Abstract
Calcium influx through L-type voltage-gated calcium channels (VGCC) is required for ERK activation induced by GnRH in pituitary gonadotropes. The current studies investigate VGCC-sensitive catalytic activities that may lie upstream of ERKs within the GnRH signaling network. Ion exchange fractionation of αT3-1 cell lysates subjected to anti-phosphotyrosine Western blot analysis revealed a nifedipine-sensitive activity that colocalized with proline-rich tyrosine kinase (Pyk) 2 immunoreactivity. Phosphorylated Pyk2 was present in αT3-1 cells after GnRH agonist administration for a time course that lasted up to 4 h. Pyk2 phosphorylation was also evident in gonadotropes in vivo after administration of a bolus of GnRH. Knockdown of Pyk2 using specific small interfering RNAs revealed that Pyk2 contributed to modulation of GnRH-induced ERK but not c-Jun N-terminal kinase activation. Using pharmacological approaches, calmodulin (Cam) was also demonstrated to be required for the phosphorylation of Pyk2. Pyk2 was shown to bind specifically to a Cam agarose affinity column in a calcium-dependent manner, suggesting Cam and Pyk2 are capable of forming a complex. Specific mutation of a putative Cam binding motif within the catalytic domain of Pyk2 blocked association with Cam and uncoupled Pyk2’s ability to activate ERK-dependent gene transcription. Thus, GnRH induces Pyk2 tyrosine phosphorylation dependent upon calcium flux within gonadotropes. Furthermore, association of Pyk2 and Cam may be required to mediate the effects of calcium on Pyk2 phosphorylation and subsequent activation of ERKs by GnRH.
HYPOTHALAMIC SYNTHESIS and secretion of GnRH and expression of the type I GnRH receptor (GnRHR) in pituitary gonadotropes are central to the regulation of the hypothalmo-pituitary-gonadal axis and are required for normal reproductive function in mammals. In the absence of GnRH stimulation to the anterior pituitary, hypogonadism results due to a lack of gonadotropic stimulation to the gonads (1,2,3). The GnRHR is a member of a superfamily of G protein-coupled receptors (GPCR), classically identified by seven transmembrane-spanning domains. However, unlike other GPCRs studied to date, the GnRHR is unique in that following transmembrane domain 7, this receptor does not have an extensive carboxyl-terminal tail extending into the cytosol of the gonadotrope. This observation and subsequent studies supported speculation that this unique tailless attribute of the GnRHR leads to slowed internalization and desensitization kinetics (4,5). Slowed desensitization kinetics relative to tailed GPCRs (such as the type II GnRHR) may also contribute to the complex signaling network induced by GnRH in gonadotrope cell models. GnRH induces the activation of a number of different second messengers and intracellular catalytic activities, including phospholipase Cβ, diacylglycerol, and inositol 1,4,5-trisphosphate, protein kinase C iso-zymes; release of intracellular calcium; and influx of extracellular calcium leading to activation of multiple MAPKs. MAPK activities induced by GnRH include the ERKs 1 and 2, c-Jun N-terminal kinase (JNK), and the p38 MAPK (reviewed in Ref. 6). Key links between these pathways and activation of several genes necessary for the differentiated function of the gonadotrope have emerged over the past decade. These include regulation of the glycoprotein hormone α-subunit gene via putative Ets factors and activating transcription factor-3 (7,8), the LHβ subunit and MKP-2 genes via alterations in early growth response factor (Egr)-1 activity (9,10,11), the FSHβ subunit and the GnRHR genes via regulation by activator protein-1 activity, all potentially linked to ERK and/or JNK activity induced by GnRH (12,13,14,15).
We have explored the regulation of MAPK pathways by GnRH in both the αT3-1 cell model as well as in rat pituitary cells in primary culture (16,17). These studies have led to the hypothesis that calcium flux from both intra- and extracellular stores are critical for the activation of several components of this signaling network, particularly regarding activation of the ERK and JNK modules. Most notable is the specific requirement for influx of extracellular calcium through L-type voltage-gated calcium channels (VGCC) on activation of the ERK cascade. Dihydropyridine receptor antagonists such as nifedipine or acute withdrawal of extracellular calcium were shown to specifically block GnRH-induced ERK (but not JNK) activation in both the αT3-1 cell gonadotrope model and in rat pituitaries in primary culture. The impact of calcium flux on the ERK cascade may be due in part to the apparent association between the calcium-binding protein calmodulin (Cam) and c-Raf kinase, an upstream activator of MAP/ERK kinase (MEK1) and subsequently ERKs (18). Taken together, these data suggested a model whereby localized or spatially restricted calcium signals may be playing a fundamental role in regulation of MAPK pathways within the GnRH signaling network.
The central aim of the current studies was to identify possible catalytic activities capable of mediating the effects of calcium influx through VGCCs on the activation of the ERK pathway by GnRH. These studies revealed that GnRHR stimulation results in calcium- dependent activation of proline-rich tyrosine kinase 2 (Pyk2) in the homologous gonadotrope cell line αT3-1 and in vivo in mouse pituitary gonadotropes. Specific knockdown of Pyk2 using small interfering RNAs (siRNAs) resulted in specific loss of ERK but not JNK activation. Pyk2 was determined to be a Cam-interacting protein, and Cam association with Pyk2 appears to occur via a specific Cam binding site within the catalytic domain of Pyk2.
RESULTS
GnRH Treatment Induces Tyrosine Phosphorylation of Pyk2 in αT3-1 Cells
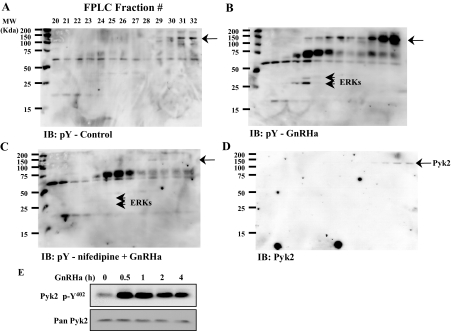
Our previous studies demonstrated that inhibition of L-type VGCCs or withdrawal of extracellular calcium immediately before GnRH agonist (GnRHa) (buserelin) treatment specifically blocked GnRHa-induced ERK activation in αT3-1 cells and rat pituitary cells in primary culture (16,17). We examined the possibility that specific upstream activators of the ERK cascade may be revealed by examining global tyrosine phosphorylation induced by GnRHa in the absence or presence of VGCC blockade by nifedipine. αT3-1 cells were serum starved, pretreated with control solution or nifedipine, and then treated with saline or GnRHa. Whole-cell lysates were prepared and fractionated on Mono Q ion exchange chromatography. Column fractions were then subjected to Western blot analysis using an anti-phosphotyrosine antibody (Fig. 1, A–C). GnRHa induced a number of tyrosine-phosphorylated proteins including ERK1 and -2. Nifedipine pretreatment blocked GnRHa-induced ERK phosphorylation as well as tyrosine phosphorylation of several other proteins including a rather strong activity at approximately 115–120 kDa in fractions 29–32. Because Pyk2 activity has been shown to be regulated by calcium and the molecular mass of Pyk2 was consistent with our observations, blots were stripped and reprobed with a pan-specific anti-Pyk2 monoclonal antibody (Fig. 1D). The nifedipine-sensitive tyrosine-phosphorylated band at 115–120 kDa colocalized with Pyk2 immunoreactivity.
Figure 1.
Detection of Nifedipine-Sensitive Tyrosine Phosphorylation Implicates Pyk2 in the GnRHa Signaling Network in αT3-1 Cells
αT3-1 whole-cell lysates were fractionated by FPLC using an ion-exchange Mono Q column. Before fractionation, cells were treated with control solution (A), GnRHa buserelin (10 nm, 15 min; B), or nifedipine (2 μm) followed by GnRHa (nifedipine + GnRHa; C). Mono Q fractions 20–32 were resolved by SDS-PAGE and subjected to phosphotyrosine immunoblot analysis (IB: pY; A–C). The molecular markers are listed to the left of each panel. Short arrowheads identify tyrosine phosphorylation of ERKs in B and C. In D, blots were stripped and reprobed with antibody against Pyk2. Longer arrows identify tyrosine phosphorylation and colocalization with Pyk2 immunoreactivity. GnRHa treatment (100 nm) induced phosphorylation at Pyk2 Y402 as determined in whole-cell αT3-1 lysates over a 4-h time course (E) using a phosphospecific antibody and Western blot analysis. A Pyk2 antibody (Pan Pyk2) was used to demonstrate equivalent lane loading in E. MW, Molecular weight.
To confirm Pyk2 tyrosine phosphorylation in Mono Q fractions 29–32, fractions were reexamined by Western blot analysis using a phosphospecific antibody recognizing Pyk2 phosphorylated at Y402. GnRHa treatment clearly induced increased Pyk2 Y402 immunoreactivity in these Mono Q fractions compared with similar fractions obtained from saline-treated cells (data not shown). GnRH induced Pyk2 Y402 phosphorylation over a 4-h time course (Fig. 1E), and this effect could be mimicked by addition of a phorbol ester as a direct pharmacological activator of protein kinase C (data not shown).
GnRHa Induces Phosphorylation of Multiple Tyrosine Residues on Pyk2
Structurally, Pyk2 includes a central kinase domain, and tyrosine residues at positions Y402, Y580, and Y881 are key sites of phosphorylation (19). Using a series of phosphospecific antibodies in Western blotting studies, GnRHa administration induced phosphorylation at all three tyrosine residues within Pyk2. Pretreatment with nifedipine abolished Y402 and Y580 phosphorylation and greatly reduced phosphorylation at Y881 (data not shown). Consistent with the effects of nifedipine on GnRHa-induced Pyk2 phosphorylation, isotonic substitution of magnesium for calcium ions in the cell culture media immediately before GnRHa administration also blocked GnRHa-induced phosphorylation of Pyk2 at Y402 (data not shown). This same paradigm also blocked GnRHa-induced ERK phosphorylation as shown previously (16).
GnRHa Treatment Induces Pyk2 Phosphorylation in Mouse Gonadotropes in Vivo
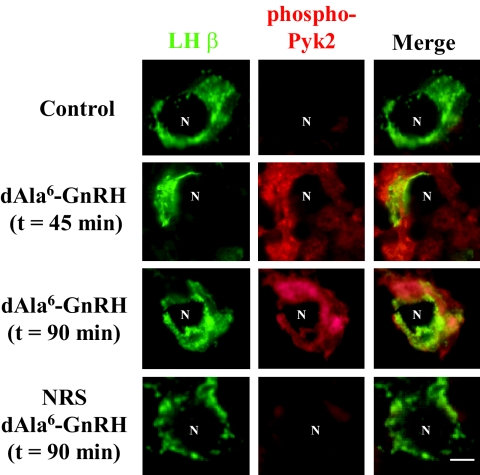
To confirm an in vivo correlate to our αT3-1 cell gonadotrope cell model, we used a GnRH immunoneutralization paradigm to examine the effects of a GnRH analog on Pyk2 phosphorylation in vivo in the context of a physiological milieu (20). Mature female mice were ovariectomized and allowed a 14-d recovery period. Mice were then administered an antiserum against endogenous GnRH. Three days later, mice received either saline injection or 100 ng of a non-immunoreactive analog of GnRH (d-Ala6-GnRH), and mice were killed at 45 and 90 min after injection of GnRHa. Trunk blood was collected at the time of exsanguination and LH measured in a pilot study after 60 min of d-Ala6-GnRH administration. In this preliminary study, mean concentrations of LH in serum at 1 h after saline or d-Ala6-GnRH administration were 0.53 ± 0.31 and 53.2 ± 13.2 ng/ml, respectively. Double-label fluorescent immunocytochemistry studies indicated an increase in phospho-Pyk2 (Y881) immunoreactivity in individual gonadotropes in pituitary sections from animals receiving 45 and 90 min of d-Ala6-GnRH treatment compared with controls (Fig. 2). Phospho-Pyk2 Y881 was compartmentalized primarily to the cytosol and colocalized with LHβ immunoreactivity to identify gonadotrope populations within the anterior pituitary. Preimmune rabbit serum was used as a negative control in lieu of the phospho-Pyk2 (Y881) antiserum. These studies confirm that Pyk2 becomes phosphorylated by GnRHa administration in vivo and supports the utility of the αT3-1 cell model in our studies.
Figure 2.
GnRH Induces Pyk2 Phosphorylation in Vivo
Mature ovariectomized mice received anti-GnRH antiserum (250 μl) ip to immunoneutralize endogenous GnRH. Three days later, immunoneutralized mice received either saline or an analog of GnRH (d-Ala6-GnRH; 100 ng ip) and were killed 45 or 90 min later. Pituitaries were dissected out, fixed, sectioned, and examined for Pyk2 (pY881) phosphorylation or LHβ protein by double-labeled fluorescent immunocytochemistry. The first column depicts labeling for LHβ (green), the second column of images phospho-Pyk2 (pY881; red), and the final column of images is a merge of the two images. The first row depicts results from cells collected from mice receiving saline for 60 min (control). The second and third rows of images depict cells collected 45 and 90 min (respectively) after d-Ala6-GnRH administration. The fourth row of images is immunocytochemical analysis using normal rabbit serum substituted for the phospho-Pyk2 antibody on tissues collected at 90 min after d-Ala6-GnRH treatment. The figure reflects a representative example from three or four mice per treatment. n, Nucleus. Bar, 5 μm.
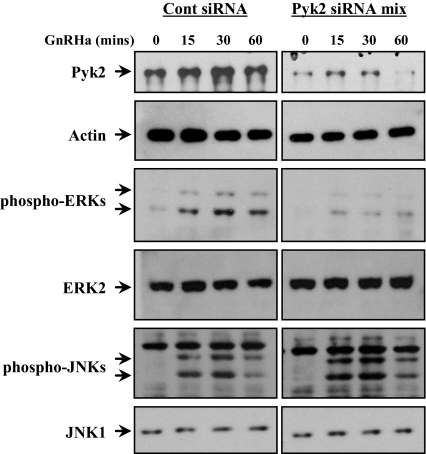
Knockdown of Pyk2 Using siRNA Results in Modulation of GnRHa-Induced ERK Phosphorylation
Thus far, our data suggest the possibility that Pyk2 may potentially lie upstream of the ERK pathway in gonadotropes. In an effort to determine the functional consequence of Pyk2 in the GnRH signaling network, we designed a series of siRNAs against Pyk2. Four stable cell lines were created that overexpressed either a control scrambled siRNA or three distinct siRNAs for mouse Pyk2. All three Pyk2 siRNA cell lines had reduced levels of Pyk2 protein; however, none of these siRNAs used alone produced knockdown greater that 20–30% (data not shown). To increase the efficacy of the Pyk2 knockdown, a stable cell line was created using all three siRNAs as a mixture. The resultant cell line demonstrated Pyk2 knockdown of 52.0 ± 6.0% over all studies (P < 0.05; Fig. 3). Using this cell line (along with the control siRNA cell line), we examined the phosphorylation state of ERK and JNK proteins after GnRHa treatment. GnRHa-induced ERK phosphorylation was blunted (P < 0.05) in the Pyk2 siRNA cell line compared with the control siRNA cell line (Fig. 2). For example, using the control siRNA cell line, ERK phosphorylation increased 10.4-fold at the 30-min time point. ERK phosphorylation induced by GnRHa in the Pyk2 siRNA cell line was increased by 3-fold. In contrast, GnRHa-induced phosphorylation of JNK was not appreciably altered (P > 0.05) comparing these cell lines (Fig. 3). Importantly, control and Pyk2-specific siRNAs did not affect expression levels of actin, ERK2, or JNK1 over all of the experiments, suggesting the effects of the Pyk2 siRNA mixture were specific. These results support the conclusion that specific disruption of Pyk2 protein levels in αT3-1 cells uncouples the ability of the GnRHR to effectively activate the ERK cascade.
Figure 3.
Knockdown of Pyk2 Using siRNA Uncouples GnRHa Stimulation with Activation of the ERK But Not JNK Pathway
Preliminary studies identified three separate Pyk2 siRNAs that all had partial knockdown effects when transfected into αT3-1 cells. A stably transfected population of αT3-1 cells was created using a mixture of all three Pyk2 siRNAs (Pyk2 siRNA mix). A second stable cell line was transfected with a control siRNA. After selection, GnRHa (10 nm) was administered over a 60-min time course to both cell lines, and whole-cell lysates were prepared and subjected to Western blot analysis using antibodies for Pan-Pyk2, actin, phospho-ERKs, ERK2, phospho-JNK, and JNK1. Actin, ERK2, and JNK1 immunoreactivity was used to confirm the specificity of the siRNAs used as well as lane loading controls.
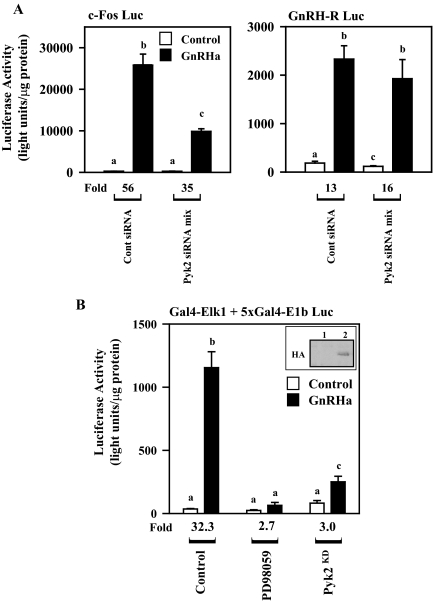
These Pyk2 knockdown studies were further corroborated by transfection studies examining the ability of GnRHa to induce known ERK- and JNK-dependent reporter gene systems (Fig. 4A). The c-Fos gene promoter has been shown to be ERK dependent within the GnRH signaling network (10,16,21), and expression of this reporter gene construct after GnRHa administration was markedly reduced (P < 0.05) in the Pyk2 siRNA cell line (Fig. 4A). In contrast, the GnRHR promoter-luciferase reporter has been shown to be sensitive to JNK pathway activity and relatively insensitive to ERK activity in αT3-1 cells (22). Consistent with these observations, GnRHa-induced (fold) activation of the GnRHR promoter-luciferase reporter was similar in all siRNA cell lines. A reduction in basal expression of the GnRHR luciferase reporter was detected in the Pyk2 siRNA cell line compared with the control cell line (P < 0.05).
Figure 4.
Knockdown of Pyk2 or Overexpression of a Dominant-Negative Pyk2 in αT3-1 Cells Results in Inhibition of ERK- But Not JNK-Dependent Gene Transcription
A, c-Fos and GnRHR luciferase reporter genes (c-Fos-Luc and GnRHR-Luc, respectively; 1 μg/reporter) were transfected into stable cell lines expressing control (Cont siRNA) or Pyk2 (Pyk2 siRNA mix) siRNAs as described in Fig. 3. After transfection, cells received control solution (saline) or GnRHa for 6 h. Transfected cells were then lysed, and luciferase activity was determined per microgram total cell protein. Fold induction relative to saline-treated controls is shown. B, αT3-1 cells were transfected with an expression vector for a Gal4 DNA binding domain-Elk1 fusion protein along with a luciferase reporter containing five copies of the Gal4 binding site cloned upstream of the E1b minimal promoter (Gal4-Elk1 + 5xGal4–E1b Luc; 500 ng of each plasmid). Some transfected cells received dimethylsulfoxide or PD98059 (50 μm) 30 min before administration of either saline (control) or GnRHa for 6 h. Some transfections included a control expression vector (pKH3) or an expression vector for an HA-tagged dominant-negative kinase dead Pyk2 (Pyk2KD; 5 μg). Luciferase activity was assayed as described above. Fold induction relative to control is shown. In addition, expression level of Pyk2KD was determined by Western blot analysis using an HA antibody (see inset: lane 1, pKH3 control plasmid; lane 2, Pyk2KD).
To provide additional corroboration to the Pyk2 siRNA data, studies examined the ability of a dominant-negative form of Pyk2 to interfere with GnRHa-induced expression of a known ERK target, the transcription factor Elk1 (Fig. 4B). GnRHa induced a robust activation of a Gal4-Elk1 fusion protein that was nearly abolished with pretreatment using the MEK1 inhibitor PD98059 (P < 0.05). Overexpression of a dominant-negative form of Pyk2 also reduced (P < 0.05) the ability of GnRH signaling to transactivate the Gal4-Elk1 fusion protein. Combined, these studies provide strong evidence that activation of Pyk2 within the GnRH signaling network is necessary for ERK pathway activation and subsequent ERK-dependent gene promoter activity.
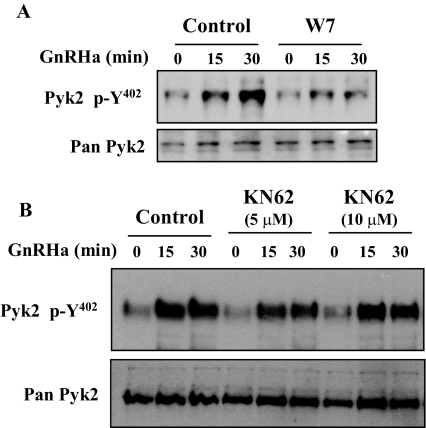
Inhibition of Cam But Not Cam-Dependent Kinase (CamK) II Blocks GnRHa-Induced Pyk2 Phosphorylation in αT3-1 Cells
We have previously demonstrated that pharmacological perturbation of Cam resulted in blockade in GnRH-induced signaling to ERK (18). Because Pyk2 appears to reside within the GnRH pathway leading to ERK activation (Figs. 3 and 4) and Pyk2 is known to be calcium-dependent, it was logical to consider the possibility that Cam may be playing a role as a calcium sensor mediating the effects of VGCC calcium on Pyk2 activity and subsequently ERK phosphorylation. To assess the potential contribution of Cam in the regulation of Pyk2 phosphorylation by GnRHa, W7 (a relatively nonspecific Cam inhibitor) was used to inhibit Cam (Fig. 5A). Pretreatment of αT3-1 cells with W7 for 30 min greatly diminished (P < 0.05) Pyk2 Y402 phosphorylation induced by GnRHa. For example, 30 min after GnRHa administration, ERK phosphorylation was increased 3.0 ± 1.0-fold in control treated cells; GnRHa administration induced a 1.3 ± 0.27-fold increase in cells pretreated with W7. Similar results were obtained using another Cam inhibitor, W13 (data not shown). In contrast, KN62 (a Cam inhibitor with greater specificity toward Cam-dependent protein kinases) did not disrupt GnRHa-induced Pyk2 phosphorylation (Fig. 5B).
Figure 5.
GnRHa-Induced Pyk2 Phosphorylation Is Blocked by Cam Inhibition
αT3-1 cells were pretreated with saline (control) or the Cam inhibitor W7 (15 μm) for 30 min. Cells then received GnRHa for 15 or 30 min, and whole-cell lysates were prepared. Western blot analysis was used to determine the phosphorylation status of Pyk2 (p-Y402). A, The Pan-Pyk2 antibody was used to determine lane loading. B, A similar study was performed except cells were pretreated with increasing doses of the CamK II-specific inhibitor KN62 (5 or 10 μm), and Western blots were used to determine phosphorylation status of Pyk2 as described above.
The Catalytic Domain of Pyk2 Binds to Cam Directly
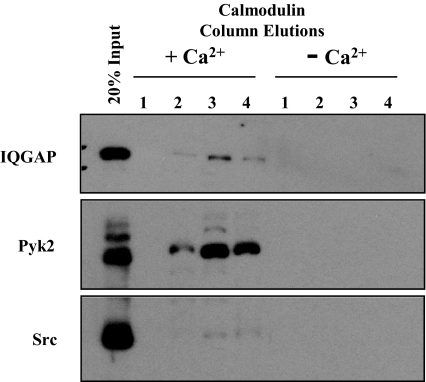
To examine the possibility that Cam and Pyk2 may interact directly, αT3-1 cell lysates were prepared and applied to a Cam agarose affinity column in the absence or presence of calcium. The column was washed extensively and Cam-binding proteins were eluted by chelating calcium within the column. Analysis of the elution fractions revealed the presence of IQ domain-containing GTPase activating protein (IQGAP1) as a positive control (Fig. 6). Notably, Pyk2 was retained on the Cam affinity column in a calcium-dependent manner, whereas the related tyrosine kinase, Src, did not bind to this column, providing evidence that Pyk2 interaction with the Cam affinity column was specific and calcium dependent.
Figure 6.
Pyk2 Binds to a Cam Agarose Affinity Column
αT3-1 whole-cell lysates were prepared (see Materials and Methods) and subjected to Cam agarose affinity chromatography in the presence or absence of Ca2+ followed by column elution with EGTA. Input lysates (20%; positive control for Western blots) and fractions 1–4 were resolved on SDS-PAGE, and Western blot analysis was used to determine the presence of IQGAP, Pyk2, and Src.
To determine whether the activity state of Pyk2 may affect Cam binding, we synthesized recombinant wild-type and mutant forms of full-length Pyk2 with mutations in either the kinase domain (catalytically inactive) or at residue Y402. 35S-labeled proteins were subjected to Cam agarose binding in the absence or presence of calcium. As observed with endogenously expressed Pyk2 derived from αT3-1 cells, recombinant Pyk2 bound Cam agarose in a calcium-dependent manner. All of the forms of Pyk2 tested bound the Cam column in a calcium-dependent manner, suggesting that kinase activity or activation state related to phosphorylation at Y402 does not determine the ability of Pyk2 to form complexes with Cam (data not shown).
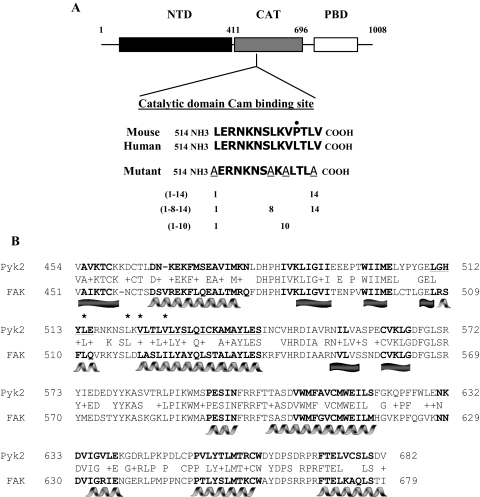
We next investigated the possibility that a putative Cam binding site may be identifiable within the primary amino acid sequence of Pyk2. This search revealed the presence of a potential Cam binding site within the catalytic domain of Pyk2 that was conserved in mouse and human (Fig. 7A). Potential Cam binding sites are generally characterized by IQ domains or the presence of hydrophobic residues within amphiphilic α-helices where the hydrophobic residues are spaced in positions 1–10, 1–14, or 1–16 or some variant of this positioning (as reviewed in Ref. 23). Within the Pyk2 catalytic domain, putative Cam binding sites included a 1–14, 1–8-14, and 1–10 motif (Fig. 7A). Making use of web-based protein structure prediction programs (ProteinPredict and nnPredict), the hydrophobic residues at positions 1, 10, and 14 (positions 514, 523, and 527 of mouse Pyk2) within the putative Cam binding site aligned with predicted α-helices. Although the crystal structure of the catalytic domain of Pyk2 has not been solved, the catalytic domain of a closely related family member, focal adhesion kinase (FAK) has been crystallized and solved (24). Using this as a model, the predicted positioning of α-helices within the catalytic domain of Pyk2 and FAK (using ProteinPredict and nnPredict) were consistent with the crystal structure in the region of the putative Cam binding site (Fig. 7B).
Figure 7.
A Putative Cam Binding Site Is Present within the Catalytic Domain of Pyk2
A, Schematic of mouse Pyk2 highlighting the amino-terminal domain (NTD), the catalytic domain (CAT), and the paxillin-binding domain (PBD). Amino acid sequences for mouse and human Pyk2 were subjected to a web-based search engine to identify potential Cam binding sites (Calmodulin Target Database). The 14 residues shown are about 93% conserved between mouse and human and match potential (1–14; [FILVW]xxxxxxxxxxxx[FILVW]) or (1–8-14; [FILVW]xxxxxx [FAILVW]xxxxx[FILVW]) or (1–10; [FILVW]xxxxxxxx[FILVW]) Cam binding sites. Within these putative sites, alanine was substituted for key hydrophobic residues at positions 1, 8, 10, and 14 (Mutant). B, Alignment of the catalytic domains of human Pyk2 and FAK. This alignment revealed a 64% identity within this region. The positioning of the FAK secondary structures [β-sheets (flat ribbon shape) and α-helices (curled ribbon shape)] based upon crystallography studies (24) is shown. Asterisks identify the positioning of the hydrophobic residues within the putative Cam binding site. The residues shown in bold for Pyk2 and FAK indicate structural predictions using nnPredict and ProteinPredict (ca.expasy.org).
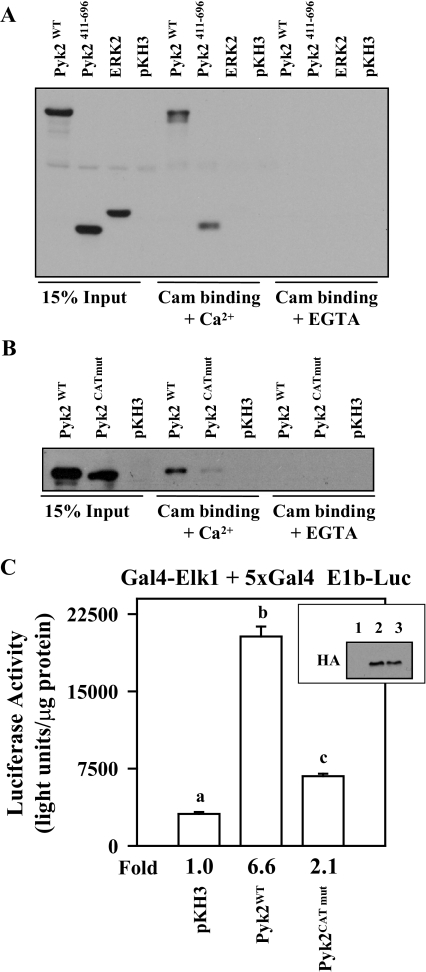
We then determined whether the catalytic domain of Pyk2 alone was sufficient to bind Cam in a calcium-dependent manner. For these studies, αT3-1 cells were transiently transfected with expression vectors for hemagglutinin (HA)-tagged full-length wild-type Pyk2, a truncated form of Pyk2 (Pyk2411–696) that specifically represented the catalytic domain and an expression vector for HA-tagged ERK2 as a negative control (Fig. 8A). Transfected cells were cultured for 24 h, lysed, and subjected to the Cam agarose binding assay described above. As predicted, full-length Pyk2 clearly bound Cam as did the Pyk2 catalytic domain in a calcium-dependent fashion. HA-tagged ERK2 did not bind the Cam agarose. Thus, the catalytic domain of Pyk2 alone was sufficient for Cam interactions. Based upon these studies, we prepared alanine substitution mutations within the putative Cam binding site in the catalytic domain (Fig. 7A) to determine whether disruption of this site in the context of full-length Pyk2 would interfere with Cam binding. Transient transfection overexpression studies were carried out in αT3-1 cells. Substituting alanines for the hydrophobic residues within the putative Cam binding site nearly abolished Cam binding (Fig. 8B). To determine the potential functional consequences of mutations within the Cam binding domain of Pyk2, αT3-1 cells were transfected with the Gal4-Elk1–5xGal4-E1B luciferase reporter system demonstrated earlier to be ERK dependent and sensitive to a kinase dead form of Pyk2 (Fig. 4B). In this system, simple overexpression of wild-type Pyk2 was sufficient to increase Gal4-Elk1 transcriptional activity more than 6-fold, consistent with the observations of others that overexpressed Pyk2 is catalytically active (25). In contrast, full-length Pyk2 harboring the mutation within the Cam binding site in the catalytic domain was not sufficient to induce a similar transcriptional activation. Collectively, these studies provide important evidence that a Cam binding site is present within the catalytic domain of Pyk2 based upon structural models of the closely related FAK. Mutations in the Cam binding site of Pyk2 reduce calcium-dependent Cam binding correlated with a reduction in Pyk2 activation of the ERK signaling pathway.
Figure 8.
Mutations within the Putative Cam Binding Site in the Pyk2 Catalytic Domain Block Cam Binding and Reduce Pyk2 Activity
αT3-1 cells were transfected with expression vectors (2 μg each) for HA-tagged wild-type Pyk2 (Pyk2WT), the Pyk2 catalytic domain (Pyk2411–696), full-length HA-tagged ERK2, or the pKH3 parent vector. Twenty-four hours after transfection, cells were lysed and clarified by centrifugation. A portion of the lysate was used for input (15%), and the remainder was split into two binding reactions with Cam agarose in the presence (+Ca2+) or absence (+EGTA) of calcium. A, The binding reactions were washed, resolved using SDS-PAGE, and Western blotted using the HA antibody. B, A similar overexpression study was carried out in αT3-1 cells using wild-type Pyk2 (Pyk2WT) and full-length Pyk2 with the mutation within the putative Cam binding site (Pyk2CATmut). C, The Gal4-Elk1 + 5xGal4-E1b Luc reporter system (500 ng of each plasmid) was transfected into αT3-1 cells with either pKH3 parent vector (5 μg), wild-type Pyk2 (Pyk2WT; 5 μg) or Pyk2 with the mutation within the putative Cam binding site (Pyk2CATmut; 5 μg). Twenty-four hours later, cells were lysed, and luciferase activity was determined. Aliquots from the transfected and lysed cells were subjected to immunoblot analysis using the HA antibody to determine the expression level of the wild-type and mutant Pyk2 proteins (inset: lane 1, pKH3 transfection; lane 2, Pyk2WT; lane 3, Pyk2CATmut).
DISCUSSION
Our original goal for these studies was to determine the presence of upstream catalytic activities induced by GnRHa administration that were affected by blockade of L-type VGCCs and thus could potentially lie upstream of ERK activation in the gonadotrope αT3-1 cell model. GnRHa was clearly sufficient to induce Pyk2 phosphorylation in a calcium-dependent manner on multiple tyrosine residues. Others have demonstrated GnRH-induced Pyk2 phosphorylation in the GnRH neuron model GT1-7 cells and in a heterologous HEK293 cell model expressing the GnRHR (26,27). In GT1-7 cells, GnRH induced a rapid phosphorylation of Pyk2 that could be mimicked by protein kinase C activation, consistent with the present studies. Because the time course used in the GT1-7 studies did not extend beyond about 30 min, it is presently unclear whether GnRH action induced similar kinetics of Pyk2 phosphorylation seen in the present studies. Pyk2 activity induced by GnRH was critical for ERK activation in GT1-7 cells; however, this mechanisms did not involve GnRHR-dependent shedding of epidermal growth factor isoforms (28). In the heterologous HEK293 cell model where GnRHR is overexpressed, treatment with GnRH induced a rapid phosphorylation of Pyk2 and translocation into the nuclear compartment; however, the role of nuclear Pyk2 is presently not clear. Interestingly, Pyk2 phosphorylation in HEK293 cells did not appear to contribute to ERK activation by GnRH (27). In the present studies, GnRHa induced Pyk2 phosphorylation for an extended time course (up to 4 h), and phosphorylation of Y881 was localized primarily to the cytosol in vivo in pituitary gonadotropes. Consistent with the GT1-7 and HEK293 cell studies, GnRHa-induced Pyk2 phosphorylation in the homologous αT3-1 cell line could be mimicked by the addition of phorbol ester via diacylglycerol-dependent protein kinase C isozymes. Most recently, Pamela Brown and her colleagues (29) provided important new evidence that GnRH activation of the ERK pathway was indeed linked to phosphorylation of Pyk2 in the αT3-1 and LβT2 gonadotrope cell models. The present studies extend these findings in several key areas. First, the present studies provide important corroboration of Pyk2 phosphorylation on multiple specific tyrosine residues in the gonadotrope-derived cell line αT3-1. Our studies provide evidence for the fidelity of these cell lines because Pyk2 appears to be phosphorylated in vivo in response to GnRH administration using our GnRH immunoneutralization mouse model (Fig. 2). Our Pyk2 knockdown studies provide evidence for the role of Pyk2 upstream of the ERK but not JNK modules with the GnRH signaling network (Figs. 3 and 4). Lastly, our studies define a critical interface between the calcium-binding protein Cam and Pyk2 through a discrete interaction within the catalytic domain of Pyk2 (Figs. 6–8).
Our original prediction was that Pyk2 would be an important intermediate effectively linking GnRH-induced ERK activation with influx of extracellular calcium through L-type VGCCs consistent with earlier studies (16,17). A number of studies have implicated Pyk2 as a signaling intermediate upstream of ERKs in a diverse array of cell types. In the central nervous system, Pyk2 is widely expressed and thought to be a functional link between calcium and MAPK signaling (30). In cardiac fibroblasts, angiotensin II induces a calcium/Cam-dependent increase in Pyk2 phosphorylation that is functionally linked to downstream activation of the ras/ERK cascade (31). Our initial studies examining the effects of nifedipine on influx of calcium through VGCCs and Pyk2 phosphorylation induced by GnRHa were consistent with the hypothesis that Pyk2 was potentially upstream of ERK activation in the GnRH signaling network.
The specific mechanism(s) by which calcium mediates Pyk2 activation is not currently clear. Activation of Pyk2 appears to involve a complex mechanism of trans-acting autophosphorylation of Y402, followed by phosphorylation on additional tyrosine residues that lead to full activation of Pyk2, capable of phosphorylation of downstream substrates (32). In the present studies, GnRH-induced Pyk2 phosphorylation in αT3-1 cells clearly required mobilization of cell calcium and could be blocked by Cam inhibitors. We reasoned that Cam may serve as a calcium sensor in this system, and our studies implicate a role for Cam in this capacity. Others have demonstrated a requirement for calcium/Cam-dependent kinases in calcium-mediated Pyk2 phosphorylation. In studies using carbachol and angiotensin II as agonists, Pyk2 phosphorylation was clearly calcium/Cam dependent in kidney cells (33). Similar observations were reported for KCl/depolarization- and bradykinin-stimulated PC12 cells (34). In the latter study, the signaling link between calcium influx and Pyk2 phosphorylation induced by membrane depolarization was dependent upon CamK II, whereas the effects of bradykinin appeared to be independent of CamK II but fully dependent on Cam. Other studies examining the effects of cerebral ischemia on Pyk2 phosphorylation in rats (35) or the induction of Pyk2 phosphorylation with ionomycin in vascular smooth muscle cells (36) demonstrate sensitivity to or requirement for CamKs to mediate Pyk2 phosphorylation. The role of GnRH-induced CamK II activity was recently demonstrated in LβT2 cells and in rat pituitary cells, demonstrating that inhibition of CamK II affected the regulation of gonadotropin subunit gene expression (37,38). Thus, although GnRH is clearly capable of activating CamK II, its role in the calcium/Cam-dependent phosphorylation of Pyk2 appears to be negligible based upon limited pharmacological studies presented here. It will be interesting to determine whether the trans-acting autophosphorylation of Pyk2 recently reported (32) is sensitive to Cam disruption, suggesting a possible role for calcium and Cam in the initial activation steps leading to Pyk2 catalytic activity via aggregation of Pyk2.
Corroborating the pharmacology studies described above was the observation that Pyk2 could be retained on a Cam agarose column, and this association was dependent upon the presence of calcium, suggesting the formation of a putative Cam-Pyk2 complex. Another nonreceptor tyrosine kinase, Src, did not associate with the Cam agarose column, whereas a known Cam-interacting protein, IQGAP, was retained on the column, contributing to the notion that Pyk2 association with the Cam agarose affinity column was indeed specific. Because Pyk2 and Src have been shown to specifically interact associated with integrin stimulation (reviewed in Ref. 19) and in the context of the GnRH signaling network (29), it is interesting to note a lack of Src binding to the Cam agarose column in conjunction with Pyk2. It is certainly plausible that Src cannot bind Cam directly, and the conditions used in the present studies were incompatible with Pyk2-Src association in the binding assays. Additional studies will be necessary to determine the precise interactions between Pyk2, Cam, and potentially Src.
The solved crystal structure of the catalytic domain of FAK (24) was instrumental to our prediction of a specific Cam binding interface within the catalytic domain of Pyk2. Modeling studies provide the basis for the prediction that key α-helices and hydrophobic residues adjacent to the putative catalytic cleft (predicted based upon FAK structure) form the interface for Cam association. Two separate structure prediction databases (ProteinPredict and nnPredict) accurately predicted α-helices within the Pyk2 catalytic domain that were entirely consistent with the FAK crystal structure (Fig. 7B). Indeed, the catalytic domain alone was sufficient to bind Cam, suggesting that the folding of this domain was likely correct. The predicted Cam binding site within the catalytic domain of Pyk2 was characterized by either a 1–10 or 1–14 motif. Unique to Pyk2 was that the α-helix containing the hydrophobic residue in position 1 in either motif was separated from the hydrophobic residue at position 10 or 14 by a nonhelical stretch of amino acids (Fig. 7B). Generally, the 1–10 or 1–14 motif resides within an uninterrupted α-helix. Mutations within this putative Cam binding site were sufficient to block Cam-Pyk2 physical interactions and Pyk2 functional activity (Fig. 8). The potential caveat here is that alanine substitution mutations within the putative Cam binding site resulted in aberrant folding of full-length Pyk2 in our studies. However, this seems unlikely because modeling the Pyk2 catalytic domain (based upon the FAK structure) with these alanine substitutions did not alter the prediction of α-helices within this domain (data not shown).
The substrates for Pyk2 catalytic activity within gonadotropes are currently not known, limiting our understanding of the functional consequence of Pyk2 activation within the GnRH signaling network. Remarkable tyrosine phosphorylation of intracellular proteins is evident after GnRH administration to αT3-1 cells, presumably some of which reflects substrates of activated Pyk2 (Fig. 1). Indirectly, Pyk2 substrates would include any of the putative gene targets for ERK activation. This would be consistent with the recent findings that Pyk2 perturbation reduced GnRH-induced LHβ subunit gene expression in LβT2 cells (29). Consistent with this finding are studies from our group suggesting that Cam activity is required for GnRH-induced up-regulation of the immediate-early gene, Egr-1 (18), a required transcription factor supporting LHβ subunit gene expression in vivo (9). Several potential direct substrates of Pyk2 have also been described. In a CHO cell model, Pyk2 was shown to directly tyrosine phosphorylate glycogen synthase kinase 3B, a serine threonine kinase known to play an important role in many cellular functions (39). Metabotropic glutamate receptor 1 activity appears to be modulated by Pyk2/Src activity in cortical neurons (40). The large-conductance, calcium-activated K (BKCa) channel is directly phosphorylated by Src family members including Pyk2 functionally enhancing BKCa channel activity (41). And likely one of the best characterized substrates of Pyk2, gelsolin directly interacts with Pyk2 where Pyk2 tyrosine phosphorylates gelsolin, modulating its bioactivity in regulating the actin cytoskeleton in osteoclasts (42). Certainly, a limitation of the current studies is ascertaining whether GnRH-induced tyrosine-phosphorylated proteins are direct substrates of Pyk2, and the challenge for future studies will be to identify these tyrosine-phosphorylated proteins by proteomic approaches.
MATERIALS AND METHODS
Reagents
Antibodies for phosphotyrosine (titer 1:2000), ERK2 (titer 1:1000), JNK1 (titer 1:1000), IQGAP (titer 1:1000), actin (titer 1:1000), and all horseradish peroxidase-coupled secondary antibodies (titer 1:5000) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA) and were used according to the manufacturer’s instructions. Antibody for pan-Pyk2 (titer 1:1000) was purchased from BD Biosciences (Palo Alto, CA) and was used according to manufacturer’s instructions. The phospho-Pyk2 antibodies (Y402, Y580, and Y881; titer 1:1000) and the Src antibody (titer 1:500) were purchased from Biosource (Camarillo, CA) and were used according to the manufacturer’s instructions. The HA epitope tag antibody (YPYDVPDYA; titer 1:1000) was purchased from Berkeley Antibody Co. (Berkeley, CA) and used according to the manufacturer’s instructions. Antibodies against phosphorylated forms of ERK and JNK (titer 1:1000) were obtained from Cell Signaling Technologies (Danvers, MA) and were used according to the manufacturer’s instructions. The GnRHa buserelin was purchased from Sigma Chemical Co. (St. Louis, MO) and was used at 10 nm for all studies. Nifedipine, W7, and KN62 were obtained from CalBiochem (San Diego, CA) and were used at 1, 15, and 10 μm, respectively. Cam agarose was purchased from Stratagene (La Jolla, CA), and the matrix was prepared according to the manufacturer’s instructions. Expression vector for ERK2 was generously provided by Melanie Cobb (University of Texas, Southwestern, Dallas, TX).
Cell Culture and siRNA Stable Cell Selection
αT3-1 cells were generously provided by Dr. Pamela Mellon (University of California, San Diego) (43,44). Cells were cultured in monolayers in the presence of DMEM containing 10% fetal bovine serum and supplemented with penicillin and streptomycin. Cells were maintained at 37 C in a 5% CO2, humidified atmosphere. For all studies, αT3-1 cells were split within 2 d of experimentation and used as subconfluent cultures.
In some specific knockdown studies, control and Pyk2 siRNAs were designed and cloned into the expression vector pSuper-Retro-Neo using the same strategy described previously (45). The control siRNA contained the following sequence: 5′-TTCTCCGAACGTGTC ACGT-3′. Three mouse Pyk2-specific siRNA target sequences were identified as follows: 5′-GAACATGGCTGATCTCATA-3′, 5′-CTACCT GGAACGAAATAAA-3′, and 5′-TTGAGGACG AAGACTATTA-3′. Each siRNA in pSuper-Retro-Neo was expressed as a hairpin looped siRNA structure. Briefly, αT3-1 cells were plated at 30–40% confluence in 100-mm dishes and were transfected the following day with pSuper-Retro-Neo-Pyk2 or control siRNAs using Fugene transfection reagent (Roche Applied Science, Indianapolis, IN) following the manufacturer’s instructions. After 18 h of transfection, stably transfected cells were selected by geneticin treatment (Invitrogen, Carlsbad, CA) at 500 μg/ml in DMEM. After 3 wk of neomycin selection, the stable cell populations were ready for use in experiments. Originally, the three Pyk2 siRNAs were tested individually in stable cell lines to identify the effectiveness of knockdown of Pyk2. Although all three Pyk2 siRNAs resulted in a modest level of knockdown at the protein level, the combination of all three Pyk2 siRNA in the same stable cell population proved most effective for Pyk2 knockdown.
Mono Q Chromatography
αT3-1 cell lysates were prepared and subjected to fractionation as described previously (46). Briefly, cells were serum starved for 2 h and then pretreated with either control solution or nifedipine for 30 min. Some cells then received GnRHa for 15 min. Cells were then lysed with gentle agitation in a buffer containing 0.5% Triton X-100. Lysates were cleared of debris using centrifugation and equal amounts of cellular protein (1–2 mg total protein; equal within an individual replicate) were loaded on a Mono Q column. Fractions were collected and subjected to Western blot analysis outlined below. Mono Q fractionation procedures were completed on two separate occasions with identical results.
Preparation of Whole-Cell Lysates and Western Blot Analysis
For all Western blot studies, cells were serum starved for 2 h followed by treatment application. After treatments (for example buserelin time-course studies) within individual experiments, cells were lysed in a standard RIA immunoprecipitation (RIPA) buffer as described (10,21). Lysates were clarified by centrifugation and denatured by boiling in an equal volume of buffer containing 100 mm Tris (pH 6.8), 4% sodium dodecyl sulfate (SDS), 20% glycerol, and 200 mm dithiothreitol (SDS-loading buffer). Samples were resolved on 10% polyacrylamide gels (SDS-PAGE), transferred to polyvinylidene difluoride or nitrocellulose membranes, blocked in either 5% BSA (phosphotyrosine antibody) or nonfat dried milk in Tris-buffered saline (pH 7.5)/0.1% Tween 20. Primary antibodies were added at the appropriate dilution and incubated at 4 C overnight with constant agitation. Blots were washed in Tris-buffered saline (pH 7.5)/0.1% Tween 20 and then exposed to secondary antibody for 1–2 h at room temperature and washed again, and protein bands were visualized using enhanced chemiluminescence reagents (PerkinElmer, Boston, MA). Lane loading was determined using the pan-specific antibodies to the corresponding phosphospecific antisera used in individual studies. For all Western blot analyses, studies were carried out at least three times. ImageJ (http://rsb.info.nih.gov/ij/) was used to quantitate band intensity. Representative experiments are shown.
Animal Studies and Double-Labeling Fluorescent Immunocytochemistry
All animal studies were completed in accordance with the Cornell University Institutional Animal Care and Use Committee. NSA (CF-1) female mice (Harlan, Indianapolis, IN) were ovariectomized and allowed 14 d to recover. All ovariectomized females were administered 0.25 ml ovine anti-GnRH serum ip and were maintained for 3 d to immunosuppress endogenous GnRH. On the fourth day, control animals (n = 3) received an ip injection of sterile saline (50 μl), whereas treated animals (n = 4 per group) received 100 ng (in 50 μl saline) of a nonimmunoreactive analog of GnRH (d-Ala6-GnRH). GnRH analog-treated animals were then killed 45 and 90 min after injection. Control animals were killed at approximately 60 min after saline injection. Whole pituitaries were dissected free and fixed in 4% paraformaldehyde. Fixed tissues were sectioned at 7 μm. Endogenous peroxides were blocked by incubating sections in methanol containing 0.6% H2O2. Slides were then rinsed one time with 70% ethanol and three times over 15 min in 150 mm NaCl, 40 mm K2HPO4, 10 mm KH2PO4 (pH 7.4) (KPBS). Next, tissue sections were put through an antigen retrieval method using trypsin (Sigma). Sections were incubated in a 0.1% trypsin solution containing 0.1% CaCl2 and 20 mm Tris (pH 7.6). Sections then were rinsed with KPBS and incubated in primary antibody (anti-Pyk2 Y881) at 4 C overnight. The next morning, the tissue sections were rinsed with KPBS and then incubated for 30 min at room temperature in biotinylated donkey antirabbit IgG (Vector Laboratories, Burlingame, CA) at a concentration of 1:5000 in KPBS. Sections were rinsed and then incubated in an avidin-biotin complex solution (1.12 μl/ml KPBS, Elite ABC kit; Vector) for 30 min at room temperature. After additional rinsing, sections were incubated in streptavidin Texas Red (Vector) for 45 min at 37 C. Rinsing followed, and then the sections were incubated in the second primary antibody (LHβ obtained from the National Hormone and Peptide Program, National Institute of Diabetes and Digestive and Kidney Diseases and Dr. A. F. Parlow, Torrance, CA) for 6 h at room temperature. Tissue was rinsed again and then incubated in Cy2-donkey antirabbit IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) for 45 min at 37 C. After rinsing with KPBS, sections were dehydrated in graded ethanols, cleared in xylene, and coverslipped with Krystalon (Harleco, EM Science, Gibbstown, NJ).
In a pilot study to determine the effectiveness of the immunosuppression model on LH secretion, control (n = 4) and GnRHa-treated (n = 4) animals were prepared as described above, and trunk blood was collected after exsanguination at 60 min after ip injection of control saline or GnRHa. Serum was collected and assayed for mouse LH using a specific LH RIA (Colorado State University Reproductive Endocrinology Laboratory, Fort Collins, CO).
Transient Transfection and Reporter Gene Assay
For transient transfections, αT3-1 cells were plated at 60–70% confluence in 60-mm plates and were transiently transfected with a c-Fos promoter-luciferase fusion (500 ng) (46), a GnRHR promoter-luciferase fusion (15,47,48), or a Gal-Elk1 and 5xGal4-EIB luciferase reporter (500 ng of each plasmid) (7) using lipofection reagents (either Fugene from Roche Applied Science or Lipofectamine 2000 from Invitrogen) following the manufacturer’s instructions. In specific experiments, reporter systems were cotransfected with 5 μg empty parent vector (pKH3), wild-type Pyk2 (Pyk2WT), or catalytically inactive Pyk2 (Pyk2KD) (25,49). In other studies, site-directed mutagenesis was used to prepare a mutation within the putative Cam binding site in the catalytic domain in full-length Pyk2 (Pyk2cat-mut). The mutation was constructed by replacing the leucine residues with alanine as described in Fig. 7A using a PCR-based strategy. To facilitate cloning into the pKH3 vector, EcoR1 and Cla1 restriction sites were engineered to the forward and reverse primers, respectively. The mutation was verified by nucleotide sequence analysis. Eighteen to twenty hours after transfection, cells were collected for luciferase activity as previously described (45). All data are presented as mean ± sem for luciferase activity standardized by total protein as measured by Bradford assay.
Cam Agarose Affinity Chromatography
αT3-1 cell lysates were prepared in a buffer containing 50 mm Tris (pH 7.5), 1.0% Triton X-100, 5 mm EDTA, 250 mm NaCl, 1 mm sodium vanadate, 25 mm β-glycerophosphate, 5 mm benzamidine, and 0.2 mm phenylmethylsulfonyl fluoride (PMSF) (referred to as buffer A). This buffer was supplemented with a protease inhibitor cocktail (Sigma; catalog number P-8340). Lysates were subjected to two freeze-thaw cycles (−80 C, then thawed on ice) and clarified by centrifugation. To prepare the Cam agarose, beads were sedimented by low-speed centrifugation, the storage buffer was removed, and beads were washed four times in a buffer containing 20 mm Tris (pH 7.5), 4 mm MgCl2, 2 mm CaCl2, 10 mm KCl, and 2 mm PMSF (buffer B +Ca2+), or in some cases, buffer B was supplemented with 10 mm EGTA and is referred to as buffer B −Ca2+. Cell lysates in buffer A were diluted 10-fold in buffer B +Ca2+ or in some cases buffer B −Ca2+. Diluted lysates were then mixed with washed Cam agarose beads (in the presence or absence of calcium) for 3–4 h at 4 C with constant mixing. The lysate/agarose solutions were then loaded into a disposable column, and the retained Cam agarose matrix was washed with 20 column volumes of buffer B +Ca2+ or in some cases buffer B −Ca2+. The columns were eluted with a buffer containing 20 mm Tris (pH 7.5), 4 mm MgCl2, 10 mm EGTA, 10 mm KCl, and 1 mm PMSF. Fractions were collected in approximately 250-μl volumes. Equal volumes of fractions were used for Western blot analysis. In some overexpression studies, control plasmid (pKH3) or HA-tagged wild-type and Pyk2 mutants (2 μg each) were transiently transfected into αT3-1 cells as described above, and cell lysates were isolated and subjected to Cam agarose affinity chromatography. Chromatography experiments using Cam agarose were carried out at least three times with similar results.
FAK Structural Modeling
Expasy (ca.expansy.org) was used to identify the domains for human FAK and Pyk2 based upon the sequences published in accession numbers Q05397 (FAK) and Q14289 (Pyk2). The catalytic domains of each protein were identified and aligned using NCBI Blast. The FAK structure (24) was manipulated using Deep View/Swiss-PDB Viewer version 3.7. The FAK structure was obtained from the Swiss Protein Databank where the ID number is 1MP8. Primary amino acid sequence data for the catalytic domain of human FAK and Pyk2 were subjected to two different secondary structure prediction tools (nnPredict and ProteinPredict). Predicted structures of each sequence were compared between the two search engines, and sequences common to both prediction tools as well as consistency with the solved FAK structure were identified. Similar analyses were carried out substituting critical hydrophobic residues within the putative Cam binding site in the Pyk2 catalytic domain with alanine residues to determine whether predicted secondary structure would be altered.
Statistical Analysis
Data sets were subjected to an ANOVA, followed by individual pairwise comparisons using the Tukey honestly significant difference test. P < 0.05 was deemed significant. Data are reported as the treatment group means ± sem for representative studies. Studies were carried out at least three times on separate occasions.
Acknowledgments
We are grateful to Colin Clay, Pamela Mellon, Melanie Cobb, and Terry Nett for helpful reagents. We are indebted to Dr. Bill Horne at Cornell University for helpful discussions in the development of this manuscript.
Footnotes
This work was supported by a grant from the National Institutes of Child Health and Human Development (R01 HD34722) to M.S.R.
Disclosure Statement: The authors have nothing to disclose.
First Published Online July 17, 2008
Abbreviations: Cam, Calmodulin; CamK, Cam-dependent kinase; Egr, early growth response factor; FAK, focal adhesion kinase; GnRHa, GnRH agonist; GnRHR, GnRH receptor; GPCR, G protein-coupled receptor; HA, hemagglutinin; IQGAP1, IQ domain-containing GTPase activating protein; JNK, c-Jun N-terminal kinase; MEK1, MAP/ERK kinase; PMSF, phenylmethylsulfonyl fluoride; Pyk2, proline-rich tyrosine kinase 2; VGCC, voltage-gated calcium channel.
References
- Fink G, Sheward WJ, Charlton HM 1982 Priming effect of luteinizing hormone releasing hormone in the hypogonadal mouse. J Endocrinol 94:283–287 [DOI] [PubMed] [Google Scholar]
- Fink G, Sheward WJ, Plant TM 1984 The hypogonadal mouse pituitary contains bioactive LH. J Reprod Fertil 70:277–280 [DOI] [PubMed] [Google Scholar]
- Mason AJ, Pitts SL, Nikolics K, Szonyi E, Wilcox JN, Seeburg PH, Stewart TA 1986 The hypogonadal mouse: reproductive functions restored by gene therapy. Science 234:1372–1378 [DOI] [PubMed] [Google Scholar]
- Hislop JN, Madziva MT, Everest HM, Harding T, Uney JB, Willars GB, Millar RP, Troskie BE, Davidson JS, McArdle CA 2000 Desensitization and internalization of human and Xenopus gonadotropin-releasing hormone receptors expressed in αT4 pituitary cells using recombinant adenovirus. Endocrinology 141:4564–4575 [DOI] [PubMed] [Google Scholar]
- Vrecl M, Heding A, Hanyaloglu A, Taylor PL, Eidne KA 2000 Internalization kinetics of the gonadotropin-releasing hormone (GnRH) receptor. Pflugers Arch 439:R19–R20 [PubMed] [Google Scholar]
- Ruf F, Fink MY, Sealfon SC 2003 Structure of the GnRH receptor-stimulated signaling network: insights from genomics. Front Neuroendocrinol 24:181–199 [DOI] [PubMed] [Google Scholar]
- Roberson MS, Misra-Press A, Laurance ME, Stork PJ, Maurer RA 1995 A role for mitogen-activated protein kinase in mediating activation of the glycoprotein hormone α-subunit promoter by gonadotropin-releasing hormone. Mol Cell Biol 15:3531–3539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Bliss SP, Nett TM, Ebersole BJ, Sealfon SC, Roberson MS 2005 Transcript profiling of immediate early genes reveals a unique role for activating transcription factor 3 in mediating activation of the glycoprotein hormone α-subunit promoter by gonadotropin-releasing hormone. Mol Endocrinol 19:2624–2638 [DOI] [PubMed] [Google Scholar]
- Lee SL, Sadovsky Y, Swirnoff AH, Polish JA, Goda P, Gavrilina G, Milbrandt J 1996 Luteinizing hormone deficiency and female infertility in mice lacking the transcription factor NGFI-A (Egr-1). Science 273:1219–1221 [DOI] [PubMed] [Google Scholar]
- Zhang T, Wolfe MW, Roberson MS 2001 An early growth response protein (Egr) 1 cis-element is required for gonadotropin-releasing hormone-induced mitogen-activated protein kinase phosphatase 2 gene expression. J Biol Chem 276:45604–45613 [DOI] [PubMed] [Google Scholar]
- Tremblay JJ, Drouin J 1999 Egr-1 is a downstream effector of GnRH and synergizes by direct interaction with Ptx1 and SF-1 to enhance luteinizing hormone β gene transcription. Mol Cell Biol 19:2567–2576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coss D, Jacobs SB, Bender CE, Mellon PL 2004 A novel AP-1 site is critical for maximal induction of the follicle-stimulating hormone β gene by gonadotropin-releasing hormone. J Biol Chem 279:152–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahl BD, Huang HJ, Sebastian J, Ghosh BR, Miller WL 1998 Transcriptional activation of the ovine follicle-stimulating hormone β-subunit gene by gonadotropin- releasing hormone: involvement of two activating protein-1-binding sites and protein kinase C. Endocrinology 139:4455–4465 [DOI] [PubMed] [Google Scholar]
- Strahl BD, Huang HJ, Pedersen NR, Wu JC, Ghosh BR, Miller WL 1997 Two proximal activating protein-1-binding sites are sufficient to stimulate transcription of the ovine follicle-stimulating hormone-β gene. Endocrinology 138:2621–2631 [DOI] [PubMed] [Google Scholar]
- Duval DL, Nelson SE, Clay CM 1997 The tripartite basal enhancer of the gonadotropin-releasing hormone (GnRH) receptor gene promoter regulates cell-specific expression through a novel GnRH receptor activating sequence. Mol Endocrinol 11:1814–1821 [DOI] [PubMed] [Google Scholar]
- Mulvaney JM, Zhang T, Fewtrell C, Roberson MS 1999 Calcium influx through L-type channels is required for selective activation of extracellular signal-regulated kinase by gonadotropin-releasing hormone. J Biol Chem 274:29796–29804 [DOI] [PubMed] [Google Scholar]
- Mulvaney JM, Roberson MS 2000 Divergent signaling pathways requiring discrete calcium signals mediate concurrent activation of two mitogen-activated protein kinases by gonadotropin-releasing hormone. J Biol Chem 275:14182–14189 [DOI] [PubMed] [Google Scholar]
- Roberson MS, Bliss SP, Xie J, Navratil AM, Farmerie TA, Wolfe MW, Clay CM 2005 Gonadotropin-releasing hormone induction of extracellular-signal regulated kinase is blocked by inhibition of calmodulin. Mol Endocrinol 19:2412–2423 [DOI] [PubMed] [Google Scholar]
- Avraham H, Park SY, Schinkmann K, Avraham S 2000 RAFTK/Pyk2-mediated cellular signalling. Cell Signal 12:123–133 [DOI] [PubMed] [Google Scholar]
- Duval DL, Farris AR, Quirk CC, Nett TM, Hamernik DL, Clay CM 2000 Responsiveness of the ovine gonadotropin-releasing hormone receptor gene to estradiol and gonadotropin-releasing hormone is not detectable in vitro but is revealed in transgenic mice. Endocrinology 141:1001–1010 [DOI] [PubMed] [Google Scholar]
- Zhang T, Mulvaney JM, Roberson MS 2001 Activation of mitogen-activated protein kinase phosphatase 2 by gonadotropin-releasing hormone. Mol Cell Endocrinol 172:79–89 [DOI] [PubMed] [Google Scholar]
- Ellsworth BS, White BR, Burns AT, Cherrington BD, Otis AM, Clay CM 2003 c-Jun N-terminal kinase activation of activator protein-1 underlies homologous regulation of the gonadotropin-releasing hormone receptor gene in αT3-1 cells. Endocrinology 144:839–849 [DOI] [PubMed] [Google Scholar]
- O’Day DH, Myre MA 2004 Calmodulin-binding domains in Alzheimer’s disease proteins: extending the calcium hypothesis. Biochem Biophys Res Commun 320:1051–1054 [DOI] [PubMed] [Google Scholar]
- Nowakowski J, Cronin CN, McRee DE, Knuth MW, Nelson CG, Pavletich NP, Rogers J, Sang BC, Scheibe DN, Swanson RV, Thompson DA 2002 Structures of the cancer-related Aurora-A, FAK, and EphA2 protein kinases from nanovolume crystallography. Structure 10:1659–1667 [DOI] [PubMed] [Google Scholar]
- Ueda H, Abbi S, Zheng C, Guan JL 2000 Suppression of Pyk2 kinase and cellular activities by FIP200. J Cell Biol 149:423–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah BH, Soh JW, Catt KJ 2003 Dependence of gonadotropin-releasing hormone-induced neuronal MAPK signaling on epidermal growth factor receptor transactivation. J Biol Chem 278:2866–2875 [DOI] [PubMed] [Google Scholar]
- Farshori PQ, Shah BH, Arora KK, Martinez-Fuentes A, Catt KJ 2003 Activation and nuclear translocation of PKCδ, Pyk2 and ERK1/2 by gonadotropin releasing hormone in HEK293 cells. J Steroid Biochem Mol Biol 85:337–347 [DOI] [PubMed] [Google Scholar]
- Shah BH, Farshori MP, Catt KJ 2004 Neuropeptide- induced transactivation of a neuronal epidermal growth factor receptor is mediated by metalloprotease-dependent formation of heparin-binding epidermal growth factor. J Biol Chem 279:414–420 [DOI] [PubMed] [Google Scholar]
- Maudsley S, Naor Z, Bonfil D, Davidson L, Karali D, Pawson AJ, Larder R, Pope C, Nelson N, Millar RP, Brown P 2007 Proline-rich tyrosine kinase 2 mediates gonadotropin-releasing hormone signaling to a specific extracellularly regulated kinase-sensitive transcriptional locus in the luteinizing hormone β-subunit gene. Mol Endocrinol 21:1216–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev S, Moreno H, Martinez R, Canoll P, Peles E, Musacchio JM, Plowman GD, Rudy B, Schlessinger J 1995 Protein tyrosine kinase PYK2 involved in Ca2+-induced regulation of ion channel and MAP kinase functions. Nature 376:737–745 [DOI] [PubMed] [Google Scholar]
- Murasawa S, Mori Y, Nozawa Y, Masaki H, Maruyama K, Tsutsumi Y, Moriguchi Y, Shibasaki Y, Tanaka Y, Iwasaka T, Inada M, Matsubara H 1998 Role of calcium-sensitive tyrosine kinase Pyk2/CAKβ/RAFTK in angiotensin II induced Ras/ERK signaling. Hypertension 32:668–675 [DOI] [PubMed] [Google Scholar]
- Park SY, Avraham HK, Avraham S 2004 RAFTK/Pyk2 activation is mediated by trans-acting autophosphorylation in a Src-independent manner. J Biol Chem 279:33315–33322 [DOI] [PubMed] [Google Scholar]
- Espiritu DJ, Bernardo AA, Robey RB, Arruda JA 2002 A central role for Pyk2-Src interaction in coupling diverse stimuli to increased epithelial NBC activity. Am J Physiol Renal Physiol 283:F663–F670 [DOI] [PubMed] [Google Scholar]
- Zwick E, Wallasch C, Daub H, Ullrich A 1999 Distinct calcium-dependent pathways of epidermal growth factor receptor transactivation and PYK2 tyrosine phosphorylation in PC12 cells. J Biol Chem 274:20989–20996 [DOI] [PubMed] [Google Scholar]
- Guo J, Meng F, Fu X, Song B, Yan X, Zhang G 2004 N-methyl-d-aspartate receptor and L-type voltage-gated Ca2+ channel activation mediate proline-rich tyrosine kinase 2 phosphorylation during cerebral ischemia in rats. Neurosci Lett 355:177–180 [DOI] [PubMed] [Google Scholar]
- Ginnan R, Singer HA 2002 CaM kinase II-dependent activation of tyrosine kinases and ERK1/2 in vascular smooth muscle. Am J Physiol Cell Physiol 282:C754–C761 [DOI] [PubMed] [Google Scholar]
- Haisenleder DJ, Burger LL, Aylor KW, Dalkin AC, Marshall JC 2003 Gonadotropin-releasing hormone stimulation of gonadotropin subunit transcription: evidence for the involvement of calcium/calmodulin-dependent kinase II (Ca/CAMK II) activation in rat pituitaries. Endocrinology 144:2768–2774 [DOI] [PubMed] [Google Scholar]
- Haisenleder DJ, Ferris HA, Shupnik MA 2003 The calcium component of gonadotropin-releasing hormone-stimulated luteinizing hormone subunit gene transcription is mediated by calcium/calmodulin-dependent protein kinase type II. Endocrinology 144:2409–2416 [DOI] [PubMed] [Google Scholar]
- Hartigan JA, Xiong WC, Johnson GV 2001 Glycogen synthase kinase 3β is tyrosine phosphorylated by PYK2. Biochem Biophys Res Commun 284:485–489 [DOI] [PubMed] [Google Scholar]
- Heidinger V, Manzerra P, Wang XQ, Strasser U, Yu SP, Choi DW, Behrens MM 2002 Metabotropic glutamate receptor 1-induced upregulation of NMDA receptor current: mediation through the Pyk2/Src-family kinase pathway in cortical neurons. J Neurosci 22:5452–5461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling S, Sheng JZ, Braun AP 2004 The calcium-dependent activity of large conductance, calcium-activated K+ channels is enhanced by Pyk2 and Hck-induced tyrosine phosphorylation. Am J Physiol Cell Physiol 287:C698–C706 [DOI] [PubMed] [Google Scholar]
- Wang Q, Xie Y, Du QS, Wu XJ, Feng X, Mei L, McDonald JM, Xiong WC 2003 Regulation of the formation of osteoclastic actin rings by proline-rich tyrosine kinase 2 interacting with gelsolin. J Cell Biol 160:565–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle JJ, Weiner RI, Mellon PL 1990 Cell lines of the pituitary gonadotrope lineage derived by targeted oncogenesis in transgenic mice. Mol Endocrinol 4:597–603 [DOI] [PubMed] [Google Scholar]
- Horn F, Bilezikjian LM, Perrin MH, Bosma MM, Windle JJ, Huber KS, Blount AL, Hille B, Vale W, Mellon PL 1991 Intracellular responses to gonadotropin-releasing hormone in a clonal cell line of the gonadotrope lineage. Mol Endocrinol 5:347–355 [DOI] [PubMed] [Google Scholar]
- Xie J, Roberson MS 2008 3′,5′-Cyclic adenosine 5′-monophosphate response element-dependent transcriptional regulation of the secretogranin II gene promoter depends on gonadotropin-releasing hormone-induced mitogen- activated protein kinase activation and the transactivator activating transcription factor 3. Endocrinology 149:783–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson MS, Zhang T, Li HL, Mulvaney JM 1999 Activation of the p38 mitogen-activated protein kinase pathway by gonadotropin-releasing hormone. Endocrinology 140:1310–1318 [DOI] [PubMed] [Google Scholar]
- Duval DL, Nelson SE, Clay CM 1997 A binding site for steroidogenic factor-1 is part of a complex enhancer that mediates expression of the murine gonadotropin-releasing hormone receptor gene. Biol Reprod 56:160–168 [DOI] [PubMed] [Google Scholar]
- Cherrington BD, Farmerie TA, Lents CA, Cantlon JD, Roberson MS, Clay CM 2005 Activin responsiveness of the murine gonadotropin-releasing hormone receptor gene is mediated by a composite enhancer containing spatially distinct regulatory elements. Mol Endocrinol 19:898–912 [DOI] [PubMed] [Google Scholar]
- Zheng C, Xing Z, Bian ZC, Guo C, Akbay A, Warner L, Guan JL 1998 Differential regulation of Pyk2 and focal adhesion kinase (FAK). The C-terminal domain of FAK confers response to cell adhesion. J Biol Chem 273:2384–2389 [DOI] [PubMed] [Google Scholar]