Abstract
The receptors for IGF-I (IGF-IR) and insulin (IR) have been implicated in physiological cardiac growth, but it is unknown whether IGF-IR or IR signaling are critically required. We generated mice with cardiomyocyte-specific knockout of IGF-IR (CIGF1RKO) and compared them with cardiomyocyte-specific insulin receptor knockout (CIRKO) mice in response to 5 wk exercise swim training. Cardiac development was normal in CIGF1RKO mice, but the hypertrophic response to exercise was prevented. In contrast, despite reduced baseline heart size, the hypertrophic response of CIRKO hearts to exercise was preserved. Exercise increased IGF-IR content in control and CIRKO hearts. Akt phosphorylation increased in exercise-trained control and CIRKO hearts and, surprisingly, in CIGF1RKO hearts as well. In exercise-trained control and CIRKO mice, expression of peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) and glycogen content were both increased but were unchanged in trained CIGF1RKO mice. Activation of AMP-activated protein kinase (AMPK) and its downstream target eukaryotic elongation factor-2 was increased in exercise-trained CIGF1RKO but not in CIRKO or control hearts. In cultured neonatal rat cardiomyocytes, activation of AMPK with 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) prevented IGF-I/insulin-induced cardiomyocyte hypertrophy. These studies identify an essential role for IGF-IR in mediating physiological cardiomyocyte hypertrophy. IGF-IR deficiency promotes energetic stress in response to exercise, thereby activating AMPK, which leads to phosphorylation of eukaryotic elongation factor-2. These signaling events antagonize Akt signaling, which although necessary for mediating physiological cardiac hypertrophy, is insufficient to promote cardiac hypertrophy in the absence of myocardial IGF-I signaling.
CARDIAC HYPERTROPHY DEVELOPS in response to a variety of extrinsic and intrinsic stimuli. For the physiological stimulus of endurance exercise (e.g. swim training), hemodynamic stress results in adaptive myocyte growth with preserved contractile function. Conversely, pathological stimuli such as pressure overload (e.g. aortic stenosis or hypertension) induce hypertrophy, which often progresses to heart failure (1). The functional outcomes of physiological or pathological cardiac hypertrophy are likely due to differences in proximal signaling pathways. It is generally accepted that activation of G protein-coupled receptors are necessary for inducing pathological hypertrophy, whereas insulin or IGF-I coupled to the phosphatidylinositol 3-kinase (PI3K)/Akt1 pathway has been associated with physiological growth of the heart (2,3). Inhibition of PI3K or genetic ablation of Akt1 prevents exercise-induced hypertrophy (4,5,6,7), suggesting that PI3K/Akt is required for compensatory growth in the heart.
Multiple lines of evidence suggest that IGF-I may be involved in the development of physiological cardiac hypertrophy. Cardiac IGF-I levels are increased in swim-trained rats (8); and increased cardiac IGF-I production was associated with physiological cardiac hypertrophy in athletes (9). Despite evidence for a role of IGF-I signaling in physiological cardiac growth, transgenic overexpression studies show conflicting results. Persistent local IGF-I expression in the heart and skeletal muscle results in physiological cardiac hypertrophy at early time points but ultimately leads to cardiac dysfunction (10). In contrast, transgenic mice overexpressing IGF-I receptors (IGF-IR) in cardiomyocytes show cardiac hypertrophy without evidence of fibrosis (11).
In addition to its metabolic effects, insulin also regulates cardiac growth and function. We have previously reported that mice with cardiomyocyte- restricted knockout (KO) of the insulin receptor (CIRKO) exhibit reduced heart size and mildly impaired contractile function (12,13,14). Thus, insulin signaling appears to be an important physiological regulator of postnatal cardiac growth and function. In CIRKO hearts, insulin receptor (IR) recombination likely begins as early as embryonic d 11.5 (15), which is consistent with a developmental role. No studies, however, have directly demonstrated whether IGF-I or IRs are necessary upstream regulatory signals for exercise-induced cardiac hypertrophy. In the current study, we generated mice with cardiomyocyte-specific IGF-IR gene ablation (CIGF1RKO) to determine the impact of IGF-IR loss of function in cardiomyocytes on the hypertrophic and contractile response of the heart to long-term swimming exercise and compared their response to that of similarly treated CIRKO mice. We found that CIGF1RKO mice exhibited normal cardiac development but blunted hypertrophic responses to swimming exercise training. This is in contrast to CIRKO mice that show a developmental reduction in cardiac size but a normal hypertrophic response to swim training. The mechanism for the absence of cardiac hypertrophy in IGF-IR-deficient hearts is not due to reduced Akt signaling but may be related to energetic stress that activates AMP-activated protein kinase (AMPK), which we postulate antagonizes Akt-mediated cardiac hypertrophy. Thus, IGF-IR but not IR signaling is an important regulator of exercise-induced, physiological cardiac hypertrophy.
RESULTS
Characterization of CIGF1RKO Mice
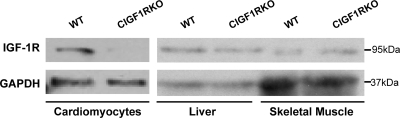
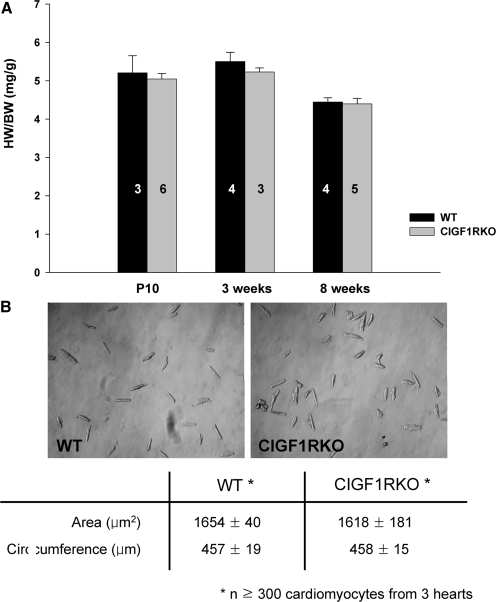
In CIGF1RKO mice, deletion of IGF-IR protein in isolated cardiomyocytes was nearly complete, but expression of IGF-IR in liver and skeletal muscle was preserved (Fig. 1). From postnatal d 10 to 8 wk of age, there was no difference in heart weight (HW) to body weight (BW) ratio between the CIGF1RKO and wild-type (WT) mice (Fig. 2A). At 8 wk of age, myocyte area and circumference were not different between the two groups (Fig. 2B).
Figure 1.
Cardiomyocyte Deletion of IGF-IR
Western blot analysis of IGF-IR protein (molecular mass 95 kDa) obtained from lysates of isolated cardiomyocytes (40 μg), liver (60 μg), and skeletal muscle (60 μg) from WT and CIGF1RKO mice. GAPDH is the loading control.
Figure 2.
Impact of IGF-IR Deletion on Cardiac Growth
A, HW/BW ratio in male WT and CIGF1RKO mice at postnatal d 10 and 3 or 8 wk of age, respectively. Numbers of mice in each group are indicated on the bars. B, Representative phase contrast images of isolated cardiomyocytes from hearts of 8-wk-old male WT and CIGF1RKO mice. Magnification, ×10. Myocyte area and circumference (at least 100 cells in each of three mice) are shown in the table. Data are mean ± sem.
Attenuation of Exercise-Induced Cardiac Hypertrophy by IGF-IR Deletion But Not by Insulin Receptor Deletion
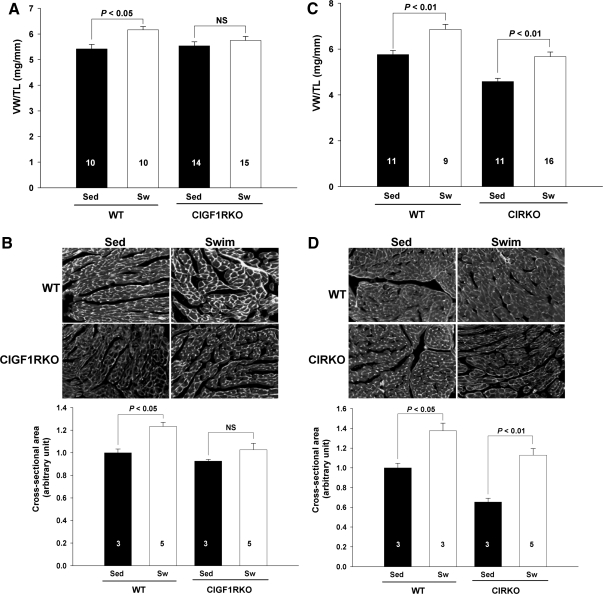
To determine whether IGF-I signaling is required for physiological hypertrophy, CIGF1RKO and control mice were exposed to 36 d swimming exercise. There were no differences in serum IGF-I concentrations between WT and CIGF1RKO mice before exercise, and there was no significant change in either group after exercise (supplemental Fig. S1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org). Ventricular weight (VW) normalized to tibia length (TL) ratios increased by 13.8% in WT mice after swim training (5.42 ± 0.18 vs. 6.17 ± 0.13, P < 0.05), but by a nonsignificant 3.8% in swim-trained CIGF1RKO mice (5.54 ± 0.16 vs. 5.75 ± 0.16, P = 0.75) (Fig. 3A). The fold increase in VW/TL was significantly greater in swim-trained WT vs. swim-trained CIGF1RKO (P < 0.05). Cross-sectional area of individual myocytes increased by 23.3% in swim-trained WT mice (P < 0.05) and by 10.9% in swim-trained CIGF1RKO mice (P = 0.46) compared with sedentary controls (Fig. 3B). Despite attenuated hypertrophy, left ventricular (LV) systolic function and LV cavity dimensions were preserved in exercise-trained CIGF1RKO mice (supplemental Table 1A, published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend. endojournals.org). Parallel studies were performed in CIRKO and littermate controls. Both trained WT and CIRKO mice developed significant cardiac hypertrophy. Relative to sedentary mice, VW/TL ratios increased by 19.1% in WT mice after swim training (5.76 ± 0.18 vs. 6.86 ± 0.22, P < 0.01) and by 23.5% in swim-trained CIRKO mice (4.59 ± 0.14 vs. 5.67 ± 0.20, P < 0.01) (Fig. 3C). Cross-sectional area of individual myocytes was increased by 37.6% in swim-trained WT (P < 0.05) and by 72.7% in swim-trained CIRKO (P < 0.01) (Fig. 3D). LV systolic function was maintained in both swim-trained groups (supplemental Table 1B).
Figure 3.
Attenuated Cardiac Hypertrophy in Swim-Trained CIGF1RKO Mice
A, VW/TL ratio in exercise-trained (Sw) and sedentary (Sed) WT and CIGF1RKO mice. Numbers of ventricles are indicated on the bars. B, Immunostaining of ventricular sections with FITC-conjugated wheat germ agglutinin (magnification, ×40) and quantification of cross-sectional area from at least 100 myocytes per ventricle in randomly selected fields of sections from WT and CIGF1RKO hearts. Numbers of sections are indicated on the bars. C, VW/TL in exercise-trained (Sw) and sedentary (Sed) WT and CIRKO mice. Numbers of ventricles are indicated on the bars. D, Immunostaining of ventricular sections with FITC-conjugated wheat germ agglutinin (magnification, ×40) and quantitation of cross-sectional area in WT and CIRKO mice. Numbers of sections are indicated on the bars. NS, Not significant.
Increased Cardiac IGF-IR Expression in Trained Mice
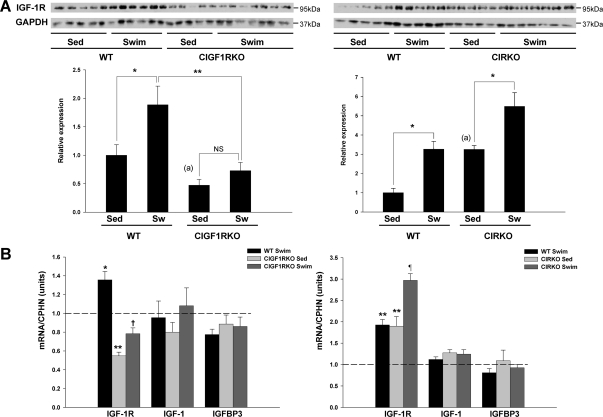
In whole heart homogenates, IGF-IR protein was reduced by more than 50% in sedentary CIGF1RKO mouse hearts (P < 0.05) (Fig. 4A). The residual levels likely reflect expression in other cell types such as fibroblasts and endothelial cells. In whole hearts, IGF-IR mRNA and protein were coordinately increased in swim-trained WT mice (P < 0.05) (Fig. 4, A and B). In contrast, swim training failed to increase IGF-IR expression in CIGF1RKO hearts (P = 0.83). In CIRKO hearts, a compensatory increase in IGF-IR protein and mRNA was observed (P < 0.05), and in contrast to CIGF1RKO mice, exercise further increased IGF-IR protein and mRNA levels in WT and CIRKO mice (Fig. 4, A and B). In distinction to IGF-IR gene expression, levels of IGF-I or IGF-binding protein-3, which can be generated in cardiac muscle and which may modulate IGF-I activity (16), were not changed by exercise training (Fig. 4B). Moreover, there was no compensatory change in IR content in CIGF1RKO hearts, and there was no increase in IR expression in WT or CIGF1RKO hearts after exercise training (supplemental Fig. S2). Taken together, these data suggest that transcriptional up-regulation of IGF-IR but not IR expression in response to exercise represents an important myocardial adaptation to exercise-induced cardiac hypertrophy.
Figure 4.
IGF-IR Expression
A, IGF-IR expression in the hearts of sedentary (Sed) and swim-trained (Sw) WT, CIGF1RKO, and CIRKO mice. Upper panels are representative blots, and lower panel is densitometry of results from five to seven hearts per group. *, P < 0.05; **, P < 0.01; NS, not significant. a, P < 0.05 vs. WT Sed (by Student’s t test). B, mRNA levels expressed as fold change compared with sedentary WT. n = 7–8 per group. *, P < 0.05; **, P < 0.01 vs. WT Sed; †, P < 0.001 vs. WT swim; ¶, P < 0.001 vs. CIRKO Sed.
Loss of IGF-IR or IR Signaling Does Not Limit Akt Activation in Exercise-Trained Hearts
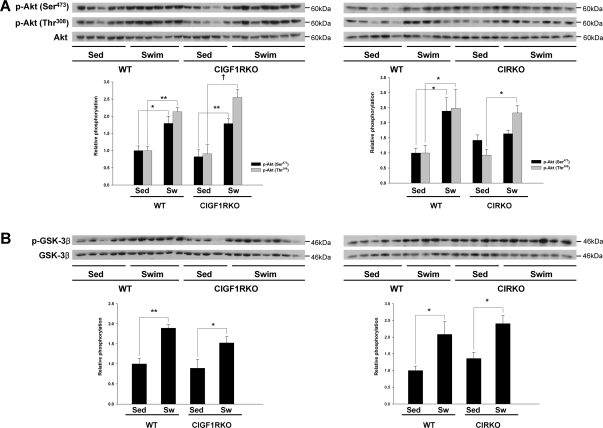
It is widely accepted that activation of PI3K/Akt signaling are critical mediators of physiological cardiac hypertrophy (5,7,17,18). Thus, we hypothesized that the increase in IGF-IR expression with exercise might correlate with activation of Akt. Phosphorylation of Akt was significantly increased at Ser473 (1.8-fold, P < 0.05) and Thr308 (2.1-fold, P < 0.01) in trained WT vs. sedentary controls (Fig. 5A). Unexpectedly, Akt phosphorylation at the Ser473 and Thr308 sites was also increased in trained CIGF1RKO mice vs. sedentary controls (2.2- and 2.8-fold; P < 0.01 and P < 0.001, respectively) (Fig. 5A). Consistent with the changes in Akt phosphorylation, phosphorylation of glycogen synthase kinase-3β (GSK3β) was significantly increased (P < 0.05) in both trained WT (1.9-fold) and CIGF1RKO (1.7-fold) mice relative to sedentary controls (Fig. 5B). Phosphorylation of Akt was significantly increased at Thr308 (2.5-fold, P < 0.05) but not at Ser473 (1.2-fold, P = 0.72) in swim-trained CIRKO hearts (Fig. 5A), whereas phosphorylation of GSK3β was increased in swim-trained WT and CIRKO mice relative to sedentary controls (P < 0.05 both) (Fig. 5B). Thus exercise-induced Akt activation in the heart is independent of upstream signals that are mediated via IRs or IGF-IRs, respectively.
Figure 5.
Akt-Mediated Signaling
Total and phosphorylated Akt (A) and total and phosphorylated GSK3β (B) in sedentary (Sed) and swim-trained (Sw) CIGF1RKO and CIRKO and their respective controls. Upper panels are representative blots, and lower panel is densitometry of results from five to six hearts per group. *, P < 0.05; **, P < 0.01; †, P < 0.001.
Potential Mechanisms for Attenuation of Cardiac Hypertrophy in Swim-Trained CIGF1RKO Mice
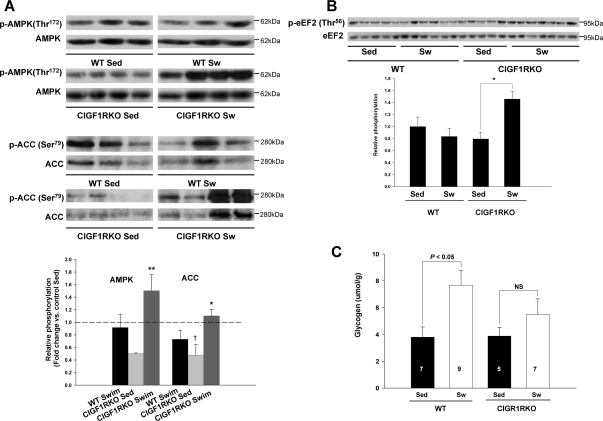
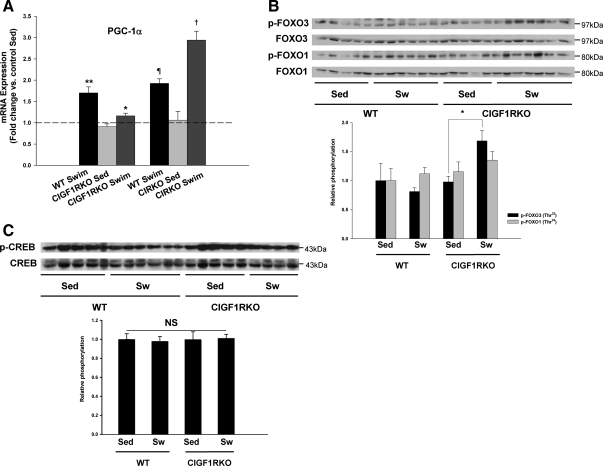
The question arises why activation of Akt was not sufficient to induce cardiac hypertrophy in swim-trained CIGF1RKO mice. To address this, we examined AMPK signaling pathways. In general, AMPK is not considered to be an intrinsic component of hypertrophic signaling cascades (19). However, it has been reported that activation of AMPK inhibited hypertrophic growth via phosphorylation of eukaryotic elongation factor-2 (eEF2) kinase (20). Basal AMPK phosphorylation tended to be lower in sedentary CIGF1RKO vs. sedentary WT (P = 0.08). However, swim training resulted in a significant increase in AMPK phosphorylation in CIGF1RKO (3-fold vs. sedentary KO, P = 0.002) but was not increased in swim-trained WT. Relative to swim-trained WT, phosphorylated AMPK was 64% greater in trained CIGF1RKO (P < 0.05) (Fig. 6A). Phosphorylation of the AMPK target, acetyl coenzyme A carboxylase (ACC), exhibited a similar pattern and was increased by 2.3-fold (P = 0.006) in trained vs. sedentary CIGF1RKO but was not increased in trained WT (Fig. 6A). AMPK-mediated phosphorylation of eEF2 kinase, which leads to phosphorylation and inhibition of eEF2 at Thr56, was unchanged in control animals but was increased by 1.7-fold (P < 0.05) in trained CIGF1RKO mice (Fig. 6B). These data support the hypothesis that increased eEF2 phosphorylation could lead to decreased protein synthesis, thereby blunting the hypertrophic response in swim-trained CIGF1RKO hearts. In contrast, phosphorylation of AMPK and eEF2 were not changed in swim-trained CIRKO hearts (supplemental Fig. S3, A and B). The increase in AMPK activation suggested that CIGF1RKO were subjected to increased energetic stress, as was supported by lower concentrations of glycogen after exercise (Fig. 6C). We therefore determined levels of peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α), which mediates the exercise-induced increase in mitochondrial function in the heart (17) and postexercise glycogen accumulation in skeletal muscle (21). The induction of PGC-1α expression evident in exercise-trained CIRKO and WT mice was severely blunted in exercise-trained CIGF1RKO hearts (Fig. 7A). We then examined levels of two proteins that have been reported to regulate the PGC-1α promoter, namely forkhead box class O (FOXO) transcription factors and cAMP response element-binding protein (CREB) (22,23). Exercise training was associated with a significant increase in phosphorylation of FOXO3 in CIGF1RKO but not in controls (Fig. 7B). There were no differences in the relative content of phosphorylated CREB (Fig. 7C).
Figure 6.
AMPK Signaling and Glycogen Content
A, Total and phosphorylated AMPK and ACC. Upper panels are representative blots, and lower panel is densitometry of results from three to four hearts per group expressed as fold change relative to sedentary WT. **, P < 0.01 vs. CIGF1RKO Sed, and P < 0.05 vs. WT swim for p-AMPK; *, P < 0.01 vs. CIGF1RKO Sed; †, P < 0.05 vs. WT Sed for p-ACC. B, Total and phosphorylated eEF2. Upper panel shows a representative blot, and the lower panel is densitometry of results from five to seven hearts per group. *, P < 0.05. C, Myocardial glycogen content in sedentary or swim-trained WT or CIGF1RKO mice. Numbers of ventricles are indicated on the bars.
Figure 7.
PGC-1α Expression
A, PGC-1α mRNA in sedentary or swim-trained mice, expressed as fold change relative to sedentary WT. *, P < 0.05 vs. WT swim; **, P < 0.001 vs. WT Sed; ¶, P < 0.01 vs. WT Sed; †, P < 0.001 vs. CIRKO Sed. or vs. WT swim. B and C, Total and phosphorylated FOXO3 and FOXO1 (B) and total and phosphorylated CREB (C) in sedentary (Sed) and swim-trained (Sw) CIGF1RKO and their respective controls. *, P < 0.05 vs. Sed.
ERK1/2 signaling has been implicated in physiological cardiac hypertrophy. Transgenic activation of MAPK kinase-1 (MEK1) and its downstream target ERK1/2 produced compensated concentric cardiac hypertrophy (24). Although it is unclear whether activated AMPK can directly suppress ERK phosphorylation in the setting of physiological hypertrophy, adiponectin-stimulated AMPK activation suppressed endothelin-1-induced ERK phosphorylation in cultured cardiomyocytes (25). Swim training did not alter phosphorylation levels of MEK and ERK1/2 in WT hearts. Interestingly, phosphorylation ratios of MEK and ERK1/2 were significantly decreased in response to swim training in CIGF1RKO hearts relative to sedentary controls (supplemental Fig. S4, A and B). In contrast, no changes were observed in phosphorylation levels of MEK and ERK1/2 in hearts from swim-trained CIRKO mice (supplemental Fig. S5, A and B). Thus, impaired ERK activation could represent another mechanism leading to attenuated cardiac hypertrophy in CIGF1RKO mice.
AICAR Treatment Blocks IGF-I-Stimulated Hypertrophy of Neonatal Rat Cardiomyocytes
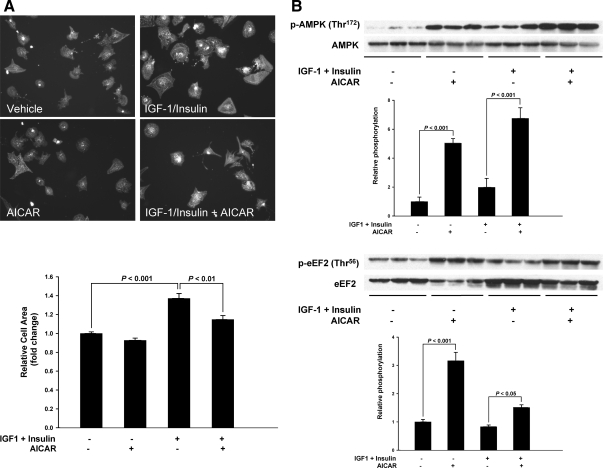
To independently evaluate whether AMPK activation could inhibit growth factor-mediated cardiomyocyte hypertrophy, we treated cultured neonatal rat cardiomyocytes with IGF-I and insulin in the presence or absence of 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) and measured cell size. IGF-I and insulin treatment for 72 h induced hypertrophic cell growth by 30%. Compared with cardiomyocytes treated with IGF-I and insulin, AICAR activated AMPK and eEF2 phosphorylation and suppressed the IGF-I and insulin-induced increase in cell surface area (Fig. 8, A and B). Thus, activation of AMPK suppresses physiological cardiomyocyte growth in vitro.
Figure 8.
AICAR Inhibits IGF-I/Insulin-Mediated Cardiomyocyte Hypertrophy
A, Upper panel shows representative fluorescence micrographs of neonatal rat ventricular cardiomyocytes treated with or without 100 nm insulin plus 10 nm IGF-I for 72 h in the presence of absence of 1 mm AICAR and immunostained with α-actinin (magnification, ×20), and lower panel shows image quantification (mean ± sem) of data from four independent experiments where at least 100 cells were measured per condition. B, Representative immunoblots showing total and phosphorylated AMPK and eEF2, respectively. Densitometry is shown below each blot. Significant differences are indicated on each figure.
DISCUSSION
The present study was designed to determine the potential roles of myocardial insulin and IGF-I signaling in physiological cardiac hypertrophy induced by exercise training. Although it is widely accepted that activation of Akt and PI3K are obligate signals for physiological cardiac hypertrophy (5,6,7,17,18), the nature of the upstream activators of these pathways remains incompletely understood. Earlier studies suggested that insulin signal transduction might regulate postnatal heart size in part via Akt-dependent mechanisms (14). Moreover, activation of IGF-IR signaling also promoted PI3K/Akt-dependent hypertrophic remodeling in the heart that mimicked physiological LV hypertrophy (11). The availability of conditional mutants lacking either insulin or IGF-IRs afforded the unique ability to directly evaluate the role of these upstream activators of PI3K/Akt signaling in physiological cardiac hypertrophy induced by exercise training. Our study demonstrated that IGF-IR expression is increased in cardiomyocytes in response to exercise training and that genetic deletion of IGF-IRs but not IRs significantly attenuated exercise-induced cardiac hypertrophy. The mechanism for the attenuation of the hypertrophic response in hearts that lack IGF-IR was unexpected. Our initial expectation was that impaired up-regulation of IGF-IR in response to exercise training would limit exercise-induced Akt phosphorylation if indeed activation of IGF-IR signaling via paracrine/autocrine or even endocrine mechanisms played a pivotal role. Contrary to this hypothesis, Akt activation was not impaired in exercise-trained CIGF1RKO mice, which indicates that alternative upstream mechanisms are responsible for Akt activation in response to exercise training.
We were still faced with the observation that cardiac hypertrophy was significantly attenuated in CIGF1RKO hearts despite robust activation of Akt. This led us to hypothesize that growth signaling downstream of Akt was being antagonized. We turned our attention initially to AMPK because of recent studies mainly in cultured cells that suggest that AMPK signaling could prevent mammalian target of rapamycin (mTOR) and S6 kinase-mediated cardiomyocyte hypertrophy via its effect on eEF2, an important cofactor in mTOR-mediated peptide elongation (20). EF2 kinase phosphorylates eEF2 and inhibits its activity. EF2 kinase is inactivated via TOR-mediated phosphorylation. Thus, activation of mTOR signaling promotes eEF2 activity by inhibiting its phosphorylation by EF2 kinase. It was recently shown that AMPK phosphorylates EF2 kinase on a novel site (Ser398) and increases its activity, which in turn increases the phosphorylation of eEF2. AMPK-mediated phosphorylation of eEF2 therefore inhibits its activity (26), which limits peptide elongation. Our observation of increased AMPK activation and increased phosphorylation of eEF2 in CIGF1RKO hearts after exercise training is consistent with the hypothesis that the increase in AMPK activity might be limiting the hypertrophic response in IGF-IR-deficient hearts. Previous studies have shown that activation of AMPK is sufficient to limit protein synthesis in cells that are exposed to phenylephrine or that are transduced with a constitutively active Akt adenovirus (20). Our in vitro studies also show that AMPK activation is sufficient to block insulin- and IGF-I-mediated hypertrophy in neonatal cardiomyocytes, lending further support for an anti-hypertrophic effect of AMPK activation in IGF-IR-deficient hearts. We propose that this signaling mechanism is specific for exercise-induced cardiac hypertrophy, because we observed no reduction in LV hypertrophy in CIGF1RKO hearts after transverse aortic constriction (data not shown).
The question arises as to mechanisms responsible for the increased activation of AMPK in swim-trained CIGF1RKO hearts. It has previously been shown that short-term exercise increases AMPK activation in normal hearts (27). However, the effect of endurance training on myocardial AMPK activation is unknown. We observed no consistent increase in AMPK phosphorylation in WT hearts after 5 wk swim training, which suggests that any exercise-induced increase in myocardial AMPK content might be transient. In contrast, AMPK phosphorylation (an index of AMPK activation), was increased in hearts of swim-trained CIGF1RKO mice. We interpret this increase in AMPK activation to be consistent with energetic stress within the myocardium, and this was supported by a relative reduction in postexercise glycogen stores. To address potential mechanisms that could limit the energetic reserves of exercise-trained CIGF1RKO hearts, we examined the exercise-mediated induction of PGC-1α, which is an important mediator of the increase in mitochondrial metabolic capacity that accompanies physiological cardiac hypertrophy and plays an important role in glycogen resynthesis in skeletal muscle after exercise (17,21). Remarkably, the induction of PGC-1α expression was blunted in exercise-trained CIGF1RKO mice but not in exercise-trained WT or CIRKO hearts. Impaired induction of PGC-1α would be predicted to limit the exercise-associated increase in myocardial fatty acid oxidative capacity and mitochondrial function (17). We hypothesize that this limitation in exercise-induced mitochondrial adaptations will limit myocardial high-energy reserves sufficiently to activate AMPK. We have not yet conducted a comprehensive analysis of mitochondrial biogenesis or mitochondrial energetics and high-energy phosphate content, and we have not yet determined substrate oxidation rates in the hearts of exercise-trained CIGF1RKO mice. This is a limitation of the present study.
The mechanisms by which exercise increases PGC-1α expression in the heart are incompletely understood. PGC-1α gene expression is regulated by a variety of transcriptional regulators that include forkhead transcription factors (FOXOs), CREB, monocyte enhancer-factor-2 isoforms, calcium/calmodulin protein kinases, and calcineurin (22,23,28,29,30). We initially focused on CREB and FOXO1/FOXO3, which are highly expressed in the heart. Interestingly, there was a significant increase in FOXO3 phosphorylation after exercise only in exercise-trained CIGF1RKO hearts. Phosphorylation of FOXO3 will lead to its nuclear exclusion, thereby reducing the transactivation of the PGC-1α promoter. This could therefore be one potential mechanism for the blunted up-regulation of PGC-1α expression in exercise-trained CIGF1RKO hearts. The mechanisms for the selective increase in FOXO3 phosphorylation in exercise-trained CIGFIRKO but not WT hearts remain to be elucidated. Exercise increased the phosphorylation of Akt, which is one potential mediator of FOXO3 phosphorylation in exercise-trained WT and CIGF1RKO hearts. However, the degree of Akt induction was somewhat greater in exercise-trained CIGF1RKO hearts. Although these differences were not sufficient to alter the degree of GSK3β phosphorylation, the possibility exists that FOXO3 phosphorylation could be more susceptible to differences in Akt activation. There are other kinases such as the serum and glucocorticoid-inducible kinases SGK1, which also phosphorylates FOXO3 on the Thr32 site that was recognized by the antibody used (31) and could represent an Akt-independent mechanism that could account for the increased phosphorylation of FOXO3 in the exercise-trained CIGF1RKO hearts. The possibility also remains that additional mechanisms could also be responsible for blunting PGC-1α expression. For example, cross talk between IGF-I and calcineurin signaling has been described (32). Thus, future studies will be required to determine whether the dependence of the exercise-training induction of PGC-1α on IGF-I signaling is also mediated in part via changes in calcineurin activity. IGF-I signaling has been shown to play a role in the prevention of muscle atrophy, in part by activating Akt-mediated phosphorylation of forkhead transcription factors such as FOXO3 (33). We did not examine atrophic signaling pathways in the present study. Although the increase in FOXO3 phosphorylation would be predicted to limit atrophic signaling, a recent report suggested that increased cytokine signaling could promote muscle atrophy even in the face of increased FOXO3 phosphorylation (34). Thus, it will be important to determine whether activation of atrophic signaling pathways contributed to the limited hypertrophic response in exercise-trained CIGF1RKO mouse hearts.
We previously demonstrated that perinatal deletion of IRs resulted in a reduction in cardiomyocyte size (12,14). However, despite the dramatic effect of impaired insulin signaling on postnatal cardiac size, loss of insulin signaling did not limit subsequent hypertrophy after exercise training. Deletion of IRs in CIRKO mice likely occurs between embryonic d 12 and birth (15). The predominant IR splice variant in neonatal life (IRa) is a selective receptor for IGF-II, whereas the adult splice variant (IRb) has a much higher affinity for insulin (35). Because the splicing event occurs in exon 11, both isoforms would be deleted in CIRKO mice. Given the important role of IGF-I signaling in physiological cardiac hypertrophy in the postnatal heart, it is interesting to speculate that the basis for the reduction in baseline cardiac size that we observed in CIRKO mice could be the result of loss of IGF-II signaling in utero.
In summary, the present study has identified an essential role for IGF-I signaling in exercise-induced cardiac hypertrophy via mechanisms that are independent of Akt signaling. IGF-I signaling plays an important role in the transcriptional regulation of PGC-1α during exercise, which contributes to the coordinate increase in mitochondrial capacity. In the absence of IGF-IRs, there is increased activation of AMPK, which likely sustained myocardial energy metabolism given that cardiac function was preserved. The increased activity of AMPK, in turn, potently inhibits exercise-induced cardiac hypertrophy. Thus, IGF-I signaling in the heart may regulate physiological cardiac hypertrophy by coordinating signaling pathways that modulate mitochondrial energy metabolism with pathways such as Akt that are required for cellular hypertrophy (Fig. 9).
Figure 9.
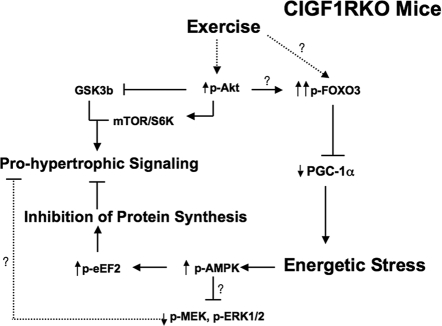
Proposed Model for the Signaling Mechanisms Responsible for the Blunted Hypertrophic Response in Mouse Hearts that Lack IGF-IR (CIGF1RKO)
In WT and CIGF1RKO hearts, exercise training leads to increased phosphorylation of Akt (p-Akt), which promotes cardiac hypertrophy by phosphorylating and inhibiting GSK3β and by activating mTOR and S6 kinase (S6K). In CIGF1RKO mice, there is increased activation of FOXO3 by mechanisms that might be Akt dependent or Akt independent. FOXO3 is a positive regulator of PGC-1α expression. Nuclear exclusion of FOXO3 by phosphorylation reduces the FOXO3-dependent activation of the PGC-1α gene. Reduced PGC-1α expression limits the mitochondrial adaptations to exercise training, thereby promoting energetic stress, which leads to activation of AMPK. AMPK activation will increase the phosphorylation of eEF2, which inhibits protein synthesis and antagonizes pro-hypertrophic signaling pathways. Increased AMPK phosphorylation might also antagonize MAPK signaling which is also pro-hypertrophic.
MATERIALS AND METHODS
Generation of Animals
CIGF1RKO mice were generated by crossing mice that were homozygous for floxed IGF-IR alleles in which loxP sites flank exon 3 of the IGF-IR gene (IGF-IRlox/lox) (36,37) with IGF-IRlox/lox transgenic mice in which cardiac-specific expression of Cre recombinase was driven by the α-myosin heavy chain (α-MHC) promoter (38). CIRKO mice were generated as previously described (12). Animals were fed standard chow and housed in temperature-controlled facilities with a 12-h light, 12-h dark cycle (lights on at 0600 h). Mice were maintained on a mixed C57BL6J/129Sv/FVB background, and littermate controls were used. Animals were killed for tissue isolations between 1100–1200 h. All animal experiments were conducted in accordance with guidelines approved by the Institutional Animal Care and Use Committee of the University of Utah.
Measurement of Cardiomyocyte Size
Cardiomyocytes were isolated from 8-wk-old male CIGF1RKO mice and their control littermates using our previously described protocols (12). Myocyte area and circumference were calculated by measuring at least 100 cells per mouse heart in three mice using NIH ImageJ analysis software.
Swimming Exercise
Eight- to 10-wk-old male CIGF1RKO and CIRKO and their control littermates were subjected to swim training as described previously (7,17). This protocol has been used by many groups to induce sustained LV hypertrophy after as little as 21 d of swim training (5,6,7,39,40). Briefly, each day consisted of two swimming sessions, separated by at least a 4-h interval, beginning with 10 min duration on the first day. The swim duration was increased by 10 min/d until mice were swimming 90 min twice a day. Mice were trained for 28 additional days (36 d total). In all study groups, one day before killing, in vivo cardiac function was assessed by echocardiography. Three to five hours after the final swimming session, mouse hearts were harvested and weighed. VW, TL, and BW were measured so that VW/TL and VW/BW could be calculated. Because some mice lost weight after 36 d swim training, we elected to normalize VW to TL.
Echocardiographic Studies
Transthoracic M-mode and two-dimensional echocardiography examinations were performed in mice under isoflurane anesthesia using an ultrasound machine (Vivid 7; GE Healthcare, Tampa, FL) with a 13-MHz transducer. One operator (blinded to genotype and treatment) performed all echocardiograms. The echocardiograms were stored digitally and analyzed offline.
Measurement of Serum IGF-I Concentrations
Serum IGF-I levels were determined using the Quantikine mouse IGF-I immunoassay (MG100; R&D Systems, Minneapolis, MN). Briefly, blood was collected right before killing and serum obtained after brief centrifugation. Mouse serum was then diluted 1/500 with calibrator diluent RD5-38 and assayed for IGF-I levels according to manufacturer’s recommendations. OD at 450 nm was corrected with OD at 540 nm.
Histological Analysis
Immediately after harvest, a midventricular slice of myocardium was fixed in 4% paraformaldehyde and then protected in a sequential series of 10, 20, and 30% sucrose/PBS solutions, oriented in OCT-filled (Tissue Tek) molds, rapidly frozen, and then sectioned at 8 μm on a Cryostat microtome (Leica Instruments, Bannockburn, IL) at −20 C as described (41). For measurement of myocyte cross-sectional area, sections were stained for membranes with fluorescein isothiocyanate (FITC)-conjugated wheat germ agglutinin (Invitrogen Corp., Carlsbad, CA). The slides were examined using an Olympus IX71 inverted microscope (Olympus, Center Valley, PA) equipped with a fluorescence filter at ×40 magnification, and myocyte area was measured using ImageJ.
Western Blot Analysis
Total protein lysates were extracted from frozen hearts as described previously (17). Proteins were resolved by SDS-PAGE and electrotransferred onto nitrocellulose membranes (GE Healthcare, Piscataway, NJ). The antibodies used were IGF-IRβ, IR-β, phospho-Akt (Thr308), phospho-Akt (Ser473), Akt, phospho-GSK-3β, phospho-MEK, MEK, phospho-ERK, ERK, phospho-AMPK (Thr172), AMPK, phospho-ACC (Ser79), ACC, phospho-eEF2 (Thr56), eEF2, GAPDH, CREB, phospho-CREB (Ser133), FOXO1, phospho-FOXO1 (Thr24), phospho-FOXO3a (Thr32) (Cell Signaling Technology, Danvers, MA), GSK-3β, and FOXO3a (Santa Cruz Biotechnology, Santa Cruz, CA). Protein detection was carried out with the appropriate horseradish peroxidase-conjugated secondary antibody and SuperSignal West Femto Maximum Sensitivity Substrate (ThermoFisher Scientific, Pittsburgh, PA). Blots were exposed to blue autoradiography film (ISC BioExpress, Kaysville, UT) for 1–10 min. Films were scanned, and densitometric quantification of protein bands was achieved using an AlphaImager 2200 (Alpha Innotech Corp., San Leandro, CA).
Gene Expression
mRNA was quantified by real-time PCR using an ABI Prism 7900HT instrument in 384-well plate format as described previously (42). Cyclophilin (CPHN) was used as a template normalizer because CPHN levels were unchanged between groups. Primer sequences are IGF-IR, CTGCGGCGATGAAGAGAAGAAAA/TACCGGTGCCACGTTATGATGATT; IGF-I, GTGGACCGAGGGGCTTTTACTTC/TTTGCAGCTTCGTTTTCTTGTTTG; and IGFBP3, AATGGCCGCGGGTTCTGC/TTCTGGGTGTCTGTGCTTTGAG.
Glycogen Measurement
Within 4 h after final swimming, mouse hearts were harvested and analyzed as previously described (21). Briefly, snap-frozen tissue was powdered under liquid nitrogen and homogenized in a 0.3 m perchloric acid solution. This extract was assayed with and without amyloglucosidase digestion (A7420; Sigma-Aldrich, St., Louis, MO) in 50 mm Na acetate (pH 5.5) and 0.02% BSA. Resulting changes in absorption at 340 nm were compared with a standard of 0–80 μm glucose. Results are presented as glucose released from glycogen corrected to tissue weight.
Cell Culture
Primary cultures of neonatal rat ventricular cardiomyocytes were made from 1- to 3-d-old Sprague Dawley rats as described previously with some modification (17). Cardiomyocytes were seeded at 105 cells per well onto Permanox two-well chamber slides (Nunc Nalge International, Rochester, NY) precoated with 0.1% gelatin in serum- and growth factor-free DMEM/M199 (Invitrogen). After 24 h recovery, cells were incubated with or without 100 nm insulin, 10 nm IGF-I (National Hormone and Peptide Program, Torrance, CA) for 72 h in the presence or absence of 1 mm AICAR (Toronto Research Chemicals Inc., Toronto, Canada), a pharmacological activator of AMPK. Media and treatments were refreshed every 24 h. Cells were fixed, and immunofluorescence studies were performed by applying antibodies against α-actinin (clone EA53; Sigma-Aldrich) to stain cardiomyocytes. The areas of at least 100 individual cells per condition from randomly selected fields were examined from four individual cell preparations using ImageJ software.
Statistical Analysis
All values are presented as the mean ± sem. Statistical analyses were performed using either a Student’s t test or a one-way ANOVA with Fisher’s protected least significance squares or Tukey’s post hoc test using SPSS for Windows, version 11.5 (SPSS, Chicago, IL) or Statview 5.0.1 (SAS Institute Inc., Cary, NC). A two-tailed P < 0.05 was considered significant.
Supplementary Material
Footnotes
This study was supported by National Institutes of Health Grants RO1HL070070 to E.D.A. who is an Established Investigator of the American Heart Association (AHA), and 5T32 HL007576 (to A.R.W.), who now holds an AHA postdoctoral fellowship.
Disclosure Statement: The authors have nothing to disclose.
First Published Online September 18, 2008
Abbreviations: ACC, Acetyl coenzyme A carboxylase; AICAR, 5-Aminoimidazole-4-carboxamide-1-β-d-ribofuranoside; AMPK, AMP-activated protein kinase; CIRKO, cardiomyocyte-restricted knockout of the insulin receptor; BW, body weight; CIGF1RKO, cardiomyocyte-specific IGF-IR gene ablation; CREB, cAMP response element-binding protein; eEF2, eukaryotic elongation factor-2; FITC, fluorescein isothiocyanate; FOXO, forkhead box class O; GSK3β, glycogen synthase kinase-3β; HW, heart weight; IGF-IR, IGF-I receptor; IR, insulin receptor; KO, knockout; LV, left ventricular; mTOR, mammalian target of rapamycin; PGC-1α, peroxisome proliferator-activated receptor-γ coactivator-1α; PI3K, phosphatidylinositol 3-kinase; TL, tibia length; VW, ventricular weight; WT, wild type.
References
- Heineke J, Molkentin JD 2006 Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol 7:589–600 [DOI] [PubMed] [Google Scholar]
- Dorn 2nd GW 2007 The fuzzy logic of physiological cardiac hypertrophy. Hypertension 49:962–970 [DOI] [PubMed] [Google Scholar]
- Shiojima I, Walsh K 2006 Regulation of cardiac growth and coronary angiogenesis by the Akt/PKB signaling pathway. Genes Dev 20:3347–3365 [DOI] [PubMed] [Google Scholar]
- Debosch B, Sambandam N, Weinheimer C, Courtois M, Muslin AJ 2006 Akt2 regulates cardiac metabolism and cardiomyocyte survival. J Biol Chem 281:32841–32851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBosch B, Treskov I, Lupu TS, Weinheimer C, Kovacs A, Courtois M, Muslin AJ 2006 Akt1 is required for physiological cardiac growth. Circulation 113:2097–2104 [DOI] [PubMed] [Google Scholar]
- Luo J, McMullen JR, Sobkiw CL, Zhang L, Dorfman AL, Sherwood MC, Logsdon MN, Horner JW, DePinho RA, Izumo S, Cantley LC 2005 Class IA phosphoinositide 3-kinase regulates heart size and physiological cardiac hypertrophy. Mol Cell Biol 25:9491–9502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMullen JR, Shioi T, Zhang L, Tarnavski O, Sherwood MC, Kang PM, Izumo S 2003 Phosphoinositide 3-kinase(p110α) plays a critical role for the induction of physiological, but not pathological, cardiac hypertrophy. Proc Natl Acad Sci USA 100:12355–12360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinowitz M, Kessler-Icekson G, Freimann S, Zimmermann R, Schaper W, Golomb E, Savion N, Eldar M 2003 Short- and long-term swimming exercise training increases myocardial insulin-like growth factor-I gene expression. Growth Horm IGF Res 13:19–25 [DOI] [PubMed] [Google Scholar]
- Neri Serneri GG, Boddi M, Modesti PA, Cecioni I, Coppo M, Padeletti L, Michelucci A, Colella A, Galanti G 2001 Increased cardiac sympathetic activity and insulin-like growth factor-I formation are associated with physiological hypertrophy in athletes. Circ Res 89:977–982 [DOI] [PubMed] [Google Scholar]
- Delaughter MC, Taffet GE, Fiorotto ML, Entman ML, Schwartz RJ 1999 Local insulin-like growth factor I expression induces physiologic, then pathologic, cardiac hypertrophy in transgenic mice. FASEB J 13:1923–1929 [DOI] [PubMed] [Google Scholar]
- McMullen JR, Shioi T, Huang WY, Zhang L, Tarnavski O, Bisping E, Schinke M, Kong S, Sherwood MC, Brown J, Riggi L, Kang PM, Izumo S 2004 The insulin-like growth factor 1 receptor induces physiological heart growth via the phosphoinositide 3-kinase(p110α) pathway. J Biol Chem 279:4782–4793 [DOI] [PubMed] [Google Scholar]
- Belke DD, Betuing S, Tuttle MJ, Graveleau C, Young ME, Pham M, Zhang D, Cooksey RC, McClain DA, Litwin SE, Taegtmeyer H, Severson D, Kahn CR, Abel ED 2002 Insulin signaling coordinately regulates cardiac size, metabolism, and contractile protein isoform expression. J Clin Invest 109:629–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sena S, Rasmussen IR, Wende AR, McQueen AP, Theobald HA, Wilde N, Pereira RO, Litwin SE, Berger JP, Abel ED 2007 Cardiac hypertrophy caused by peroxisome proliferator-activated receptor-γ agonist treatment occurs independently of changes in myocardial insulin signaling. Endocrinology 148:6047–6053 [DOI] [PubMed] [Google Scholar]
- Shiojima I, Yefremashvili M, Luo Z, Kureishi Y, Takahashi A, Tao J, Rosenzweig A, Kahn CR, Abel ED, Walsh K 2002 Akt signaling mediates postnatal heart growth in response to insulin and nutritional status. J Biol Chem 277:37670–37677 [DOI] [PubMed] [Google Scholar]
- Yin Z, Jones GN, Towns 2nd WH, Zhang X, Abel ED, Binkley PF, Jarjoura D, Kirschner LS 2008 Heart-specific ablation of Prkar1a causes failure of heart development and myxomagenesis. Circulation 117:1414–1422 [DOI] [PubMed] [Google Scholar]
- Knudtson KL, Boes M, Sandra A, Dake BL, Booth BA, Bar RS 2001 Distribution of chimeric IGF binding protein (IGFBP)-3 and IGFBP-4 in the rat heart: importance of C-terminal basic region. Endocrinology 142:3749–3755 [DOI] [PubMed] [Google Scholar]
- O'Neill B T, Kim J, Wende AR, Theobald HA, Tuinei J, Buchanan J, Guo A, Zaha VG, Davis DK, Schell JC, Boudina S, Wayment B, Litwin SE, Shioi T, Izumo S, Birnbaum MJ, Abel ED 2007 A conserved role for phosphatidylinositol 3-kinase but not Akt signaling in mitochondrial adaptations that accompany physiological cardiac hypertrophy. Cell Metab 6:294–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioi T, Kang PM, Douglas PS, Hampe J, Yballe CM, Lawitts J, Cantley LC, Izumo S 2000 The conserved phosphoinositide 3-kinase pathway determines heart size in mice. EMBO J 19:2537–2548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyck JR, Lopaschuk GD 2006 AMPK alterations in cardiac physiology and pathology: enemy or ally? J Physiol 574:95–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AY, Soltys CL, Young ME, Proud CG, Dyck JR 2004 Activation of AMP-activated protein kinase inhibits protein synthesis associated with hypertrophy in the cardiac myocyte. J Biol Chem 279:32771–32779 [DOI] [PubMed] [Google Scholar]
- Wende AR, Schaeffer PJ, Parker GJ, Zechner C, Han DH, Chen MM, Hancock CR, Lehman JJ, Huss JM, McClain DA, Holloszy JO, Kelly DP 2007 A role for the transcriptional coactivator PGC-1α in muscle refueling. J Biol Chem 282:36642–36651 [DOI] [PubMed] [Google Scholar]
- Daitoku H, Yamagata K, Matsuzaki H, Hatta M, Fukamizu A 2003 Regulation of PGC-1 promoter activity by protein kinase B and the forkhead transcription factor FKHR. Diabetes 52:642–649 [DOI] [PubMed] [Google Scholar]
- Herzig S, Long F, Jhala US, Hedrick S, Quinn R, Bauer A, Rudolph D, Schutz G, Yoon C, Puigserver P, Spiegelman B, Montminy M 2001 CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature 413:179–183 [DOI] [PubMed] [Google Scholar]
- Bueno OF, De Windt LJ, Tymitz KM, Witt SA, Kimball TR, Klevitsky R, Hewett TE, Jones SP, Lefer DJ, Peng CF, Kitsis RN, Molkentin JD 2000 The MEK1-ERK1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic mice. EMBO J 19:6341–6350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka D, Kawabata K, Saito Y, Kobayashi T, Nakamura T, Kodama Y, Takano H, Obata JE, Kitta Y, Umetani K, Kugiyama K 2006 Role of adiponectin receptors in endothelin-induced cellular hypertrophy in cultured cardiomyocytes and their expression in infarcted heart. Am J Physiol Heart Circ Physiol 290:H2409–H2416 [DOI] [PubMed] [Google Scholar]
- Browne GJ, Finn SG, Proud CG 2004 Stimulation of the AMP-activated protein kinase leads to activation of eukaryotic elongation factor 2 kinase and to its phosphorylation at a novel site, serine 398. J Biol Chem 279:12220–12231 [DOI] [PubMed] [Google Scholar]
- Coven DL, Hu X, Cong L, Bergeron R, Shulman GI, Hardie DG, Young LH 2003 Physiologic role of AMP-activated protein kinase (AMPK) in the heart: graded activation during exercise. Am J Physiol Endocrinol Metab 285:E629–E636 [DOI] [PubMed] [Google Scholar]
- Czubryt MP, McAnally J, Fishman GI, Olson EN 2003 Regulation of peroxisome proliferator-activated receptor gamma coactivator 1α (PGC-1α) and mitochondrial function by MEF2 and HDAC5. Proc Natl Acad Sci USA 100:1711–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer PJ, Wende AR, Magee CJ, Neilson JR, Leone TC, Chen F, Kelly DP 2004 Calcineurin and calcium/calmodulin-dependent protein kinase activate distinct metabolic gene regulatory programs in cardiac muscle. J Biol Chem 279:39593–39603 [DOI] [PubMed] [Google Scholar]
- Wright DC, Geiger PC, Han DH, Jones TE, Holloszy JO 2007 Calcium induces increases in peroxisome proliferator-activated receptor γ coactivator-1α and mitochondrial biogenesis by a pathway leading to p38 mitogen-activated protein kinase activation. J Biol Chem 282:18793–18799 [DOI] [PubMed] [Google Scholar]
- Brunet A, Park J, Tran H, Hu LS, Hemmings BA, Greenberg ME 2001 Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a). Mol Cell Biol 21:952–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musaro A, McCullagh KJ, Naya FJ, Olson EN, Rosenthal N 1999 IGF-1 induces skeletal myocyte hypertrophy through calcineurin in association with GATA-2 and NF-ATc1. Nature 400:581–585 [DOI] [PubMed] [Google Scholar]
- Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, Gonzalez M, Yancopoulos GD, Glass DJ 2004 The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell 14:395–403 [DOI] [PubMed] [Google Scholar]
- Dehoux M, Gobier C, Lause P, Bertrand L, Ketelslegers JM, Thissen JP 2007 IGF-I does not prevent myotube atrophy caused by proinflammatory cytokines despite activation of Akt/Foxo and GSK-3β pathways and inhibition of atrogin-1 mRNA. Am J Physiol Endocrinol Metab 292:E145–E150 [DOI] [PubMed] [Google Scholar]
- White MF 2006 Regulating insulin signaling and β-cell function through IRS proteins. Can J Physiol Pharmacol 84:725–737 [DOI] [PubMed] [Google Scholar]
- Cadoret A, Desbois-Mouthon C, Wendum D, Leneuve P, Perret C, Tronche F, Housset C, Holzenberger M 2005 c-myc-induced hepatocarcinogenesis in the absence of IGF-I receptor. Int J Cancer 114:668–672 [DOI] [PubMed] [Google Scholar]
- Desbois-Mouthon C, Wendum D, Cadoret A, Rey C, Leneuve P, Blaise A, Housset C, Tronche F, Le Bouc Y, Holzenberger M 2006 Hepatocyte proliferation during liver regeneration is impaired in mice with liver-specific IGF-1R knockout. FASEB J 20:773–775 [DOI] [PubMed] [Google Scholar]
- Abel ED, Kaulbach HC, Tian R, Hopkins JC, Duffy J, Doetschman T, Minnemann T, Boers ME, Hadro E, Oberste-Berghaus C, Quist W, Lowell BB, Ingwall JS, Kahn BB 1999 Cardiac hypertrophy with preserved contractile function after selective deletion of GLUT4 from the heart. J Clin Invest 104:1703–1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMullen JR, Amirahmadi F, Woodcock EA, Schinke-Braun M, Bouwman RD, Hewitt KA, Mollica JP, Zhang L, Zhang Y, Shioi T, Buerger A, Izumo S, Jay PY, Jennings GL 2007 Protective effects of exercise and phosphoinositide 3-kinase(p110α) signaling in dilated and hypertrophic cardiomyopathy. Proc Natl Acad Sci USA 104:612–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan ML, Cheslow Y, Vikstrom K, Malhotra A, Geenen DL, Nakouzi A, Leinwand LA, Buttrick PM 1994 Cardiac adaptations to chronic exercise in mice. Am J Physiol 267:H1167–H1173 [DOI] [PubMed] [Google Scholar]
- Frank DU, Fotheringham LK, Brewer JA, Muglia LJ, Tristani-Firouzi M, Capecchi MR, Moon AM 2002 An Fgf8 mouse mutant phenocopies human 22q11 deletion syndrome. Development 129:4591–4603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudina S, Sena S, Theobald H, Sheng X, Wright JJ, Hu XX, Aziz S, Johnson JI, Bugger H, Zaha VG, Abel ED 2007 Mitochondrial energetics in the heart in obesity-related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes 56:2457–2466 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.