Abstract
Until recently, the study of nuclear receptor (NR) function in breast cancer biology has been largely limited to estrogen and progesterone receptors. The development of reliable gene expression arrays, real-time quantitative RT-PCR, and immunohistochemical techniques for studying NR superfamily members in primary human breast cancers has now revealed the presence and potential importance of several additional NRs in the biology of breast cancer. These include receptors for steroid hormones (including androgens and corticosteroids), fat-soluble vitamins A and D, fatty acids, and xenobiotic lipids derived from diet. It is now clear that after NR activation, both genomic and nongenomic NR pathways can coordinately activate growth factor signaling pathways. Advances in our understanding of both NR functional networks and epithelial cell growth factor signaling pathways have revealed a frequent interplay between NR and epithelial cell growth factor family signaling that is clinically relevant to breast cancer. Understanding how growth factor receptors and their downstream kinases are activated by NRs (and vice-versa) is a central goal for maximizing treatment opportunities in breast cancer. In addition to the estrogen receptor, it is predicted that modulating the activity of other NRs will soon provide novel prevention and treatment approaches for breast cancer patients.
CLINICALLY, AN APPRECIATION of the connection between nuclear receptor (NR) function and breast cancer biology can be traced to Sir Thomas Beatson’s demonstration in 1896 that the removal of ovaries from young women with advanced breast cancer could cause tumor regression (1). In the last 30 yr, the standardization of ligand-binding (2) and immunohistochemical assays (3) for estrogen receptor (ER)-α and progesterone receptor (PR) expression in breast cancer specimens, coupled with the successful implementation of large-scale clinical trials, has established a positive correlation between ER and PR expression and response to antiestrogen therapy. Systemic antiendocrine therapy is currently used in two clinical situations: 1) in the adjuvant setting (i.e. either after surgery to halt the growth of metastatic cancer cells, or pre-surgically, to shrink a large primary breast tumor) and 2) in the metastatic setting (Stage IV) where tumor response is commonly assessed by two-dimensional radiographic measurements of metastatic breast cancer. Antiestrogen therapies include LH-releasing hormone agonists that suppress ovarian function in premenopausal patients, aromatase inhibitors (AIs) that effectively block the production of estrogens from androgens in postmenopausal women, and selective estrogen modulators (SERMs) such as tamoxifen that can be effective in both groups of patients. However, the recognition that those patients with ER/PR-negative breast cancers do not benefit from these antiestrogen therapies, and that approximately 40%–50% of ER/PR-positive breast cancers are also insensitive to initial endocrine therapy, has provided an important clinical context for researchers to identify signaling pathways that may be alternatively targeted for therapy. As a result, both growth factor-signaling pathways acting through membrane-bound tyrosine kinase receptors such as the human epidermal growth factor receptor2 (Her2) as well as several nonestrogen NR-signaling pathways are being intensively studied in the context of ER-positive and ER-negative breast cancer (Fig. 1).
Figure 1.
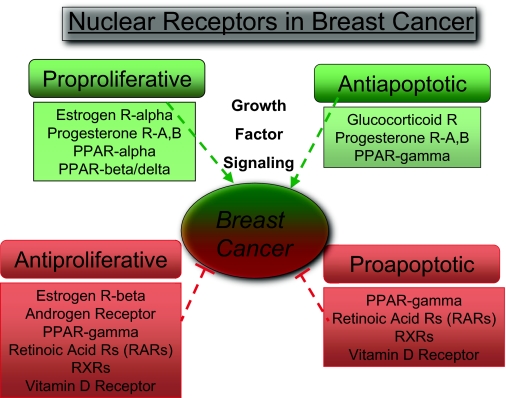
NR Function in Breast Cancer
Those NRs that have been studied in primary human breast cancers are listed. NRs are categorized according to their predominant growth-related function after ligand-mediated activation, i.e. NRs are listed as proproliferative, antiapoptotic, antiproliferative, and/or proapoptotic.
The development of reliable real-time quantitative RT-PCR and immunohistochemical (IHC) techniques for studying the human NR superfamily in primary breast cancers has recently revealed the presence and potential importance of several NRs beyond the ERs and PRs (Table 1). These include receptors for steroid hormones (e.g. androgen and corticosteroid), fat-soluble vitamins A and D, fatty acids, and xenobiotic lipids derived from the diet (4). In addition, some members of the NR family classified as “orphan receptors” (because their specific ligands remain unknown) have important functions in breast cancer (5). Furthermore, the simultaneous advances in our understanding of NR superfamily functional networks (6) and epithelial cell growth factor signaling (7) have revealed a frequent interplay between NR and growth signaling that is clinically relevant to breast cancer. This review will focus on observations in NR biology that have specific implications for breast cancer biology and will highlight how basic findings in NR biology impact on breast cancer prevention and treatment. It should be noted that a distinction is made between those aspects of NR biology that may be predictive of a tumor’s response to a particular therapy, vs. those that have prognostic value, i.e. that contribute to a tumor’s growth characteristics independently of a specific treatment.
Table 1.
NRs in Breast Cancer
| Nuclear Receptor | Expression in Breast Cancer | NR Activation—Phenotypes
|
||
|---|---|---|---|---|
| Proliferation | Apoptosis | Relevance to Breast Cancer Therapy | ||
| Steroid Hormone Receptors | ||||
| ER-α | Overexpression common ∼70% | Proproliferative | Antiapoptotic | Expression determines utility of therapy with SERM or AI (3) |
| ER-β | Not routinely assessed; likely low | Antiproliferative | Unknown | Increased expression may be a favorable marker (27,28) |
| PR-A | Expression frequent with ER-α | Proproliferative | Antiapoptotic | May be regulated by ER-α and reflect Tam sensitivity (50) |
| PR-B | Not routinely assessed | Unknown | Unknown | High PR-A:PR-B ratio increases relapse risk with Tam Rx (48) |
| AR | Overepression ∼48%–78% | Antiproliferative | Unknown | Appears to be favorable when expressed (48) |
| GR-α | Overexpression ∼50% | Antiproliferative | Antiapoptotic | Unknown |
| Coregulators | ||||
| AIB1/SRC-3 | Overexpression (amplification) | Proproliferative | Unknown | High expression may promote Tam resistance (38) |
| N-CoR | Variable | Antiprolferative | Unknown | Low N-Cor is associated with shorter relapse-free survival (38) |
| PPARs | ||||
| PPAR-α | Unknown | Proproliferative | Unknown | Unknown |
| PPAR-β/δ | Unknown | Proproliferative | Antiapoptotic | Unknown |
| PPAR-γ | Overexpression | Antiproliferative | Proapoptotic | Increased PPAR-γ expression correlates with survival (83) |
| RAR and RXR | ||||
| RAR-α | Variable | Antiproliferative | Unknown | The synthetic RAR ligand, fenritinide, reduces second breast cancers in premenopausal women (111) |
| RAR-β | Down-regulated | Antiproliferative | Unknown | |
| RAR-γ | Variable | Antiproliferative | Unknown | Unknown |
| RXRs | Variable | Antiproliferative | Proapoptotic | Unknown |
| VDR | ||||
| VDR | Variable | Antiproliferative | Proapoptotic | Vitamin D and calcium intake may inversely correlate with the risk of breast cancer in women (103) |
| Orphan Receptors (ERRs) | ||||
| ERR-α | Overexpression | Proproliferative | Unknown | Correlated with overexpression of Her2 and poor prognosis (126) |
| ERR-β | Overexpression | Unknown | Unknown | |
| ERR-γ | Overexpression | Proproliferative | Unknown | Correlated with hormonally responsive ER/PR positivity (125) |
Tam, Tamoxifen; Rx, therapy.
ESTROGEN RECEPTORS (ERs)
As mentioned above, the link between estrogen-mediated signaling via the ovaries and breast cancer biology was recognized at least as early as the 19th century. In modern day epidemiological studies, exposure to estrogen has been consistently linked to an increase in breast cancer risk (8). Furthermore, a requirement for ER-α in normal mammary gland differentiation and growth is strongly supported by knockout mouse models (9). There are two ERs encoded on different chromosomes: ER-α, cloned in 1986 (10,11); and ER-β, which was discovered in 1996 (12). ER-α is expressed in as many as 70% of breast cancers and remains a very effective biological target for the treatment of breast cancer. Interestingly, a comprehensive meta-analysis of the epidemiological literature concluded that reproduction-related exposures (i.e. estrogen) tend to be associated with an increased risk of ER-positive and not ER-negative breast cancers (13). This suggests that ER activation may be driving the critical events that lead to ER-positive breast cancer; other risk factors (including genetics and diet) may be important in ER-negative (and ER-positive) breast cancer.
Currently, the treatment of nonmetastatic breast cancer includes local therapy, i.e. breast and axillary lymph node surgery and radiation, along with adjuvant systemic therapy (e.g. hormonal, Her2-directed, and/or chemotherapy). The rationale for adding systemic therapy to surgery and radiation in the treatment of early-stage breast cancer is to prevent cancer cells that are undetectable at diagnosis from surviving and later growing into clinically significant metastases. Thus, antiestrogen treatment of pre- and postmenopausal breast cancer patients with SERMs such as tamoxifen, as well as treatment of postmenopausal women with AIs, remains a mainstay of therapy for women with ER-α-expressing breast cancers.
As with all types of adjuvant therapy used in breast cancer, the absolute benefit in terms of reducing the risk of recurrent tumor growth is directly proportional to the initial likelihood of tumor recurrence, i.e. the higher the risk of relapse, the greater the absolute reduction in risk of relapse afforded by adding adjuvant treatment. Predicting the initial likelihood of recurrence on the basis of a tumor’s molecular characteristics is of intense interest in clinical oncology; the question is perhaps particularly relevant to women with ER-α-positive breast cancer for whom the decision of whether or not to add toxic chemotherapy to their treatment plan is often difficult. Recently, the first breast cancer multigene expression assay, using real-time quantitative RT-PCR to evaluate a panel of 21 genes implicated in breast cancer biology, was approved by the Food and Drug Administration for women with ER-α-positive, early-stage invasive breast cancer (14). In this test, quantitative RT-PCR is performed from RNA extracted from a patient’s paraffin-embedded tumor samples; the expression data are then used in an algorithm that generates a likelihood of recurrence score with prognostic value regarding the overall likelihood of breast cancer recurrence. This score also has a predictive value for the benefit of adding standard adjuvant chemotherapy to hormonal therapy. Several other gene expression tests are in various stages of clinical development as molecular prognostic and predictive tests for both ER-α-positive and -negative breast cancers (15).
In the context of ER-α biology and tumor cell sensitivity to treatment, it should also be noted that when compared with ER-negative tumors, ER-α-positive breast cancers have long been considered relatively resistant to traditional chemotherapeutic drugs (16). Consistent with these much earlier observations, a recent meta-analysis of several large multi-institution clinical trials by Berry et al. (17) found that the absolute benefit from adjuvant chemotherapy was significantly worse for patients with ER-positive tumors: only 7.0% more ER-positive patients survived to 5 yr disease free if receiving adjuvant chemotherapy vs. 22.8% ER-negative patients; corresponding improvements for overall survival were 4.0% for ER-negative vs. 16.7% for ER-positive patients. Similarly, Carey et al. (18) recently reported that women with ER-α-positive tumors have a significantly lower response to preoperative chemotherapy than women with ER-negative breast cancers. The molecular mechanisms for the relative chemoresistance of ER-α-positive tumors are not known; however, the reduced sensitivity of these tumors to chemotherapy-induced cell death appears to be consistent in experimental systems and across large groups of patients receiving various combinations of adjuvant chemotherapy.
Another long-standing question concerning the biology of ER-positive vs. ER-negative breast cancers is related to the molecular mechanisms responsible for silencing ER expression in receptor-negative tumors. This question has important clinical implications because of the theoretical possibility that allowing reexpression, or at least increased expression, of ER-α might permit hormone-resistant tumors to become sensitive to an antiestrogen agent. There is convincing evidence that ESR1 is an epigenetically regulated gene that frequently undergoes promoter methylation; however, up to 35% of ER/PR-positive tumors also exhibit substantial ESR1 methylation, suggesting that methylation alone does not determine ER-α expression (19).
An alternative explanation for the loss of ER-α expression has been suggested from the results of cell-based assays analyzing histone function as a determinant of gene expression. In the ER-negative human breast cancer lines MDA-MB-231 and MDA-MB-435, treatment with a histone deacetylase (HDAC) inhibitor (LBH589) for 24 h restores ER-α mRNA and protein expression without concomitant demethylation of CpG islands in the promoter, suggesting that HDAC inhibitors might increase ESR1 gene expression by reorganizing the heterochromatin-associated proteins without demethylation (20). An increase in ER-α expression by an HDAC inhibitor also restores 4-hydroxytamoxifen sensitivity in MDA-MB-231 cells. This possibility is being explored clinically in a current Phase II trial (http://clinicaltrials.gov/ct/show/NCT00365599) of an HDAC inhibitor in combination with tamoxifen for patients with originally ER-positive advanced breast cancer whose tumors have progressed despite hormonal therapy with either an aromatase inhibitor or a minimum 12-month course of adjuvant tamoxifen. After HDAC/tamoxifen inhibitor treatment on the trial, the investigators will determine whether or not the tumor becomes sensitive to tamoxifen therapy and/or exhibits increased expression of ER-α and decreased histone acetylation.
The discovery of the gene encoding ER-β (ESR2), found on a separate chromosome from ESR1, has illuminated the complexity of estrogen signaling in the breast and other tissues. Based on the observation that overexpression of ER-β decreases ER-α transcriptional activity as measured by estradiol stimulation of endogenous ER-α target genes such as IGFBP4 (21), ER-β appears to act as a negative modulator of ER-α action. On a molecular level, ER-β has been shown to modulate ER-α-mediated transcriptional activation by altering the recruitment of c-Fos and c-Jun to estrogen-responsive promoters and to also cause a further reduction in ER-α protein levels after estradiol treatment of cells (22). The ratio of ER-α to ER-β may also be important in human breast cancer, because functional ER-α/β heterodimers result in unique patterns of gene regulation, many of which are distinct from the genes regulated by the ER-α or -β homodimers (23,24). Consistent with the described antiproliferative effects of ER-β activity on the growth-promoting functions via ER-α, ER-β expression exhibits decreased frequency in the pathological progression of ER-α-positive tumors (reviewed in Ref. 25). In addition, there are now several retrospective clinical trials supporting the hypothesis that increased expression of ER-β in ER-α-expressing tumors is associated with an increased likelihood of response to endocrine therapy (reviewed in Ref. 26). For example, using immunohistochemistry (IHC) to examine 50 tumors from women treated with adjuvant tamoxifen, Esslimani-Sahla et al. (27) found that low ER-β levels are an independent marker for predicting tamoxifen resistance (i.e. relapse) in breast cancer patients. On the other hand, Cappalletti et al. (28) concluded that in the preoperative treatment of 47 ER-α positive tumors, levels of ESR2 mRNA were neither predictive of a response to preoperative toremifene (a SERM) nor provided additional information to knowing ESR1 mRNA levels, the latter of which were directly correlated with likelihood of response. These relatively small correlative studies illustrate the fact that determining whether or not ER-β expression levels predict endocrine responsiveness in breast cancer will likely require much larger-scale, multi-institutional clinical trials with appropriate tissue collection for translational studies.
COREGULATORS—IMPORTANT PLAYERS IN BREAST CANCER BIOLOGY
NRs are classically defined as ligand-activated transcription factors that complex directly or indirectly to response elements in noncoding regions of target genes. Transcriptional activity is regulated by both the availability of NR ligands and by interactions with protein coactivators and corepressors (collectively called “coregulators”) that are recruited into transcriptional complexes and subsequently modulate gene expression (reviewed in Ref. 29). Because coactivators affect chromatin configuration and recruit protein complexes to serve as a link between the NR and the transcriptional apparatus, they are critical fine-tuning proteins for many aspects of classic NR transcriptional function and when coregulator expression goes awry, pathogenesis can occur. Reflecting their importance in NR signaling, steroid receptor coactivators [e.g. steroid receptor coactivator-1 (SRC-1), amplified in breast cancer 1 (AIB1 or SRC-3), and transcription intermediary factor 2 and the NR corepressor (N-CoR), have all been examined extensively in breast cancer cell lines, animal models of breast cancer, and in primary human breast tumors (30).
Perhaps the most studied coactivator in breast cancer is AIB1/SRC-3, a coregulator that interacts with NRs as well as certain other transcription factors. AIB1/SRC-3 recruits histone acetyl transferases (additional coactivators) and methyltransferases for chromatin remodeling and facilitates target gene transcription (31). AIB1/SRC-3 is also amplified and overexpressed in a subgroup of primary breast tumors, including ER-α-negative tumors, suggesting that the role of A1B/SRC-3 in breast cancer may extend beyond the actions on ER-α activity (32). Increased levels of AIB1/SRC-3 have been shown to favor the functional interaction of ER-α with the cyclin D1 promoter (33), increasing the estrogen-dependent mitogenic stimulation of breast cancer cells. Clinically, Osborne et al. (34) demonstrated that high AIB1 expression in breast cancers from patients not receiving adjuvant tamoxifen therapy is associated with a better prognosis and longer disease-free survival. In contrast, for patients with metastatic breast cancer receiving tamoxifen therapy, high AIB1 expression is associated with a worse disease-free survival, suggesting that high AIB1 is predictive of tamoxifen resistance.
There is also evidence that AIB1 expression can function independently of ER-α to promote breast cancer cell growth. For example, using the ER-negative MDA-MB-231 cells, Oh et al. (35) demonstrated that AIB1 is required for IGF-I-induced proliferation, signaling, cell survival, and gene expression in human breast cancer cells, independently of its role in ER-α signaling. In agreement with this finding, Yan et al. (36) demonstrated in prostate cancer cells that recruitment of AIB1 to the promoter regions of two ER-α target genes, IRS-2 and IGF-I, requires the transcription factor activator protein-1 independently of ligand. Therefore, AIB1 appears to have growth-promoting activities outside of the classical ligand-dependent model of ER action.
Several NR corepressors have been identified that also exhibit altered expression in breast cancer, including nuclear receptor corepressors (N-CoRs). For example, studies by Lavinsky et al. (37) suggest that decreased N-CoR levels correlate with the acquisition of resistance to tamoxifen by human MCF-7 breast cancer cells in a mouse model. In a clinical-translational study of human tumors, low N-CoR expression (vs. intermediate or high expression) was associated with significantly shorter relapse-free survival, suggesting that diminished N-CoR activity is an independent predictor of tamoxifen resistance in patients with ER-α-positive breast tumors (38). Consistent with an adverse biological effect from low N-CoR expression, Brown and colleagues (39) showed that reducing N-CoR and silencing mediator of retinoid and thyroid hormone receptor (SMRT) levels increases the ability of tamoxifen to stimulate proliferation in breast cancer cells without activating the ER-α target genes c-myc, cyclin D1, or stromal cell-derived factor 1, all of which play a role in estrogen-induced proliferation. This finding suggests that N-CoR and SMRT may be functionally important in repressing a subset of prooncogenic target genes involved in ER-α function and cell proliferation. Furthermore, an evaluation of 160 cases of invasive breast cancer demonstrated that patients with high levels of expression of N-CoR1 mRNA have a better prognosis than those diagnosed with tumors having low N-CoR1 mRNA expression (40). On the other hand, a recent study by Green et al. (41) found that N-CoR 2/SMRT expression is associated with poor patient outcome, independent of other prognostic factors. Taken together, these studies consistently support the hypothesis that expression levels of coactivators and corepressors can moderate tumor cell responsiveness to endocrine therapy and suggest that assessing NR coregulator expression in human breast cancer may improve prediction of response to antiestrogen therapy (42). Furthermore, targeting coregulator function could be considered as a treatment strategy in conjunction with or independently of selective NR modulation.
PROGESTERONE RECEPTORS (PRs)
The PR has two predominant isoforms, denoted as PR-A and PR-B, which are expressed from a single gene by alternative promoter usage (43). A less prominent isoform, PR-C, is truncated within the DNA-binding domain and can inhibit or modify PR-B activities (44). Clinically, PR expression is routinely assessed in breast cancer specimens by IHC, most frequently using an antibody that recognizes both PR-A and PR-B. These isoforms have similar steroid hormone and DNA binding activities, but PR-B has a much higher transcriptional activating potential (45). PR-A (94 kDa) differs from PR-B (120 kDa) because it lacks the N-terminal 164 amino acids of PR-B (46); the result is a unique activation function 3 region at the far N terminus of PR-B. This activation function 3 region plays a critical modulatory role; therefore PR-B function appears to be regulated differently from PR-A (47). Interestingly, a retrospective study found that tamoxifen-treated patients with PR-positive tumors expressing high PR-A:PR-B ratios were 2.76 times more likely to relapse than patients with tumors having lower ratios, indicating a resistance to tamoxifen in tumors with low PR-B expression (48). One interpretation of this observation is that the level of PR-B expression may reflect the degree of ER-α functionality; therefore, those tumors with low PR-B expression (i.e. a high PR-A:PR-B ratio) may have disrupted ER-α function and are therefore less likely to respond to tamoxifen.
Overall, although PR expression is frequently positively correlated with ER-α expression, recent studies that have measured expression of both receptors in human breast cancer samples have offered interesting insights into the consequence of absent PR expression in ER-positive tumors. For example, the anastrazole, tamoxifen, alone or in combination (ATAC) trial randomly assigned postmenopausal women to adjuvant treatment with the AI anastrazole, tamoxifen, or both (49). Analysis of the outcome data demonstrated an inferior outcome in ER-positive/PR-negative subgroups that was largely restricted to the tamoxifen-containing arms and suggested that the overall benefit seen from anastrazole over tamoxifen may be from the superior efficacy of anastrazole in ER-positive/PR-negative breast cancers (50).
Several groups have also reported that increased growth factor signaling is frequently associated with PR loss in ER-positive/PR-negative breast cancers, raising the possibility that tamoxifen resistance seen in these tumors is actually a consequence of growth factor signaling that cannot be overcome by AIs. For example, PR-negative tumors have been shown to have a higher percentage (25%) of HER-2 overexpression compared with ER/PR-positive tumors (10%) (51). Higher levels of epidermal growth factor receptor-1 expression are also associated with PR-negative tumors (52). Phosphatase and tensin analog loss, which results in constitutive phosphatidylinositol 3-kinase (PI3-K) activation, has also been correlated with a reduced PR expression (53). The association between growth factor pathway activation and PR down-regulation may be mechanistically linked: IGF-I, epidermal growth factor, and heregulin were independently found to result in decreased PR gene expression through a PI3-K-dependent mechanism even though ER-α’s measured activity toward other target genes was not affected (54). Overall, these studies raise the interesting possibility that activated growth factor signaling selectively alters ER’s transcriptional function toward PR; alternatively, growth factors may repress PR gene expression through an as yet unknown, ER-α-independent mechanism.
ANDROGEN RECEPTORS (ARs)
The AR is essential for the development of male reproductive organs and is also expressed in a significant subset of both ER-α-positive and -negative breast cancers. For example, using IHC, Agoff et al. (55) found high levels of AR expression in 49% (34/69) of ER-negative and 89% (17/19) of ER-positive cases. AR expression has recently been examined in several subtypes of breast cancer from patients with clinical follow-up. In these cases, AR expression by IHC was shown to be associated with a good prognosis in ER/PR-negative cancers (56); conversely, loss of AR was associated with a poor prognosis in lymph node-positive ER/PR/Her2-negative breast cancers (57). These findings are consistent with cell-based assays, where AR activation with the agonist 5 α-dihydrotestosterone (58) or dehydroepiandrosterone sulfate (DHEAS) (59) inhibits cell growth in AR-positive breast cancer cell lines, suggesting that AR initiates a growth inhibitory signal in breast cancer. Interestingly, Wilson and colleagues (60) have found that AR function can be inhibited by low doses of progestins (<10 nm), which are similar to doses achieved in the serum of women taking hormone replacement therapy. This finding has raised concern that perhaps agents such as medroxyprogesterone may negate the protective effects of androgen signaling in the breast (61).
GLUCOCORTICOID RECEPTORS (GRs)
The human GR is expressed predominantly as two alternatively spliced C-terminal isoforms, α and β (62). In addition, translational regulatory mechanisms can generate N-terminal GR isoforms from a single GR mRNA species, although the extent of expression of these isoforms in normal mammary tissue vs. breast cancer is not known (63). Using ligand-binding assays, however, it has been long recognized that about 50% of invasive breast cancers and many breast cancer cell lines express GR (64). Recently, the availability of improved antibodies against the GR has revealed that the most commonly used breast cancer cell lines express varying levels of GR-α, including both the ER-α positive MCF7 cell line and triple-negative (ER, PR and Her2-negative) cell lines such as MDA-MB-231 (65).
Although GR activation is associated with inducing apoptosis in lymphocytes and lymphomas, our laboratory and others have demonstrated that in both breast epithelial and cancer cell lines, GR activation paradoxically inhibits the apoptotic response to several cell stressors including growth factor withdrawal (66) and chemotherapy treatment (67). To promote breast epithelial cell survival, the GR requires transcriptional activation of genes encoding proteins in both the PI3-K and MAPK pathways; many of these genes have also been reported to be induced as part of the cellular stress response independently of GR activation (68). For example, induction of MAPK phosphatase-1 (MKP-1 or DUSP1), a direct GR target, is also seen during the cellular response to oxidative damage and chemotherapy-induced stress (69). Understanding the mechanisms underlying GR’s antiapoptotic signaling in breast cancer has potential physiological relevance because biobehavioral stressors that increase endogenous cortisol responses have been linked to breast cancer progression (70). In addition, the routine administration of fairly high doses of synthetic glucocorticoids as premedication for chemotherapy treatment has the potential to activate GR-mediated tumor cell survival pathways, thereby diminishing chemotherapy’s effectiveness (71). The current pipeline of selective GR modulators under development (72) may provide new options for prevention of chemotherapy-associated side effects without inducing cell survival mechanisms in breast cancer cells.
PEROXISOME PROLIFERATOR-ACTIVATED RECEPTORS (PPARs)
PPARs consist of a subfamily of three different isoforms: PPAR-γ, PPAR-α, and PPAR-β/δ, the ligands of which include long-chain polyunsaturated fatty acids, eicosanoid derivatives, and oxidized lipids (73). PPARs can also form heterodimers with the retinoid X receptor (RXR) (discussed below). The connection between PPARs and fatty acids is of potential importance in considering dietary prevention strategies for breast cancer because of both cell line data (74) and animal data (75) suggesting that long-chain n-3 polyunsaturated fatty acids inhibit mammary tumor growth and metastasis, respectively. Consistent with these experimental observations, mounting epidemiological evidence exists for a significant protective effect of n-3 fatty acids on breast cancer risk (76).
All PPAR isoforms appear to play an important part in regulating cellular metabolism, including lipid biosynthesis and glucose metabolism (reviewed in Ref. 77). Interestingly, PPAR-γ synthetic ligands both inhibit proliferation (78) and cause differentiation (79) of human breast cancer cell lines in vitro. In cell-based studies, the specific PPAR-γ agonist rosiglitazone promotes growth arrest and apoptosis in MCF7 cells, at least in part, through a cross talk between p53 and PPAR-γ (80). Furthermore, ER-α can bind to PPAR response elements and repress PPAR-γ transactivation (81). PPAR agonists were also shown to promote TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis of breast cancer in an in vivo xenograft model of human breast cancer using MDA-MB-431 cells (82). Consistent with having an antitumorigenic effect, PPAR-γ expression assessed by IHC in human tumors was found to be positively associated with an improved clinical outcome of breast carcinoma patients, and multivariate analysis demonstrated that high PPAR-γ expression by IHC was also an independent prognostic factor for overall survival in ER-α-positive patients (n = 238 human breast carcinoma tissues) (83). Taken together, these cell-based and clinical correlative studies suggest that PPAR-γ plays a predominantly antitumorigenic role in human breast cancer.
Further in vivo results concerning PPAR-γ activation in rodent models of human breast cancer have raised additional questions. The use of PPAR-γ agonists, such as GW7845, has generally supported an antitumorigenic role for PPAR-γ. For example, in rat models, GW7845 administration inhibits rat mammary carcinogenesis using the classic model of nitrosomethylurea-induced mammary gland carcinogenesis (84). Similarly, GW7845 treatment was shown to cause a moderate delay in tumor formation in ductal carcinomas in FVB/N mice treated with medroxyprogesterone acetate and 7,12-dimethylbenz(a)anthracene to initiate carcinogenesis (85). Consistent with a role for PPAR-γ activity in delaying carcinogen-initiated rodent mammary gland cancer, reduced PPAR-γ expression in (+/−) heterozygote mice resulted in an almost 3-fold increase in mammary adenocarcinomas in dimethylbenz(a)anthracene-mediated carcinogenesis (86). However, Evans and colleagues (87) found that transgenic expression of a constitutively active PPAR-γ targeted to the mammary epithelium had a protumorigenic effect, even though these aggressive tumors appeared more differentiated. In these experiments, transgenic mice with mammary epithelial PPAR-γ expression had no obvious glandular phenotype, but when bred to mice expressing mammary epithelial polyoma virus middle T antigen (MMTV-PyV), the bigenic mice had accelerated tumor growth kinetics compared with MMTV-PyV mice. This interesting result suggests that in the context of strong tumor initiation, PPAR-γ signaling in the epithelium may act as a tumor promoter, rather than as an inhibitor. It is also possible that in this transgenic model, overexpression of PPAR-γ exclusively in the mammary epithelial cells is not comparable to the situation in which either PPAR-γ is activated in all cell types using GW7845 treatment, or in which PPAR-γ expression is reduced in all cell types of (+/−) heterozygote mice. Overall, the results from the heterozygote and transgenic PPAR-γ mice emphasize the need to distinguish the role of NR function and expression in stromal, adipose, and epithelial cells of the mammary gland, all of which express NRs and can strongly influence tumorigenesis (88).
Despite the preclinical evidence that PPAR-γ activation may inhibit breast tumor growth through a direct effect on tumor cell apoptosis, cell cycle, differentiation, and angiogenesis, the first published clinical trial using a PPAR-γ ligand, rosiglitazone, in patients with metastatic breast cancer failed to show a significant clinical response (n = 22 patients) (89). In this trial, no objective responses were observed, although three patients had stable disease at 8 wk. A more recent trial examining PPAR-γ expression in tumors from women after rosiglitazone therapy demonstrated that down-regulation of nuclear PPAR-γ expression occurred after PPAR-γ ligand administration (90). These results suggest that PPAR-γ can be modulated in breast tissue through the use of clinically available compounds; given the preclinical results suggesting a role for PPAR-γ activation in glandular differentiation, PPAR-γ ligand administration may be useful as a strategy for treating precancerous lesions or much earlier stages of breast cancer.
In comparison with PPAR-γ, less is known about PPAR-α and -β/δ expression patterns and their functions in human breast cancer. However, it does appear that the effects of PPAR-α and PPAR-γ activation in human breast cancer cell lines are opposing; PPAR-α activation using Wy14643 and clofibrate significantly increases proliferation in MDA-MB-231 cells and MCF-7 cells, whereas PPAR-γ agonists inhibit proliferation (91). Although there is very little information on PPAR-β/δ expression in the human breast, a study in mouse mammary glands observed roughly equivalent PPAR-α, -β/δ, and -γ mRNA expression based on Northern blot analysis; during pregnancy and lactation, PPAR β/δ mRNA remained relatively unchanged whereas PPAR-α and -γ mRNA expression decreased (92). PPAR-β/δ activation has been most studied in colonic epithelium and has been implicated in colorectal carcinogenesis by various molecular genetic observations (93). Consistent with the proproliferative effect of PPAR-β/δ in colonic epithelium, activation of PPAR-β/δ in T47D breast cancer cells resulted in increased expression of the proliferation marker Cdk2, vascular endothelial growth factor α, and its receptor, FLT-1 (88). In vivo, FVB mice treated with the PPAR-β/δ agonist GW501516 showed accelerated tumor formation in a progestin- and carcinogen-induced mouse mammary tumorigenesis model (85). Tumors from these mice also exhibited increased PPAR-δ levels and activated phosphoinositide-dependent protein kinase-1 (PDK-1). Surprisingly, PDK-1 and PPAR-δ coassociated, suggesting a possible link between the known oncogenic activity of PDK-1 in mammary gland tissues and PPAR-β/δ activation.
Overall, experiments to date suggest that activation of PPARs may play important and likely opposing roles in mammary tumor formation, and that the pharmacological activation of these receptors is likely to have important consequences in both the stromal cells (including adipocytes) and in the mammary epithelium itself. The use of PPAR isoform-specific agonists and antagonists, along with cell type-specific genetic mouse models will be required to dissect the complexity of these important receptors in breast cancer biology.
RETINOIC ACID (RA) AND RETINOID X RECEPTORS (RXRs)
The physiological actions of retinoids are mediated through two distinct NR families: the RA receptors (RAR-α, RAR-β, and RAR-γ), each of which bind to both all-trans-retinoic acid (ATRA) or 9-cis-retinoic acid (9-cis-RA), and the RXRs (RXR-α, RXR-β, and RXR-γ), which preferentially bind 9-cis-RA (94). Most breast carcinomas and breast cancer cell lines show loss or down-regulation of RAR-β receptor expression, whereas RAR-α and -γ as well as the RXRs exhibit variable expression in both normal and tumor cells (reviewed in Ref. 95).
Initial cell-based assays demonstrated that 9-cis-RA inhibits the growth of monolayer cultures of several ER-α-positive (but not ER-negative) cell lines in a dose-dependent manner (96). ATRA was found to cause cell cycle arrest of normal human mammary epithelial cells expressing endogenous RAR-β receptor (97). In vivo work demonstrated that 9-cis-RA suppresses mammary tumor development in the C3(1)-simian virus 40 large T-antigen mouse model of breast cancer, in which mice display a loss of ER-α expression during tumor progression (98). However, when tested in patients in Phase I clinical trials, 9-cis-RA was poorly tolerated due to elevated triglyceride levels and moderate to severe liver and skin toxicity (99).
Synthetic receptor-selective retinoids were subsequently developed and found to be as effective as 9-cis-RA in suppressing the growth of human mammary epithelial cells and estrogen receptor-positive (ER-positive) breast cancer cells (100). The predominant factor that leads to growth arrest in either 9-cis-RA or synthetic retinoid treatment is G1 cell cycle blockade; this blockade appears to result from the down-regulation of Cyclin D1 and Cyclin D3, which in turn causes retinoblastoma hypophosphorylation (100,101).
Because of the potential for retinoids to block cellular division, ATRA has also been tested in patients with advanced breast cancer; however, ATRA does not have significant activity in patients with hormone-refractory, metastatic breast cancer (102). In the setting of chemoprevention, the synthetic amide of retinoic acid, N-(4-hydroxyphenyl) retinamide, or fenretinide, has been widely studied because of its favorable toxicity profile. An updated analysis of the largest chemoprevention trial with retinoids in breast cancer was recently reported after a median follow-up of 14.6 yr using a subgroup of 1739 women followed up in a single center and representing 60% of the initial multicenter cohort (103). This multicenter randomized chemoprevention clinical trial, coordinated by the National Tumor Institute in Milan, was started in 1987 to evaluate the effect of fenretinide on the incidence of second breast cancers. The results indicate that fenretinide induced a borderline significant reduction in second breast cancer incidence in women overall (17%), but a statistically significant reduction in second breast cancer incidence in the subset of premenopausal women (38%). Notably, the younger the women, the greater the risk reduction associated with fenretinide; the risk reduction reached 50% in women aged 40 yr or younger and disappeared after age 55 yr.
Activation of the RXRs also appears to be antiproliferative. Synthetic RXR-selective ligands (also termed “rexinoids”) can cause regression of mammary carcinoma in N-Nitroso-N-methylurea-treated rats (104). RXR-selective retinoids were shown to suppress tumorigenesis with minimal toxicity compared with RAR-selective ligands. Interestingly, the RXR-selective retinoid LGD1069, or bexarotene, appears to suppress both ER-positive and ER-negative tumor development with minimal toxicity, as observed in the N-Nitroso-N-methylurea-induced rat mammary tumor model (105). Because the RXRs are common heterodimeric partners for several NRs, and RXR homodimers can selectively bind to functional PPAR response elements and induce transactivation of PPAR target genes (106), combinations of rexinoids with other NR-selective modulators may become promising combination regimens for the prevention of breast cancer.
VITAMIN D RECEPTOR (VDR)
There has been increasing clinical interest in the role of vitamin D and its receptor (VDR) in breast cancer biology, partially based on evidence suggesting that vitamin D and calcium intake may inversely correlate with a risk for breast cancer (103). In cell-based assays, the active metabolite of vitamin D3, 1,25-dihydroxyvitamin D3 [1,25(OH)2D3], has been shown to inhibit breast cancer cell growth (107). Both the VDR and vitamin D 1-hydroxylase, the enzyme that generates 1,25(OH)2D3, are expressed in the normal mouse mammary gland and human breast. Recent evidence suggests that in human breast cancers, breast tissue-specific expression of 1[α]-hydroxylase may act as an important mechanism linking vitamin D status (25-hydroxy vitamin D3 levels) with the anticancer effects of 1,25(OH)2D3 (108).
The induction of cell cycle arrest and apoptosis in breast cancer cells by 1,25(OH)2D3 and other VDR agonists is dependent on the expression of nuclear VDR. Cells lacking VDR remain sensitive to growth arrest mediated by 9-cis-RA, a ligand for the RXR that can heterodimerize with the VDR (109). Moreover, mammary cells derived from wild-type mice are growth inhibited by 1,25(OH)2D3; however, cells derived from VDR −/− mice are completely unresponsive to 1,25(OH)2D3 (110). In vivo, the antitumor activity of 1,24(OH)2D3 has been assessed using MCF-7 xenografts in nude mice. 1,24(OH)2D3 inhibited the growth of VDR MCF-7 xenografts by 50% after 5 wk. Interestingly, tumor morphology in treated animals was consistent with replacement of epithelial cells by stromal tissue (111). Furthermore, in a transgenic mouse model of breast cancer that overexpresses LH, treatment with a 1,25(OH)2D3 analog, EB1089, had the ability to limit hormone-induced proliferation of endogenous mammary epithelial cells and reduce the growth rate of a subset of spontaneous mammary tumors in vivo (112). These preclinical models strongly suggest that VDR signaling through active vitamin D could play an important negative regulatory role in breast cancer biology.
ESTROGEN-RELATED RECEPTORS (ERRs)
The orphan NR subfamily includes ERR-α, -β, and -γ. These NRs are closely related to ER-α and ER-β and can share target genes, coregulatory proteins, ligands, and response elements (113). Although the transcriptional activity of ERR is not regulated directly by estrogens, it has been demonstrated that ERRs can affect ER-mediated signal transduction through common estrogen-responsive elements (114). In addition, ER-α and ERR-α can compete directly for promoter binding in MCF7 cells by recruiting corepressor proteins that actively suppress the expression of ER-α responsive target genes (115).
Consistent with a role for ERRs in suppressing ER-α gene expression, an initial examination of ERR-α expression in breast cancers suggested that ERR-α might be a marker of unfavorable clinical outcome and, possibly, hormonal insensitivity. On the other hand, ERR-γ overexpression was found to be associated with hormonally responsive ER-negative/PR-positive status (116). A more recent examination of clinical breast cancer specimens confirmed that ERR-α appears to be a prognostic biomarker of unfavorable clinical outcome (117). In this study, ERR-α was also the most abundant NR in a subset of tumors that expressed Her2 at high levels. Consequently, ERR-α may potentiate constitutive transcription of estrogen-responsive element-containing genes independently of ER-α and SERMs in Her2-positive tumors. It is likely that the discovery of physiologically relevant and specific ERR ligands will allow preclinical testing of the roles of ERRs in experimental breast cancers that have functional and nonfunctional ER-α and ER-β expression. Furthermore, large-scale studies of ERR expression in primary breast cancer specimens from patients receiving uniform treatments on clinical trials will assist in dissecting the consequences of the complex interactions of ERRs with ERs and other NRs in breast cancer.
EXTRANUCLEAR ACTIVITIES OF NRs: RELEVANCE TO BREAST CANCER
Probably the best-studied of the rapid extranuclear functions of NRs in breast cancer are via ER-α- (118) and PR-mediated signaling (119). For example, estradiol treatment of breast cancer cells causes a rapid and transient activation of MAPK signaling that is ER-α dependent but is independent of ER-α’s transcriptional activity (120). Whereas the genomic effects of steroid hormone treatment manifest over hours (after transcription and translation), the extranuclear, or nongenomic, effects occur in only a few minutes. There is good evidence that both ER and PR can mediate signaling cascades at the membrane and in the cytoplasm via various second messengers, such as receptor-mediated protein kinases. These nongenomic effects of ER and PR signaling may have important consequences in breast cancer prevention and treatment, where errant activation of growth factor signaling can now be targeted with effective results using specific small molecule inhibitors.
In primary breast cancer, endogenous ER-α is thought to exist in all three major compartments: the plasma membrane, cytosol, and nucleus. Membrane-associated ER-α can bind in a ligand-dependent manner to the p85α-regulatory subunit of PI3-K (121); here, ER-α has been shown to initiate PI3-K signaling and subsequent changes in gene expression (122). In addition to PI3-K signaling, ER-α can associate directly with Shc and Src (123). The interaction with Shc appears to be required for the formation of a complex between ER-α and the IGF-1 receptor (IGF-1R) that can lead to MAPK signaling and subsequent pro-proliferative gene expression (124). The ER-α coactivator, proline-, glutamic acid-, and leucine-rich protein (PELP), also known as modulator of norgenomic actions of the ER (MNAR) can act as a nongenomic adaptor protein between ER-α and Src, thereby allowing 17β-estradiol-dependent activation of Src and the downstream ERK/MAPK signaling cascade (125). In addition, this coactivator can act in the nucleus as a classical ER-α coactivator and, therefore, may play a role in integrating extranuclear signaling with genomic mechanisms of ER-α activation (126).
Similarly to the ERs, PR-B also interacts functionally with Src and mediates rapid (]lt]5 min) activation of Src and downstream MAPK signaling independently of PR’s transcriptional activity (127). However, in the case of PR-B, the receptor interacts directly through an SH3-binding domain, subsequently activating MAPK-mediated gene transcription (128). Interestingly, although Src/MAPK can be activated by the PR-B isoform found in the cytoplasm and nucleus, Edwards and colleagues (129) found that PR-A that was predominantly nuclear did not activate Src/MAPK. In addition to the rapid and transient PR-mediated signaling, Faivre and Lange (130) recently reported a more sustained (6–72 h) ERK1/2 activation initiated by PR-B through Wnt transactivation and subsequent epidermal growth factor receptor activation. The resultant more sustained MAPK and Cyclin D expression, which is also Src dependent, is an example of how classic nuclear transactivation functions combined with rapid extranuclear actions of PR-B may coordinate to result in amplified PR-mediated proliferative effects.
There is relatively limited evidence that ER and PR exist outside the nucleus in primary human breast cancers, although functional membrane-associated ER-α was first reported more than 30 yr ago (131). Recently, ER-α expression was reported to be in an extranuclear distribution in four of 219 breast cancers; extranuclear PR expression was seen in 20 of 219 of these specimens (132). Interestingly, extranuclear expression was seen relatively commonly in ER-positive/PR-negative tumors (40%), suggesting a possible cross talk, as mentioned above, between PR negativity and activation of the PI3-K or Her2 pathways.
In summary, after NR activation, both genomic and nongenomic NR pathways coordinately activate growth factor signaling pathways that are central to breast cancer biology. It follows that selective modulation of these NR pathways, in combination with inhibition of upstream and downstream activated growth factor-signaling pathways, will lead to more effective treatments (133). Indeed, there are several ongoing randomized clinical trials currently attempting to improve response rates in patients who might benefit from such combined treatments. Understanding how growth factor receptors and their associated kinases are activated by NRs (and vice versa) will be critical for identifying the appropriate tumors for novel treatment with specific combinations. Really significant progress in treating breast cancer will also require an integration of basic and preclinical experimental models with genomic and proteomic tumor characterization as well as cutting-edge clinical trial design. Requisite to the success of this process will be an ongoing dialogue between NR biologists and clinical scientists who together can integrate their current working models into innovative approaches to the prevention and treatment of human breast cancer.
Acknowledgments
I thank members of my laboratory and Drs. Geoffrey Greene and Gini Fleming for helpful discussions. I regret being unable to cite many outstanding contributions to this field due to space limitations.
Footnotes
This work was supported by the National Institutes of Health Grants CA089208 and ES012382.
Disclosure statement: The author of this manuscript has no conflict to disclose.
First Published Online April 16, 2008
Abbreviations: AI, Aromatase inhibitor; AIB1, amplified in breast cancer 1; AR, androgen receptor; ATRA, all-trans-retinoic acid; 9-cis-RA, 9-cis-retinoic acid; ER, estrogen receptor; ERR, estrogen-related receptor; GR, glucocorticoid receptor; HDAC, histone deacetylase; Her2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; N-CoR, nuclear receptor corepressor; NR, nuclear receptor; 1,25(OH)2D3, 1,25-dihydroxyvitamin D3; PDK-1, phosphoinositide-dependent protein kinase-1; PI3-K, phosphatidylinositol 3-kinase; PPAR, peroxisome proliferator-activated receptor; PR, progesterone receptor; RAR, retinoic acid receptor; RXR, retinoid X receptor; SERM, selective estrogen modulator; SMRT, silencing mediator of retinoid and thyroid hormone receptor; SRC, steroid receptor coactivator; VDR, vitamin D receptor.
References
- Beatson G 1896 On the treatment of inoperable cases of carcinoma of the mamma: suggestions for a new method of treatment, with illustrative cases. Lancet 2:104–107 [PMC free article] [PubMed] [Google Scholar]
- McGuire WL, Clark GM 1989 Prognostic factors for recurrence and survival in axillary node-negative breast cancer. J Steroid Biochem 34:145–148 [DOI] [PubMed] [Google Scholar]
- Harvey JM, Clark GM, Osborne CK, Allred DC 1999 Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol 17:1474–1481 [DOI] [PubMed] [Google Scholar]
- Willson TM, Moore JT 2002 Genomics versus orphan nuclear receptors—a half-time report. Mol Endocrinol 16:1135–1144 [DOI] [PubMed] [Google Scholar]
- Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ 2001 Nuclear receptors and lipid physiology: opening the X-files. Science 294:1866–1870 [DOI] [PubMed] [Google Scholar]
- Bookout AL, Jeong Y, Downes M, Yu RT, Evans RM, Mangelsdorf DJ 2006 Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell 126:789–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange CA, Gioeli D, Hammes SR, Marker PC 2007 Integration of rapid signaling events with steroid hormone receptor action in breast and prostate cancer. Annu Rev Physiol 69:171–199 [DOI] [PubMed] [Google Scholar]
- Kelsey JL, Bernstein L 1996 Epidemiology and prevention of breast cancer. Annu Rev Public Health 17:47–67 [DOI] [PubMed] [Google Scholar]
- Hewitt SC, Harrell JC, Korach KS 2005 Lessons in estrogen biology from knockout and transgenic animals. Annu Rev Physiol 67:285–308 [DOI] [PubMed] [Google Scholar]
- Green S, Walter P, Greene G, Krust A, Goffin C, Jensen E, Scrace G, Waterfield M, Chambon P 1986 Cloning of the human oestrogen receptor cDNA. J Steroid Biochem 24:77–83 [DOI] [PubMed] [Google Scholar]
- Greene GL, Gilna P, Waterfield M, Baker A, Hort Y, Shine J 1986 Sequence and expression of human estrogen receptor complementary DNA. Science 231:1150–1154 [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA 1996 Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA 93:5925–5930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Althuis MD, Fergenbaum JH, Garcia-Closas M, Brinton LA, Madigan MP, Sherman ME 2004 Etiology of hormone receptor-defined breast cancer: a systematic review of the literature. Cancer Epidemiol Biomarkers Prev 13:1558–1568 [PubMed] [Google Scholar]
- Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, Baehner FL, Walker MG, Watson D, Park T, Hiller W, Fisher ER, Wickerham DL, Bryant J, Wolmark N 2004 A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 351:2817–2826 [DOI] [PubMed] [Google Scholar]
- Pusztai L, Cristofanilli M, Paik S 2007 New generation of molecular prognostic and predictive tests for breast cancer. Semin Oncol 34:S10–S16 [DOI] [PubMed] [Google Scholar]
- Allegra JC, Lippman ME, Thompson EB, Simon R 1978 An association between steroid hormone receptors and response to cytotoxic chemotherapy in patients with metastatic breast cancer. Cancer Res 38:4299–4304 [PubMed] [Google Scholar]
- Berry DA, Cirrincione C, Henderson IC, Citron ML, Budman DR, Goldstein LJ, Martino S, Perez EA, Muss HB, Norton L, Hudis C, Winer EP 2006 Estrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive breast cancer. JAMA 295:1658–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey LA, Dees EC, Sawyer L, Gatti L, Moore DT, Collichio F, Ollila DW, Sartor CI, Graham ML, Perou CM 2007 The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res 13:2329–2334 [DOI] [PubMed] [Google Scholar]
- Lapidus RG, Ferguson AT, Ottaviano YL, Parl FF, Smith HS, Weitzman SA, Baylin SB, Issa JP, Davidson NE 1996 Methylation of estrogen and progesterone receptor gene 5′ CpG islands correlates with lack of estrogen and progesterone receptor gene expression in breast tumors. Clin Cancer Res 2:805–810 [PubMed] [Google Scholar]
- Yang X, Ferguson AT, Nass SJ, Phillips DL, Butash KA, Wang SM, Herman JG, Davidson NE 2000 Transcriptional activation of estrogen receptor α in human breast cancer cells by histone deacetylase inhibition. Cancer Res 60:6890–6894 [PubMed] [Google Scholar]
- Omoto Y, Eguchi H, Yamamoto-Yamaguchi Y, Hayashi S 2003 Estrogen receptor (ER) β1 and ERβcx/β2 inhibit ERα function differently in breast cancer cell line MCF7. Oncogene 22:5011–5020 [DOI] [PubMed] [Google Scholar]
- Matthews J, Wihlen B, Tujague M, Wan J, Strom A, Gustafsson JA 2006 Estrogen receptor (ER) β modulates ERα-mediated transcriptional activation by altering the recruitment of c-Fos and c-Jun to estrogen-responsive promoters. Mol Endocrinol 20:534–543 [DOI] [PubMed] [Google Scholar]
- Monroe DG, Secreto FJ, Subramaniam M, Getz BJ, Khosla S, Spelsberg TC 2005 Estrogen receptor α and β heterodimers exert unique effects on estrogen- and tamoxifen-dependent gene expression in human U2OS osteosarcoma cells. Mol Endocrinol 19:1555–1568 [DOI] [PubMed] [Google Scholar]
- Chang EC, Frasor J, Komm B, Katzenellenbogen BS 2006 Impact of estrogen receptor β on gene networks regulated by estrogen receptor α in breast cancer cells. Endocrinology 147:4831–4842 [DOI] [PubMed] [Google Scholar]
- Bardin A, Boulle N, Lazennec G, Vignon F, Pujol P 2004 Loss of ERβ expression as a common step in estrogen-dependent tumor progression. Endocr Relat Cancer 11:537–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CE, Carder PJ, Lansdown MR, Speirs V 2006 Steroid hormone receptor expression in male breast cancer. Eur J Surg Oncol 32:44–47 [DOI] [PubMed] [Google Scholar]
- Esslimani-Sahla M, Simony-Lafontaine J, Kramar A, Lavaill R, Mollevi C, Warner M, Gustafsson JA, Rochefort H 2004 Estrogen receptor β (ER β) level but not its ER β cx variant helps to predict tamoxifen resistance in breast cancer. Clin Cancer Res 10:5769–5776 [DOI] [PubMed] [Google Scholar]
- Cappelletti V, Celio L, Bajetta E, Allevi A, Longarini R, Miodini P, Villa R, Fabbri A, Mariani L, Giovanazzi R, Galante E, Greco M, Grazia Daidone M 2004 Prospective evaluation of estrogen receptor-β in predicting response to neoadjuvant antiestrogen therapy in elderly breast cancer patients. Endocr Relat Cancer 11:761–770 [DOI] [PubMed] [Google Scholar]
- O’Malley BW 2007 Coregulators: from whence came these “master genes.” Mol Endocrinol 21:1009–1013 [DOI] [PubMed] [Google Scholar]
- Hudelist G, Czerwenka K, Kubista E, Marton E, Pischinger K, Singer CF 2003 Expression of sex steroid receptors and their co-factors in normal and malignant breast tissue: AIB1 is a carcinoma-specific co-activator. Breast Cancer Res Treat 78:193–204 [DOI] [PubMed] [Google Scholar]
- Liao L, Kuang SQ, Yuan Y, Gonzalez SM, O’Malley BW, Xu J 2002 Molecular structure and biological function of the cancer-amplified nuclear receptor coactivator SRC-3/AIB1. J Steroid Biochem Mol Biol 83:3–14 [DOI] [PubMed] [Google Scholar]
- Anzick SL, Kononen J, Walker RL, Azorsa DO, Tanner MM, Guan XY, Sauter G, Kallioniemi OP, Trent JM, Meltzer PS 1997 AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science 277:965–968 [DOI] [PubMed] [Google Scholar]
- Planas-Silva MD, Shang Y, Donaher JL, Brown M, Weinberg RA 2001 AIB1 enhances estrogen-dependent induction of cyclin D1 expression. Cancer Res 61:3858–3862 [PubMed] [Google Scholar]
- Osborne CK, Bardou V, Hopp TA, Chamness GC, Hilsenbeck SG, Fuqua SA, Wong J, Allred DC, Clark GM, Schiff R 2003 Role of the estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer. J Natl Cancer Inst 95:353–361 [DOI] [PubMed] [Google Scholar]
- Oh A, List HJ, Reiter R, Mani A, Zhang Y, Gehan E, Wellstein A, Riegel AT 2004 The nuclear receptor coactivator AIB1 mediates insulin-like growth factor I-induced phenotypic changes in human breast cancer cells. Cancer Res 64:8299–8308 [DOI] [PubMed] [Google Scholar]
- Yan J, Tsai SY, Tsai MJ 2006 SRC-3/AIB1: transcriptional coactivator in oncogenesis. Acta Pharmacol Sin 27:387–394 [DOI] [PubMed] [Google Scholar]
- Lavinsky RM, Jepsen K, Heinzel T, Torchia J, Mullen TM, Schiff R, Del-Rio AL, Ricote M, Ngo S, Gemsch J, Hilsenbeck SG, Osborne CK, Glass CK, Rosenfeld MG, Rose DW 1998 Diverse signaling pathways modulate nuclear receptor recruitment of N-CoR and SMRT complexes. Proc Natl Acad Sci USA 95:2920–2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girault I, Lerebours F, Amarir S, Tozlu S, Tubiana-Hulin M, Lidereau R, Bieche I 2003 Expression analysis of estrogen receptor α coregulators in breast carcinoma: evidence that NCOR1 expression is predictive of the response to tamoxifen. Clin Cancer Res 9:1259–1266 [PubMed] [Google Scholar]
- Keeton EK, Brown M 2005 Cell cycle progression stimulated by tamoxifen-bound estrogen receptor-α and promoter-specific effects in breast cancer cells deficient in N-CoR and SMRT. Mol Endocrinol 19:1543–1554 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Yamashita H, Toyama T, Sugiura H, Ando Y, Mita K, Hamaguchi M, Hara Y, Kobayashi S, Iwase H 2006 NCOR1 mRNA is an independent prognostic factor for breast cancer. Cancer Lett 237:123–129 [DOI] [PubMed] [Google Scholar]
- Green AR, Burney C, Granger CJ, Paish EC, El-Sheikh S, Rakha EA, Powe DG, Macmillan RD, Ellis IO, Stylianou E, The prognostic significance of steroid receptor co-regulators in breast cancer: co-repressor NCOR2/SMRT is an independent indicator of poor outcome. Breast Cancer Res Treat, in press [DOI] [PubMed] [Google Scholar]
- Keeton EK, Brown M 2003 Coregulator expression and breast cancer: improving the predictive power of estrogen receptor α. Clin Cancer Res 9:1229–1230 [PubMed] [Google Scholar]
- Jeltsch JM, Krozowski Z, Quirin-Stricker C, Gronemeyer H, Simpson RJ, Garnier JM, Krust A, Jacob F, Chambon P 1986 Cloning of the chicken progesterone receptor. Proc Natl Acad Sci USA 83:5424–5428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei LL, Hawkins P, Baker C, Norris B, Sheridan PL, Quinn PG 1996 An amino-terminal truncated progesterone receptor isoform, PRc, enhances progestin-induced transcriptional activity. Mol Endocrinol 10:1379–1387 [DOI] [PubMed] [Google Scholar]
- Takimoto GS, Graham JD, Jackson TA, Tung L, Powell RL, Horwitz LD, Horwitz KB 1999 Tamoxifen resistant breast cancer: coregulators determine the direction of transcription by antagonist-occupied steroid receptors. J Steroid Biochem Mol Biol 69:45–50 [DOI] [PubMed] [Google Scholar]
- Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, Chambon P 1990 Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J 9:1603–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung L, Abdel-Hafiz H, Shen T, Harvell DM, Nitao LK, Richer JK, Sartorius CA, Takimoto GS, Horwitz KB 2006 Progesterone receptors (PR)-B and -A regulate transcription by different mechanisms: AF-3 exerts regulatory control over coactivator binding to PR-B. Mol Endocrinol 20:2656–2670 [DOI] [PubMed] [Google Scholar]
- Hopp TA, Weiss HL, Hilsenbeck SG, Cui Y, Allred DC, Horwitz KB, Fuqua SA 2004 Breast cancer patients with progesterone receptor PR-A-rich tumors have poorer disease-free survival rates. Clin Cancer Res 10:2751–2760 [DOI] [PubMed] [Google Scholar]
- Buzdar AU 2004 The ATAC (arimidex, tamoxifen, alone or in combination) trial: an update. Clin Breast Cancer 5(Suppl 1):S6–S12 [DOI] [PubMed] [Google Scholar]
- Cui X, Schiff R, Arpino G, Osborne CK, Lee AV 2005 Biology of progesterone receptor loss in breast cancer and its implications for endocrine therapy. J Clin Oncol 23:7721–7735 [DOI] [PubMed] [Google Scholar]
- Dowsett M, Cuzick J, Howell A, Jackson I 2001 Pharmacokinetics of anastrozole and tamoxifen alone, and in combination, during adjuvant endocrine therapy for early breast cancer in postmenopausal women: a sub-protocol of the ‘Arimidex and tamoxifen alone or in combination’ (ATAC) trial. Br J Cancer 85:317–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpino G, Weiss H, Lee AV, Schiff R, De Placido S, Osborne CK, Elledge RM 2005 Estrogen receptor-positive, progesterone receptor-negative breast cancer: association with growth factor receptor expression and tamoxifen resistance. J Natl Cancer Inst 97:1254–1261 [DOI] [PubMed] [Google Scholar]
- Shi W, Zhang X, Pintilie M, Ma N, Miller N, Banerjee D, Tsao MS, Mak T, Fyles A, Liu FF 2003 Dysregulated PTEN-PKB and negative receptor status in human breast cancer. Int J Cancer 104:195–203 [DOI] [PubMed] [Google Scholar]
- Cui X, Zhang P, Deng W, Oesterreich S, Lu Y, Mills GB, Lee AV 2003 Insulin-like growth factor-I inhibits progesterone receptor expression in breast cancer cells via the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin pathway: progesterone receptor as a potential indicator of growth factor activity in breast cancer. Mol Endocrinol 17:575–588 [DOI] [PubMed] [Google Scholar]
- Agoff SN, Swanson PE, Linden H, Hawes SE, Lawton TJ 2003 Androgen receptor expression in estrogen receptor-negative breast cancer. Immunohistochemical, clinical, and prognostic associations. Am J Clin Pathol 120:725–731 [DOI] [PubMed] [Google Scholar]
- Kuenen-Boumeester V, Van der Kwast TH, Claassen CC, Look MP, Liem GS, Klijn JG, Henzen-Logmans SC 1996 The clinical significance of androgen receptors in breast cancer and their relation to histological and cell biological parameters. Eur J Cancer 32A:1560–1565 [DOI] [PubMed] [Google Scholar]
- Rakha EA, El-Sayed ME, Green AR, Lee AH, Robertson JF, Ellis IO 2007 Prognostic markers in triple-negative breast cancer. Cancer 109:25–32 [DOI] [PubMed] [Google Scholar]
- de Launoit Y, Dauvois S, Dufour M, Simard J, Labrie F 1991 Inhibition of cell cycle kinetics and proliferation by the androgen 5α-dihydrotestosterone and antiestrogen N,n-butyl-N-methyl-11-[16′ α-chloro-3′,17β-dihydroxy-estra-1′,3′,5′-(10′)triene-7′ α-yl] undecanamide in human breast cancer ZR-75–1 cells. Cancer Res 51:2797–2802 [PubMed] [Google Scholar]
- Hardin C, Pommier R, Calhoun K, Muller P, Jackson T, Pommier S 2007 A new hormonal therapy for estrogen receptor-negative breast cancer. World J Surg 31:1041–1046 [DOI] [PubMed] [Google Scholar]
- Kemppainen JA, Langley E, Wong CI, Bobseine K, Kelce WR, Wilson EM 1999 Distinguishing androgen receptor agonists and antagonists: distinct mechanisms of activation by medroxyprogesterone acetate and dihydrotestosterone. Mol Endocrinol 13:440–454 [DOI] [PubMed] [Google Scholar]
- Birrell SN, Butler LM, Harris JM, Buchanan G, Tilley WD 2007 Disruption of androgen receptor signaling by synthetic progestins may increase risk of developing breast cancer. FASEB J 21:2285–2293 [DOI] [PubMed] [Google Scholar]
- Duma D, Jewell CM, Cidlowski JA 2006 Multiple glucocorticoid receptor isoforms and mechanisms of post-translational modification. J Steroid Biochem Mol Biol 102:11–21 [DOI] [PubMed] [Google Scholar]
- Lu NZ, Cidlowski JA 2005 Translational regulatory mechanisms generate N-terminal glucocorticoid receptor isoforms with unique transcriptional target genes. Mol Cell 18:331–342 [DOI] [PubMed] [Google Scholar]
- Horwitz KB, Costlow ME, McGuire WL 1975 MCF-7; a human breast cancer cell line with estrogen, androgen, progesterone, and glucocorticoid receptors. Steroids 26:785–795 [DOI] [PubMed] [Google Scholar]
- Mikosz CA, Brickley DR, Sharkey MS, Moran TW, Conzen SD 2001 Glucocorticoid receptor-mediated protection from apoptosis is associated with induction of the serine/threonine survival kinase gene, sgk-1. J Biol Chem 276:16649–16654 [DOI] [PubMed] [Google Scholar]
- Moran TJ, Gray S, Mikosz CA, Conzen SD 2000 The glucocorticoid receptor mediates a survival signal in human mammary epithelial cells. Cancer Res 60:867–872 [PubMed] [Google Scholar]
- Huang Y, Johnson KR, Norris JS, Fan W 2000 Nuclear factor-κB/IκB signaling pathway may contribute to the mediation of paclitaxel-induced apoptosis in solid tumor cells. Cancer Res 60:4426–4432 [PubMed] [Google Scholar]
- Wu W, Pew T, Zou M, Pang D, Conzen SD 2005 Glucocorticoid receptor-induced MAPK phosphatase-1 (MPK-1) expression inhibits paclitaxel-associated MAPK activation and contributes to breast cancer cell survival. J Biol Chem 280:4117–4124 [DOI] [PubMed] [Google Scholar]
- Laderoute KR, Mendonca HL, Calaoagan JM, Knapp AM, Giaccia AJ, Stork PJ 1999 Mitogen-activated protein kinase phosphatase-1 (MKP-1) expression is induced by low oxygen conditions found in solid tumor microenvironments. A candidate MKP for the inactivation of hypoxia-inducible stress-activated protein kinase/c-Jun N-terminal protein kinase activity. J Biol Chem 274:12890–12897 [DOI] [PubMed] [Google Scholar]
- Antoni MH, Lutgendorf SK, Cole SW, Dhabhar FS, Sephton SE, McDonald PG, Stefanek M, Sood AK 2006 The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat Rev Cancer 6:240–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agadir A, Shealy YF, Hill DL, Zhang X 1997 Retinyl methyl ether down-regulates activator protein 1 transcriptional activation in breast cancer cells. Cancer Res 57:3444–3450 [PubMed] [Google Scholar]
- Miner JN, Tyree C, Hu J, Berger E, Marschke K, Nakane M, Coghlan MJ, Clemm D, Lane B, Rosen J 2003 A nonsteroidal glucocorticoid receptor antagonist. Mol Endocrinol 17:117–127 [DOI] [PubMed] [Google Scholar]
- Issemann I, Green S 1990 Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature 347:645–650 [DOI] [PubMed] [Google Scholar]
- Maggiora M, Bologna M, Ceru MP, Possati L, Angelucci A, Cimini A, Miglietta A, Bozzo F, Margiotta C, Muzio G, Canuto RA 2004 An overview of the effect of linoleic and conjugated-linoleic acids on the growth of several human tumor cell lines. Int J Cancer 112:909–919 [DOI] [PubMed] [Google Scholar]
- Rose DP, Connolly JM, Coleman M 1996 Effect of ω-3 fatty acids on the progression of metastases after the surgical excision of human breast cancer cell solid tumors growing in nude mice. Clin Cancer Res 2:1751–1756 [PubMed] [Google Scholar]
- Shannon J, King IB, Moshofsky R, Lampe JW, Gao DL, Ray RM, Thomas DB 2007 Erythrocyte fatty acids and breast cancer risk: a case-control study in Shanghai, China. Am J Clin Nutr 85:1090–1097 [DOI] [PubMed] [Google Scholar]
- Michalik L, Auwerx J, Berger JP, Chatterjee VK, Glass CK, Gonzalez FJ, Grimaldi PA, Kadowaki T, Lazar MA, O’Rahilly S, Palmer CN, Plutzky J, Reddy JK, Spiegelman BM, Staels B, Wahli W 2006 International Union of Pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacol Rev 58:726–741 [DOI] [PubMed] [Google Scholar]
- Crowe DL, Chandraratna RA 2004 A retinoid X receptor (RXR)-selective retinoid reveals that RXR-α is potentially a therapeutic target in breast cancer cell lines, and that it potentiates antiproliferative and apoptotic responses to peroxisome proliferator-activated receptor ligands. Breast Cancer Res 6:R546–R555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller E, Sarraf P, Tontonoz P, Evans RM, Martin KJ, Zhang M, Fletcher C, Singer S, Spiegelman BM 1998 Terminal differentiation of human breast cancer through PPAR γ. Mol Cell 1:465–470 [DOI] [PubMed] [Google Scholar]
- Bonofiglio D, Aquila S, Catalano S, Gabriele S, Belmonte M, Middea E, Qi H, Morelli C, Gentile M, Maggiolini M, Ando S 2006 Peroxisome proliferator-activated receptor-γ activates p53 gene promoter binding to the nuclear factor-κB sequence in human MCF7 breast cancer cells. Mol Endocrinol 20:3083–3092 [DOI] [PubMed] [Google Scholar]
- Bonofiglio D, Gabriele S, Aquila S, Catalano S, Gentile M, Middea E, Giordano F, Ando S 2005 Estrogen receptor α binds to peroxisome proliferator-activated receptor response element and negatively interferes with peroxisome proliferator-activated receptor γ signaling in breast cancer cells. Clin Cancer Res 11:6139–6147 [DOI] [PubMed] [Google Scholar]
- Lu M, Kwan T, Yu C, Chen F, Freedman B, Schafer JM, Lee EJ, Jameson JL, Jordan VC, Cryns VL 2005 Peroxisome proliferator-activated receptor γ agonists promote TRAIL-induced apoptosis by reducing survivin levels via cyclin D3 repression and cell cycle arrest. J Biol Chem 280:6742–6751 [DOI] [PubMed] [Google Scholar]
- Suzuki T, Hayashi S, Miki Y, Nakamura Y, Moriya T, Sugawara A, Ishida T, Ohuchi N, Sasano H 2006 Peroxisome proliferator-activated receptor γ in human breast carcinoma: a modulator of estrogenic actions. Endocr Relat Cancer 13:233–250 [DOI] [PubMed] [Google Scholar]
- Suh N, Wang Y, Williams CR, Risingsong R, Gilmer T, Willson TM, Sporn MB 1999 A new ligand for the peroxisome proliferator-activated receptor-γ (PPAR-γ), GW7845, inhibits rat mammary carcinogenesis. Cancer Res 59:5671–5673 [PubMed] [Google Scholar]
- Yin Y, Russell RG, Dettin LE, Bai R, Wei ZL, Kozikowski AP, Kopelovich L, Glazer RI 2005 Peroxisome proliferator-activated receptor δ and γ agonists differentially alter tumor differentiation and progression during mammary carcinogenesis. Cancer Res 65:3950–3957 [DOI] [PubMed] [Google Scholar]
- Nicol CJ, Yoon M, Ward JM, Yamashita M, Fukamachi K, Peters JM, Gonzalez FJ 2004 PPARγ influences susceptibility to DMBA-induced mammary, ovarian and skin carcinogenesis. Carcinogenesis 25:1747–1755 [DOI] [PubMed] [Google Scholar]
- Saez E, Rosenfeld J, Livolsi A, Olson P, Lombardo E, Nelson M, Banayo E, Cardiff RD, Izpisua-Belmonte JC, Evans RM 2004 PPAR γ signaling exacerbates mammary gland tumor development. Genes Dev 18:528–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen RL, Gustafsson MC, Jarvis M, Tatoud R, Marshall BR, Knight D, Ehrenborg E, Harris AL, Wolf CR, Palmer CN 2004 Activation of peroxisome proliferator-activated receptor δ stimulates the proliferation of human breast and prostate cancer cell lines. Cancer Res 64:3162–3170 [DOI] [PubMed] [Google Scholar]
- Burstein HJ, Demetri GD, Mueller E, Sarraf P, Spiegelman BM, Winer EP 2003 Use of the peroxisome proliferator-activated receptor (PPAR) γ ligand troglitazone as treatment for refractory breast cancer: a phase II study. Breast Cancer Res Treat 79:391–397 [DOI] [PubMed] [Google Scholar]
- Yee LD, Williams N, Wen P, Young DC, Lester J, Johnson MV, Farrar WB, Walker MJ, Povoski SP, Suster S, Eng C 2007 Pilot study of rosiglitazone therapy in women with breast cancer: effects of short-term therapy on tumor tissue and serum markers. Clin Cancer Res 13:246–252 [DOI] [PubMed] [Google Scholar]
- Suchanek KM, May FJ, Robinson JA, Lee WJ, Holman NA, Monteith GR, Roberts-Thomson SJ 2002 Peroxisome proliferator-activated receptor α in the human breast cancer cell lines MCF-7 and MDA-MB-231. Mol Carcinog 34:165–171 [DOI] [PubMed] [Google Scholar]
- Gimble JM, Pighetti GM, Lerner MR, Wu X, Lightfoot SA, Brackett DJ, Darcy K, Hollingsworth AB 1998 Expression of peroxisome proliferator activated receptor mRNA in normal and tumorigenic rodent mammary glands. Biochem Biophys Res Commun 253:813–817 [DOI] [PubMed] [Google Scholar]
- Gupta RA, Wang D, Katkuri S, Wang H, Dey SK, DuBois RN 2004 Activation of nuclear hormone receptor peroxisome proliferator-activated receptor-δ accelerates intestinal adenoma growth. Nat Med 10:245–247 [DOI] [PubMed] [Google Scholar]
- Germain P, Chambon P, Eichele G, Evans RM, Lazar MA, Leid M, De Lera AR, Lotan R, Mangelsdorf DJ, Gronemeyer H 2006 International Union of Pharmacology. LXIII. Retinoid X receptors. Pharmacol Rev 58:760–772 [DOI] [PubMed] [Google Scholar]
- Pavan B, Biondi C, Dalpiaz A 2006 Nuclear retinoic acid receptor β as a tool in chemoprevention trials. Curr Med Chem 13:3553–3563 [DOI] [PubMed] [Google Scholar]
- Rubin M, Fenig E, Rosenauer A, Menendez-Botet C, Achkar C, Bentel JM, Yahalom J, Mendelsohn J, Miller Jr WH 1994 9-Cis retinoic acid inhibits growth of breast cancer cells and down-regulates estrogen receptor RNA and protein. Cancer Res 54:6549–6556 [PubMed] [Google Scholar]
- Seewaldt VL, Kim JH, Caldwell LE, Johnson BS, Swisshelm K, Collins SJ 1997 All-trans-retinoic acid mediates G1 arrest but not apoptosis of normal human mammary epithelial cells. Cell Growth Differ 8:631–641 [PubMed] [Google Scholar]
- Wu K, Kim HT, Rodriquez JL, Munoz-Medellin D, Mohsin SK, Hilsenbeck SG, Lamph WW, Gottardis MM, Shirley MA, Kuhn JG, Green JE, Brown PH 2000 9-cis-retinoic acid suppresses mammary tumorigenesis in C3(1)-simian virus 40 T antigen-transgenic mice. Clin Cancer Res 6:3696–3704 [PubMed] [Google Scholar]
- Miller VA, Rigas JR, Benedetti FM, Verret AL, Tong WP, Kris MG, Gill GM, Loewen GR, Truglia JA, Ulm EH, Warrell Jr RP 1996 Initial clinical trial of the retinoid receptor pan agonist 9-cis retinoic acid. Clin Cancer Res 2:471–475 [PubMed] [Google Scholar]
- Wu K, DuPre E, Kim H, Tin UC, Bissonnette RP, Lamph WW, Brown PH 2006 Receptor-selective retinoids inhibit the growth of normal and malignant breast cells by inducing G1 cell cycle blockade. Breast Cancer Res Treat 96:147–157 [DOI] [PubMed] [Google Scholar]
- Donato LJ, Suh JH, Noy N 2007 Suppression of mammary carcinoma cell growth by retinoic acid: the cell cycle control gene Btg2 is a direct target for retinoic acid receptor signaling. Cancer Res 67:609–615 [DOI] [PubMed] [Google Scholar]
- Sutton LM, Warmuth MA, Petros WP, Winer EP 1997 Pharmacokinetics and clinical impact of all-trans retinoic acid in metastatic breast cancer: a phase II trial. Cancer Chemother Pharmacol 40:335–341 [DOI] [PubMed] [Google Scholar]
- Zanardi S, Serrano D, Argusti A, Barile M, Puntoni M, Decensi A 2006 Clinical trials with retinoids for breast cancer chemoprevention. Endocr Relat Cancer 13:51–68 [DOI] [PubMed] [Google Scholar]
- Bischoff ED, Gottardis MM, Moon TE, Heyman RA, Lamph WW 1998 Beyond tamoxifen: the retinoid X receptor-selective ligand LGD1069 (TARGRETIN) causes complete regression of mammary carcinoma. Cancer Res 58:479–484 [PubMed] [Google Scholar]
- Gottardis MM, Lamph WW, Shalinsky DR, Wellstein A, Heyman RA 1996 The efficacy of 9-cis retinoic acid in experimental models of cancer. Breast Cancer Res Treat 38:85–96 [DOI] [PubMed] [Google Scholar]
- Ijpenberg A, Tan NS, Gelman L, Kersten S, Seydoux J, Xu J, Metzger D, Canaple L, Chambon P, Wahli W, Desvergne B 2004 In vivo activation of PPAR target genes by RXR homodimers. EMBO J 23:2083–2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narvaez CJ, Zinser G, Welsh J 2001 Functions of 1α,25-dihydroxyvitamin D3 in mammary gland: from normal development to breast cancer. Steroids 66:301–308 [DOI] [PubMed] [Google Scholar]
- Townsend K, Banwell CM, Guy M, Colston KW, Mansi JL, Stewart PM, Campbell MJ, Hewison M 2005 Autocrine metabolism of vitamin D in normal and malignant breast tissue. Clin Cancer Res 11:3579–3586 [DOI] [PubMed] [Google Scholar]
- Zinser GM, McEleney K, Welsh J 2003 Characterization of mammary tumor cell lines from wild type and vitamin D3 receptor knockout mice. Mol Cell Endocrinol 200:67–80 [DOI] [PubMed] [Google Scholar]
- Welsh J, Wietzke JA, Zinser GM, Smyczek S, Romu S, Tribble E, Welsh JC, Byrne B, Narvaez CJ 2002 Impact of the vitamin D3 receptor on growth-regulatory pathways in mammary gland and breast cancer. J Steroid Biochem Mol Biol 83:85–92 [DOI] [PubMed] [Google Scholar]
- Zinser GM, Tribble E, Valrance M, Urben CM, Knutson JC, Mazess RB, Strugnell SA, Welsh I 2005 1,24(S)-dihydroxyvitamin D2, and endogenous vitamin D2 metabolite, inhibits growth of breast cancer cells and tumors. Anticancer Res 25:235–241 [PubMed] [Google Scholar]
- Milliken EL, Zhang X, Flask C, Duerk JL, MacDonald PN, Keri RA 2005 EB1089, a vitamin D receptor agonist, reduces proliferation and decreases tumor growth rate in a mouse model of hormone-induced mammary cancer. Cancer Lett 229:205–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giguere V, Yang N, Segui P, Evans RM 1988 Identification of a new class of steroid hormone receptors. Nature 331:91–94 [DOI] [PubMed] [Google Scholar]
- Johnston SD, Liu X, Zuo F, Eisenbraun TL, Wiley SR, Kraus RJ, Mertz JE 1997 Estrogen-related receptor α1 functionally binds as a monomer to extended half-site sequences including ones contained within estrogen-response elements. Mol Endocrinol 11:342–352 [DOI] [PubMed] [Google Scholar]
- Kraus RJ, Ariazi EA, Farrell ML, Mertz JE 2002 Estrogen-related receptor α1 actively antagonizes estrogen receptor-regulated transcription in MCF-7 mammary cells. J Biol Chem 277:24826–24834 [DOI] [PubMed] [Google Scholar]
- Ariazi EA, Clark GM, Mertz JE 2002 Estrogen-related receptor α and estrogen-related receptor γ associate with unfavorable and favorable biomarkers, respectively, in human breast cancer. Cancer Res 62:6510–6518 [PubMed] [Google Scholar]
- Ariazi EA, Jordan VC 2006 Estrogen-related receptors as emerging targets in cancer and metabolic disorders. Curr Top Med Chem 6:203–215 [DOI] [PubMed] [Google Scholar]
- Levin ER 2005 Integration of the extranuclear and nuclear actions of estrogen. Mol Endocrinol 19:1951–1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonyaratanakornkit V, Edwards DP 2004 Receptor mechanisms of rapid extranuclear signaling initiated by steroid hormones. Essays Biochem 40:105–120 [DOI] [PubMed] [Google Scholar]
- Marino M, Acconcia F, Bresciani F, Weisz A, Trentalance A 2002 Distinct nongenomic signal transduction pathways controlled by 17β-estradiol regulate DNA synthesis and cyclin D(1) gene transcription in HepG2 cells. Mol Biol Cell 13:3720–3729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoncini T, Hafezi-Moghadam A, Brazil DP, Ley K, Chin WW, Liao JK 2000 Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature 407:538–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Aitkenhead M, Hughes CC, Levin ER 2002 Integration of the non-genomic and genomic actions of estrogen. Membrane-initiated signaling by steroid to transcription and cell biology. J Biol Chem 277:50768–50775 [DOI] [PubMed] [Google Scholar]
- Song RX, Barnes CJ, Zhang Z, Bao Y, Kumar R, Santen RJ 2004 The role of Shc and insulin-like growth factor 1 receptor in mediating the translocation of estrogen receptor α to the plasma membrane. Proc Natl Acad Sci USA 101:2076–2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Kumar R, Santen RJ, Song RX 2004 The role of adapter protein Shc in estrogen non-genomic action. Steroids 69:523–529 [DOI] [PubMed] [Google Scholar]
- Wong CW, McNally C, Nickbarg E, Komm BS, Cheskis BJ 2002 Estrogen receptor-interacting protein that modulates its nongenomic activity-crosstalk with Src/Erk phosphorylation cascade. Proc Natl Acad Sci USA 99:14783–14788 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Vadlamudi RK, Kumar R 2007 Functional and biological properties of the nuclear receptor coregulator PELP1/MNAR. Nucl Recept Signal 5:e004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio A, Piccolo D, Castoria G, Di Domenico M, Bilancio A, Lombardi M, Gong W, Beato M, Auricchio F 1998 Activation of the Src/p21ras/Erk pathway by progesterone receptor via cross-talk with estrogen receptor. EMBO J 17:2008–2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonyaratanakornkit V, Scott MP, Ribon V, Sherman L, Anderson SM, Maller JL, Miller WT, Edwards DP 2001 Progesterone receptor contains a proline-rich motif that directly interacts with SH3 domains and activates c-Src family tyrosine kinases. Mol Cell 8:269–280 [DOI] [PubMed] [Google Scholar]
- Boonyaratanakornkit V, McGowan E, Sherman L, Mancini MA, Cheskis BJ, Edwards DP 2007 The role of extranuclear signaling actions of progesterone receptor in mediating progesterone regulation of gene expression and the cell cycle. Mol Endocrinol 21:359–375 [DOI] [PubMed] [Google Scholar]
- Faivre EJ, Lange CA 2007 Progesterone receptors upregulate Wnt-1 to induce epidermal growth factor receptor transactivation and c-Src-dependent sustained activation of Erk1/2 mitogen-activated protein kinase in breast cancer cells. Mol Cell Biol 27:466–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietras RJ, Szego CM 1977 Specific binding sites for oestrogen at the outer surfaces of isolated endometrial cells. Nature 265:69–72 [DOI] [PubMed] [Google Scholar]
- Kim R, Kaneko M, Arihiro K, Emi M, Tanabe K, Murakami S, Osaki A, Inai K 2006 Extranuclear expression of hormone receptors in primary breast cancer. Ann Oncol 17:1213–1220 [DOI] [PubMed] [Google Scholar]
- Johnston SR, Martin LA, Leary A, Head J, Dowsett M 2007 Clinical strategies for rationale combinations of aromatase inhibitors with novel therapies for breast cancer. J Steroid Biochem Mol Biol 106:180–186 [DOI] [PubMed] [Google Scholar]