Abstract
An involvement of molecular chaperones in the action and well-being of steroid receptors was recognized early in the molecular era of hormone research. However, this has continued to be a topic of much enquiry and some confusion. All steroid receptors associate with heat shock protein 90, the main character of a series of multiprotein chaperone complexes generally referred to as the “heat shock protein 90 chaperoning machine.” Receptor association with chaperones occurs in an ordered, step-wise fashion and is necessary for the maintenance of unliganded receptor in a state ready to bind and respond to hormone. Chaperones additionally modulate how receptors respond to hormone and activate target genes. Although much is known about the participants in this chaperoning process and the consequences of chaperoning, many key questions remain unanswered, particularly those concerning molecular mechanisms, cellular dynamics, and the functions of an array of cochaperone proteins. Here, we point out several areas in need of investigation to encourage new ideas and participants in this burgeoning field.
TWENTY-THREE YEARS AGO the Baulieu, Pratt, and Toft laboratories identified heat shock protein 90 (Hsp90) as a component of steroid receptor complexes (1,2,3,4). This was shortly after the first recognized Hsp90 association with v-Src tyrosine kinase (5). At the time, these discoveries drew attention not only from those studying steroid receptors and kinases, but also from investigators studying heat shock proteins, because the functions and endogenous partners of these heat-inducible proteins were poorly understood. About the same time, scientists were first realizing that heat shock proteins can chaperone the folding and assembly of various proteins (6). Hsp70 and Hsp40 were soon shown to cooperate in protein folding (7), and Hsp90 was found to be adept at preventing irreversible aggregation of thermally denatured proteins (8). Later, several Hsp90-associated cochaperones were found individually to also inhibit aggregation (9,10). Thus, heat shock proteins found in steroid receptor complexes are major players in the molecular chaperone machinery that supports homeostatic folding of the proteome as well as recovery of the proteome from denaturing stresses. Within the general proteome, we now understand that a sizeable number of proteins involved in diverse cellular signaling pathways, i.e. transcription factors, kinases, enzymes, are obligate substrates, or clients, for Hsp90, Hsp70, and Hsp40 plus an array of cochaperone proteins (for an up-to-date listing of clients and co-chaperones see http://www.picard.ch/downloads/Hsp90interactors.pdf). These signaling clients are characterized by metastable conformations that correlate closely with conformational and functional transitions of the client protein. The chaperone machinery clearly affords such metastability, but one can debate whether client metastability reflects an inherent limitation of a protein’s refoldability, whether metastability is a client adaptation that co-opts the chaperone machinery to assist in regulating client activity, or some combination of the two.
Steroid receptors are one class of chaperone client protein that have been studied in great detail. Steroid hormones are vital regulators of reproductive, growth, and homeostatic processes in vertebrates, but hormonal stimulation of the cognate receptors for estrogens [estrogen receptor (ER)α in particular], progestins [progesterone receptor (PR)], androgens [androgen receptor (AR)], glucocorticoids [glucocorticoid receptor (GR)], and mineralocorticoids [mineralocorticoid receptor (MR)] are filtered through the molecular chaperone machinery in physiologically meaningful ways that are still neither fully appreciated nor understood. Characterizations of chaperone interactions with steroid receptors and chaperone-chaperone interactions within the context of steroid receptor assembly have provided fundamental insights that reveal the range of ways by which chaperones cooperate with or antagonize one another. These diverse chaperone interactions with steroid receptors have helped reveal functional elaborations provided by a rich diversity of cochaperone proteins and form a comparative basis for understanding chaperone roles in unrelated client complexes. Our primary goal here is to take what is known about the intimate relationship between steroid receptors and molecular chaperones to underscore remaining questions and opportunities we see for further research.
RECEPTOR CONFORMATION AND CHAPERONE BINDING
Before binding hormone, the steroid receptors (ER-α, PR, AR, GR, and MR) are typically found associated with Hsp90 and other chaperone proteins. This contrasts with other classes of nuclear receptor that display no ready affinity for Hsp90. If the steroid receptors simply have an unstable conformation that requires chaperone support, this would seem to be a property peculiar to the steroid receptor class, which evolved much later than other nuclear receptor classes (11). Do steroid receptors have less stable conformations than the more numerous, more ancient members of the nuclear receptor superfamily? Structural studies do not support such a distinction for steroid receptors (12). Chaperone interactions localize to the ligand-binding domain (LBD) of steroid receptors (13,14), and the hormone-free LBD has a more loosely ordered structure than hormone-bound LBD; however, the same holds true for the LBD of other nuclear receptors, as attested by the general difficulty in crystallizing nuclear receptor LBDs in the absence of ligand. Another suggestion that chaperones are not simply attracted to general misfolded features in steroid receptors are studies showing that the N-terminal domain of steroid as well as other nuclear receptors characteristically has a metastable structure (12), no doubt contributing to the inability of crystallographers to obtain a structure for this domain. However, chaperone binding has not been observed at the N-terminal domain of any nuclear receptor but only to the C-terminal LBD of steroid receptors.
One consideration is that nuclear receptors in general might transit quickly through metastable states in the cellular environment, perhaps assisted by chaperones. One study showed that vertebrate retinoic acid receptor function, both hormone binding and transcriptional activation, was impaired in a yeast cellular background deficient for Hsp90 (15); this study did not distinguish whether Hsp90 was required only for nascent receptor polypeptide folding or over a more extended period, as is the case for steroid receptors, but it hints at more general nuclear receptor requirements for chaperones. Hsp90 association with several nonsteroid nuclear receptors has been observed in mammalian cells (16,17,18,19,20,21), but it is unclear as yet how comparable these interactions are to those readily observed with steroid receptors. Attempts at in vitro assembly of nonsteroid nuclear receptors have not yielded complexes similar to those obtained with steroid receptors (Ref. 22 and Smith, D. F., unpublished observations). It is noteworthy that nonsteroid nuclear receptors, whether bound or not by hormonal ligand, are typically associated with DNA and transcriptional cofactors; for those nuclear receptors having cognate ligands, ligand binding stimulates a conformational change that results in altered affinities for DNA and protein partners and thereby a change in transcriptional activity. Steroid receptors differ in that DNA binding and association with transcriptional cofactors predominantly occurs only in response to ligand binding or, in some cases, covalent modification. Chaperone associations effectively prevent DNA binding (2) and maintain receptor in a soluble complex until ligand binding stimulates a receptor conformational change, release of receptor from chaperones, and receptor associations with DNA and transcriptional complexes. This silencing property of chaperone interactions with steroid receptors is readily demonstrated by artificial chimeric proteins in which the activity of partner domain fused to a steroid receptor LBD is induced by hormone-dependent dissociation of chaperones from the LBD (23). One must consider that unliganded steroid receptors, rather than simply being more unstable than other nuclear receptors, in fact have evolved to maintain chaperone interactions as a mechanism for regulating receptor activity and target gene expression.
HORMONE BINDING ABILITY AND CHAPERONES
Chaperones are required to establish and maintain the hormone binding ability of steroid receptors, consistent with the concept of an unstable LBD in the absence of ligand. Upon removal of Hsp90, GR and PR quickly lose hormone-binding ability at temperatures of 25 C or above whereas hormone binding is readily restored at temperatures up to 37 C in the presence of Hsp90, its supporting cast of chaperones, and ATP (24,25). ERα is interesting in that its hormone-binding ability is stable in the absence of Hsp90 (26,27), yet ERα assembles with Hsp90 in a manner very similar to GR and PR. AR and MR have not been studied in as much detail as the other steroid receptors, but they appear to be more similar to GR and PR in their reliance on Hsp90 for hormone binding (28,29,30). Therefore, at least in the case of ERα, one can separate a strict reliance on Hsp90 binding from hormone-binding ability. Another telling feature of chaperone interactions with the LBD is that, as discussed below, there appear to be discrete binding sites for chaperones within the LBD. If the overall LBD conformation is unstable, one might expect chaperones to bind at various sites, but assembly appears to be a well-ordered process directed by specific features within the LBD. One of us speculated (25) that during evolution an ancestral steroid receptor adapted to retain chaperone interactions for regulatory purposes; subsequently in evolution, receptors could have lost an ability to bind hormone independently due to relaxation of internal LBD scaffolding that was compensated by external scaffolding provided by Hsp90. A recent report from the laboratory of Darimont and co-workers (31) demonstrated that point mutation of specific sites within the GR LBD relaxed dependence on Hsp90 for hormone binding. One suggestion from their observations is that GR could readily adapt to improve hormone binding without having to rely on Hsp90; therefore, Hsp90 interaction appears to serve other evolutionarily conserved roles in regulating GR activity. As suggested by one of the anonymous reviewers of this minireview, it would likely be very informative to determine the phenotype of a mouse model in which wild-type GR is replaced by a mutant with independent hormone-binding ability. Reducing receptor-chaperone interactions simply to a matter of steroid receptor misfolding potentially obscures more interesting evolutionary pathways and physiological roles for these interactions. Modern bioinformatic and computational techniques have just begun to be applied to understanding evolutionary relationships among receptor-associated chaperones (32), and there are many additional untapped opportunities for advancing our understanding of receptor-chaperone interactions via comparative approaches.
Investigators have commonly referred to the ability of chaperones to refold the receptor LBD into a conformation competent for hormone binding. It is not clear that refolding per se is the driving force behind chaperone assembly with the LBD because, as noted above, ERα attracts similar chaperone assemblies even while maintaining its hormone-binding ability. An LBD conformational change in steroid receptors is the likely explanation for gain of hormone-binding ability, but there are few structural data that speak more precisely to the nature of the presumed conformational change. The unliganded LBD of most nuclear receptors appears to have a flexible structure, as attested by persistent challenges in obtaining x-ray crystallographic structures of an LBD-lacking hormone. In the Protein Data Bank there are LBD crystal structures with bound ligand for more than 30 nuclear receptors, but companion apo-receptor structures exist for only four of these receptors, none of which are steroid receptors. Assuming that conformational changes induced by ligand binding, as revealed by companion crystal structures, are typical of other nuclear receptors, there is significant compaction of the LBD around bound ligand; additionally, it is clear that the ultimate orientation of the C-terminal helix 12 is highly sensitive to the particular ligand bound, which in turn dictates transcriptional coactivator vs. corepressor interactions with the LBD.
Unfortunately, there is no cocrystal structure for an Hsp90-bound LBD, whether in the presence or absence of ligand, so one can only speculate on the conformational state of pre- and post-Hsp90 binding that switches hormone-binding ability. Refolding perhaps implies a rather profound conformational change when, in fact, the functionally key rearrangement that renders the LBD competent for hormone binding could be structurally minor. Technical advances in structural biology combined with imaginative application of these capabilities to steroid receptor complexes will one day provide much-needed visualization of the changes in receptor conformational states that occur as a result of chaperone interactions and ligand binding.
MULTISTEP PATHWAY FOR ASSEMBLY OF Hsp90 WITH RECEPTOR
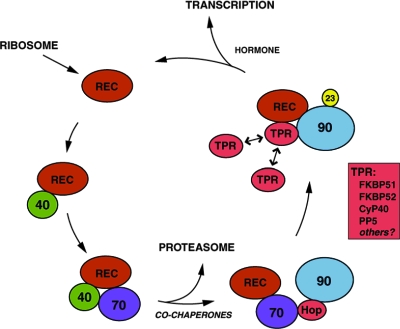
Hsp90 in isolation does not bind the receptor LBD; instead, other chaperones must have established prior interactions on the LBD to recruit Hsp90 for direct binding (see pathway outlined in Fig. 1). In vitro assemblies with purified proteins were used to define Hsp40, Hsp70, Hop, Hsp90, and p23 as the minimal system for efficient assembly of GR or PR complexes that have robust hormone-binding ability (33,34). Hsp40 is the only chaperone in isolation that readily binds the LBD (35), and binding of Hsp40 is a prerequisite for efficient recruitment of Hsp70 (35,36). With facilitation by Hsp40, Hsp70 forms a direct interaction with the LBD that attracts the cochaperone Hop in complex with Hsp90 (33,37). Only with the ordered assistance of Hsp40, Hsp70, and Hop does Hsp90 ultimately bind the LBD and stimulate hormone-binding ability. Additionally, the Hsp90 cochaperone p23 serves to stabilize Hsp90 conformation and extend Hsp90 binding to maximize receptor hormone-binding ability (38,39). The physiological importance of p23 in steroid receptor pathways is supported by mouse p23 gene disruption models (40), which display a perinatal lethality that is similar to GR knockout mice (41).
Figure 1.
Cyclic Assembly of Steroid Receptors with Molecular Chaperones
Nascent receptor polypeptides enter the chaperone assembly cycle through an initial interaction with Hsp40 that, in turn, recruits Hsp70 and Hsp90 to the receptor complex. Multiple cochaperones participate along the way, including Hop, a TPR protein that links Hsp70 and Hsp90, p23, a cochaperone that stabilizes Hsp90 binding to receptor in functionally mature complexes, and any of several TPR proteins that dynamically compete for a common binding site on Hsp90 within the mature complex. Additional cochaperones influence receptor assembly or favor receptor degradation at the proteasome. Hormone binding disrupts the chaperone assembly cycle and promotes activation of receptor as a transcription factor. REC, Receptor.
Hormone binding and an associated LBD conformational change do not directly stimulate Hsp90 release (25). In general, Hsp90 release from substrates is stimulated by hydrolysis of ATP at the nucleotide-binding domain of Hsp90 (42,43,44). Hsp90 dissociation from receptor, in the presence or absence of hormone, occurs spontaneously at a rate similar to the rate of Hsp90-dependent ATP hydrolysis (25), and ligand-dependent dissociation of Hsp90 from receptor requires ATP (45). Hormone-dependent escape of receptor from repetitive rounds of chaperone assembly may occur at the level of Hsp40 binding, the requisite initial step in reassembly of receptor complexes. Thus, a key structural transition in response to hormone binding could be loss of the Hsp40-binding site in the LBD and consequent arrest of further chaperone assembly cycles. Currently, the LBD site for Hsp40 binding is unknown, as are the precise sites for Hsp70 and Hsp90 binding. Investigations that identify these sites will assist in deciphering the structural code that underlies ordered chaperone interactions and conformational changes in the LBD.
Hormone-dependent release of chaperones is further complicated by a poorly understood need for dynamics in chaperone interactions. Whereas assembly of PR complexes with the minimal five-protein system regenerates PR hormone-binding ability, the minimally assembled complexes differ in some striking ways from PR complexes formed and maintained in more complete systems such as crude rabbit reticulocyte lysate. In the complete system, PR complexes are more dynamic; Hsp40 dissociates soon after recruitment of Hsp70, Hsp70 levels reach a maximum, and then, as Hsp90 is recruited and directly associates with the LBD, Hsp70 levels drop off (25). Additionally, as noted above, hormone binding to the mature receptor complex prevents further Hsp40 binding so there is no reassembly after Hsp90 dissociates. In contrast, minimal PR complexes lack the dynamics of the complete system as reflected by stoichiometric recovery of Hsp40 and Hsp70 in Hsp90-bound complexes and a failure of hormone binding to stimulate receptor release from the chaperone machinery. Clearly, there are important factors missing from the minimal system that are critical for chaperone dynamics. We have found that supplementing the minimal system with a small volume of crude reticulocyte lysate does restore dynamics (Toft, D. O., unpublished findings), but fractionation of lysate has failed to identify relevant individual factors. Efforts to enhance the minimal system with additional purified chaperone components known to exist in native receptor complexes, i.e. Hip, Bag1, FK506 binding protein (FKBP)52, FKBP51, cyclophilin (CyP)40, and protein phosphatase 5 (PP5), have likewise failed to restore iterative binding and release of chaperones. Presumably, there is a combination of factors that animate chaperone interactions with receptor. Another important avenue for future investigation is to define the full repertoire of factors and conditions that underlie normal assembly and disassembly of receptor complexes.
COCHAPERONES AND MODULATION OF STEROID RECEPTOR ACTIVITY
The Hsp90 cochaperone proteins FKBP52, FKBP51, CyP40, and PP5 compete for a common Hsp90-binding site, and each can probably be identified at some level in all steroid receptor complexes (46). A common feature of these cochaperones is a tetratricopeptide repeat (TPR) domain that targets binding to a TPR-binding site in the C-terminal region of Hsp90. In vitro measurements have demonstrated that the TPR cochaperones dynamically exchange on Hsp90 while it resides in receptor complexes (46); as a consequence, the receptor samples its environment for cochaperones, and mature receptor complexes exist in a dynamic mixture the proportions of which are determined by the relative abundance and affinity of each TPR cochaperone for the receptor-Hsp90 complex. Potentially, receptor behavior could differ depending on which TPR cochaperone is bound to Hsp90. We know that each steroid receptor displays a unique order of preference for the different TPR cochaperones (46). For example, PP5 is recovered at relatively high levels in GR complexes (47); FKBP51 is recovered in preference to FKBP52 in PR, GR, and MR complexes; and CyP40 is relatively preferred in ERα complexes (46,48). How receptor differentially senses the Hsp90-bound cochaperones is unknown. These cochaperones are not required for Hsp90 binding to receptor, for establishing the core hormone-binding ability of receptor, or for iterative binding and release of chaperones from minimal receptor complexes, yet the TPR cochaperones can play physiologically critical roles in the cellular responses to hormone.
When present in PR, GR, or AR complexes, FKBP52 elevates hormone-binding affinity 2- to 5-fold as compared with complexes containing FKBP51, CyP40, or PP5 (49,50,51,52); FKBP52 does not similarly affect ERα (49) or MR (53) activity even though FKBP52 assembles with these receptor complexes. Conversely, FKBP51 attenuates hormone responsiveness in MR and GR complexes (53,54,55); in addition, FKBP51 can offset FKBP52-dependent potentiation of GR and PR complexes (49,51). The physiological relevance of FKBP52-mediated potentiation of AR and PR responses to hormone has been demonstrated by knocking out expression of the FKBP52 gene in mouse models. Male 52 knockout mice display androgen resistance and defects in development of several secondary sexual features (56,57). Female 52 knockout mice are infertile due to uterine progesterone resistance (51,58,59). FKBP51 gene knockout mouse models do not display similar reproductive abnormalities (56,57), supporting a specific role for FKBP52 in reproductive processes. The physiological importance of FKBP51 has been suggested, however, by analysis of glucocorticoid resistance in New World primates. All New World primates, such as squirrel monkey, have markedly higher levels of circulating glucocorticoids than Old World primates (60), and GR in lymphocytes from squirrel monkey and other species has a lower affinity for glucocorticoids (61). Scammell and colleagues (54,62) have demonstrated that FKBP51 in New World primate cells contributes significantly to lowered GR hormone-binding affinity and glucocorticoid resistance. Relative to FKBP51 in Old World monkey and human cells, the steady-state level of FKBP51 protein is severalfold higher in New World monkey cells, and FKBP51 from New World primates further diminishes GR hormone-binding affinity. Why New World primates have adapted different endocrine properties is unknown, but the potential for FKBP51 to influence GR activity is highlighted in these animals.
How does FKBP52 selectively elevate hormone-binding affinity in some steroid receptors? Initially, the mechanism appeared to relate to the peptidyl-prolyl isomerase (PPIase) activity of FKBP52 (49), perhaps through an FKBP52-stimulated change in the isomeric state of an LBD proline with resulting conformational and functional change in the LBD. Recently, however, it was shown that FKBP52 PPIase enzymatic activity is unimportant, although individual amino acids in the PPIase domain are critical (52). For example, conversion of a single amino acid (L119 in FKBP51, P119 in FKBP52) in the PPIase domain of FKBP51 confers an FKBP52-like ability on the mutant FKBP51 (L119P) to stimulate AR and GR activities. One hypothesis is that the PPIase domain of FKBP52 forms a specific, direct contact with the LBD that favors a reorientation of the LBD relative to Hsp90 and enhances hormone-binding affinity. Testing this hypothesis and others will ultimately reveal how FKBP52 is acting. The mechanism by which FKBP51 inhibits GR hormone-binding affinity is even less well understood. In part, competitive displacement of FKBP52 could account for apparent inhibition of GR activity, but there also appears to be a more direct means by which FKBP51 attenuates GR response to hormone. Given the close relationship of FKBP52 and FKBP51, it seems likely that their opposing influences on receptor activity will share some mechanistic basis. Understanding how the FKBP cochaperones are acting in steroid receptor complexes could lead to novel insights into clinically relevant defects in steroid signaling pathways and provide rationales for therapeutically intervening in steroid-dependent processes.
In addition to those involving the FKBPs, are there alternative cochaperone-receptor pairings that have significant functional impacts on hormone-dependent processes? We suspect that further study will provide an affirmative answer. PP5 is a protein phosphatase, so it would be reasonable to test for PP5-mediated dephosphorylation events in known phosphoprotein components of receptor complexes, i.e. Hsp90, p23, and FKBP52, or the receptor itself. Studies suggest that PP5 is important for GR activity in cells (63,64), although it is unclear whether this effect is phosphatase dependent. Another report (65) provides evidence for direct association of PP5 to either ERα and ERβ with corresponding changes in ER phosphorylation status and activity. CyP40 is another PPIase, albeit one unrelated to the FKBP class of PPIase and thus having the potential for unique substrate specificities within receptor complexes. As noted above, CyP40 is preferentially associated with ERα complexes, but its functional importance in ERα or other steroid receptor complexes is unknown.
We have focused on cochaperones that are known to exist in steroid receptor complexes; however, there are more than 20 known cochaperones for the Hsp90 chaperoning machine, most of which are proteins with TPR domains (66,67). Many of these have been identified through studies on Hsp90 clients other than steroid receptors, and even among those that interact with receptors, we do not anticipate that there will be relevant functional consequences to all cochaperone/receptor pairings. Instead, we suspect that the multiplicity of Hsp90 cochaperones that dynamically compete for a common binding site on Hsp90 reflects a general mechanism that allows any Hsp90 client protein, whether steroid receptor or one of the many other types of client, to efficiently sample the cochaperone environment for potentially productive interaction. During the sampling process, most pairings will neither be advantageous nor necessarily disadvantageous, but the sampling process itself, introducing a broad array of Hsp90 clients with an assortment of cochaperone activities, could be a robust adaptation that requires only a few productive pairings for conservation. Based on such a sampling mechanism, there is the opportunity to set up experimental assays that allow one to methodically screen for functionally productive pairings out of many possible combinations. The value of this approach would be to quickly narrow one’s sights to Hsp90 client-cochaperone matches that are most likely to have biological consequence.
Considering the broad array of receptor-associated cochaperones and the valuable insights already obtained from studying a limited number of possible interactions, we feel expansive opportunities remain for future research into, and potential exploitation of, receptor-cochaperone interactions.
CHAPERONING RECEPTOR THROUGH CELLULAR PATHWAYS
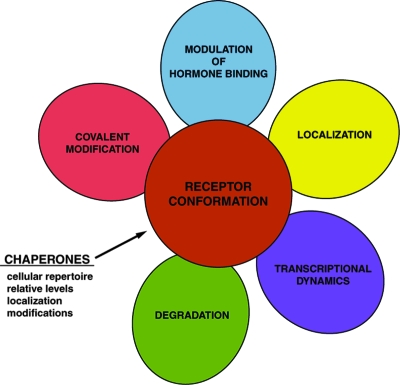
The Hsp90 machinery as a whole supports several aspects of receptor activity beyond establishing and maintaining the hormone-binding ability of steroid receptors (Fig. 2). For many years, the chaperoning of steroid receptors was thought to persist only until hormone binding, and the receptor was then freed to act independently of chaperones. This now appears not to be the case. In fact, steroid receptors may never act independently of chaperones. This is supported by observations that Hsp90 inhibitors cause a loss of receptor activity when applied either before or subsequent to hormone binding (68,69,70,71). Another study has shown that chaperone activity is necessary for faithful hormone-dependent gene activation by PR in an in vitro system (72). The persistence of chaperoning is indicated more directly by studies on the mobility of GR and PR in the nucleus. Studies with permeabilized cells have shown that receptor translocation to the nucleus and receptor mobility within the nucleus require the presence of the Hsp90 machine with at least some of the same chaperone proteins involved in the earlier maintenance of receptors (71,73,74). Additionally, Hsp90 and the cochaperone p23 have been shown to have complex effects on steroid receptors within transcriptionally active chromatin complexes (75,76,77). A range of studies indicate that steroid receptors active in gene regulation naturally exist in short-lived complexes with DNA, coregulators, and auxiliary proteins, and chaperones appear to be critical in this process. Upon hormone binding, receptor assembly with certain transcriptional coregulators may occur during, and as part of, the process of liberation from chaperones; a somewhat reciprocal process may limit the duration of receptor activity at any one gene locus. These findings provide a first glimpse into ongoing chaperone actions within transcription complexes and raise many questions as to the chaperone participants and mechanisms involved in receptor transitions that occur within the nucleus.
Figure 2.
Chaperone Influences on Cellular Actions of Steroid Receptors
Chaperone activity varies based on the repertoire of chaperone components expressed in the cell, the relative levels of each component, and posttranslational modifications. Primarily through alterations of receptor conformation, the chaperones influence receptor hormone binding properties, receptor posttranslational modifications, subcellular localization of receptor, receptor interactions with chromatin and transcriptional cofactors, as well as receptor proteolytic half-life.
It is now quite clear that steroid receptors can have important nongenomic actions to regulate functions in target cells. An example is the PR that, upon hormone treatment, has been shown to interact with c-Src to induce a phosphorylation cascade (78,79,80). As c-Src is itself an Hsp90 client (81), the receptor-kinase interaction is likely to involve coordinated transitions between receptor and kinase complexes containing common and distinct chaperone components. Steroid receptors can also be activated by hormone-independent mechanisms involving receptor phosphorylation (reviewed in Ref. 82). Again, relevant kinases stand a good chance of being Hsp90 clients. In both nongenomic actions and hormone-independent activation, the processes must contend with receptor-associated chaperones, yet this issue has hardly been examined and is in great need of investigation.
The Hsp90 machinery prolongs receptor proteolytic half-life because pharmacological inhibition of Hsp90, which short circuits the assembly cycle, promotes rapid receptor degradation in cells (69). Yet although chaperone assembly stabilizes steroid receptors against proteolysis, at least one cochaperone observed in receptor complexes can shunt the receptor toward the proteasome for degradation. CHIP (carboxyl terminus of Hsp70-interacting protein) is a TPR cochaperone of both Hsp70 and Hsp90 that also has ubiquitin E3 ligase activity (83). Entry of CHIP into receptor complexes promotes receptor ubiquitinylation and diversion of receptor to the proteasome. Steady-state concentration of receptor in different cell types could be determined in part by the cellular level and activity of CHIP. Furthermore, CHIP could play a role in down-regulation of receptor function subsequent to transcriptional activation. CHIP is perhaps not physiologically critical for steroid receptor function because CHIP knockout mice develop normally, are fertile, and do not display other phenotypes consistent with defects in steroid receptor function (84); however, this does not preclude that other ubiquitin ligase activities are compensating for loss of CHIP in mutant mice. Again, chaperone participation in receptor accumulation and degradation is another area requiring additional study.
As noted previously, FKBP52 and FKBP51 can potentiate or attenuate, respectively, receptor affinity for hormone. In addition to FKBP effects on hormone-binding affinity, evidence has been presented that the FKBP, as well as CyP40 and PP5, can influence subcellular transport of receptor complexes (reviewed in Ref. 85). FKBP52, CyP40, and PP5 each bind the microtubule-associated dynein motor complex, although FKBP51 does not. Rapid movement of GR from the cytoplasmic to nuclear compartment in response to hormone binding might rely on tethering of receptor complexes to dynein, although a clear demonstration that dynein-binding ability of the cochaperones is strictly related to receptor movements and activity has not been demonstrated. Ideally, one could generate cochaperone point mutants that discretely lose binding to dynein to assess the functional importance of this interaction in cells, but such studies are complicated by the apparent redundancy of dynein binding among at least three TPR cochaperones that are endogenously expressed at relatively high levels in cells. Novel system-oriented studies are needed in each of the above areas, i.e. transcriptional dynamics, proteolytic turnover, and transport, to determine the extent of chaperone influences on receptor behavior in the full cellular context.
COCHAPERONE EXPRESSION/MODIFICATION AND RECEPTOR ACTIVITY
As specific functional roles for chaperones and cochaperones in steroid receptor complexes are coming into focus, one must consider the potential for regulating steroid responsiveness in tissues through expression and modification of relevant chaperones. Hsp90 is expressed at constitutively high levels in cells, likely due to its involvement with so many clients and affiliated pathways; therefore, varying the level of Hsp90 protein is probably not a natural mechanism for selectively regulating receptor activity. However, transient modification of Hsp90 within subcellular compartments could be a mechanism for regulating receptor activity. Hsp90 has long been recognized as a phosphoprotein with multiple phosphorylation sites (5), although functional consequences of differential phosphorylation in regard to steroid receptor activity have not been delineated. Additionally, reversible acetylation of Hsp90 regulates its activity. Inhibition of histone deacetylase 6 promotes acetylation of Hsp90, which inhibits cochaperone interactions and prevents maturation and transcriptional activation of GR (86). Mutation of a relevant acetylation site in Hsp90 also blocks Hsp90 activity, suggesting that acetylation/deacetylation of Hsp90 is critical for Hsp90 function in client complexes (87). Differential acetylation of histones, as well as multiple components of transcription complexes, dynamically regulates gene transcription; therefore, it is not surprising that acetylation of Hsp90, and perhaps Hsp70 (88), adds another layer of regulation to steroid-dependent transcription.
Posttranslational modifications have been identified in several other components of the chaperone machinery. The cochaperone p23, which stabilizes Hsp90 binding to receptor, is readily phosphorylated (89,90). Some members of the Hsp40 gene family can be prenylated (91), potentially altering Hsp40 activity and localization; modification of Hsp40 could influence Hsp40-mediated initiation of receptor complex assembly. Finally, FKBP52, which is critical for some physiological responses to progestins and androgens, has casein kinase II phosphorylation sites (92), one of which is near the PPIase domain involved in potentiation of receptor activity. An initial report provided evidence that phosphorylation of this site could block FKBP52 binding to Hsp90 (92). More recently, evidence was presented that phosphorylation of this site could block receptor potentiation without disrupting Hsp90 binding (93). In the case of FKBP52 as well as other modified chaperones, further investigations are needed to resolve when modifications are physiologically placed, and in response to what signals, as a means for altering hormonal signaling.
In addition to posttranslational modification of chaperones, patterns of chaperone expression could also influence receptor activity. In one study of adult male mouse tissues (56), four androgen target organs were compared for relative protein expression levels of nine receptor-associated chaperones; although all nine chaperones were detected in each organ, the pattern of expression levels was unique for each organ. It seems reasonable to speculate that AR activity, and that of other chaperone client proteins, differs based on the chaperone cohort in that particular organ. Additionally, altering the relative concentrations of cochaperones would be expected to shift the balance of Hsp90 complexes, which could indirectly affect clients and processes that are sensitive to particular Hsp90 complexes.
One of the early reports on FKBP52 in GR complexes noted that FKBP52 expression is heat shock inducible (94) (the designation for FKBP52 in this and several following publications was Hsp56). Presumably, elevated levels of FKBP52, which potentiates GR activity, would complement physiological stress responses stimulated by glucocorticoid release. In another example, FKBP51 gene expression is highly inducible by glucocorticoids, progestins, or androgens (Ref. 95 and references therein); because FKBP51 can inhibit receptor activity or antagonize FKBP52-dependent potentiation, hormone-inducible expression of FKBP51 would appear to be a feedback mechanism for down-modulating tissue responses to secondary hormone exposures (54). These observations hint at the potential importance of chaperone expression and activity as determinants of cellular responses to steroid hormones in different physiological and tissue contexts. The paucity of research in these physiological contexts limits our biological understanding of chaperone-steroid receptor interactions.
POTENTIAL FOR TARGETING RECEPTOR-ASSOCIATED CHAPERONES
Because chaperones are critical for steroid receptor function, targeting individual chaperones holds prospects for clinically modifying tissue responses to steroid hormones. Several chaperones are current drug targets and others are under study. Hsp90 is an established drug target, and the Hsp90 inhibitor geldanamycin effectively blocks hormone-binding ability of some steroid receptors and promotes receptor degradation (68,69,96). However, geldanamycin and other classes of Hsp90 inhibitor are not selective toward steroid receptors and generally heighten proteolytic degradation of the whole spectrum of Hsp90 client proteins (Ref. 97; for a recent review see Ref. 98). Hsp90 inhibitors are showing promise in cancer clinical trials where they appear to act primarily by inducing degradation of cancer-related kinase clients of Hsp90 (99).
The multitude of Hsp90 cochaperones (now more than 20) are believed to provide the Hsp90 machine with additional activities needed for a particular client or subgroup of clients. Thus, the development of drugs that target a specific cochaperone may provide a selective manipulation of certain clients while allowing the functioning of Hsp90 for other purposes, and thus, reducing toxicity. A fairly established example concerns the members of the FKBP family of proteins that are the immediate targets for immunosuppressive drugs related to FK506 and rapamycin, widely used in transplantation and autoimmune disorders. Although receptor-associated FKBP52 and FKBP51 do not appear to be the most relevant family members for immunosuppression, FK506 binds the PPIase domain of these proteins, and FK506 has been shown to block FKBP52-dependent potentiation of steroid receptor activity (49). Cyclosporin A is another important immunosuppressive drug that targets members of the cyclophilin family of PPIase such as receptor-associated CyP40. In cellular studies, FK506 or cyclosporine A has been reported to have a mixture of effects on steroid-induced reporter gene expression (50,100,101,102), but it is unclear what portion of drug effect is through receptor-associated cochaperones as opposed to indirect effects through non-receptor-bound FKBP and cyclophilins. Still, the well-established drugability of FKBP family members and the observation that FK506 can inhibit FKBP52 actions in cellular models of steroid receptor function provide an at-tractive rationale to pursue novel compounds that selectively block FKBP52 activity without inducing immunosuppression. Similar efforts toward other cochaperone proteins have not been made, and this remains an area worthy of further investigation.
CLOSING
Much progress has been made over the past 20 yr toward characterizing molecular chaperones and parsing out their roles in steroid receptor function. At the same time, vast attention has been devoted to other mechanisms by which steroid receptors activate or inhibit gene transcription. Surprisingly, there has been relatively little cross talk between these two fields of investigation. Clearly, the time is right, and perhaps somewhat tardy, for novel experimental paradigms that better incorporate chaperone actions with transcriptional and nongenomic signaling through steroid receptors. We have attempted to highlight some of the main aspects of chaperone/steroid receptor research that are key and in need of investigation and resolution. Although we have learned much, this field is at a stage where it still seems to generate more questions than answers. In this review we hope to have stimulated the imagination of others and encouraged their participation in this very rewarding field of enquiry.
Acknowledgments
We thank the anonymous reviewers for helpful comments and regret that format limitations prevented citation of many relevant publications.
Footnotes
Recent work in the authors’ laboratories has been supported by National Institutes of Health Grants R01-DK44923 (to D.F.S.), R01-DK48218 (to D.F.S.), and R01-DK46249 (to D.O.T.) and by the Mayo Foundation.
Disclosure Statement: The authors have nothing to disclose.
First Published Online May 1, 2008
Abbreviations: AR, Androgen receptor; CHIP, carboxyl terminus of Hsp70-interacting protein; CyP, cyclophilin; ER, estrogen receptor; FKBP, FK506 binding protein; GR, glucocorticoid receptor; Hsp, heat shock protein; LBD, ligand-binding domain; MR, mineralocorticoid receptor; PP5, protein phosphatase 5; PPIase, peptidyl-prolyl isomerase; PR, progesterone receptor; TPR, tetratricopeptide repeat.
References
- Joab I, Radanyi C, Renoir M, Buchou T, Catelli MG, Binart N, Mester J, Baulieu EE 1984 Common non-hormone binding component in non-transformed chick oviduct receptors of four steroid hormones. Nature 308:850–853 [DOI] [PubMed] [Google Scholar]
- Catelli MG, Binart N, Jung-Testas I, Renoir JM, Baulieu EE, Feramisco JR, Welch WJ 1985 The common 90-kd protein component of non-transformed ‘8S’ steroid receptors is a heat-shock protein. EMBO J 4:3131–3135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez ER, Toft DO, Schlesinger MJ, Pratt WB 1985 Evidence that the 90-kDa phosphoprotein associated with the untransformed L-cell glucocorticoid receptor is a murine heat shock protein. J Biol Chem 260:12398–12401 [PubMed] [Google Scholar]
- Schuh S, Yonemoto W, Brugge J, Bauer VJ, Riehl RM, Sullivan WP, Toft DO 1985 A 90,000-dalton binding protein common to both steroid receptors and the Rous sarcoma virus transforming protein, pp60v-src. J Biol Chem 260:14292–14296 [PubMed] [Google Scholar]
- Brugge JS 1986 Interaction of the Rous sarcoma virus protein pp60src with the cellular proteins pp50 and pp90. Curr Top Microbiol Immunol 123:1–22 [DOI] [PubMed] [Google Scholar]
- Pelham HR 1986 Speculations on the functions of the major heat shock and glucose-regulated proteins. Cell 46:959–961 [DOI] [PubMed] [Google Scholar]
- Wickner S, Hoskins J, McKenney K 1991 Function of DnaJ and DnaK as chaperones in origin-specific DNA binding by RepA. Nature 350:165–167 [DOI] [PubMed] [Google Scholar]
- Wiech H, Buchner J, Zimmermann R, Jakob U 1992 Hsp90 chaperones protein folding in vitro. Nature 358:169–170 [DOI] [PubMed] [Google Scholar]
- Bose S, Weikl T, Bugl H, Buchner J 1996 Chaperone function of Hsp90-associated proteins. Science 274:1715–1717 [DOI] [PubMed] [Google Scholar]
- Freeman BC, Morimoto RI 1996 The human cytosolic molecular chaperones hsp90, hsp70 (hsc70) and hdj-1 have distinct roles in recognition of a non-native protein and protein refolding. EMBO J 15:2969–2979 [PMC free article] [PubMed] [Google Scholar]
- Escriva H, Bertrand S, Laudet V 2004 The evolution of the nuclear receptor superfamily. Essays Biochem 40:11–26 [DOI] [PubMed] [Google Scholar]
- Bain DL, Heneghan AF, Connaghan-Jones KD, Miura MT 2007 Nuclear receptor structure: implications for function. Annu Rev Physiol 69:201–220 [DOI] [PubMed] [Google Scholar]
- Pratt WB, Jolly DJ, Pratt DV, Hollenberg SM, Giguere V, Cadepond FM, Schweizer-Groyer G, Catelli MG, Evans RM, Baulieu EE 1988 A region in the steroid binding domain determines formation of the non-DNA-binding, 9 S glucocorticoid receptor complex. J Biol Chem 263:267–273 [PubMed] [Google Scholar]
- Scherrer LC, Picard D, Massa E, Harmon JM, Simons Jr SS, Yamamoto KR, Pratt WB 1993 Evidence that the hormone binding domain of steroid receptors confers hormonal control on chimeric proteins by determining their hormone-regulated binding to heat-shock protein 90. Biochemistry 32:5381–5386 [DOI] [PubMed] [Google Scholar]
- Holley SJ, Yamamoto KR 1995 A role for Hsp90 in retinoid receptor signal transduction. Mol Biol Cell 6:1833–1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig TA, Lutz WH, Kumar R 1999 Association of prokaryotic and eukaryotic chaperone proteins with the human 1α,25-dihydroxyvitamin D3 receptor. Biochem Biophys Res Commun 260:446–452 [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Sueyoshi T, Inoue K, Moore R, Negishi M 2003 Cytoplasmic accumulation of the nuclear receptor CAR by a tetratricopeptide repeat protein in HepG2 cells. Mol Pharmacol 64:1069–1075 [DOI] [PubMed] [Google Scholar]
- Sumanasekera WK, Tien ES, Turpey R, Vanden Heuvel JP, Perdew GH 2003 Evidence that peroxisome proliferator-activated receptor α is complexed with the 90-kDa heat shock protein and the hepatitis virus B X-associated protein 2. J Biol Chem 278:4467–4473 [DOI] [PubMed] [Google Scholar]
- Yoshinari K, Kobayashi K, Moore R, Kawamoto T, Negishi M 2003 Identification of the nuclear receptor CAR:HSP90 complex in mouse liver and recruitment of protein phosphatase 2A in response to phenobarbital. FEBS Lett 548:17–20 [DOI] [PubMed] [Google Scholar]
- Squires EJ, Sueyoshi T, Negishi M 2004 Cytoplasmic localization of pregnane X receptor and ligand-dependent nuclear translocation in mouse liver. J Biol Chem 279:49307–49314 [DOI] [PubMed] [Google Scholar]
- Angelo G, Lamon-Fava S, Sonna LA, Lindauer ML, Wood RJ 2008 Heat shock protein 90β: a novel mediator of vitamin D action. Biochem Biophys Res Commun 367:578–583 [DOI] [PubMed] [Google Scholar]
- Dalman FC, Sturzenbecker LJ, Levin AA, Lucas DA, Perdew GH, Petkovitch M, Chambon P, Grippo JF, Pratt WB 1991 Retinoic acid receptor belongs to a subclass of nuclear receptors that do not form “docking” complexes with hsp90. Biochemistry 30:5605–5608 [DOI] [PubMed] [Google Scholar]
- Picard D, Salser SJ, Yamamoto KR 1988 A movable and regulable inactivation function within the steroid binding domain of the glucocorticoid receptor. Cell 54:1073–1080 [DOI] [PubMed] [Google Scholar]
- Scherrer LC, Dalman FC, Massa E, Meshinchi S, Pratt WB 1990 Structural and functional reconstitution of the glucocorticoid receptor-hsp90 complex. J Biol Chem 265:21397–21400 [PubMed] [Google Scholar]
- Smith DF 1993 Dynamics of heat shock protein 90-progesterone receptor binding and the disactivation loop model for steroid receptor complexes. Mol Endocrinol 7:1418–1429 [DOI] [PubMed] [Google Scholar]
- Picard D, Khursheed B, Garabedian MJ, Fortin MG, Lindquist S, Yamamoto KR 1990 Reduced levels of hsp90 compromise steroid receptor action in vivo. Nature 348:166–168 [DOI] [PubMed] [Google Scholar]
- Aumais JP, Lee HS, Lin R, White JH 1997 Selective interaction of hsp90 with an estrogen receptor ligand-binding domain containing a point mutation. J Biol Chem 272:12229–12235 [DOI] [PubMed] [Google Scholar]
- Alnemri ES, Litwack G 1993 The steroid binding domain influences intracellular solubility of the baculovirus overexpressed glucocorticoid and mineralocorticoid receptors. Biochemistry 32:5387–5393 [DOI] [PubMed] [Google Scholar]
- Nemoto T, Ohara-Nemoto Y, Sato N, Ota M 1993 Dual roles of 90-kDa heat shock protein in the function of the mineralocorticoid receptor. J Biochem (Tokyo) 113:769–775 [PubMed] [Google Scholar]
- Fang Y, Fliss AE, Robins DM, Caplan AJ 1996 Hsp90 regulates androgen receptor hormone binding affinity in vivo. J Biol Chem 271:28697–28702 [DOI] [PubMed] [Google Scholar]
- Ricketson D, Hostick U, Fang L, Yamamoto KR, Darimont BD 2007 A conformational switch in the ligand-binding domain regulates the dependence of the glucocorticoid receptor on Hsp90. J Mol Biol 368:729–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers SA, Fares MA 2007 Functional coevolutionary networks of the Hsp70-Hop-Hsp90 system revealed through computational analyses. Mol Biol Evol 24:1032–1044 [DOI] [PubMed] [Google Scholar]
- Dittmar KD, Hutchison KA, Owens-Grillo JK, Pratt WB 1996 Reconstitution of the steroid receptor·hsp90 heterocomplex assembly system of rabbit reticulocyte lysate. J Biol Chem 271:12833–12839 [DOI] [PubMed] [Google Scholar]
- Kosano H, Stensgard B, Charlesworth MC, McMahon N, Toft D 1998 The assembly of progesterone receptor-hsp90 complexes using purified proteins. J Biol Chem 273:32973–32979 [DOI] [PubMed] [Google Scholar]
- Hernandez MP, Chadli A, Toft DO 2002 HSP40 binding is the first step in the HSP90 chaperoning pathway for the progesterone receptor. J Biol Chem 277:11873–11881 [DOI] [PubMed] [Google Scholar]
- Cintron NS, Toft D 2006 Defining the requirements for Hsp40 and Hsp70 in the Hsp90 chaperone pathway. J Biol Chem 281:26235–26244 [DOI] [PubMed] [Google Scholar]
- Chen S, Prapapanich V, Rimerman RA, Honore B, Smith DF 1996 Interactions of p60, a mediator of progesterone receptor assembly, with heat shock proteins hsp90 and hsp70. Mol Endocrinol 10:682–693 [DOI] [PubMed] [Google Scholar]
- Hutchison KA, Stancato LF, Owens-Grillo JK, Johnson JL, Krishna P, Toft DO, Pratt WB 1995 The 23-kDa acidic protein in reticulocyte lysate is the weakly bound component of the hsp foldosome that is required for assembly of the glucocorticoid receptor into a functional heterocomplex with hsp90. J Biol Chem 270:18841–18847 [DOI] [PubMed] [Google Scholar]
- Johnson JL, Toft DO 1995 Binding of p23 and hsp90 during assembly with the progesterone receptor. Mol Endocrinol 9:670–678 [DOI] [PubMed] [Google Scholar]
- Grad I, McKee TA, Ludwig SM, Hoyle GW, Ruiz P, Wurst W, Floss T, Miller III CA, Picard D 2006 The Hsp90 co-chaperone p23 is essential for perinatal survival. Mol Cell Biol 26:8976–8983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole TJ, Blendy JA, Monaghan AP, Krieglstein K, Schmid W, Aguzzi A, Fantuzzi G, Hummler E, Unsicker K, Schutz G 1995 Targeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maturation. Genes Dev 9:1608–1621 [DOI] [PubMed] [Google Scholar]
- Grenert JP, Sullivan WP, Fadden P, Haystead TAJ, Clark J, Mimnaugh E, Krutzsch H, Ochel HJ, Schulte TW, Sausville E, Neckers LM, Toft DO 1997 The amino-terminal domain of heat shock protein 90 (hsp90) that binds geldanamycin is an ATP/ADP switch domain that regulates hsp90 conformation. J Biol Chem 272:23843–23850 [DOI] [PubMed] [Google Scholar]
- Panaretou B, Prodromou C, Roe SM, O’Brien R, Ladbury JE, Piper PW, Pearl LH 1998 ATP binding and hydrolysis are essential to the function of the Hsp90 molecular chaperone in vivo. EMBO J 17:4829–4836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermann WM, Sondermann H, Russo AA, Pavletich NP, Hartl FU 1998 In vivo function of Hsp90 is dependent on ATP binding and ATP hydrolysis. J Cell Biol 143:901–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DF, Stensgard BA, Welch WJ, Toft DO 1992 Assembly of progesterone receptor with heat shock proteins and receptor activation are ATP mediated events. J Biol Chem 267:1350–1356 [PubMed] [Google Scholar]
- Barent RL, Nair SC, Carr DC, Ruan Y, Rimerman RA, Fulton J, Zhang Y, Smith DF 1998 Analysis of FKBP51/FKBP52 chimeras and mutants for Hsp90 binding and association with progesterone receptor complexes. Mol Endocrinol 12:342–354 [DOI] [PubMed] [Google Scholar]
- Silverstein AM, Galigniana MD, Chen MS, Owens-Grillo JK, Chinkers M, Pratt WB 1997 Protein phosphatase 5 is a major component of glucocorticoid receptor·hsp90 complexes with properties of an FK506-binding immunophilin. J Biol Chem 272:16224–16230 [DOI] [PubMed] [Google Scholar]
- Ratajczak T, Hlaing J, Brockway MJ, Hahnel R 1990 Isolation of untransformed bovine estrogen receptor without molybdate stabilization. J Steroid Biochem 35:543–553 [DOI] [PubMed] [Google Scholar]
- Riggs DL, Roberts PJ, Chirillo SC, Cheung-Flynn J, Prapapanich V, Ratajczak T, Gaber R, Picard D, Smith DF 2003 The Hsp90-binding peptidylprolyl isomerase FKBP52 potentiates glucocorticoid signaling in vivo. EMBO J 22:1158–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies TH, Ning YM, Sanchez ER 2005 Differential control of glucocorticoid receptor hormone-binding function by tetratricopeptide repeat (TPR) proteins and the immunosuppressive ligand FK506. Biochemistry 44:2030–2038 [DOI] [PubMed] [Google Scholar]
- Tranguch S, Cheung-Flynn J, Daikoku T, Prapapanich V, Cox MB, Xie H, Wang H, Das SK, Smith DF, Dey SK 2005 Cochaperone immunophilin FKBP52 is critical to uterine receptivity for embryo implantation. Proc Natl Acad Sci USA 102:14326–14331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs DL, Cox MB, Tardif HL, Hessling M, Buchner J, Smith DF 2007 Noncatalytic role of the FKBP52 peptidyl-prolyl isomerase domain in the regulation of steroid hormone signaling. Mol Cell Biol 27:8658–8669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo LI, Ghini AA, Pilipuk GP, Galigniana MD 2007 Differential recruitment of tetratricorpeptide repeat domain immunophilins to the mineralocorticoid receptor influences both heat-shock protein 90-dependent retrotransport and hormone-dependent transcriptional activity. Biochemistry 46:14044–14057 [DOI] [PubMed] [Google Scholar]
- Reynolds PD, Ruan Y, Smith DF, Scammell JG 1999 Glucocorticoid resistance in the squirrel monkey is associated with overexpression of the immunophilin FKBP51. J Clin Endocrinol Metab 84:663–669 [DOI] [PubMed] [Google Scholar]
- Denny WB, Valentine DL, Reynolds PD, Smith DF, Scammell JG 2000 Squirrel monkey immunophilin FKBP51 is a potent inhibitor of glucocorticoid receptor binding. Endocrinology 141:4107–4113 [DOI] [PubMed] [Google Scholar]
- Cheung-Flynn J, Prapapanich V, Cox MB, Riggs DL, Suarez-Quian C, Smith DF 2005 Physiological role for the cochaperone FKBP52 in androgen receptor signaling. Mol Endocrinol 19:1654–1666 [DOI] [PubMed] [Google Scholar]
- Yong W, Yang Z, Periyasamy S, Chen H, Yucel S, Li W, Lin LY, Wolf IM, Cohn MJ, Baskin LS, Sanchez ER, Shou W 2007 Essential role for co-chaperone Fkbp52 but not Fkbp51 in androgen receptor-mediated signaling and physiology. J Biol Chem 282:5026–5036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Wolf IM, Chen H, Periyasamy S, Chen Z, Yong W, Shi S, Zhao W, Xu J, Srivastava A, Sanchez ER, Shou W 2006 FK506-binding protein 52 is essential to uterine reproductive physiology controlled by the progesterone receptor A isoform. Mol Endocrinol 20:2682–2694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranguch S, Wang H, Daikoku T, Xie H, Smith DF, Dey SK 2007 FKBP52 deficiency-conferred uterine progesterone resistance is genetic background and pregnancy stage specific. J Clin Invest 117:1824–1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP, Renquist D, Brandon D, Eil C, Pugeat M, Vigersky R, Cutler Jr GB, Loriaux DL, Lipsett MB 1982 Glucocorticoid hormone resistance during primate evolution: receptor-mediated mechanisms. Proc Natl Acad Sci USA 79:2036–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP, Loriaux DL, Tomita M, Brandon DD, Renquist D, Albertson B, Lipsett MB 1986 The New World primates as animal models of glucocorticoid resistance. Adv Exp Med Biol 196:129–144 [DOI] [PubMed] [Google Scholar]
- Scammell JG, Denny WB, Valentine DL, Smith DF 2001 Overexpression of the FK506-binding immunophilin FKBP51 is the common cause of glucocorticoid resistance in three New World primates. Gen Comp Endocrinol 124:152–165 [DOI] [PubMed] [Google Scholar]
- Chen MS, Silverstein AM, Pratt WB, Chinkers M 1996 The tetratricopeptide repeat domain of protein phosphatase 5 mediates binding to glucocorticoid receptor heterocomplexes and acts as a dominant negative mutant. J Biol Chem 271:32315–32320 [DOI] [PubMed] [Google Scholar]
- Dean DA, Urban G, Aragon IV, Swingle M, Miller B, Rusconi S, Bueno M, Dean NM, Honkanen RE 2001 Serine/threonine protein phosphatase 5 (PP5) participates in the regulation of glucocorticoid receptor nucleocytoplasmic shuttling. BMC Cell Biol 2:6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K, Ogawa S, Tsukui T, Horie-Inoue K, Ouchi Y, Kato S, Muramatsu M, Inoue S 2004 Protein phosphatase 5 is a negative regulator of estrogen receptor-mediated transcription. Mol Endocrinol 18:1131–1143 [DOI] [PubMed] [Google Scholar]
- Smith DF 2004 Tetratricopeptide repeat cochaperones in steroid receptor complexes. Cell Stress Chaperones 9:109–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Te J, Jia L, Rogers J, Miller A, Hartson SD 2007 Novel subunits of the mammalian Hsp90 signal transduction chaperone. J Proteome Res 6:1963–1973 [DOI] [PubMed] [Google Scholar]
- Smith DF, Whitesell L, Nair SC, Chen S, Prapapanich V, Rimerman RA 1995 Progesterone receptor structure and function altered by geldanamycin, an hsp90-binding agent. Mol Cell Biol 15:6804–6812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitesell L, Cook P 1996 Stable and specific binding of heat shock protein 90 by geldanamycin disrupts glucocorticoid receptor function in intact cells. Mol Endocrinol 10:705–712 [DOI] [PubMed] [Google Scholar]
- Czar MJ, Galigniana MD, Silverstein AM, Pratt WB 1997 Geldanamycin, a heat shock protein 90-binding benzoquinone ansamycin, inhibits steroid-dependent translocation of the glucocorticoid receptor from the cytoplasm to the nucleus. Biochemistry 36:7776–7785 [DOI] [PubMed] [Google Scholar]
- Liu J, DeFranco DB 1999 Chromatin recycling of glucocorticoid receptors: implications for multiple roles of heat shock protein 90. Mol Endocrinol 13:355–365 [DOI] [PubMed] [Google Scholar]
- Thackray VG, Toft DO, Nordeen SK 2003 Novel activation step required for transcriptional competence of progesterone receptor on chromatin templates. Mol Endocrinol 17:2543–2553 [DOI] [PubMed] [Google Scholar]
- Yang J, DeFranco DB 1996 Assessment of glucocorticoid receptor-heat shock protein 90 interactions in vivo during nucleocytoplasmic trafficking. Mol Endocrinol 10:3–13 [DOI] [PubMed] [Google Scholar]
- Elbi C, Walker DA, Romero G, Sullivan WP, Toft DO, Hager GL, DeFranco DB 2004 Molecular chaperones function as steroid receptor nuclear mobility factors. Proc Natl Acad Sci USA 101:2876–2881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman BC, Yamamoto KR 2002 Disassembly of transcriptional regulatory complexes by molecular chaperones. Science 296:2232–2235 [DOI] [PubMed] [Google Scholar]
- Stavreva DA, Muller WG, Hager GL, Smith CL, McNally JG 2004 Rapid glucocorticoid receptor exchange at a promoter is coupled to transcription and regulated by chaperones and proteasomes. Mol Cell Biol 24:2682–2697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijsing SH, Elbi C, Luecke HF, Hager GL, Yamamoto KR 2007 The ligand binding domain controls glucocorticoid receptor dynamics independent of ligand release. Mol Cell Biol 27:2442–2451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio A, Piccolo D, Castoria G, Di Domenico M, Bilancio A, Lombardi M, Gong W, Beato M, Auricchio F 1998 Activation of the Src/p21ras/Erk pathway by progesterone receptor via cross-talk with estrogen receptor. EMBO J 17:2008–2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonyaratanakornkit V, Scott MP, Ribon V, Sherman L, Anderson SM, Maller JL, Miller WT, Edwards DP 2001 Progesterone receptor contains a proline-rich motif that directly interacts with SH3 domains and activates c-Src family tyrosine kinases. Mol Cell 8:269–280 [DOI] [PubMed] [Google Scholar]
- Shupnik MA 2004 Crosstalk between steroid receptors and the c-Src-receptor tyrosine kinase pathways: implications for cell proliferation. Oncogene 23:7979–7989 [DOI] [PubMed] [Google Scholar]
- Xu Y, Singer MA, Lindquist S 1999 Maturation of the tyrosine kinase c-src as a kinase and as a substrate depends on the molecular chaperone Hsp90. Proc Natl Acad Sci USA 96:109–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel NL, Moore NL 2007 Steroid receptor phosphorylation: a key modulator of multiple receptor functions. Mol Endocrinol 21:2311–2319 [DOI] [PubMed] [Google Scholar]
- Connell P, Ballinger CA, Jiang J, Wu Y, Thompson LJ, Hohfeld J, Patterson C 2001 The co-chaperone CHIP regulates protein triage decisions mediated by heat-shock proteins. Nat Cell Biol 3:93–96 [DOI] [PubMed] [Google Scholar]
- Dai Q, Zhang C, Wu Y, McDonough H, Whaley RA, Godfrey V, Li HH, Madamanchi N, Xu W, Neckers L, Cyr D, Patterson C 2003 CHIP activates HSF1 and confers protection against apoptosis and cellular stress. EMBO J 22:5446–5458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt WB, Galigniana MD, Harrell JM, DeFranco DB 2004 Role of hsp90 and the hsp90-binding immunophilins in signalling protein movement. Cell Signal 16:857–872 [DOI] [PubMed] [Google Scholar]
- Kovacs JJ, Murphy PJ, Gaillard S, Zhao X, Wu JT, Nicchitta CV, Yoshida M, Toft DO, Pratt WB, Yao TP 2005 HDAC6 regulates Hsp90 acetylation and chaperone-dependent activation of glucocorticoid receptor. Mol Cell 18:601–607 [DOI] [PubMed] [Google Scholar]
- Scroggins BT, Robzyk K, Wang D, Marcu MG, Tsutsumi S, Beebe K, Cotter RJ, Felts S, Toft D, Karnitz L, Rosen N, Neckers L 2007 An acetylation site in the middle domain of Hsp90 regulates chaperone function. Mol Cell 25:151–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wang SY, Zhang XH, Zhao M, Hou CM, Xu YJ, Du ZY, Yu XD 2007 FK228 inhibits Hsp90 chaperone function in K562 cells via hyperacetylation of Hsp70. Biochem Biophys Res Commun 356:998–1003 [DOI] [PubMed] [Google Scholar]
- Johnson JL, Beito TG, Krco CJ, Toft DO 1994 Characterization of a novel 23-kilodalton protein of unactive progesterone receptor complexes. Mol Cell Biol 14:1956–1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Nakatani Y, Tanioka T, Tsujimoto M, Nakajo S, Nakaya K, Murakami M, Kudo I 2004 Regulation of cytosolic prostaglandin E synthase by phosphorylation. Biochem J 381:59–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan AJ, Tsai J, Casey PJ, Douglas MG 1992 Farnesylation of YDJ1p is required for function at elevated growth temperatures in Saccharomyces cerevisiae. J Biol Chem 267:18890–18895 [PubMed] [Google Scholar]
- Miyata Y, Chambraud B, Radanyi C, Leclerc J, Lebeau MC, Renoir JM, Shirai R, Catelli MG, Yahara I, Baulieu EE 1997 Phosphorylation of the immunosuppressant FK506-binding protein FKBP52 by casein kinase II: regulation of HSP90-binding activity of FKBP52. Proc Natl Acad Sci USA 94:14500–14505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox MB, Riggs DL, Hessling M, Schumacher F, Buchner J, Smith DF 2007 FK506-binding protein 52 phosphorylation: a potential mechanism for regulating steroid hormone receptor activity. Mol Endocrinol 21:2956–2967 [DOI] [PubMed] [Google Scholar]
- Sanchez ER 1990 Hsp56: a novel heat shock protein associated with untransformed steroid receptor complexes. J Biol Chem 265:22067–22070 [PubMed] [Google Scholar]
- Hubler TR, Denny WB, Valentine DL, Cheung-Flynn J, Smith DF, Scammell JG 2003 The FK506-binding immunophilin FKBP51 is transcriptionally regulated by progestin and attenuates progestin responsiveness. Endocrinology 144:2380–2387 [DOI] [PubMed] [Google Scholar]
- Bamberger CM, Wald M, Bamberger AM, Schulte HM 1997 Inhibition of mineralocorticoid and glucocorticoid receptor function by the heat shock protein 90-binding agent geldanamycin. Mol Cell Endocrinol 131:233–240 [DOI] [PubMed] [Google Scholar]
- Whitesell L, Mimnaugh EG, De Costa B, Myers CE, Neckers LM 1994 Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc Natl Acad Sci USA 91:8324–8328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckers L 2007 Heat shock protein 90: the cancer chaperone. J Biosci 32:517–530 [DOI] [PubMed] [Google Scholar]
- Workman P, Burrows F, Neckers L, Rosen N 2007 Drugging the cancer chaperone HSP90: combinatorial therapeutic exploitation of oncogene addiction and tumor stress. Ann NY Acad Sci 1113:202–216 [DOI] [PubMed] [Google Scholar]
- Milad M, Sullivan W, Diehl E, Altmann M, Nordeen S, Edwards DP, Toft DO 1995 Interaction of the progesterone receptor with binding proteins for FK506 and cyclosporin A. Mol Endocrinol 9:838–847 [DOI] [PubMed] [Google Scholar]
- Ratajczak T, Mark PJ, Martin RL, Minchin RF 1996 Cyclosporin A potentiates estradiol-induced expression of the cathepsin D gene in MCF7 breast cancer cells. Biochem Biophys Res Commun 220:208–212 [DOI] [PubMed] [Google Scholar]
- Le Bihan S, Marsaud V, Mercier-Bodard C, Baulieu EE, Mader S, White JH, Renoir JM 1998 Calcium/calmodulin kinase inhibitors and immunosuppressant macrolides rapamycin and FK506 inhibit progestin- and glucocorticosteroid receptor-mediated transcription in human breast cancer T47D cells. Mol Endocrinol 12:986–1001 [DOI] [PubMed] [Google Scholar]