Abstract
Estradiol (E2) acts as a potent feedback molecule between the ovary and hypothalamic GnRH neurons, and exerts both positive and negative regulatory actions on GnRH synthesis and secretion. However, the extent to which these actions are mediated by estrogen receptors (ERs) expressed in GnRH neurons has been controversial. In this study, Single-cell RT-PCR revealed the expression of both ERα and ERβ isoforms in cultured fetal and adult rat hypothalamic GnRH neurons. Both ERα and ERβ or individual ERs were expressed in 94% of cultured fetal GnRH neurons. In adult female rats at diestrus, 68% of GnRH neurons expressed ERs, followed by 54% in estrus and 19% in proestrus. Expression of individual ERs was found in 24% of adult male GnRH neurons. ERα exerted marked Gi-mediated inhibitory effects on spontaneous action potential (AP) firing, cAMP production, and pulsatile GnRH secretion, indicating its capacity for negative regulation of GnRH neuronal function. In contrast, increased E2 concentration and ERβ agonists increase the rate of AP firing, GnRH secretion, and cAMP production, consistent with ERβ-dependent positive regulation of GnRH secretion. Consonant with the coupling of ERα to pertussis toxin-sensitive Gi/o proteins, E2 also activates G protein-activated inwardly rectifying potassium channels, decreasing membrane excitability and slowing the firing of spontaneous APs in hypothalamic GnRH neurons. These findings demonstrate that the dual actions of E2 on GnRH neuronal membrane excitability, cAMP production, and GnRH secretion are mediated by the dose-dependent activation of ERα and ERβ expressed in hypothalamic GnRH neurons.
ESTROGEN EXERTS ITS cellular actions through estrogen receptor (ER)α and ERβ, members of the superfamily of ligand-dependent nuclear and membrane-localized receptors (1,2). In addition to its well-defined genomic actions, estrogen has rapid, nongenomic actions that are initiated at the cell membrane (3,4,5). The first observations on such rapid membrane-mediated actions of estradiol (E2) were reported almost three decades ago by Pietras and Szego (6). Subsequently, rapid increases in intracellular second messengers such as calcium, cAMP, MAPK, and phospholipase C have been observed during E2 treatment (7,8).
Estrogen has also been found to exert short-term actions on the electrical properties of neurons and to influence transmitter release (9,10,11). There has been increasing evidence for interactions between estrogen and cAMP in enhancing the growth of the mammary gland and breast cancer cells, and for cAMP induction of estrogen-like uterine growth (12). Such findings have indicated that G proteins and second messengers are involved in estrogen actions. These effects are distinct from genomic mechanisms of action and are attributable to actions of estrogens on membrane receptors or other cellular components that alter neuronal activity.
The extent to which GnRH neurons are directly responsive to steroid hormones has been a controversial issue in reproductive physiology. Recent studies have reported that ERβ, but not ERα, is expressed in GnRH neurons derived from cultured mouse nasal explants (13) and adult mouse GnRH neurons (14,15,16). However, expression of ERα was found in adult rat GnRH neurons (17). In the present study, expression of ERα and ERβ was analyzed by single-cell RT-PCR in identified rat fetal GnRH neurons and GnRH neurons derived from adult male and female rats expressing green fluorescent protein (GFP)-tagged GnRH neurons (18). These cells were found to express both ERα and ERβ. Selective activation of ERα increases G protein-activated inwardly rectifying potassium (GIRK) current, inhibits spontaneous action potential (AP) firing, decreases cAMP production, and abolishes pulsatile GnRH release. In contrast, the rate of AP firing, cAMP production, and GnRH secretion increases during selective activation of ERβ. Thus, the coexpression of ERα and ERβ in rat hypothalamic GnRH neurons, and their dose- and time-dependent activation by E2 (5), engenders the direct stimulatory and inhibitory actions of E2 on GnRH neurons in vivo.
RESULTS
Expression of ERα and ERβ in Hypothalamic GnRH Neurons
RT-PCR analysis of total RNA derived from identified cultured fetal hypothalamic GnRH neurons using gene-specific primers based on the sequences of GnRH, ERα, and ERβ gave the expected fragment size of 107 bp for GnRH, 155 bp for ERα, and 176 bp for ERβ (Fig. 1A, lines 1–8). Probing of RNA extracts from GT1–7 cells with primers for GnRH, ERα, and ERβ revealed expression of all three gene products (data not shown). The amplified products were of the expected size, and the nucleotide sequences matched the published sequences for mouse GnRH, ERα, and ERβ. No GnRH mRNA product amplification was detected in single cells that did not morphologically resemble GnRH neurons (Fig. 1A, neg.-1). No amplification of an ERα mRNA product was observed in the absence of reverse-transcribed mRNA, indicating that the RNA preparation was free of genomic DNA contamination (Fig. 1A, neg.-2). Neither ERα nor ERβ was amplified from nonneuronal cells (Fig. 1A, neg.-3 and neg.-4). Control mRNAs for ERα and ERβ were extracted from the rat uterus and ovary (Fig. 1A, cont.-1 and cont.-2).
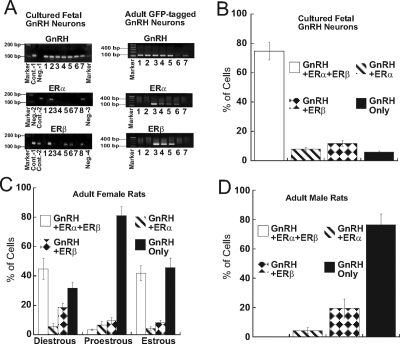
Figure 1.
Expression of ERα and ERβ in Rat Hypothalamic GnRH Neurons
A, Single-cell RT-PCR of individual fetal and adult rat GnRH neurons showing amplicons for GnRH, ERα, and ERβ (sample 1–sample 7), positive and negative controls, and ladder marker. B, Histograms showing the frequency of detection of GnRH + ERα, and ERβ, GnRH + ERα, GnRH + ERβ, and GnRH alone transcripts in cultured fetal GnRH neurons, C, Adult female rats. D, Adult male rats. Cont., Control; Neg., negative control.
Individual hypothalamic GnRH neurons were selected by differential interference contrast microscopy, which permits their morphological identification in hypothalamic cell cultures with an accuracy of more than 95% (19). After harvesting, the cytoplasmic content of each neuron was subjected to multiplex single-cell PCR to determine the presence of GnRH, ERα, and ERβ transcripts. Of the 154 GnRH-positive neurons selected from cultured hypothalamic cells by their morphology, 115 (74.7 ± 6.0%) expressed GnRH, ERα, and ERβ mRNA (Fig. 1B). Both ERα and ERβ or individual ERs were expressed in 12 (7.8 ± 1.2%), GnRH and ERβ in 18 (11.7 ± 2.0%), combined 145 (94.2%), and GnRH alone in nine (5.8 ± 0.8%) of the examined GnRH neurons (Fig. 1B).
Expression of ERα and ERβ was also examined in adult male and female rats expressing GFP-tagged GnRH neurons. Transgenic rats were kindly provided by Dr. M. Kato, Department of Physiology, Nippon Medical School, Tokyo, Japan (18). Multiplex single-cell RT-PCR confirmed that mRNAs for GnRH, ERα, and ERβ were expressed in adult GFP-tagged GnRH neurons (Fig. 1C). Adult female GFP-tagged GnRH neurons were harvested from hypothalamic slices obtained during specific phases of the estrous cycle. GFP-tagged neurons (n = 38) from three female rats during diestrus I and diestrus II were collected and probed for expression of GnRH, ERα, and ERβ. GnRH, ERα, and ERβ were expressed in 17 (44.7 ± 7.1%) of 38 GnRH-positive neurons. GnRH and ERα were expressed in two (5.3 ± 2.1%), GnRH and ERβ in seven (18.3 ± 3.0%). Both ERα and ERβ or individual ERs were expressed in 26 (68.3%), and GnRH only was expressed in 12 (31.7 ± 4.0%) of 38 examined GnRH neurons (Fig. 1C). In GFP-tagged GnRH neurons collected from three female rats (n = 32) during the afternoon of proestrus, expression of GnRH, ERα, and ERβ was found in one (3.3 ± 0.5%), GnRH and ERα in two (6.3 ± 2.6%), GnRH and ERβ in three (9.4 ± 2.3%). Both ERα and ERβ or individual ERs were expressed in six (18.9%), and GnRH only in 26 (81.18 ± 6.3%) of GFP-tagged GnRH neurons (Fig. 1C). In GFP-tagged neurons (n = 24) collected from three female rats during estrus, GnRH, ERα, and ERβ were expressed in 10 (41.8 ± 5.1%) of 24 GnRH-positive neurons. GnRH and ERα were expressed in one (4.1 ± 1.1%), GnRH and ERβ in two (8.3 ± 1.5%), combined 13 (54.1%), and GnRH only was expressed in 11 (45.8 ± 6.2%) of 24 examined GnRH neurons (Fig. 1C). In adult male GFP-tagged GnRH neurons (n = 59) ERα was expressed in two (3.4 ± 2.1%), GnRH and ERβ in 12 (20.3 ± 6.4%), combined 13 (23.7%), and GnRH alone was expressed in 45 (76.3 ± 7.2%) (Fig. 1D).
Modulation of Spontaneous AP Firing in Hypothalamic GnRH Neurons by ER Agonists and Antagonists
Whole-cell recordings from identified hypothalamic GnRH neurons consistently revealed spontaneous AP firing, with most of the cells (75%) showing irregular spiking activity. Treatment with E2 had both stimulatory and inhibitory effects on the frequency of AP firing in identified hypothalamic GnRH neurons. The frequency of AP firing decreased during E2 treatment in some GnRH neurons (Fig. 2A), and in others it was increased. Reversal of the inhibitory action and transition to the stimulatory action of E2 on AP firing was observed during treatment with an ERα antagonist (Fig. 2A). The stimulatory action of E2 on AP firing was abolished during concomitant treatment with an ERβ antagonist (Fig. 2B). Treatment of identified GnRH neurons with a selective ERα agonist caused inhibition of AP firing (Fig. 2C). Such ERα agonist-induced inhibition of AP firing was reversible, and basal firing resumed during the washout period. Treatment with an ERβ agonist increased AP firing in native hypothalamic GnRH neurons, and basal firing was resumed during washout (Fig. 2D).
Figure 2.
Spontaneous Electrical Activity and E2-Induced Activation of GIRK Current in Identified GnRH Neurons
A, Inhibition of AP firing during E2 treatment and reversal of the inhibitory action and transition to the stimulatory action of E2 on AP firing during treatment with an ERα antagonist. B, Stimulation of AP firing by E2 and transition to the stimulatory action of E2 on AP firing during treatment with an ERα antagonist. C, Inhibition of AP firing by ERα agonist and recovery of AP firing during washout period. D, ERβ agonist-induced increase in spontaneous AP firing in cultured hypothalamic GnRH neurons and recovery of AP firing during washout period. Ag., Agonist; Ant., antagonist.
Activation of GIRK Channels by Estrogen in Hypothalamic GnRH Neurons
Voltage-step commands (−120 to −20 mV; 200 msec) delivered to identified hypothalamic GnRH neurons were used to elicit GIRK currents. The magnitude of the basal GIRK current was hyperpolarization dependent and was maximal at a test potential of −120 mV. The GIRK current decreased in a hyperpolarization-dependent manner and was close to zero at −40 mV (Fig. 3A). Treatment of identified GnRH neurons with 200 pm E2 during the voltage ramp caused a substantial rise in GIRK current, which increased by 25.6 ± 2.6% (P < 0.01; n = 5) for all voltage steps negative to potassium equilibrium. During the washout period, the GIRK current fell almost to the basal level (Fig. 3B).
Figure 3.
E2-Induced Activation of GIRK Current in Identified GnRH Neurons
A, E2-induced potentiation of GIRK current (open circles) and pertussis toxin-induced inhibition of E2-activated GIRK current (triangles). B, Current-voltage relationships of basal GIRK current (open squares), E2-activated (open circles), and pertussis toxin-induced inhibition of E2-activated GIRK current (open triangles). ms, Millisecond.
Effects of ER Subtypes on cAMP Production of GnRH Secretion in GT1–7 Neurons
Treatment of GT1–7 cells with E2 had a biphasic effect on cAMP production. Low (picomolar) concentrations were inhibitory, whereas higher nanomolar concentrations increased cAMP production. Both effects were prevented by prior treatment with equimolar concentrations of the nonselective ER antagonist, ICI 182,780 (Fig. 4A). In E2-treated GT1–7 cells, addition of an ERα antagonist [1,3-bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]-1H-pyrazole dihydrochloride] abolished the inhibition of cAMP production and potentiated the stimulatory action of E2 on cAMP production (Fig. 4B). In contrast, stimulation of cAMP production by E2 was abolished in the presence of an ERβ antagonist (Fig. 4C). Inhibition of cAMP production was consistently observed during treatment of GT1–7 cells with a selective ERα agonist. This inhibitory action was prevented by prior treatment of GT1–7 cells with an ERα antagonist (Fig. 4D). Pretreatment with an ERβ antagonist (cyclofenil) suppressed cAMP production at all concentrations (Fig. 4E).
Figure 4.
Effects of ER Subtypes on cAMP Production in GT1–7 Cells
A, Biphasic dose-dependent cAMP responses to E2 (open circles) were inhibited by pretreatment with the ER antagonist, ICI 182,780 (open squares). B, Concentration-dependent inhibition and stimulation of cAMP by E2 in GT1–7 cells (open circles). An inhibitory action of E2 on cAMP production was prevented, and stimulatory action potentiated, in presence of selective ERα antagonist (solid circles). C, Stimulatory action of E2 on cAMP production was prevented, and inhibitory action unaltered in presence of ERβ antagonist (closed circles). D, Inhibition of cAMP production by a selective ERα agonist (open circles) and its reversal by an ERα antagonist in GT1–7 cells (solid circles). E, Stimulation of cAMP production by a selective ERβ agonist (open circles), and its reversal by an ERβ antagonist in GT1–7 cells (solid circles). E2 and ER agonist and antagonist analogs were applied in equimolar concentrations. Data are means ± ses of four experiments. Single asterisks indicate a significant difference compared with the control. Double asterisks indicate significant difference between control and treated groups. Ag, Agonist; Ant, antagonist; ICI, ICI 182,780.
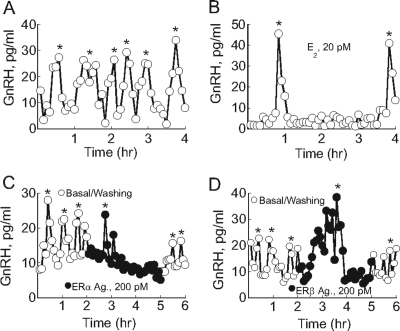
ER-Mediated Secretory Responses in Perifused GT1–7 Neurons
Perifused GT1–7 cells cultured on cytodex beads consistently exhibited pulsatile GnRH release. A representative example of pulsatile GnRH release in GT1–7 cells, with several episodic increases in GnRH release during the 4-h sampling period, is shown in Fig. 5A. Each episodic increase was statistically defined as a GnRH peak, indicating that GT1–7 cells released GnRH in a pulsatile manner (Fig. 5A). The estimated interpulse interval was 25.3 ± 3.4 min, and the mean amplitude of the GnRH pulses was 30.25 ± 3.27 pg/ml (n = 6 independent experiments). In comparison with control GT1–7 cells, those incubated with an ovulatory level of E2 showed a significant increase in GnRH pulse amplitude and a decrease in pulse frequency. Pulse amplitude rose from 30.2 ± 3.27 pg/ml in control cells (Fig. 5B) to 48.1 ± 5.1 pg/ml (n = 3; P < 0.05) in E2-treated cells (Fig. 5B), and the mean peak interval increased from 25.3 ± 3.4 min to 127.6 ± 16.2 min (n = 3; P < 0.01). To dissect the effects of ERα and ERβ on GnRH pulsatility, GT1–7 cells were perifused with selective ERα and ERβ agonists. Treatment with an ERα receptor agonist caused profound inhibition of pulsatile GnRH release, which resumed during the washout period (Fig. 5C). Conversely, treatment with an ERβ receptor agonist significantly increased the amplitude of GnRH pulse (Fig. 5D).
Figure 5.
Neurosecretory Actions of E2 in Perifused GT1–7 Cells
A, Basal pulsatile GnRH release from perifused GT1–7 cells. B, E2-induced transient stimulation and prolonged inhibition of pulsatile GnRH secretion. C, Inhibition of pulsatile GnRH release by a selective ERα agonist. D, Stimulation of pulsatile GnRH release by a selective ERβ agonist. Representative traces of four independent experiments are shown, and detected GnRH pulses are indicated by asterisks. Ag, Agonist.
DISCUSSION
Several plausible mechanisms have been proposed to generate the pulsatile release of GnRH that is essential for mammalian reproduction. These include calcium and cAMP regulation (20,21); an autocrine regulatory component (22); GnRH concentration-dependent switching in coupling of specific G proteins to the GnRH receptor (23,24); and expression of several other G protein-coupled and steroid receptors (13,17,25,26,27).
The regulation of GnRH neuronal function by ERα and ERβ has been a controversial topic over the last decade, due to the plethora of conflicting evidence about the expression and functions of these receptors in GnRH neurons. Early studies by Shivers et al. (28) found no evidence of E2 concentration in the nuclei of LHRH-immunoreactive neurons. However, Kelly et al. (29) observed estrogen-responsive LHRH neurons in the guinea-pig hypothalamus. Conversely, Langub and Watson (30) found that most LHRH neurons in the guinea-pig were not directly estrogen responsive. Likewise, Herbison and Theodosis (31) did not find ERs in preoptic nucleus containing LHRH in male and female rats. Subsequently, Roy et al. (32) reported the expression of both ERα and ERβ in immortalized GnRH (GT1–7) neuronal cells.
The first evidence of ER expression in rat GnRH neurons, using immunoprecipitation and double-label immunohistochemistry with an ERα-selective antibody, was reported by Butler et al. (17). Shortly after, Skynner et al. (15) using single-cell RT-PCR observed ERα expression in more than 50% of prepubertal GnRH neurons. The presence of ERα expression in GnRH neurons has been controversial since the original positive findings by Skynner et al. (15) were retracted after subsequent experiments by the authors did not detect ERα expression in mouse GnRH neurons (14,27).
To define the extent to which the two ERs are expressed in hypothalamic GnRH neurons, the present analysis was performed on identified fetal hypothalamic GnRH neurons in culture, and in adult GnRH neurons obtained from transgenic rats expressing GFP-tagged GnRH neurons (18). These studies have unequivocally revealed the expression of GnRH, ERα, and ERβ in cultured rat fetal hypothalamic GnRH neurons. The majority (74%) of GnRH neurons expressed both ERα and ERβ. Significantly fewer GnRH neurons expressed individual ERα and/or ERβ. Complete lack of ERs expression was found in only 6% of GnRH-positive neurons. In contrast to our findings, premigratory GnRH neurons derived from nasal explants of 11.5-d-old fetal mice express only ERβ (13), suggesting possible developmental and species differences in ERα expression. The same group reported lack of expression of ERα in GT1–7 cells, whereas others (5,32,33) found expression and translation of both ERα and ERβ in these cells, suggesting that culture conditions and/or technical factors may contribute to the differential expression of ERs.
In adult female rats, we observed that expression of both ERα and ERβ in GnRH neurons was dependent on the stage of the estrous cycle. During the afternoon of proestrus, 18% of GnRH neurons were found to express ERα and ERβ. The number of GnRH neurons expressing ERα and ERβ increased to 54% during estrus, and was maximal (67%) during diestrus. Similar estrous cycle dependence of expression of ERβ and ERRα, initially believed to be ERα, was found in adult female mice (15). This fluctuating pattern of ER mRNA expression is also similar to that reported previously in the rat preoptic area using quantitative in situ hybridization techniques and is thought to depend upon circulating gonadal steroid concentrations (34,35). Such estrous cycle-dependent expression of ERα and ERβ was also found in dispersed pituitary cells (36). The relatively high number of GnRH neurons expressing ERα and ERβ in neurons cultured in steroid-free medium, and in diestrus and estrus, when the circulating E2 level is low, and the relatively low number of GnRH neurons expressing ERα and ERβ during proestrus, when E2 is high, might suggest a similar E2-dependent regulation of the number of GnRH neurons expressing ER mRNAs.
Treatment with E2 had both inhibitory and stimulatory effects on the frequency of AP firing in identified hypothalamic GnRH neurons. The inhibitory action of E2 on AP firing was prevented by concomitant treatment with a selective ERα antagonist, and treatment of identified hypothalamic GnRH neurons with an ERα agonist caused marked inhibition of spontaneous AP firing. This effect was reversible, and spontaneous firing of APs recovered during washout of the ERα agonist. The inhibitory action of ERα activation on spontaneous AP firing in hypothalamic GnRH neurons could be related to the release of βγ-subunits from Gi (or Go), with consequent actions on plasma membrane GIRK channels. These findings, together with the inhibitory actions of an ERα-selective agonist on cAMP production and abolition of pulsatile GnRH release, indicate the capacity of ERα for negative regulation of GnRH neuronal function. The negative regulation of GnRH mRNA in αER-knockout mice indicates that ERα is the principal receptor mediating the suppressive effect of E2 on GnRH neuronal function. Because mouse GnRH neurons appear not to express ERα, it was proposed that cells other than the GnRH neurons are involved in E2-induced suppression of GnRH gene expression (37). In contrast, expression of ERα in neurons of the rostral periventricular regions of the mouse hypothalamus that project neuronal afferents to GnRH neurons mediates positive action on GnRH neuronal function (38). Thus, the expression of ERα and ERβ in rat hypothalamic GnRH neurons provides for integration of both stimulatory and inhibitory actions of E2 on GnRH neuronal function.
The increase in electrical activity of cultured hypothalamic GnRH neurons during treatment with an ERβ agonist, as well as cAMP production and GnRH secretion, is consistent with positive regulation of GnRH neuronal function. The increase in spontaneous AP firing was associated with membrane depolarization, increased bursting activity, and the appearance of lower-amplitude broad APs. This effect could be attributable to increased cAMP production, because both native and immortalized GnRH neurons express cyclic nucleotide-gated channels (39). Stimulation of AP firing was also observed during the treatment of primate GnRH neurons with E2 (40).
The biphasic effects of E2 on pulsatile GnRH release are mediated by activation of both ERα and ERβ expressed in hypothalamic GnRH neurons. Selective activation of ERα increases GIRK current, inhibits spontaneous AP firing, decreases cAMP production, and abolishes pulsatile GnRH release. In contrast, the rates of AP firing, cAMP production, and GnRH secretion are increased during selective activation of ERβ. Thus, the coexpression of ERα and ERβ in rat hypothalamic GnRH neurons, and the dose- and time-dependent activation of ERα and/or ERβ by E2, provides for the direct stimulatory and inhibitory actions of E2 on GnRH neurons. These factors contribute to the control of the pulsatile mode of neuropeptide secretion that is characteristic of GnRH neuronal function.
MATERIALS AND METHODS
Tissue and Cell Culture
Hypothalamic tissue was removed from 18-d fetuses of pregnant Sprague Dawley rats as previously described (41). The borders of the excised hypothalami were delineated by the anterior margin of the optic chiasm, the posterior margin of the mamillary bodies, and laterally by the hypothalamic sulci. The neuronal tissue was dispersed in 0.2% collagenase containing 0.4% BSA, 0.2% glucose, and 0.05% deoxyribonuclease I. After incubation for 60 min, the tissue was gently triturated by repeated aspiration into a smooth-tipped Pasteur pipette, incubated for another 30 min, and again dispersed. The cell suspension was passed through sterile mesh and sedimented by centrifugation for 10 min at 200 × g, then washed once in PBS and once in culture medium consisting of 500 ml DMEM containing 0.584 g/liter l-glutamate and 4.5 g/liter glucose, mixed with 500 ml F-12 medium containing 0.146 g/liter l-glutamine, 1.8 g/liter glucose, 100 μg/ml gentamicin, 2.5 g/liter sodium bicarbonate, and 10% heat-inactivated fetal bovine serum. Each dispersed hypothalamus yielded about 1.5 × 106 cells. Immortalized GnRH neurons (GT1–7 cells) were provided by Dr. Richard Weiner (University of California at San Francisco) (42) and were cultured under the same conditions as primary hypothalamic cells.
Cell Perifusion Procedure and Hormone Measurement
Bead-attached GT1–7 cells were perifused at a flow rate of 0.15 ml/min at 37 C. Fractions were collected at 5-min intervals and stored at −20 C before RIA. GnRH was measured using [125I]GnRH (Amersham, Chicago, IL), GnRH standards (Peninsula Laboratories, Belmont, CA), and primary antibody donated by Dr. V. D. Ramirez (University of Illinois, Urbana, IL). The intra- and interassay coefficients of variation at 50% binding in standard samples (15 pg/ml) were 5% and 7%, respectively. Individual point and sds were calculated using a power function variance model from the experimental duplicates. A 2 × 2 cluster configuration and a t statistic of 2 for the upstroke and downstroke were used to maintain false-positive and false-negative error rates below 10%. The statistical significance of the pulse parameters was tested by one-way ANOVA (43). In static experiments, statistical differences for cAMP production and GnRH release were tested by one-way ANOVA.
cAMP Production
For studies on cAMP release, cultured GT1–7 cells and hypothalamic neurons were stimulated in serum-free medium (1:1 DMEM/F-12) containing 0.1% BSA, 30 mg/liter bacitracin, and 1 mm 3-isobutyl-1-methylxanthine. RIA of cAMP was performed as previously described, using a specific cAMP antiserum at a titer of 1:5000 (44). The intraassay coefficient of variation of the assay was 4% at 50% displacement.
Whole-Cell Recording of GnRH Neurons
Whole-cell recording were taken from identified hypothalamic GnRH neurons cultured on collagen-coated coverslips and continuously perifused with artificial extracellular solution at a rate of 0.6 ml/min. The extracellular solution contained (in millimolar concentration): 140 NaCl, 5 KCl, 10 HEPES, 10 d-glucose, 2 CaCl2, 1 MgCl2. pH was adjusted to 7.4 with NaOH. 30 μm bicuculline and 5 μm 6-cyano-7-nitroquinoxaline-2,3-dione disodium salt were used to block excitatory and inhibitory synaptic transmission. For GIRK current recording, extracellular KCl was increased to 30 mm and Na+ was replaced by 115 mm N-methyl-d-glucamine (NMDH). In addition, calcium current was blocked by 1.8 mm CoCl2, chloride current was blocked by 20 μm 5-nitro-2-(3-phenylpropylamino)benzoic acid, and sodium current was blocked by 0.5 μm tetrodotoxin. All recordings were done at room temperature (23–25 C), using patch pipettes (3–5 mΩ) pulled from thick-wall borosilicate capillary glass (1.5-mm outer diameter and 0.86-mm inner diameter, WPI, Inc., Sarasota, FL) on a Flaming/Brown puller model P-87 (Sutter Instruments Co., Novato, CA). The pipette solution contained (in millimolar concentration): 20 KCl, 110 K gluconate, 10 Na2 creatine phosphate, 0.1 CaCl2, 2 MgCl2, 10 HEPES, 2 K2ATP (ATP potassium salt), 0.2 Na2GTP, 1 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid, pH adjusted to 7.2 with KOH. ZD7288 [4-(N-ethyl-N-phenylamino)-1,2-dimethyl-6-(methylamino) pyridinium chloride (50 μm)] was added in the pipette solution to block Ihcurrent. An Ag/AgCl pellet was used as the reference electrode. Spontaneous activities were recorded under the I-clamp mode with a Multi-Clamp700A amplifier (Axon Instruments, Foster City, CA), filtered at 2 KHz, digitized at 10 KHz with Digidata 1320A (Axon Instruments). In voltage-clamp mode, the serial resistance was compensated by 70%. Leak current subtraction was done automatically during recording by Multiclamp 700A. Individual hypothalamic neurons were selected by differential interference contrast microscopy, which permits their morphological identification in cultured hypothalamic cells with an accuracy of more than 95%. After recording, the cytoplasmic contents of each neuron were harvested under visual control and subjected to single-cell RT-PCR to confirm the presence of GnRH transcripts as reported previously (19). As controls, the contents of hypothalamic cells that did not show typical GnRH neuronal morphology were also analyzed by RT-PCR.
Expression of ER Subtypes in Cultured Hypothalamic GnRH Neurons
Dispersed fetal hypothalamic cells were maintained in culture for up to 2 wk. Before cell harvesting, the cultures were washed with ice-cold PBS, and GnRH neurons identified by their morphological characteristics were individually harvested with a polished glass pipette. A modification of the protocol described by (15) was used to complete cDNA conversion. Cellular contents were expelled into 8.5 μl of a cDNA conversion mixture containing 50 mm Tris-HCl (pH 8.3), 75 mm KCl, 3 mm MgCl2, 20 mm dithiothreitol, 0.5 mm deoxynucleotide triphosphates, 100 ng random hexamer primers, 200 ng oligo (deoxythymidine)12–15, and 20 U RNaseOUT (hexamers, oligo(deoxythymidine), and RNaseOUT; Invitrogen, Carlsbad, CA). Contents were collected by brief centrifugation, heated to 65 C for 5 min, and placed on ice for 1 min, followed by brief centrifugation. SuperScript II RNA H- (Invitrogen) and RNaseOUT were mixed in equal amounts and 2 μl added to each single-cell cDNA mixture. cDNA synthesis was performed at room temperature for 5 min and then at 42 C for 1 h. Contents were collected by centrifugation, frozen on dry ice, and stored at −70 C before multiplex RT-PCR.
Single-Cell Multiplex RT-PCR for GnRH, ERα, and ERβ Transcripts
First-round PCR was performed on 10 μl of reverse-transcribed product from each cell using oligonucleotide pairs in a 100-μl reaction containing 50 mm KCl, 10 mm Tris-HCl (pH 9.0), 1.5 mm MgCl2, 0.2 mm deoxynucleotide triphosphates, 2.5 U DNA Platinum Taq polymerase (Invitrogen), and optimized primer concentrations of GnRH, ERα, and ERβ.
First-round amplification (36 cycles) was performed using a MyCycler Thermal Cycler (Bio-Rad Laboratories, Inc., Hercules, CA) in thin-walled 0.2 ml PCR tubes according to the following protocol: first cycle at 95 C (3 min), 58 C (2 min), and 72 C (3 min) followed by 35 cycles at 95 C (40 sec), 58 C (85 sec), and 72 C (1 min). A final 5-min incubation was performed at 72 C. Second-round nested PCR was performed using 10-μl aliquots of the first round amplified products. PCR for GnRH (GenBank accession no. M31670), ERα (GenBank accession no. NM 023992), and ERβ (GenBank accession no. AY196983) was performed in 20-μl reaction capillaries with their respective secondary nested primer sequences utilizing a FastStart DNA Master SYBR Green 1 Kit according to the manufacturer’s instructions and the LightCycler (Roche Applied Sciences, Indianapolis, IN). The first cycle at 95 C (10 min) was followed by 40 cycles at 95 C (6 sec), 50 C (10 sec), and 72 C (9 sec). Melting curve analysis and cooling cycle were performed. The resulting products were resolved on 2% Tris-borate-EDTA-agarose gels stained with ethidium bromide. The product identities for GnRH, ERα, and ERβ were confirmed by cDNA sequencing. No GnRH, ERα, and ERβ mRNA product amplification was detected in single nonneuronal cells (Fig. 1A, Negative).
Materials
[125I]GnRH (2200 Ci/mmol) was obtained from Amersham Pharmacia Biotech (Arlington Heights, IL); collagenase (149 U/mg) was from Worthington Biochemical Corp. (Freehold, NJ); deoxyribonuclease I, trypsin, bacitracin, 3-isobutyl-1-methylxanthine, BSA, CTP, GDP, cGMP, cAMP, DMEM/F12 without phenol red, pertussis toxin, E2, triamcinolone acetonide, and forskolin were procured from Sigma Chemical Co. (St. Louis, MO); ICI 182,780 was from Tocris Cookson, Inc. (Ellisville, MO); cytodex beads were from Pharmacia Biotech (Piscataway, NJ); DMEM/F12 1:1 powder, eLONGase amplification system, SuperScript preamplification system, and Ready-Load 100-bp DNA ladder were from Life Technologies (Gaithersburg, MD); QIAEX II gel extraction kit was from QIAGEN, Inc. (Valencia, CA); the perifusion system was from Acusyst-S Cellex Biosciences (Minneapolis, MN); GnRH came from Peninsula Laboratories, Inc.(Belmont, CA); [125I]cAMP was from Covance Laboratories, Inc. (Vienna, VA); and protein assay came from Pierce Chemical Co. (Rockford, IL). Other reagents, if not specified, were obtained from Sigma Chemical Co. The ERα agonist 4,4′,4′-(4-propyl-[1H]-pyrazole-1,3,5-triyl)trisphenol, EBβ agonist 2,3-bis(4-Hydroxyphenyl)-propionitrile, and ERα antagonist 1,3-bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]-1H-pyrazole dihydrochloride, were from (Tocris, Ellisville, MO). The ERβ antagonist (Cyclofenil) 4,4′-cyclohexylidenemethylenediphenol was from (Sigma-Aldrich, St. Louis, MO). Rabbit polyclonal anti-GnRH serum was generously provided by Dr. V. D. Ramirez (University of Illinois, Urbana, IL).
Footnotes
Nadia Mores was on leave from the Department of Pharmacology, Catholic University of the Sacred Heart, Rome, Italy.
Author Disclosure: The authors have nothing to disclose.
First Published Online August 13, 2008
Abbreviations: AP, Action potential; E2, estradiol; GFP, green fluorescent protein; GIRK, G protein-activated inwardly rectifying potassium.
References
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER 2005 A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 307:1625–1630 [DOI] [PubMed] [Google Scholar]
- Levin ER 2005 Integration of the extranuclear and nuclear actions of estrogen. Mol Endocrinol 19:1951–1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razandi M, Alton G, Pedram A, Ghonshani S, Webb P, Levin ER 2003 Identification of a structural determinant necessary for the localization and function of estrogen receptor α at the plasma membrane. Mol Cell Biol 23:1633–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietras RJ, Levin ER, Szego CM 2005 Estrogen receptors and cell signaling. Science 310:51–53 [DOI] [PubMed] [Google Scholar]
- Navarro CE, Abdul Saeed S, Murdock C, Martinez- Fuentes AJ, Arora KK, Krsmanovic LZ, Catt KJ 2003 Regulation of cyclic adenosine 3′,5′-monophosphate signaling and pulsatile neurosecretion by Gi-coupled plasma membrane estrogen receptors in immortalized gonadotropin-releasing hormone neurons. Mol Endocrinol 17:1792–1804 [DOI] [PubMed] [Google Scholar]
- Pietras RJ, Szego CM 1977 Specific binding sites for oestrogen at the outer surfaces of isolated endometrial cells. Nature 265:69–72 [DOI] [PubMed] [Google Scholar]
- Morley P, Whitfield JF, Vanderhyden BC, Tsang BK, Schwartz JL 1992 A new, nongenomic estrogen action: the rapid release of intracellular calcium. Endocrinology 131:1305–1312 [DOI] [PubMed] [Google Scholar]
- Ho KJ, Liao JK 2002 Non-nuclear actions of estrogen: new targets for prevention and treatment of cardiovascular disease. Mol Interv 2:219–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato A, Hiruma H, Kimura F 1994 Acute estradiol modulation of electrical activity of the LHRH pulse generator in the ovariectomized rat: restoration by naloxone. Neuroendocrinology 59:426–431 [DOI] [PubMed] [Google Scholar]
- Jarry H, Hirsch B, Leonhardt S, Wuttke W 1992 Amino acid neurotransmitter release in the preoptic area of rats during the positive feedback actions of estradiol on LH release. Neuroendocrinology 56:133–140 [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Qiu J, Wagner EJ, Ronnekleiv OK 2002 Rapid effects of estrogen on G protein-coupled receptor activation of potassium channels in the central nervous system (CNS). J Steroid Biochem Mol Biol 83:187–193 [DOI] [PubMed] [Google Scholar]
- Aronica SM, Kraus WL, Katzenellenbogen BS 1994 Estrogen action via the cAMP signaling pathway: stimulation of adenylate cyclase and cAMP-regulated gene transcription. Proc Natl Acad Sci USA 91:8517–8521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi N, Reuss AE, Wray S 2002 Prenatal LHRH neurons in nasal explant cultures express estrogen receptor β transcript. Endocrinology 143:2503–2507 [DOI] [PubMed] [Google Scholar]
- Herbison A, Skynner M, Sim J 2001 Lack of detection of estrogen receptor-α transcripts in mouse gonadotropin-releasing hormone neurons. Endocrinology 42:493 [DOI] [PubMed] [Google Scholar]
- Skynner MJ, Sim JA, Herbison AE 1999 Detection of estrogen receptor α and β messenger ribonucleic acids in adult gonadotropin-releasing hormone neurons. Endocrinology 140:5195–5201 [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Shughrue PJ, Merchenthaler I, Hajszan T, Carpenter CD, Liposits Z, Petersen SL 2000 Detection of estrogen receptor-β messenger ribonucleic acid and 125I-estrogen binding sites in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology 141:3506–3509 [DOI] [PubMed] [Google Scholar]
- Butler JA, Sjoberg M, Coen CW 1999 Evidence for oestrogen receptor α-immunoreactivity in gonadotrophin-releasing hormone-expressing neurones. J Neuroendocrinol 11:331–335 [DOI] [PubMed] [Google Scholar]
- Kato M, Ui-Tei K, Watanabe M, Sakuma Y 2003 Characterization of voltage-gated calcium currents in gonadotropin-releasing hormone neurons tagged with green fluorescent protein in rats. Endocrinology 144:5118–5125 [DOI] [PubMed] [Google Scholar]
- Martinez-Fuentes AJ, Hu L, Krsmanovic LZ, Catt KJ 2004 Gonadotropin-releasing hormone (GnRH) receptor expression and membrane signaling in early embryonic GnRH neurons: role in pulsatile neurosecretion. Mol Endocrinol 18:1808–1817 [DOI] [PubMed] [Google Scholar]
- Krsmanovic LZ, Stojilkovic SS, Catt KJ 1996 Pulsatile gonadotropin-releasing hormone release and its regulation. Trends Endocrinol Metab 7:56–59 [DOI] [PubMed] [Google Scholar]
- Weiner RI, Charles A 2001 Regulation of gonadotropin-releasing hormone release by cyclic AMP signalling pathways. Growth Horm IGF Res 11(Suppl A):S9–S15 [DOI] [PubMed] [Google Scholar]
- Krsmanovic LZ, Martinez-Fuentes AJ, Arora KK, Mores N, Navarro CE, Chen HC, Stojilkovic SS, Catt KJ 1999 Autocrine regulation of gonadotropin-releasing hormone secretion in cultured hypothalamic neurons. Endocrinology 140:1423–1431 [DOI] [PubMed] [Google Scholar]
- Krsmanovic LZ, Mores N, Navarro CE, Arora KK, Catt KJ 2003 An agonist-induced switch in G protein coupling of the gonadotropin-releasing hormone receptor regulates pulsatile neuropeptide secretion. Proc Natl Acad Sci USA 100:2969–2974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse R, Schmid A, Schoneberg T, Herrlich A, Muhn P, Schultz G, Gudermann T 2000 Gonadotropin-releasing hormone receptor initiates multiple signaling pathways by exclusively coupling to G(q/11) proteins. J Biol Chem 275:9193–9200 [DOI] [PubMed] [Google Scholar]
- Krsmanovic LZ, Mores N, Navarro CE, Saeed SA, Arora KK, Catt KJ 1998 Muscarinic regulation of intracellular signaling and neurosecretion in gonadotropin-releasing hormone neurons. Endocrinology 139:4037–4043 [DOI] [PubMed] [Google Scholar]
- Abe H, Keen KL, Terasawa E 2008 Rapid action of estrogens on intracellular calcium oscillations in primate luteinizing hormone-releasing hormone-1 neurons. Endocrinology 149:1155–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison AE, Pape JR 2001 New evidence for estrogen receptors in gonadotropin-releasing hormone neurons. Front Neuroendocrinol 22:292–308 [DOI] [PubMed] [Google Scholar]
- Shivers BD, Harlan RE, Morrell JI, Pfaff DW 1983 Absence of oestradiol concentration in cell nuclei of LHRH-immunoreactive neurones. Nature 304:345–347 [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Ronnekleiv OK, Eskay RL 1984 Identification of estrogen-responsive LHRH neurons in the guinea pig hypothalamus. Brain Res Bull 12:399–407 [DOI] [PubMed] [Google Scholar]
- Langub Jr MC, Watson Jr RE 1992 Estrogen receptor-immunoreactive glia, endothelia, and ependyma in guinea pig preoptic area and median eminence: electron microscopy. Endocrinology 130:364–372 [DOI] [PubMed] [Google Scholar]
- Herbison AE, Theodosis DT 1992 Localization of oestrogen receptors in preoptic neurons containing neurotensin but not tyrosine hydroxylase, cholecystokinin or luteinizing hormone-releasing hormone in the male and female rat. Neuroscience 50:283–298 [DOI] [PubMed] [Google Scholar]
- Roy D, Angelini NL, Belsham DD 1999 Estrogen directly represses gonadotropin-releasing hormone (GnRH) gene expression in estrogen receptor-α (ERα)- and ERβ-expressing GT1–7 GnRH neurons. Endocrinology 140:5045–5053 [DOI] [PubMed] [Google Scholar]
- Martinez-Morales JR, Morales A, Marin R, Hernandez-Jimenez JG, Acevedo A, Guerra B, Hernandez G, Lopez-Coviella I, Prieto L, Alonso R 2001 Estrogen modulates norepinephrine-induced accumulation of adenosine cyclic monophosphate in a subpopulation of immortalized luteinizing hormone-releasing hormone secreting neurons from the mouse hypothalamus. Neurosci Lett 298:61–64 [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Bushnell CD, Dorsa DM 1992 Estrogen receptor messenger ribonucleic acid in female rat brain during the estrous cycle: a comparison with ovariectomized females and intact males. Endocrinology 131:381–388 [DOI] [PubMed] [Google Scholar]
- Simerly RB, Carr AM, Zee MC, Lorang D 1996 Ovarian steroid regulation of estrogen and progesterone receptor messenger ribonucleic acid in the anteroventral periventricular nucleus of the rat. J Neuroendocrinol 8:45–56 [DOI] [PubMed] [Google Scholar]
- Childs GV, Unabia G, Komak S 2001 Differential expression of estradiol receptors α and β by gonadotropes during the estrous cycle. J Histochem Cytochem 49:665–666 [DOI] [PubMed] [Google Scholar]
- Dorling AA, Todman MG, Korach KS, Herbison AE 2003 Critical role for estrogen receptor α in negative feedback regulation of gonadotropin-releasing hormone mRNA expression in the female mouse. Neuroendocrinology 78:204–209 [DOI] [PubMed] [Google Scholar]
- Wintermantel TM, Campbell RE, Porteous R, Bock D, Grone HJ, Todman MG, Korach KS, Greiner E, Perez CA, Schutz G, Herbison AE 2006 Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron 52:271–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Majdoubi M, Weiner RI 2002 Localization of olfactory cyclic nucleotide-gated channels in rat gonadotropin-releasing hormone neurons. Endocrinology 143:2441–2444 [DOI] [PubMed] [Google Scholar]
- Abe H, Terasawa E 2005 Firing pattern and rapid modulation of activity by estrogen in primate luteinizing hormone releasing hormone-1 neurons. Endocrinology 146:4312–4320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krsmanovic LZ, Stojilkovic SS, Balla T, al-Damluji S, Weiner RI, Catt KJ 1991 Receptors and neurosecretory actions of endothelin in hypothalamic neurons. Proc Natl Acad Sci USA 88:11124–11128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon PL, Windle JJ, Goldsmith PC, Padula CA, Roberts JL, Weiner RI 1990 Immortalization of hypothalamic GnRH neurons by genetically targeted tumorigenesis. Neuron 5:1–10 [DOI] [PubMed] [Google Scholar]
- Urban RJ, Johnson ML, Veldhuis JD 1989 In vivo biological validation and biophysical modeling of the sensitivity and positive accuracy of endocrine peak detection. I. The LH pulse signal. Endocrinology 124:2541–2547 [DOI] [PubMed] [Google Scholar]
- Fujita K, Aguilera G, Catt KJ 1979 The role of cyclic AMP in aldosterone production by isolated zona glomerulosa cell. J Biol Chem 254:8567–8574 [PubMed] [Google Scholar]