Abstract
Classically, activated transcription by nuclear receptors (NRs) is due to a ligand-induced switch from corepressor- to coactivator-bound states. However, coactivators and corepressors recognize overlapping surfaces of liganded and unliganded NRs, respectively. Here we show that, at sufficiently high concentration, the NR corepressor (NCoR) influences the activity of the liver X receptor (LXR) even in the presence of a potent full agonist that destabilizes NCoR binding. Partial agonist ligands that less effectively dissociate NCoR from LXR are even more sensitive to NCoR levels, in a target gene-selective manner. Thus, differential recruitment of NCoR is a major determinant of partial agonism and selective LXR modulation of target genes.
THE LIVER X RECEPTORS, LXRα [nuclear receptor (NR1H3)] and LXRβ (NR1H2) are highly homologous members of the nuclear hormone receptor superfamily that are encoded by two distinct genes (1). LXRα displays a more limited expression profile, with high expression in the liver, adipose, intestine, kidneys, and macrophages, whereas LXRβ is ubiquitously expressed. These receptors have been shown to play important roles in cholesterol and lipid homeostasis as well as innate immunity and inflammation. Although first described as an orphan nuclear receptor, LXR was later shown to be regulated by endogenous oxysterol ligands (2,3,4). LXRs regulate transcription at target genes via binding to LXREs (liver X receptor response element) within promoters of target genes. In the unliganded state, LXR preferentially associates with the nuclear receptor corepressor (NCoR) (5). Upon ligand binding, the corepressor complex is dismissed and coactivators such as steroid receptor coactivator-1 (SRC-1) are recruited, facilitating alterations to local chromatin architecture and subsequent recruitment of the general transcription machinery.
LXR regulates whole-body cholesterol metabolism via direct transcriptional regulation of ATP-binding cassette transporter (ABC) A1 in macrophages, which promotes cellular cholesterol mobilization and efflux from macrophages to apolipoprotein A-1 and apolipoprotein E acceptors to form nascent high density lipoprotein particles (6,7). Together with other LXR target genes including apolipoprotein E, these proteins regulate a pathway for the removal of cholesterol from the periphery for transport to the liver termed reverse cholesterol transport (8,9). Additionally, in rodents LXR-α promotes the hepatic induction of cholesterol 7α, which facilitates hepato-biliary cholesterol secretion in the face of excess dietary cholesterol (10). This is dramatically illustrated by mice lacking LXR, which display severe hypercholesterolemia and hepatic steatosis on a high-fat, high-cholesterol diet (11).
Due to the potential of LXR as a drug target for dyslipidemia, several highly potent and active agonists have been synthesized, including T091317 (12). However, the therapeutic utility of LXR ligands has been limited by hepatic steatosis and hypertriglyceridemia (12,13). These effects may be due to direct induction of the lipogenic transcription factor sterol-regulatory element binding protein 1 (SREBP1) as well as other lipogenic LXR target genes including fatty acid synthase (FAS), which leads to a robust induction of fatty acid synthesis in the liver (14,15,16). The limitations imposed by the dual regulation of de novo lipogenesis and reverse cholesterol transport by LXR indicate that a target-specific approach to modulation of LXR activity will be critical for successful therapeutic intervention. It is also critical to study LXR effects in humans because the beneficial induction of cholesterol 7α may be rodent specific (11), and rodents also lack functional cholesteryl ester transport protein, which is a detrimental LXR target gene in humans (17,18).
In the case of estrogen receptor (ER), differential recruitment of coactivators and corepressors may explain why selective ER modulators (SERMs) have target gene and cell type selectivity (19). SERM binding induces ER conformations that are different from those of ER bound to potent agonists. Similarly, different conformations have been noted for LXR bound to the partial agonist 24(S), 25-epoxycholesterol and the potent synthetic agonist T091317, suggesting that it may be possible to selectively modulate the activity of LXR (20). Selective modulation could involve quantitative or qualitative differences in coactivator association with liganded LXR (21,22,23,24,25). Alternatively, although not mutually exclusively, different ligands could alter interaction with NCoR (26).
Here we show that NCoR binding plays a critical role in the differential response of LXR to a variety of ligands. Using in vitro biochemical studies and chromatin immunoprecipitation (ChIP) analysis in cells, we demonstrate that, independent of binding affinity, different LXR agonist ligands have vastly different properties in destabilizing the association of NCoR with LXR target genes. In addition, modulation of the level of NCoR protein differentially influences ligand activation of LXR and is target gene specific. Moreover, individual ligands have target gene-specific effects on NCoR dismissal from endogenous LXR targets. Thus, differential NCoR recruitment underlies gene-specific differences in activation by these ligands.
RESULTS
Partial Agonism of LXR
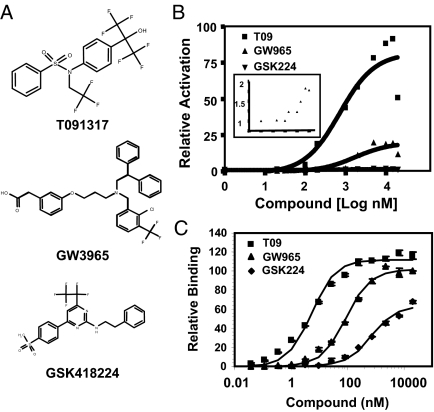
We studied two ligands that have been previously described, the sulfonamide T091317 and GW3965 (12,27), as well as a novel LXR ligand GSK418224 (Fig. 1A), referred to as T09, GW965, and GSK224. The activities of the ligands were tested by in vitro fluorescence-resonance energy transfer (FRET) assays, luciferase reporter assays using Gal-LXR or full-length LXR in human kidney 293T cells, and assays of endogenous gene expression in human liver cells. The results are summarized in Table 1. In transient transfections, T09 induced high levels of LXRα transactivation, relative to the maximum efficacy of GW965, which was only approximately 23% of that of T09 (Fig. 1B). Maximum efficacy of GSK224 was an order of magnitude lower still and approximately 1% that of T09 (Fig. 1B, see inset). Thus, both GW965 and GSK224 functioned as partial agonists relative to the full agonist T09. To determine whether the reduced efficacies at saturating concentrations were due to differences in coactivator recruitment, a cell-free FRET assay was used to measure recruitment of a biotinylated SRC-1 NR box peptide (28) in the presence of increasing concentrations of ligand. Comparisons of the relative ability to recruit coactivator at maximal ligand concentration revealed only a minor difference between T09 and GW965, whereas the effectiveness of GSK224 was less but nevertheless considerably more than would have been predicted by its low relative level of transcriptional activity (Fig. 1C).
Figure 1.
Transactivation by LXR Ligands Disproportionate to Coactivator Recruitment
A, Structures of LXR agonists. B, Activity of LXR agonists in transient transfection assay with Gal4-LXRα cotransfected with 5×UAS-SV40-luciferase reporter. EC50 values were 1.0 μm (GSK224), 2.8 μm (GW965), and 1.3 μm (T09). Inset shows a magnified view of GSK224 and vehicle data. C, FRET assay for the indicated compounds incubated with LXRα LBD and SRC-1 peptide. EC50 values were 5.90 μm (GSK224), 6.60 μm (GW965), and 7.10 μm (T09). Data shown are representative results (n = 3), and the experiment was repeated two times with similar results.
Table 1.
LXR Ligand Properties
| Ligand | Agonism | NCoR Dismissal (FRET) | Gal-LXRα | siNCoR | NCoR O/E | hABCA1-Luc | Gene Expression |
|---|---|---|---|---|---|---|---|
| GSK224 | Weak partial | −7% | ↑ | 5.2↑ | 1.3↓ | ↑ | ↑↑ABCA1 |
| ↑↑FAS | |||||||
| ↑↑SREBP1 | |||||||
| GW965 | Partial | −23% | ↑↑↑ | 3.7↑ | 1.8↓ | ↑↑ | ↑↑ABCA1 |
| ↑↑FAS | |||||||
| ↑↑SREBP1 | |||||||
| T091317 | Full | −94% | ↑↑↑↑↑ | 2.0↑ | 1.2↓ | ↑↑↑ | ↑↑ABCA1 |
| ↑↑↑FAS | |||||||
| ↑↑ SREBP1 |
NCoR O/E, Overexpression of NCoR.
LXR Agonists Display Major Differences in Dissociating Corepressor
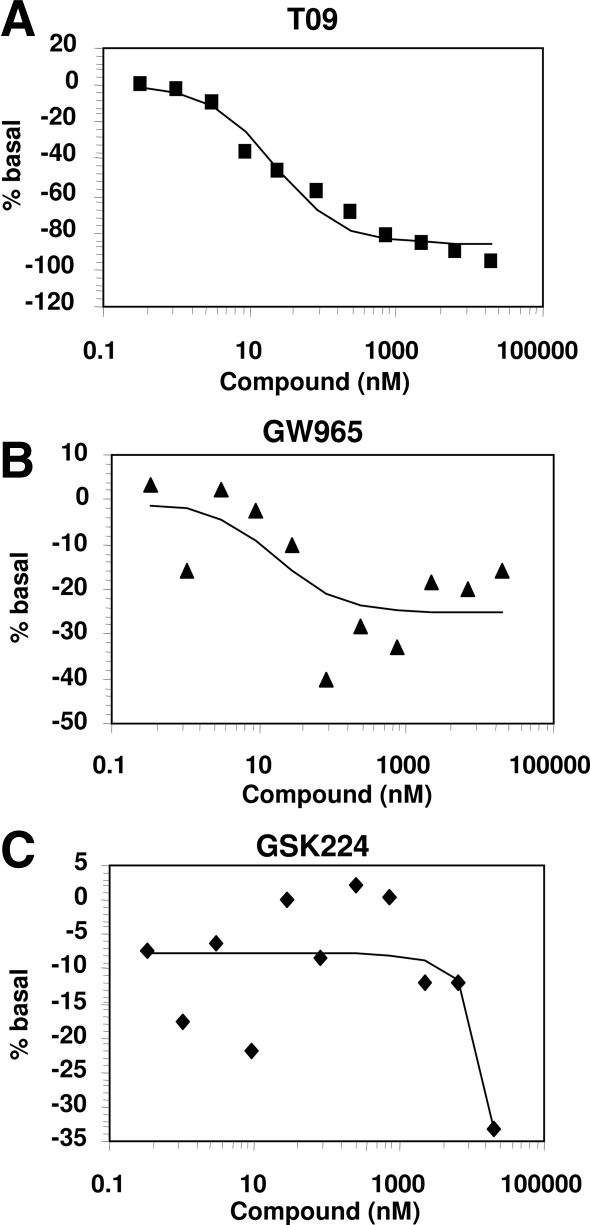
Because coactivator recruitment was insufficient to explain the large differences in transactivation by the LXR ligands, we next examined their abilities to displace a biotinylated NCoR CoRNR (corepressor NR box) peptide (29) from LXR. In this assay, T09 markedly destabilized NCoR binding (Fig. 2A), whereas GW965 was considerably less efficacious at maximal ligand concentrations (Fig. 2B), and GSK224 had a very muted effect (Fig. 2C). Thus, the degree of agonism of GW965 and GSK224 in the Gal4-LXR transactivation assay correlated with their inability to induce dissociation of NCoR from LXR.
Figure 2.
LXR Ligands Differentially Dismiss Corepressor CoRNR Peptide in Vitro
Relative FRET for the indicated compounds was determined by incubation of LXRα LBD with NCoR peptide in the presence of increasing ligand concentration. A, T0901317. B, GW965. C, GSK224. EC50 values were 5.01 μm (GSK224), 7.51 μm (GW965), and 7.53 μm (T09). Data shown are representative results, and the experiment was repeated two times with similar results.
Differential Effects of Ligands on Target Gene Recruitment of NCoR by LXR
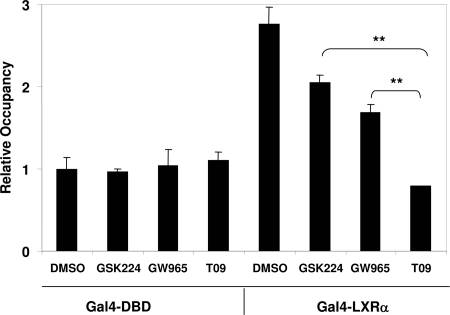
We next employed ChIP to assess the effects of LXR ligands on NCoR recruitment by Gal4-LXRα in intact cells. As expected, NCoR was robustly recruited by Gal-LXRα relative to the Gal DNA-binding domain (DBD) alone (Fig. 3). Consistent with the predictions from in vitro studies, GSK224 had little effect on NCoR recruitment, and GW965 had a moderate effect, whereas T09 led to near complete dissociation of NCoR from the reporter gene (Fig. 3).
Figure 3.
Differential Dismissal of Corepressor from LXR on an LXRα-Responsive Reporter Gene
293T cells were cotransfected with 5×UAS-SV40-luciferase and Gal-LXRα or Gal-DBD control and treated with ligand (10 μm) or vehicle overnight. ChIP assay was performed with anti-NCoR antibody as described in Materials and Methods. ChIP signal was normalized to nonspecific 36B4 gene. Gal-DBD was a negative control for NCoR recruitment by ChIP, and relative recruitment was compared with Gal-DBD, DMSO treatment. Error bars represent sem, n = 3 from three independent experiments. **, P < 0.01 calculated by Student’s t test.
LXRα Transactivation Is Modulated by Cellular NCoR Levels
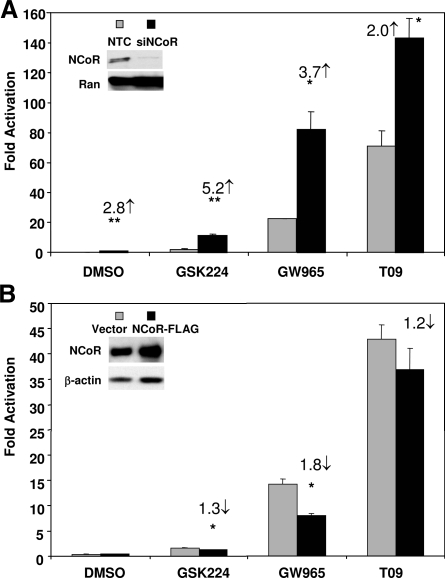
To determine the role of NCoR in modulating the activity of LXR ligands, we repeated the transactivation assays at saturating concentrations of ligands after a reduction in NCoR levels using small interfering RNA (siRNAs). Under these conditions, activation by GSK224 increased approximately 5.2-fold, and the activity of GW965 also increased markedly (∼3.7-fold, Fig. 4A). Even T09 activity was increased when NCoR levels were reduced, although the change was more modest (Fig. 4A). Overexpression of NCoR had its greatest effect on the activity of GW965, which was reduced by about 50% (Fig. 4B). GSK224 activity was decreased only approximately 1.3-fold, consistent with the previous conclusion that endogenous NCoR was already bound in this setting and the activity of the full agonist T09 was affected even less by exogenous NCoR (Fig. 4B). In sum, GW965-mediated association with NCoR was highly dynamic and thus most sensitive to the level of NCoR.
Figure 4.
Modulation of NCoR Levels Differentially Influences Ligand-Dependent Transactivation of LXRα
A, NCoR knockdown. 293T cells were cotransfected with 5×UAS-SV40-luciferase and Gal-LXRα along with siRNA against human NCoR or nontargeting control (NTC). Cells were treated with ligands (10 μm) or vehicle overnight. Relative activation was determined as fold change vs. Gal-DBD controls. *, P < 0.05 or **, P < 0.01 compared with NTC. NCoR knockdown was confirmed by immunoblotting (inset), and Ran protein level is shown as loading control. B, NCoR overexpression. 293T cells were cotransfected with 5×UAS-SV40-luciferase and Gal-LXRα along with human NCoR expression vector or empty control plasmid. Cells were treated with ligands (10 μm) or vehicle overnight. Each bar is the mean value ± se (n = 3) from three independent experiments. NCoR expression was confirmed by immunoblotting (inset), and β-actin protein level is shown as a loading control. *, P < 0.05 compared with vector control for each ligand treatment calculated by Student’s t test. siNCoR, small interfering NCoR.
Partial Agonism of LXRα/Retinoic X Receptor (RXRα) Heterodimers
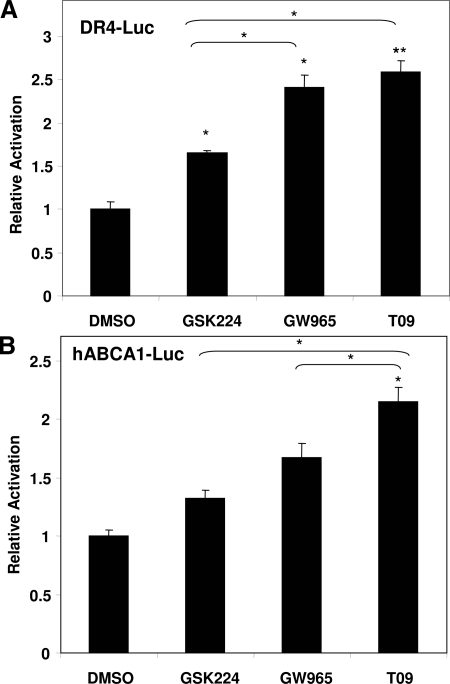
We next determined whether the ligands also differentially activated the full-length receptor LXR by cotransfecting expression plasmids for LXRα and RXRα along with LXR-responsive luciferase reporter constructs into 293T cells. Using a reporter containing three copies of an LXR-response element (DR-4) cloned upstream of the herpes simplex virus thymidine kinase promoter, T09 and GW965 induced similar levels of activation, whereas GSK224 treatment led to a lower induction (Fig. 5A). We also examined a luciferase reporter driven by the human ABCA1 promoter. 293T cells were cotransfected with a luciferase reporter driven by 1.45 kb of the proximal human ABCA1 promoter, containing the LXR-response element (30). hABCA1-driven luciferase activity was induced to the greatest extent by T09, followed by GW965, and then GSK224. Together, these results confirm that T09 is the strongest LXR agonist, with GW965 intermediate, and GSK224 a weaker agonist.
Figure 5.
Differential Activation of Full-Length LXR
A, DR-4 Element. 293T cells were cotransfected with pCIS control plasmid or 3×-(DR-4)-tk-luciferase, pCMX-LXRα, pCMX-RXRα and β-galactosidase and treated with 10 μm ligand or vehicle for 18 h. B, hABCA1 promoter activity. 293T cells were cotransfected with pGL3 control plasmid or pGL3 containing a 1.45-kb proximal promoter fragment of the human Abca1 promoter spanning the LXRE site, pCMX-LXRα, pCMX-RXRα, and β-galactosidase and treated with 10 μm ligand or vehicle for 18 h. Luciferase activity was measured and normalized to cotransfected β-galactosidase activity. Relative activation was determined by normalizing to control vector treated with corresponding vehicle or ligand. Results are representative of a minimum of three independent experiments. Activation upon treatment with DMSO was set to equal 1. Each bar is the mean value ± se of triplicate samples from three independent experiments. Statistically significant induction compared with DMSO: *, P < 0.5 or **, P < 0.01 by Student’s t test or between ligands where indicated.
Partial Agonism of LXRα in Human Liver-Derived Cells
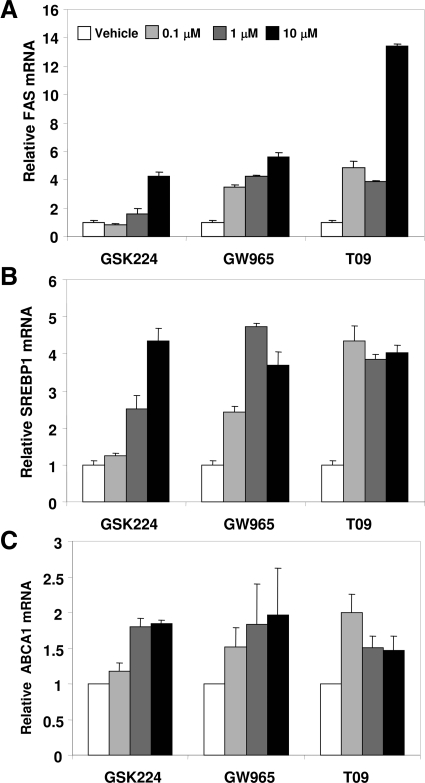
We next determined the activity of these LXR ligands on endogenous gene expression in human HepG2 hepatoma cells. Fatty acid synthase (FAS), an LXR target that may contribute to hepatic triglyceride accumulation that is an undesirable side effect for clinical LXR agonism, was dramatically induced by T09, whereas GW965 was less active at inducing FAS mRNA and GSK224 even less so (Fig. 6A). By contrast, in the same cells, the expression of another lipogenic LXR target, SREBP1, was increased to similar extents by all three ligands (Fig. 6B). Similarly all three ligands activated another LXR target, the cholesterol efflux gene ABCA1 (Fig. 6C). Thus, T09 behaved as a full agonist at all genes tested, whereas the degree of agonism of GSK224 and GW965 was gene specific.
Figure 6.
Endogenous Gene Regulation by LXR Ligands in Hepatoma Cells
HepG2 cells were seeded in 24-well plates and incubated with increasing concentrations of ligand or vehicle in media containing 10% delipidated media overnight, after which target gene expression was analyzed by quantitative real-time PCR. A, FAS gene expression. B, SREBP1 gene expression. C, ABCA1 gene expression. Results indicate mean ± se from triplicates of a representative experiment repeated three times with similar results.
NCoR Occupancy of Endogenous LXR Target Genes in Liver Cells
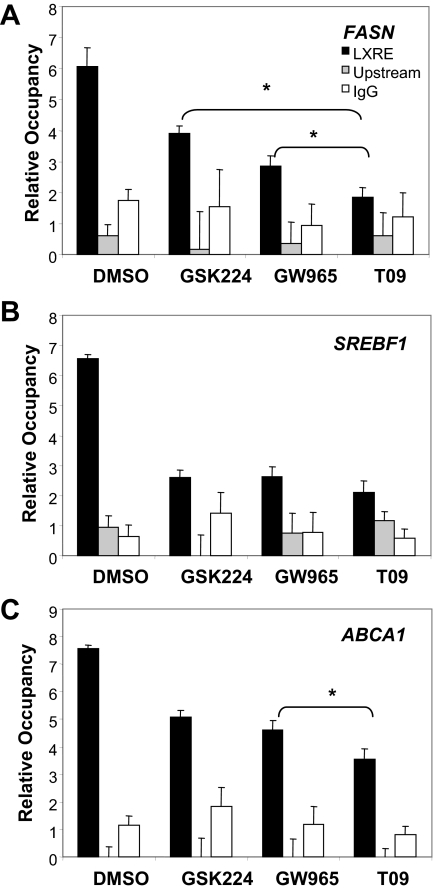
To further assess the contribution of NCoR association to relative expression levels at these LXR target genes, we performed ChIP in the vicinity of known LXREs in the endogenous FAS, SREBP1, and ABCA1 genes at saturating concentrations of each of the LXR ligands. The degree of NCoR dismissal from the fatty acid synthase gene was ligand specific and, consistent with the gene expression, reduced only modestly by GSK224, moderately by GW965, and markedly by T09 (Fig. 7A). By contrast, only a modest difference in relative NCoR recruitment was observed among the ligands at the SREBP1 promoter, consistent with the gene expression data (Fig. 7B). Examination of the LXRE at ABCA1 revealed a trend of graded occupancy; however, the only significant difference observed was between GW965 and T09. Thus, the level of NCoR occupancy correlated with the differential activation of these LXR target genes.
Figure 7.
LXR Ligands Differentially Recruit NCoR to Endogenous Target Genes
HepG2 cells were seeded in 24-well plates and incubated with ligand (10 μm) or vehicle in media containing 10% delipidated media overnight, after which ChIP analysis for NCoR was performed. A, FAS gene. B, SREBP1c gene. C, ABCA1 gene. ChIP signal was normalized to nonspecific DNA region spanning the 36B4 gene, and data represent mean ± sem (n = 3). Data are from a representative experiment repeated three times with similar results. *, P < 0.05 calculated by Student’s t test.
Knockdown of NCoR Enhances Expression of LXR Target Genes
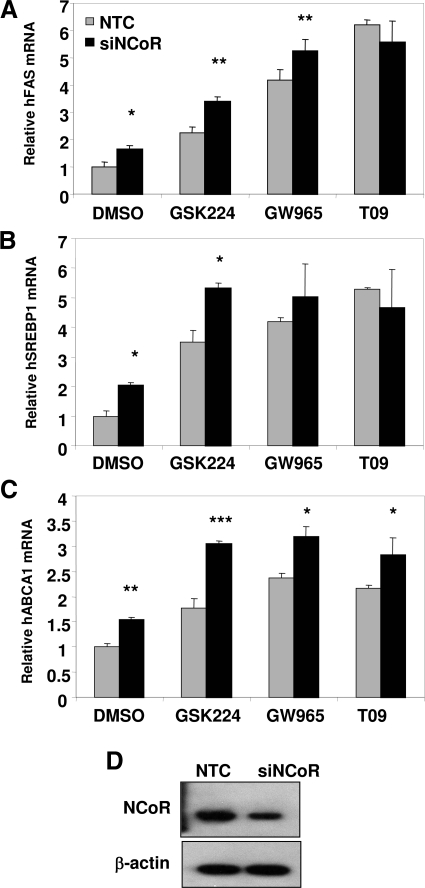
Given the gene-specific occupancy by NCoR in the presence of various LXR ligands, we explored the consequence of NCoR knockdown. Consistent with NCoR occupancy shown earlier, depletion of NCoR induced expression of the FAS gene (Fig. 8A). Reduction in NCoR levels also enhanced activation by GSK224 and GSK965, but not T09, which was also consistent with the level of NCoR occupancy in the presence of these compounds (Fig. 8A). NCoR depletion also potentiated induction of SREBP1 and ABCA1 mRNA by GSK224, and in the case of ABCA1 activation was also increased, but to a lesser extent, by the other compounds (Fig. 8, B and C). Thus, the degree of association with NCoR in the presence of LXR ligands is a target gene-specific determinant of partial agonist activity.
Figure 8.
NCoR Depletion Induces Basal Activation of LXR Target Genes and Differentially Potentiates Ligand-Dependent Gene Activation
HepG2 cells were electroporated with siRNA targeting human NCoR or NTC. Cells were treated with the indicated ligands (10 μm) or vehicle control overnight in media containing 10% delipidated serum. Relative expression was calculated by normalizing real-time PCR data to 36B4 expression, and fold change indicates relative increase over NTC vehicle-treated cells. A, Fatty acid synthase gene expression. B, SREBP1c gene expression. C, ABCA1 gene expression. Data represent mean ± sem of triplicate samples. Data are from a representative experiment repeated three times with similar results. *, P < 0.05; **, P < 0.01; or ***, P < 0.005 relative to siNTC calculated by Student’s t test. NTC, Nontargeting control; siNCoR, small interfering NCoR; siNTC, small interfering NTC.
DISCUSSION
We have described a mode of selective modulation of LXR in which the relative recruitment of the corepressor NCoR is a critical determinant of transcriptional efficacy. Two compounds that function as partial agonists relative to T09 fail to completely dissociate NCoR from LXR, with the least active compound causing almost no dissociation either in FRET assays performed in vitro or in ChIP assays in living cells. Affinity of liganded LXR for NCoR is likely to explain a portion of the reduced efficacy of these compounds. Consistent with this, removal of NCoR from cells enhanced the activity of the partial agonists. Others have previously correlated failure to dissociate NCoR with partial agonist activity (26). However, the present report is the first to demonstrate differential NCoR dismissal from endogenous genes and the first to test and confirm the NCoR hypothesis by manipulating NCoR concentrations.
It is likely that partial agonism reflects a combination of altered coactivator affinity as well as failure to completely dissociate NCoR. In this regard, it is of interest that SERMs differ from the full agonist estradiol because they alter the range of coactivator specificity as well as favoring interaction with corepressors (19). Note that, unlike LXR, ER does not have affinity for NCoR nor is it mostly bound to target genes in the unliganded state. Thus the role of corepressors is likely to be greater for LXR. This mechanism is potentially applicable to all NRs that bind corepressors, and particularly those that are bound to target genes in the absence of ligand (31).
For the development of potential therapies targeting LXR, the concomitant induction of hepatic de novo lipogenesis remains a major obstacle. Thus, it was of great interest to assess whether compounds that functioned as partial agonists in vitro and in transient transfection assays retained these properties on endogenous genes in liver cells. Indeed, GSK224 had very little effect on the lipogenic FAS gene, in part due to its failure to dissociate NCoR. Consistent with the differential effects of these ligands on NCoR dismissal at the FAS promoter, rodent studies have shown that indeed GW965 treatment results in decreased hepatic triglyceride accumulation compared with the full agonist T09 (25). However, GSK224 did activate the lipogenic SREBP1 gene, and enhanced dissociation of NCoR, about as well as the full agonist T09. Because both FAS and SREBP1 were evaluated in the same cells, these data strongly suggest that additional cis-elements in the SREBP1 gene, perhaps interacting with gene-specific factors, alter the conformation of the GSK224-bound LXR so as to recapitulate that of the full agonist-bound form. A better understanding of these factors will be critical to the rational design of selective LXR modulators that do not cause hepatic lipogenesis.
MATERIALS AND METHODS
Reagents
Compounds were provided by GlaxoSmithKline or obtained from Calbiochem (La Jolla, CA). DMSO was obtained from Sigma (St. Louis, MO). For Western blot analysis, the antibody for NCoR has been previously described (32), and antibodies for β-actin (Sigma) and Ran (BD Biosciences, San Jose, CA) were obtained. For chromatin immunoprecipitation, antibodies for NCoR were obtained from Abcam (Cambridge, MA), and control IgG antibodies were from Calbiochem. Protein A-Sepharose was from Amersham Biosciences (Piscataway, NJ).
Plasmids
pFA-CMV-Gal4-hLXRα ligand-binding domain (LBD) (162–447), pFA-CMV-Gal4-DBD, and pGL3-hABCA1-Luc were provided by GlaxoSmithKline; GAL-5XUAS-SV40-luciferase, β-galactosidase, pCMX-NCoR-FLAG, pCMX-mLXRα, and pCMX-mRXRα plasmids have been previously described (32,33). pGL3-basic was obtained from Promega (Madison, WI), and pCIS and pCIS-LXRE-Luc were obtained from Stratagene (San Diego, CA).
FRET Assay
A modified polyhistidine tag was fused in frame to the human LXR ligand-binding domain and subcloned into the expression vector pRSETa (Invitrogen) The human LXR ligand binding domain was expressed in Escherichia coli strain BL21(DE3) in Rich PO4 media with 0.1 mg/ml ampicillin at 25 C for 12 h, and 0.25 mm isopropyl-β-d-thiogalactopyranoside was added Cells were resuspended and concentrated cell slurries were stored in PBS at −80 C. Cell paste was resuspended in TBS, pH 8.0 (25 mm Tris, 150 mm NaCl). Cells were lysed by an APV Rannie MINI-lab homogenizer, and cell debris was removed by centrifugation. The cleared supernatant was filtered through coarse prefilters, and TBS, pH 8.0, containing 500 mm imidazole was added to obtain a final imidazole concentration of 50 mm. Lysate was loaded onto Sepharose (Ni++ charged) Chelation resin (Pharmacia) and preequilibrated with TBS (pH 8.0)/50 mm imidazole. The column was washed with approximately one column volume of TBS (pH −8.0) containing 95 mm imidazole. LXRLBD was eluted with a gradient from 50–500 mm imidazole. Column peak fractions were pooled immediately and diluted 5-fold with 25 mm Tris (pH 8.0), containing 5% 1,2-propanediol, 0.5 mm EDTA, and 5 mm dithiothreitol (DTT). The diluted protein sample was then loaded onto Poros HQ, washed with the dilution buffer, and the protein was eluted with a gradient from 50 −500 mm NaCl. Peak fractions were pooled and concentrated using Centri-prep 10K (Amicon) filter devices and subjected to size exclusion, using Superdex-75 resin (Pharmacia) preequilibrated with TBS (pH 8.0), containing 5% 1,2-propanediol, 0.5 mm EDTA, and 5 mm DTT. LXR protein was diluted to in PBS, and 5-fold molar excess of NHS-LC-Biotin (Pierce Chemical Co., Rockford, IL) was added in a minimal volume of PBS and incubated for 30 min. The biotinylation modification reaction was stopped by the addition of 2000× molar excess of Tris-HCl, pH 8. The modified LXR protein was dialyzed against PBS containing 5 mm DTT, 2 mm EDTA, and 2% sucrose. The biotinylated LXR protein was subjected to mass spectrometric analysis until approximately 95% of the protein had at least a single site of biotinylation; and the overall extent of biotinylation followed a normal distribution of multiple sites, ranging from one to nine. The biotinylated protein was incubated for 20–25 min at a concentration of 25 nm in assay buffer [50 mm KCl, 50 mm Tris (pH 8), 0.1 mg/ml fatty acid-free BSA; 10 mm DTT] with equimolar amounts of streptavidin-AlloPhycoCyanin (APC, Molecular Probes Inc., Eugene, OR). At the same time, the biotinylated peptide of SRC-1 or NCoR at a concentration of 25 nm was incubated in assay buffer with a ∏ molar amount of streptavidin-labeled Europium (Wallac, Inc., Boston, MA) for 20–25 min. After the initial incubations are completed, a 10 molar excess of cold biotin was added to each of the solutions to block the unattached streptavidin reagents. After 20 min the solutions were mixed yielding a concentration of 12.5 nm for the dye-labeled LXR protein and SRC-1 or NCoR peptide.
Mammalian Cell Culture and Transfection
HEK-293T and HepG2 cells were maintained in DMEM (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Hyclone Laboratories, Inc., Logan, UT) and penicillin/streptomycin (Invitrogen) at 37 C in 5% CO2. Transient transfection of 293T cells was performed with Lipofectamine 2000 (Invitrogen) according to manufacturer’s instructions. Briefly, cells were seeded in 24-well plates and allowed to adhere before transfection with 1.1 μg total of appropriate plasmids and 2.5μl of lipofectamine reagent in Opti-MEM (Invitrogen). Transfection mixture was incubated with cells overnight in DMEM containing 10% delipidated bovine serum (Intracel, Frederick, MD). The following day, the transfection mixture was aspirated and replaced with fresh delipidated medium. Cells were then treated with indicated compounds or vehicle in delipidated medium overnight for 16 h.
RNA Interference
293T cells were seeded into six-well plates and transfected with siRNA duplexes (Dharmacon, Lafayette, CO) targeting human NCoR or a nontargeting control using Lipofectamine 2000. Cells were then seeded in 24-well plates for further studies. HepG2 knockdown was carried out using the same siRNA duplexes, but cells were transfected using the Amaxa system, Cell Solution V, protocol T-28. For all knockdown studies, cells were harvested 96 h after knockdown for further analysis.
Luciferase Reporter Assay
After compound treatment for 16 h, cells were lysed in 100 μl of Passive Lysis Buffer (Promega) containing complete protease inhibitor (Roche, Indianapolis, IN). Cells were lysed by freeze-thaw at −80 C, and 5 μl of lysate was used for luciferase assay (Promega) or β-galactosidase assay. Relative light units for luciferase were normalized to β-gal activity.
Reverse Transcription and Quantitative PCR
Cells were lysed in Buffer RLT and processed using the RNeasy kit (QIAGEN, Chatsworth, CA) according to manufacturer’s instructions. Isolated RNA was reverse transcribed with oligo-dT primers using either Sprint Power Script reagents (CLONTECH Laboratories, Inc., Palo Alto, CA) or SuperScript II (Invitrogen) according to the supplied protocol. cDNA was used in Taqman reactions using commercially available primer/probe sets (Applied Biosystems, Foster City, CA) and Taqman Universal PCR mix (Applied Biosystems). Quantitative PCR was performed on the Applied Systems 7900HT or 7500 Real-Time System. Data were analyzed using the standard curve method with 36B4 serving as the housekeeping gene.
Chromatin Immunoprecipitation
293T cells were transfected as described in 10-cm dishes according to manufacturer instructions or HepG2 cells were plated in 10-cm dishes. Compounds were added in delipidated media overnight for 16 h. Cells were washed with PBS, and cell pellets were lysed in ChIP lysis buffer [50 mm HEPES/NaOH, 1% sodium dodecyl sulfate (SDS), 10 mm EDTA, 1 mm phenylmethylsulfonylfluoride, and complete protease inhibitor], incubated on ice for 10 min, and diluted in ChIP dilution buffer (50 mm HEPES/NaOH, 155 mm NaCl, 1.1% Triton X-100, 0.11% Na-deoxycholate, 1 mm phenylmethylsulfonylfluoride, and protease inhibitors with EDTA). Lysate was sonicated three times for 15 sec to yield an average DNA fragment length of approximately 500 bp. Lysates were clarified by centrifugation at 13,000 rpm for 10 min at 4 C. HepG2 samples were precleared for 2 h at 4 C with protein-A-sepharose beads containing sonicated salmon sperm DNA. Lysates were then incubated overnight at 4C with the following antibodies: anti-NCoR (Abcam) or rabbit IgG (Calbiochem) as a negative control. Immune complexes were then precipitated with protein A-sepharose for 2 h at 4 C. Complexes were subsequently washed with low-salt buffer, high-salt buffer, lithium chloride buffer, and Tris-EDTA as described by Upstate Biotechnology, Inc. (Lake Placid, NY) with minor modifications. Complexes were eluted in SDS elution buffer, and cross-links were reversed for a minimum of 6 h at 65 C. Eluted DNA was processed either by phenol/chloroform extraction or with the PCR Purification Kit (QIAGEN). DNA was used for quantitative PCR with POWER SYBR mix (Applied Biosystems) and the following primers: hFASN-forward (F)-gttactgccggtcatcgca, hFASN-reverse (R)-tctcgggtctgggttccc, hABCA1-F-acgtgctttctgctgagtga, hABCA1-R-accgagcgcagaggttacta, hSREBF1-F-tgctgcacccatattatctcc, hSREBF11-R-gctccaaccctccgtttact, SV40- F-ttccggtactgttggtaaaatgg, SV40-R accagggcgtatctcttcatagc, h36B4-F-acgctgctgaacatgctcaa, h36B4-R-gatgctgccattgtcgaaca. Upstream primer sequences are available upon request.
Immunoblotting
Lysates were prepared in passive lysis buffer for cotransfection studies or in RIPA buffer [50 mm Tris-HCl (pH 8.0), 150 mm NaCl, 1% Triton X-100, 0.4% deoxycholate, 0.1% SDS]. Lysate (15–20 μg) was loaded on 4–20% Tris-Glycine gels (Invitrogen) and electrophoresed. Transfer was performed onto Immobilon-P (Millipore Corp., Bedford, MA) membranes, and membranes were blocked with 5% nonfat milk in Tris-buffered saline with Tween 20. Membranes were incubated overnight at 4 C with primary antibodies diluted in blocking buffer. Membranes were washed extensively and incubated with appropriate secondary antibodies (Pierce). After washes, chemiluminescent signal was detected with ECL-Plus (Amersham Biosciences, Arlington Heights, IL).
Footnotes
This work was supported by National Institutes of Health Grant DK45586 and the Penn-GSK Academic Alliance Discovery Initiative.
Disclosure Summary: C.A.P., J.M.W., D.J.S., S.J., and J.T.M. have nothing to declare. J.L.C., W.J.Z., T.M.W., M.W., and M.J. are employed by GlaxoSmithKline. M.A.L. received grant support from GlaxoSmithKline.
First Published Online July 31, 2008
Abbreviations: ABC, ATP-binding cassette; ChIP, chromatin immunoprecipitation; CoRNR, corepressor NR box; DBD, DNA-binding domain; DMSO, dimethylsulfoxide; DTT, dithiothreitol; ER, estrogen receptor; FAS, fatty acid synthase; FRET, fluorescence-resonance energy transfer; LBD, ligand-binding domain; LXR, liver X receptor; LXRE, LXR response element; NCoR, nuclear receptor corepressor; NR, nuclear receptor; RXRα, retinoic X receptor; SERM, selective ER modulator; siRNA, small interfering RNA; SRC-1, steroid receptor coactivator-1; SREBP1, sterol-regulatory element binding protein 1; SV40, simian virus 40; UAS, upstream activation sequence.
References
- Willy PJ, Umesono K, Ong ES, Evans RM, Heyman RA, Mangelsdorf DJ 1995 LXR, a nuclear receptor that defines a distinct retinoid response pathway. Genes Dev 9:1033–1045 [DOI] [PubMed] [Google Scholar]
- Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ 1996 An oxysterol signalling pathway mediated by the nuclear receptor LXRα. Nature 383:728–731 [DOI] [PubMed] [Google Scholar]
- Lehmann JM, Kliewer SA, Moore LB, Smith-Oliver TA, Oliver BB, Su J-L, Sundseth SS, Winegar DA, Blanchard DE, Spencer TA, Willson TM 1997 Activation of the nuclear receptor LXR by oxysterols defines a new hormone response pathway. J Biol Chem 272:3137–3140 [DOI] [PubMed] [Google Scholar]
- Spencer TA, Li D, Russel JS, Collins JL, Bledsoe RK, Consler TG, Moore LB, Galardi CM, McKee DD, Moore JT, Watson MA, Parks DJ, Lambert MH, Willson TM 2001 Pharmacophore analysis of the nuclear oxysterol receptor LXRα. J Med Chem 44:886–897 [DOI] [PubMed] [Google Scholar]
- Hu X, Li S, Wu J, Xia C, Lala DS 2003 Liver X receptors interact with corepressors to regulate gene expression. Mol Endocrinol 17:1019–1026 [DOI] [PubMed] [Google Scholar]
- Costet P, Lalanne F, Gerbod-Giannone MC, Molina JR, Fu X, Lund EG, Gudas LJ, Tall AR 2003 Retinoic acid receptor-mediated induction of ABCA1 in macrophages. Mol Cell Biol 23:7756–7766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repa JJ, Turley SD, Lobaccaro JMA, Medina J, Li L, Lustig K, Shan B, Heyman RA, Dietschy JM, Mangelsdorf DJ 2000 Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers. Science 289:1524–1529 [DOI] [PubMed] [Google Scholar]
- Laffitte BA, Repa JJ, Joseph SB, Wilpitz DC, Kast HR, Mangelsdorf DJ, Tontonoz P 2001 LXRs control lipid-inducible expression of the apolipoprotein E gene in macrophages and adipocytes. Proc Natl Acad Sci USA 98:507–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkateswaran A, Laffitte BA, Joseph SB, Mak PA, Wilpitz DC, Edwards PA, Tontonoz P 2000 Control of cellular cholesterol efflux by the nuclear oxysterol receptor LXRα. Proc Natl Acad Sci USA 97:12097–12102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang JYL, Kimmel R, Stroup D 2001 Regulation of cholesterol 7α-hydroxylase gene (CYP7A1) transcription by the liver orphan receptor (LXRα). Gene 262:257–265 [DOI] [PubMed] [Google Scholar]
- Peet DJ, Turley SD, Ma W, Janowski BA, Lobaccaro J-MA, Hammer RE, Mangelsdorf DJ 1998 Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXRα. Cell 93:693–704 [DOI] [PubMed] [Google Scholar]
- Schultz JR, Tu H, Luk A, Repa JJ, Medina JC, Li L, Schwendner S, Wang S, Thoolen M, Mangelsdorf DJ, Lustig KD, Shan B 2000 Role of LXRs in control of lipogenesis. Genes Dev 14:2831–2838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grefhorst A, Elzinga BM, Voshol PJ, Plosch T, Kok T, Bloks VW, van der Sluijs FH, Havekes LM, Romijn JA, Verkade HJ, Kuipers F 2002 Stimulation of lipogenesis by pharmacological activation of the liver X receptor leads to production of large, triglyceride-rich very low density lipoprotein particles. J Biol Chem 277:34182–34190 [DOI] [PubMed] [Google Scholar]
- Repa JJ, Liang G, Ou J, Bashmakov Y, Lobaccaro J-MA, Shimomura I, Shan B, Brown MS, Goldstein JL, Mangelsdorf DJ 2000 Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRα and LXRβ. Genes Dev 14:2819–2830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Hao M, Luo Y, Liang C-p, Silver DL, Cheng C, Maxfield FR, Tall AR 2003 Stearoyl-CoA desaturase inhibits ATP-binding cassette transporter A1-mediated cholesterol efflux and modulates membrane domain structure. J Biol Chem 278:5813–5820 [DOI] [PubMed] [Google Scholar]
- Talukdar S, Hillgartner FB 2006 The mechanism mediating the activation of acetyl-coenzyme A carboxylase-α gene transcription by the liver X receptor agonist T0–901317. J Lipid Res 47:2451–2461 [DOI] [PubMed] [Google Scholar]
- Groot PHE, Pearce NJ, Yates JW, Stocker C, Sauermelch C, Doe CP, Willette RN, Olzinski A, Peters T, d'Epagnier D, Morasco KO, Krawiec JA, Webb CL, Aravindhan K, Jucker B, Burgert M, Ma C, Marino JP, Collins JL, Macphee CH, Thompson SK, Jaye M 2005 Synthetic LXR agonists increase LDL in CETP species. J Lipid Res 46:2182–2191 [DOI] [PubMed] [Google Scholar]
- Luo Y, Tall AR 2000 Sterol upregulation of human CETP expression in vitro and in transgenic mice by an LXR element. J Clin Invest 105:513–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y, Brown M 2002 Molecular determinants for the tissue specificity of SERMs. Science 295:2465–2468 [DOI] [PubMed] [Google Scholar]
- Williams S, Bledsoe RK, Collins JL, Boggs S, Lambert MH, Miller AB, Moore J, McKee DD, Moore L, Nichols J, Parks D, Watson M, Wisely B, Willson TM 2003 X-ray crystal structure of the liver X receptor β ligand binding domain: regulation by a histidine-tryptophan switch. J Biol Chem 278:27138–27143 [DOI] [PubMed] [Google Scholar]
- Schmidt RJ, Ficorilli JV, Zhang Y, Bramlett KS, Beyer TP, Borchert K, Dowless MS, Houck KA, Burris TP, Eacho PI, Liang G, Guo L-W, Wilson WK, Michael LF, Cao G 2006 A 15-ketosterol is a liver X receptor ligand that suppresses sterol-responsive element binding protein-2 activity. J Lipid Res 47:1037–1044 [DOI] [PubMed] [Google Scholar]
- Quinet EM, Savio DA, Halpern AR, Chen L, Miller CP, Nambi P 2004 Gene-selective modulation by a synthetic oxysterol ligand of the liver X receptor. J Lipid Res 45:1929–1942 [DOI] [PubMed] [Google Scholar]
- Traves PG, Hortelano S, Zeini M, Chao T-H, Lam T, Neuteboom ST, Theodorakis EA, Palladino MA, Castrillo A, Bosca L 2007 Selective activation of liver X receptors by acanthoic acid-related diterpenes. Mol Pharmacol 71:1545–1553 [DOI] [PubMed] [Google Scholar]
- Jaye MC, Krawiec JA, Campobasso N, Smallwood A, Qiu C, Lu Q, Kerrigan JJ, De Los Frailes Alvaro M, Laffitte B, Liu WS, Marino JP, Meyer CR, Nichols JA, Parks DJ, Perez P, Sarov-Blat L, Seepersaud SD, Steplewski KM, Thompson SK, Wang P, Watson MA, Webb CL, Haigh D, Caravella JA, Macphee CH, Willson TM, Collins JL 2005 Discovery of substituted maleimides as liver X receptor agonists and determination of a ligand-bound crystal structure. J Med Chem 48:5419–5422 [DOI] [PubMed] [Google Scholar]
- Miao B, Zondlo S, Gibbs S, Cromley D, Hosagrahara VP, Kirchgessner TG, Billheimer J, Mukherjee R 2004 Raising HDL cholesterol without inducing hepatic steatosis and hypertriglyceridemia by a selective LXR modulator. J Lipid Res 45:1410–1417 [DOI] [PubMed] [Google Scholar]
- Albers M, Blume B, Schlueter T, Wright MB, Kober I, Kremoser C, Deuschle U, Koegl M 2006 A novel principle for partial agonism of liver X receptor ligands: competitive recruitment of activators and repressors. J Biol Chem 281:4920–4930 [DOI] [PubMed] [Google Scholar]
- Collins JL, Fivush AM, Watson MA, Galardi CM, Lewis MC, Moore LB, Parks DJ, Wilson JG, Tippin TK, Binz JG, Plunket KD, Morgan DG, Beaudet EJ, Whitney KD, Kliewer SA, Willson TM 2002 Identification of a nonsteroidal liver X receptor agonist through parallel array synthesis of tertiary amines. J Med Chem 45:1963–1966 [DOI] [PubMed] [Google Scholar]
- Heery DM, Kalkhoven E, Hoare S, Parker MG 1997 A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature 387:733 [DOI] [PubMed] [Google Scholar]
- Hu X, Lazar MA 1999 The CoRNR motif controls the recruitment of corepressors by nuclear hormone receptors. Nature 402:93 [DOI] [PubMed] [Google Scholar]
- Costet P, Luo Y, Wang N, Tall AR 2000 Sterol-dependent transactivation of the ABC1 promoter by the liver X receptor/retinoid X receptor. J Biol Chem 275:28240–28245 [DOI] [PubMed] [Google Scholar]
- Hu X, Lazar MA 2000 Transcriptional repression by nuclear hormone receptors. Trends Endocrinol Metab 11:6–10 [DOI] [PubMed] [Google Scholar]
- Ishizuka T, Lazar MA 2003 The N-CoR/histone deacetylase 3 complex is required for repression by thyroid hormone receptor. Mol Cell Biol 23:5122–5131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrke M, Lebherz C, Millington SC, Guan H-P, Millar J, Rader DJ, Wilson JM, Lazar MA 2005 Diet-dependent cardiovascular lipid metabolism controlled by hepatic LXRα. Cell Metab 1:297 [DOI] [PubMed] [Google Scholar]