces behavioral and neuroanatomical phenotypes in the absence of neurodegeneration. Proc Natl Acad Sci USA 105:5614–5619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis TH, Cuellar TL, Koch SM, Barker AJ, Harfe BD, McManus MT, Ullian EM 2008 Conditional loss of Dicer disrupts cellular and tissue morphogenesis in the cortex and hippocampus. J Neurosci 28:4322–4330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Ransom JF, Li A, Vedantham V, von Drehle M, Muth AN, Tsuchihashi T, McManus MT, Schwartz RJ, Srivastava D 2007 Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1–2. Cell 129:303–317 [DOI] [PubMed] [Google Scholar]
- Chen JF, Murchison EP, Tang R, Callis TE, Tatsuguchi M, Deng Z, Rojas M, Hammond SM, Schneider MD, Selzman CH, Meissner G, Patterson C, Hannon GJ, Wang DZ 2008 Targeted deletion of Dicer in the heart leads to dilated cardiomyopathy and heart failure. Proc Natl Acad Sci USA 105:2111–2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rourke JR, Georges SA, Seay HR, Tapscott SJ, McManus MT, Goldhamer DJ, Swanson MS, Harfe BD 2007 Essential role for Dicer during skeletal muscle development. Dev Biol 311:359–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KS, Zhang Z, McManus MT, Harfe BD, Sun X 2006 Dicer function is essential for lung epithelium morphogenesis. Proc Natl Acad Sci USA 103:2208–2213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn FC, Skewes-Cox P, Kosaka Y, McManus MT, Harfe BD, German MS 2007 MicroRNA expression is required for pancreatic islet cell genesis in the mouse. Diabetes 56:2938–2945 [DOI] [PubMed] [Google Scholar]
- Harfe BD, McManus MT, Mansfield JH, Hornstein E, Tabin CJ 2005 The RNaseIII enzyme Dicer is required for morphogenesis but not patterning of the vertebrate limb. Proc Natl Acad Sci USA 102:10898–10903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiani D, Alexander JJ, O'Rourke JR, McManus M, Jadhav AP, Cepko CL, Hauswirth WW, Harfe BD, Strettoi E 2008 Dicer inactivation leads to progressive functional and structural degeneration of the mouse retina. J Neurosci 28:4878–4887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Chuva de Sousa Lopes SM, Kaneda M, Tang F, Hajkova P, Lao K, O'Carroll D, Das PP, Tarakhovsky A, Miska EA, Surani MA 2008 MicroRNA biogenesis is required for mouse primordial germ cell development and spermatogenesis. PLoS ONE 3:e1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchison EP, Stein P, Xuan Z, Pan H, Zhang MQ, Schultz RM, Hannon GJ 2007 Critical roles for Dicer in the female germline. Genes Dev 21:682–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang F, Kaneda M, O'Carroll D, Hajkova P, Barton SC, Sun YA, Lee C, Tarakhovsky A, Lao K, Surani MA 2007 Maternal microRNAs are essential for mouse zygotic development. Genes Dev 21:644–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka M, Zheng M, Hayashi M, Lee JD, Yoshino O, Lin S, Han J 2008 Impaired microRNA processing causes corpus luteum insufficiency and infertility in mice. J Clin Invest [Erratum (2008) 118:2366] 118:1944–1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamin SP, Arango NA, Mishina Y, Hanks MC, Behringer RR 2002 Requirement of Bmpr1a for Mullerian duct regression during male sexual development. Nat Genet 32:408–410 [DOI] [PubMed] [Google Scholar]
- Jeyasuria P, Ikeda Y, Jamin SP, Zhao L, De Rooij DG, Themmen AP, Behringer RR, Parker KL 2004 Cell-specific knockout of steroidogenic factor 1 reveals its essential roles in gonadal function. Mol Endocrinol 18:1610–1619 [DOI] [PubMed] [Google Scholar]
- Behringer RR, Finegold MJ, Cate RL 1994 Mullerian-inhibiting substance function during mammalian sexual development. Cell 79:415–425 [DOI] [PubMed] [Google Scholar]
- Mishina Y, Rey R, Finegold MJ, Matzuk MM, Josso N, Cate RL, Behringer RR 1996 Genetic analysis of the Mullerian-inhibiting substance signal transduction pathway in mammalian sexual differentiation. Genes Dev 10:2577–2587 [DOI] [PubMed] [Google Scholar]
- Deutscher E, Hung-Chang Yao H 2007 Essential roles of mesenchyme-derived β-catenin in mouse Mullerian duct morphogenesis. Dev Biol 307:227–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arango NA, Kobayashi A, Wang Y, Jamin SP, Lee HH, Orvis GD, Behringer RR 2008 A mesenchymal perspective of Mullerian duct differentiation and regression in Amhr2-lacZ mice. Mol Reprod Dev 75:1154–1162 [DOI] [PubMed] [Google Scholar]
- Pangas SA, Li X, Umans L, Zwijsen A, Huylebroeck D, Gutierrez C, Wang D, Martin JF, Jamin SP, Behringer RR, Robertson EJ, Matzuk MM 2008 Conditional deletion of Smad1 and Smad5 in somatic cells of male and female gonads leads to metastatic tumor development in mice. Mol Cell Biol 28:248–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgez CJ, Klysik M, Jamin SP, Behringer RR, Matzuk MM 2004 Granulosa cell-specific inactivation of follistatin causes female fertility defects. Mol Endocrinol 18:953–967 [DOI] [PubMed] [Google Scholar]
- Pangas SA, Li X, Robertson EJ, Matzuk MM 2006 Premature luteinization and cumulus cell defects in ovarian-specific Smad4 knockout mice. Mol Endocrinol 20:1406–1422 [DOI] [PubMed] [Google Scholar]
- Pangas SA, Jorgez CJ, Tran M, Agno J, Li X, Brown CW, Kumar TR, Matzuk MM 2007 Intraovarian activins are required for female fertility. Mol Endocrinol 21:2458–2471 [DOI] [PubMed] [Google Scholar]
- Petit FG, Jamin SP, Kurihara I, Behringer RR, DeMayo FJ, Tsai MJ, Tsai SY 2007 Deletion of the orphan nuclear receptor COUP-TFII in uterus leads to placental deficiency. Proc Natl Acad Sci USA 104:6293–6298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn CA, Martin L 1972 Endocrine control of the timing of endometrial sensitivity to a decidual stimulus. Biol Reprod 7:82–86 [DOI] [PubMed] [Google Scholar]
- Yin Y, Ma L 2005 Development of the mammalian female reproductive tract. J Biochem 137:677–683 [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Behringer RR 2003 Developmental genetics of the female reproductive tract in mammals. Nat Rev Genet 4:969–980 [DOI] [PubMed] [Google Scholar]
- Heikkila M, Peltoketo H, Vainio S 2001 Wnts and the female reproductive system. J Exp Zool 290:616–623 [DOI] [PubMed] [Google Scholar]
- Taylor HS, Vanden Heuvel GB, Igarashi P 1997 A conserved Hox axis in the mouse and human female reproductive system: late establishment and persistent adult expression of the Hoxa cluster genes. Biol Reprod 57:1338–1345 [DOI] [PubMed] [Google Scholar]
- Solway J, Seltzer J, Samaha FF, Kim S, Alger LE, Niu Q, Morrisey EE, Ip HS, Parmacek MS 1995 Structure and expression of a smooth muscle cell-specific gene, SM22 α. J Biol Chem 270:13460–13469 [DOI] [PubMed] [Google Scholar]
- Li L, Miano JM, Mercer B, Olson EN 1996 Expression of the SM22α promoter in transgenic mice provides evidence for distinct transcriptional regulatory programs in vascular and visceral smooth muscle cells. J Cell Biol 132:849–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees-Miller JP, Heeley DH, Smillie LB 1987 An abundant and novel protein of 22 kDa (SM22) is widely distributed in smooth muscles. Purification from bovine aorta. Biochem J 244:705–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke WW, Appelhans B, Schmid E, Freudenstein C, Osborn M, Weber K 1979 Identification and characterization of epithelial cells in mammalian tissues by immunofluorescence microscopy using antibodies to prekeratin. Differentiation 15:7–25 [DOI] [PubMed] [Google Scholar]
- Miller C, Sassoon DA 1998 Wnt-7a maintains appropriate uterine patterning during the development of the mouse female reproductive tract. Development 125:3201–3211 [DOI] [PubMed] [Google Scholar]
- Parr BA, McMahon AP 1998 Sexually dimorphic development of the mammalian reproductive tract requires Wnt-7a. Nature 395:707–710 [DOI] [PubMed] [Google Scholar]
- Miller JR 2002 The Wnts. Genome Biol 3:REVIEWS3001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milewicz DM, Guo DC, Tran-Fadulu V, Lafont AL, Papke CL, Inamoto S, Pannu H 2008 Genetic basis of thoracic aortic aneurysms and dissections: focus on smooth muscle cell contractile dysfunction. Annu Rev Genomics Hum Genet 9:283–302 [DOI] [PubMed] [Google Scholar]
- Morgan KG, Gangopadhyay SS 2001 Invited review: cross-bridge regulation by thin filament-associated proteins. J Appl Physiol 91:953–962 [DOI] [PubMed] [Google Scholar]
- van der Loop FT, Schaart G, Timmer ED, Ramaekers FC, van Eys GJ 1996 Smoothelin, a novel cytoskeletal protein specific for smooth muscle cells. J Cell Biol 134:401–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa Y, Yoshizumi M, Ishizawa K, Fujita Y, Kondo S, Kagami S, Kawazoe K, Tsuchiya K, Tomita S, Tamaki T 2007 Big mitogen-activated protein kinase 1 (BMK1)/extracellular signal regulated kinase 5 (ERK5) is involved in platelet-derived growth factor (PDGF)-induced vascular smooth muscle cell migration. Hypertens Res 30:1107–1117 [DOI] [PubMed] [Google Scholar]
- Montezano AC, Callera GE, Yogi A, He Y, Tostes RC, He G, Schiffrin EL, Touyz RM 2008 Aldosterone and angiotensin II synergistically stimulate migration in vascular smooth muscle cells through c-Src-regulated Redox-sensitive RhoA pathways. Arterioscler Thromb Vasc Biol 28:1511–1518 [DOI] [PubMed] [Google Scholar]
- Bryan J, Imai M, Lee R, Moore P, Cook RG, Lin WG 1989 Cloning and expression of a smooth muscle caldesmon. J Biol Chem 264:13873–13879 [PubMed] [Google Scholar]
- Einspanier R, Bieser B, Reischl J, Prelle K 2001 First identification of caldesmon transcripts in bovine oviduct epithelial cells in vitro by means of an RNA differential display technique examining culture-induced expression changes. Reprod Domest Anim 36:230–235 [DOI] [PubMed] [Google Scholar]
- Murphy DB, Seemann S, Wiese S, Kirschner R, Grzeschik KH, Thies U 1997 The human hepatocyte nuclear factor 3/fork head gene FKHL13: genomic structure and pattern of expression. Genomics 40:462–469 [DOI] [PubMed] [Google Scholar]
- Sendai Y, Komiya H, Suzuki K, Onuma T, Kikuchi M, Hoshi H, Araki Y 1995 Molecular cloning and characterization of a mouse oviduct-specific glycoprotein. Biol Reprod 53:285–294 [DOI] [PubMed] [Google Scholar]
- Buhi WC 2002 Characterization and biological roles of oviduct-specific, oestrogen-dependent glycoprotein. Reproduction 123:355–362 [DOI] [PubMed] [Google Scholar]
- Mayer LP, Devine PJ, Dyer CA, Hoyer PB 2004 The follicle-deplete mouse ovary produces androgen. Biol Reprod 71:130–138 [DOI] [PubMed] [Google Scholar]
- Wang H, Guo Y, Wang D, Kingsley PJ, Marnett LJ, Das SK, DuBois RN, Dey SK 2004 Aberrant cannabinoid signaling impairs oviductal transport of embryos. Nat Med 10:1074–1080 [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Burns KH, Viveiros MM, Eppig JJ 2002 Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science 296:2178–2180 [DOI] [PubMed] [Google Scholar]
- Kalidas S, Sanders C, Ye X, Strauss T, Kuhn M, Liu Q, Smith DP 2008 Drosophila R2D2 mediates follicle formation in somatic tissues through interactions with Dicer-1. Mech Dev 125:475–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branford WW, Benson GV, Ma L, Maas RL, Potter SS 2000 Characterization of Hoxa-10/Hoxa-11 transheterozygotes reveals functional redundancy and regulatory interactions. Dev Biol 224:373–387 [DOI] [PubMed] [Google Scholar]
- Umezu T, Tomooka Y 2004 An evidence of stromal cell populations functionally linked with epithelial cell populations in the mouse oviduct. Zool Sci 21:319–326 [DOI] [PubMed] [Google Scholar]
- Kurihara I, Lee DK, Petit FG, Jeong J, Lee K, Lydon JP, DeMayo FJ, Tsai MJ, Tsai SY 2007 COUP-TFII mediates progesterone regulation of uterine implantation by controlling ER activity. PLoS Genet 3:e102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyal SM, Mukherjee A, Lee KY, Li J, Li H, Demayo FJ, Lydon JP 2005 Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis 41:58–66 [DOI] [PubMed] [Google Scholar]
- Varani S, Elvin JA, Yan C, DeMayo J, DeMayo FJ, Horton HF, Byrne MC, Matzuk MM 2002 Knockout of pentraxin 3, a downstream target of growth differentiation factor-9, causes female subfertility. Mol Endocrinol 16:1154–1167 [DOI] [PubMed] [Google Scholar]
- Durlinger AL, Kramer P, Karels B, de Jong FH, Uilenbroek JT, Grootegoed JA, Themmen AP 1999 Control of primordial follicle recruitment by anti-Mullerian hormone in the mouse ovary. Endocrinology 140:5789–5796 [DOI] [PubMed] [Google Scholar]
- Pedchenko VK, Imagawa W 2000 Estrogen treatment in vivo increases keratinocyte growth factor expression in the mammary gland. J Endocrinol 165:39–49 [DOI] [PubMed] [Google Scholar]
- Griffiths-Jones S, Grocock RJ, van Dongen S, Bateman A, Enright AJ 2006 miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res 34:D140–D144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid JG, Lynn FC, Nagaraja AK, Drabek RB, Muzny DM, Shaw CA, Weiss MK, Naghavi AO, Khan MF, Zhu H, Gunaratne GH, Corry DB, Miller JN, German MS, Gibbs RA, Matzuk MM, Gunaratne PH, Mouse let-7 miRNA populations exhibit RNA editing that is constrained in the 5′-seed/cleavage/anchor regions and stabilize predicted mmu-let-7a:mRNA duplexes. Genome Res, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB 2003 Prediction of mammalian microRNA targets. Cell 115:787–798 [DOI] [PubMed] [Google Scholar]
- Krek A, Grun D, Poy MN, Wolf R, Rosenberg L, Epstein EJ, MacMenamin P, da Piedade I, Gunsalus KC, Stoffel M, Rajewsky N 2005 Combinatorial microRNA target predictions. Nat Genet 37:495–500 [DOI] [PubMed] [Google Scholar]
- John B, Enright AJ, Aravin A, Tuschl T, Sander C, Marks DS 2004 Human MicroRNA targets. PLoS Biol 2:e363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD 2001 Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Δ Δ C(T)) method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]