Abstract
Cardiovascular disease is the leading cause of mortality for both men and women in developed countries. The sex steroid hormone estrogen is required for normal vascular physiology. Estrogen functions by binding to intracellular estrogen receptors (ER), ERα and ERβ, ligand-activated transcription factors that are expressed in both vascular endothelial and smooth muscle cells. We recently demonstrated that long-term (8 d) estrogen treatment in vivo in mice recruits distinct vascular gene sets mediated by ERα and ERβ and that the promoters from these gene sets are enriched for binding sites of specific transcription factors, leading to the hypothesis that estrogen initiates a cascade of early transcriptional events that modulate gene expression in the vasculature. Here we test this hypothesis using gene expression profiling to examine initial transcriptional events (2–8 h) mediated by estrogen in blood vessels. Our data reveal that 1) estrogen regulates temporally distinct cascades of vascular gene expression, 2) initially, estrogen-mediated vascular gene repression predominates, 3) the earliest estrogen-recruited gene program is enriched in vascular transcription factors that can interact with binding sites present in estrogen-regulated vascular genes recruited subsequently, and 4) estrogen-regulated genes recruited next have specific functions, including lipid metabolism and cellular growth and proliferation that are potentially important for estrogen’s known vascular functions. In summary, estrogen directly and rapidly recruits specific transcriptional factors that then propagate distinct cascades of gene expression. These data define the temporal recruitment of specific vascular genes by estrogen and enable further analysis of the mechanisms by which estrogen directly regulates vascular function.
GENDER DIFFERENCES EXIST in the incidence and outcome of cardiovascular disease, the leading cause of morbidity and mortality in Western society. Investigation of these gender differences has demonstrated a role for estrogen in cardiovascular physiology and pathophysiology. Estrogen is a steroid hormone that acts by binding to its intracellular receptors, estrogen receptor (ER)α and ERβ, ligand-activated transcription factors (TF) of the nuclear hormone receptor superfamily. Both ERα and ERβ are expressed and function as ligand-activated TF in vascular endothelial cells (EC) and smooth muscle cells (SMC) (1) where they play a role in vascular function (2,3,4). In vitro, estrogen inhibits vascular SMC (VSMC) proliferation and enhances proliferation of vascular EC (5,6,7), resulting in protection from vascular injury and atherosclerosis in mouse models (8,9). In human clinical studies, estrogen has beneficial effects on lipid profiles, and recent analyses of the Women’s Health Initiative trial data have demonstrated a decrease in atherosclerotic vascular disease when women are treated with estrogen containing hormone replacement therapy soon after menopause (2,3,4,10). Distinct contributions of the two ER subtypes in regulating vascular function have been revealed in ERα and -β knockout mice (ERα-KO and ERβ-KO). ERα plays a role in mediating estrogen protection from vascular injury (9), and ERβ is involved in controlling systemic blood pressure (11). However, whether these vascular effects of estrogen are mediated by ERs in the vessel wall or result from secondary vascular effects of estrogen on other tissues remains to be determined. In addition, the genes regulated by estrogen that control vascular function in humans and animals are not known. An understanding of the underlying molecular pathways mediating the cardiovascular protective effects of estrogen may lead to new drug targets to prevent and treat cardiovascular disease in both men and women.
Hormone-activated vascular ER can modulate vascular gene transcription by directly binding to specific DNA response elements [estrogen-responsive elements (ERE)] in vascular cells or by altering the expression or the activity (via posttranslational modification) of other TF that subsequently modulate vascular gene transcription (2,12,13). We have recently begun to address the role of estrogen in regulating vascular gene expression by investigating the effects of steady-state in vivo estrogen treatment on vascular gene expression in wild type, ERα-KO, and ERβ-KO mice (14). We demonstrated that ERα and ERβ regulate distinct sets of vascular genes, and we identified specific TF binding sites that are enriched in the promoters of these long-term vascular estrogen-regulated genes (14). Based on these data, we hypothesized that initially, estrogen directly regulates the expression of vascular TF that consecutively initiate downstream gene expression programs thereby mediating estrogen’s long-term effects on vascular function. To test this hypothesis, we now investigate early vascular gene regulatory events in response to direct, short-term estrogen treatment of intact blood vessels. We profile gene expression changes in intact mouse aortas after 2, 4, and 8 h of direct ex vivo estrogen treatment, which avoids confounding of the data by effects of estrogen on other cells or tissues that might influence vascular gene expression. We describe the temporal and consecutive recruitment of functionally distinct vascular gene programs by estrogen and the initiation of estrogen-responsive transcriptional networks. These findings delineate the early gene regulatory events that orchestrate estrogen’s longer-term effects on vascular physiology and pathophysiology.
RESULTS
Estrogen Directly Regulates Gene Expression in the Mouse Aorta
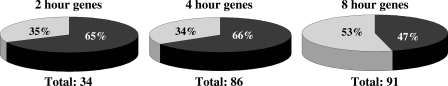
Estrogen-responsive vascular genes were identified by gene expression profiling of RNA from wild-type mouse aortas treated ex vivo with estrogen compared with vehicle for 2, 4, and 8 h. The time points were chosen based on published data in intact cells and our pilot experiments in whole vessels demonstrating that ERs bind to EREs within 45–60 min of estrogen exposure (15), resulting in detectable changes in gene expression within 2–3 h and in protein expression within 6–8 h (15,16) (data not shown). Genes with estrogen-responsive expression changes that were significant in magnitude (at least 1.5-fold up or down compared with vehicle) and highly reproducible (P ≤ 0.01) were included in all further analyses. The total number of estrogen-regulated genes increased over time with 34, 86, and 91 regulated genes at 2, 4, and 8 h, respectively (Fig. 1) (supplemental Tables S1–S3, published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org). Estrogen-mediated gene repression predominated in the first 4 h, whereas gene induction was more prominent at 8 h (Fig. 1). This trend for early estrogen-mediated gene repression followed by induction in blood vessels over time is consistent with recent reports in estrogen-treated breast cancer cells (15).
Figure 1.
Estrogen-Regulated Aortic Gene Expression Changes over Time
Summary of estrogen-mediated expression changes in the aorta (fold change ≥ 1.5; P ≤ 0.01) over a time course (2, 4, and 8 h). Shown are the total number of differentially expressed genes after estrogen treatment and the percentage of induced vs. repressed genes at each time point. Gray segments represent the percentage of estrogen-induced genes and black segments the percentage of estrogen-repressed genes.
The estrogen-stimulated microarray expression changes were validated by quantitative RT-PCR (qRT-PCR) analysis of a subset of genes in independently obtained RNA samples (Table 1). Sixteen of 23 representative genes, including genes at each time point and both estrogen-induced and estrogen-repressed genes, displayed qualitatively and quantitatively comparable levels of estrogen regulation by qRT-PCR as in the global gene profile analysis, thereby confirming, by an alternative technique, the reproducibility of the microarray data.
Table 1.
qRT-PCR Confirmation of Select Vascular Estrogen-Regulated Genes Identified by Microarray Analysis
| Gene Name | Gene Symbol | Microarray (Fold Changea ) | qRT-PCR (Fold Change ± sem) |
|---|---|---|---|
| 2 h estrogen treatment | |||
| SH2 domain binding protein 1 | Sh2bp | 1.77 | 2.26 ± 0.67 |
| TCDD-inducible poly(ADP-ribose) polymerase | Tirp | 1.67 | 1.77 ± 0.33 |
| Cholesterol 25-hydroxylase | Ch25 h | 0.4 | 0.71 ± 0.06 |
| Chemokine (C-X-C motif) ligand 5 | Cxcl5 | 0.36 | 0.56 ± 0.07 |
| D site albumin promoter binding protein | Dbp | 0.28 | 0.33 ± 0.05a |
| 4 h estrogen treatment | |||
| Matrix metallopeptidase 19 | Mmp19 | 5.52 | 6.61 ± 1.54a |
| Tropomodulin 1 | Tmod1 | 2.82 | 5.23 ± 0.78a |
| Ras responsive element binding protein 1 | Rreb1 | 2.37 | 3.12 ± 0.35a |
| ELK3, member of ETS oncogene family | Elk3 | 1.98 | 2.43 ± 0.69a |
| Myocyte enhancer factor 2A | Mef2a | 1.55 | 2.51 ± 0.26a |
| 8 h estrogen treatment | |||
| Matrix metallopeptidase 19 | Mmp19 | 3.5 | 7.88 ± 2.01 |
| GH receptor | Ghr | 2.85 | 4.45 ± 0.41a |
| Procollagen lysine, 2-oxoglutarate 5-dioxygenase 2 | Plod2 | 1.72 | 2.79 ± 0.36 |
| Kruppel-like factor 7 | Klf-7 | 0.59 | 0.46 ± 0.02a |
| Preproenkephalin 1 | Penk1 | 0.56 | 0.27 ± 0.08 |
| Decay accelerating factor 1 | Daf1 | 0.44 | 0.75 ± 0.17 |
qRT-PCR analysis was performed on RNA from a distinct set of aortas treated with estrogen or vehicle for the indicated time using gene-specific primers. Ratios represent average fold changes in normalized gene expression after estrogen treatment compared with vehicle (n = 3–6). Microarray fold changes are listed for comparison.
P < 0.05 vs. vehicle.
Temporal Pattern of Estrogen-Regulated Genes in the Mouse Aorta
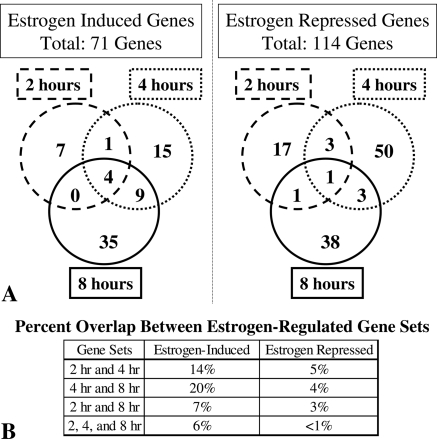
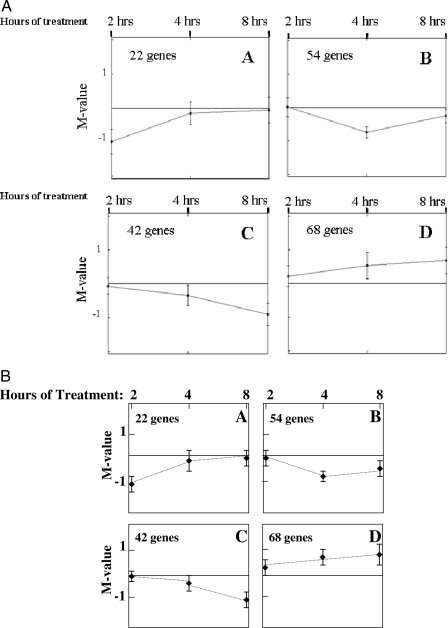
The temporal pattern of estrogen-regulated genes in the blood vessel was next investigated by analyzing the degree of overlap between gene sets at each time point (Fig. 2). There was overlap between the estrogen up-regulated gene sets at 2 and 4 h and at 4 and 8 h (14 and 20%, respectively); however, the number of genes persistently induced by estrogen over the entire 8-h time period was quite small (6%). The down-regulated gene sets were even more distinct with less than 5% overlap between any two time points and only one gene (hepatic leukemia factor) repressed by estrogen over the entire time course (<1%). Using cluster analysis to systematically group the genes based on their temporal pattern of estrogen regulation over time (17), we identified four distinct estrogen-regulated expression patterns (Fig. 3): genes down-regulated by estrogen after 2 h (Fig. 3A), genes down-regulated by estrogen after 4 h (Fig. 3B), genes down-regulated by estrogen after 8 h (Fig. 3C), and genes progressively up-regulated by estrogen over time (Fig. 3D) (supplemental Tables S4–S7). Finer cluster analysis identified two subgroups of the estrogen-induced vascular genes (cluster D), one group that is coregulated at 2 and 4 h and one group coregulated at 4 and 8 h (data not shown), suggesting that there are two consecutive waves of early estrogen-induced genes in the aorta. The cluster analysis is consistent with the qualitative data demonstrated in Fig. 2 that estrogen regulates distinct vascular gene sets over time. Specifically, estrogen represses distinct gene sets at each of the three time points, whereas there are two waves of up-regulated genes, one that spans 2–4 h estrogen treatment and a distinct group that is regulated after 4–8 h estrogen treatment.
Figure 2.
Coregulation of Estrogen-Induced and -Repressed Genes over Time
A, Venn diagrams depict numbers of estrogen-induced and -repressed genes at each time point. The dashed circle includes genes regulated after 2 h, the dotted circle includes genes regulated after 4 h, and the solid circle includes genes regulated after 8 h estrogen treatment in the aorta. B, Summary of the percent overlap between gene sets.
Figure 3.
Time-Dependent Estrogen-Responsive Vascular Gene Clusters
Four distinct gene expression patterns (A–D) were identified by metrical cluster analysis (17) of vascular genes regulated after 2, 4, and 8 h estrogen treatment. The M-value is the log2 of the estrogen-mediated fold change.
Distinct Functions of Estrogen-Regulated Vascular Gene Sets
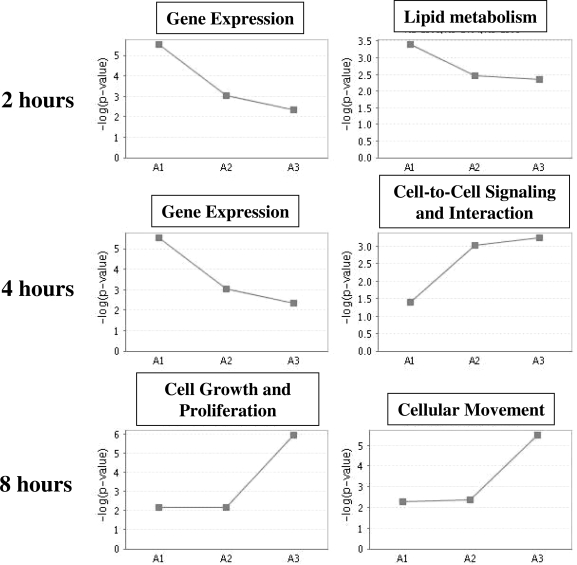
Using the Ingenuity Pathway Analysis Tool (Ingenuity Systems, www.ingenuity.com), we investigated whether temporally coregulated estrogen-responsive genes have distinct functions (Fig. 4). For vascular genes regulated by estrogen treatment for 2 and 4 h, the highest overrepresented function was gene expression, a category that includes DNA binding TF. Estrogen-regulated TF in this group include ELK3-member of ETS oncogene family (Elk3) and myocyte enhancer factor 2a (Mef2a), two TF known to be important in vascular development and biology (18,19). For the group of vascular estrogen-regulated genes at 2 h, the second highest overrepresented function was lipid metabolism, including cholesterol 25-hydroxylase and Cyp51a1, a member of the cytochrome P450 family. In the 4-h estrogen-regulated vascular gene set, the second highest overrepresented function was cell-to-cell signaling and interaction, including integrin-αV and actin-α2. αV-integrins play a role in angiogenesis and vascular remodeling, and mice genetically deficient in αV-integrin have extensive hemorrhages resulting from abnormal development of brain and intestinal vessels (20,21). α-Actin plays a role in the reversible modulation of VSMC between a synthetic and a contractile phenotype that is important for the functional plasticity of these cells (22).
Figure 4.
The Function of Estrogen-Regulated Vascular Genes over Time
For each set of estrogen-regulated vascular genes (2, 4, and 8 h), the two highest overrepresented molecular and cellular functions by Ingenuity Pathway Analysis are depicted. The y-axis indicates the −log (P value) of the overrepresentation of the depicted function at each time point compared with a random gene set. The x-axis includes the three time points (A1 = 2 h; A2 = 4 h; A8 = 8 h).
In contrast, the gene expression category was not overrepresented among vascular genes regulated by estrogen after 8 h. Instead, the two highest overrepresented functions for the 8-h estrogen-regulated vascular genes were cellular growth and proliferation and cellular movement, categories that include the genes thrombospondin 1 (TSP-1) and matrix metallopeptidase (MMP3). TSP-1, which was induced by estrogen in the vessel, is known to inhibit angiogenesis and activate TGF-β signaling, and genetic loss of TSP-1 in a mouse model of myocardial infarction results in increased and protracted myocardial inflammation (23). Matrix metallopeptidases are involved in vascular remodeling and atherosclerotic lesion formation and progression (24). MMP3, which was repressed by estrogen in the aorta, has been specifically implicated in coronary artery remodeling and is associated with unstable clinical presentation (25).
These data demonstrate that estrogen acts directly on the aorta to rapidly initiate functionally distinct gene programs over time. This cascade of gene expression changes begins with a group of transcriptional regulators but changes over 8 h to genes involved in the biology of the blood vessel.
Decrease in Predicted Direct ER Target Genes in the Vasculature over Time
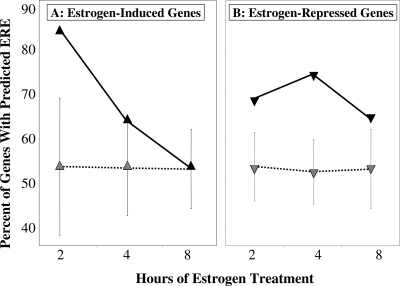
To begin to address the mechanism of estrogen-mediated regulation of vascular gene expression, we searched for the presence of ER binding sites within the promoters of the genes in each estrogen-regulated gene set. For this and all further promoter analyses, we examined 2000 bases upstream of transcription start sites (TSS) and 200 bases of the 5′-untranslated region. When the promoters of the estrogen-induced genes were analyzed, we found a time-dependent decline in the fraction of estrogen-induced genes containing a promoter ERE (from 85% at 2 h to 53% at 8 h) (Fig. 5A). This trend is not observed when the analysis is performed on random background gene sets containing the same number of genes in which we found 53% of gene promoters contained a predicted ERE, regardless of the number of genes in the gene set. The same analysis on the vascular estrogen-repressed gene promoters demonstrated a smaller but significant increase in the percentage of genes containing EREs at all times tested compared with nonregulated background gene sets of the same size (Fig. 5B). These data suggest that estrogen-induced and -repressed genes may have different transcriptional regulatory mechanisms and support the hypothesis that these specific ERE-containing genes, which are recruited first, direct subsequent recruitment of distinct vascular gene expression networks.
Figure 5.
Predicted Estrogen Response Elements in the Promoters of Estrogen-Regulated Vascular Genes
The graph demonstrates the percentage of vascular estrogen-regulated gene promoters at each time point that contain a predicted ER binding site (ERE) for estrogen-induced vascular genes (A) and estrogen-repressed vascular genes (B). In each graph, the percentage of EREs in the regulated gene set (black triangles) is compared with a random background gene set containing the same number of genes (striped triangles ± sd of permuted background set).
Estrogen-Regulated Transcriptional Networks
To test the above hypothesis, we next focused on the nine DNA-binding TF that are regulated by estrogen after 2 and 4 h (Table 2) and searched for overrepresentation of their consensus binding sites in the promoters of estrogen-regulated vascular genes. We found that binding sites for five of the estrogen-regulated DNA-binding TF [Mef2a, Dbp, Hlf, nuclear factor I/B (NfIb), and Rreb1] were overrepresented in the promoters of the vascular estrogen-regulated gene sets compared with a nonregulated background gene set (P ≤ 0.05; Table 3). The number of estrogen-regulated TF binding sites increased over time with 12, 56, and 85 sites identified in the upstream regions of the 2-, 4-, and 8-h estrogen-regulated gene sets, respectively (Table 3, functional depth ≥0.9, genes listed in supplemental Tables S9–20). These data support the hypothesis that early estrogen-regulated TF control downstream cascades of estrogen target genes in the vasculature.
Table 2.
Early Estrogen-Regulated TF in the Blood Vessel
| TF Name | Gene Symbol | Locuslink ID | Fold Change with Estrogen | P Value |
|---|---|---|---|---|
| 2 h estrogen treatment | ||||
| Thyrotroph embryonic factor | Tef | 21685 | 0.64 | 2.0 × 10−4 |
| 2 and 4 h estrogen treatment | ||||
| D site albumin promoter binding protein | Dbp | 13170 | 0.28 | 1.0 × 10−5 |
| 0.54 | 0.009 | |||
| Hepatic leukemia factor | Hlf | 217082 | 0.58 | 0.004 |
| 0.6 | 0.005 | |||
| Aryl hydrocarbon receptor nuclear translocator-like | Arntl | 11865 | 1.9 | 2.0 × 10−4 |
| 1.81 | 3.0 × 10−4 | |||
| 4 h estrogen treatment | ||||
| Nuclear factor I/B | Nfib | 18028 | 0.62 | 0.002 |
| Forkhead box C2 | Foxc2 | 14234 | 1.52 | 0.006 |
| Myocyte enhancer factor 2A | Mef2a | 17258 | 1.55 | 2.0 × 10−4 |
| ELK3, member of ETS oncogene family | Elk3 | 13713 | 1.98 | 3.0 × 10−5 |
| Ras responsive element binding protein 1 | Rreb1 | 68750 | 2.4 | 1.0 × 10−5 |
The table lists DNA-binding TF significantly regulated by estrogen in the blood vessel (1.5-fold change and P ≤ 0.01) within the first 4 h of hormone treatment.
Table 3.
Binding Sites for Estrogen-Regulated TF in the Blood Vessel Are Overrepresented in Estrogen-Regulated Vascular Genes
| Estrogen-Regulated Gene Set | Overrepresented Estrogen-Regulated TF Binding Site | TF Gene Symbol | No. of Genes with Overrepresented Binding Site | Total No. of Overrepresented Estrogen-Regulated TF Binding Sites per Time Point |
|---|---|---|---|---|
| 2 h, estrogen-repressed | Myocyte enhancer factor 2a | Mef2a | 9 | |
| 2 h, estrogen-induced | Nuclear factor I/B | NfIb | 3 | 12 |
| 4 h, estrogen-repressed | Hepatic leukemia factor | Hlf | 24 | |
| Ras responsive element binding protein 1 | Rreb1 | 14 | ||
| Myocyte enhancer factor 2a | Mef2a | 3 | ||
| Nuclear factor I/B | NfIb | 7 | ||
| 4 h, estrogen-induced | Ras responsive element binding protein 1 | Rreb1 | 8 | 56 |
| 8 h, estrogen-repressed | Myocyte enhancer factor 2a | Mef2a | 11 | |
| Ras responsive element binding protein 1 | Rreb1 | 13 | ||
| Nuclear factor I/B | NfIb | 12 | ||
| 8 h, estrogen-induced | D site albumin promoter binding protein | Dbp | 47 | |
| Myocyte enhancer factor 2a | Mef2a | 2 | 85 |
The table lists estrogen-regulated transcription factors with overrepresented binding sites in the promoters of the genes in each of six estrogen-regulated vascular gene sets (2, 4, or 8 h hormone treatment, estrogen repressed or induced). The number of genes containing the overrepresented binding site in each gene set and the total number at each time point are also listed.
Estrogen Regulation of TF Is Receptor Dependent and ER Isoform Specific
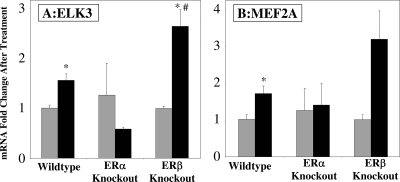
To address the mechanism of estrogen regulation of TF expression, we explored the ER dependence and isoform specificity of estrogen regulation of Elk3 and Mef2a. We chose these two TF because they are both rapidly up-regulated by estrogen in our study and are known to play a role in vascular function and because Mef2A binding sites are overrepresented in the promoters of estrogen-regulated vascular genes at all three time points (Table 3). qRT-PCR was used to investigate estrogen regulation of Elk3 and Mef2a in aortas from mice genetically deficient in either ERα or ERβ (Fig. 6). The induction of Elk3 in response to 4 h ex vivo estrogen treatment was confirmed in the aortas from wild-type mice. However, this effect was lost when ERα was not present in the aorta (Fig. 6A). This supports that Elk3 induction by estrogen in the mouse aorta is mediated by vascular ERα. When ERβ was disrupted, the mean estrogen induction of Elk3 increased compared with wild-type aortas, supporting that in the wild-type vessel, ERβ may repress Elk3 expression. The same pattern is observed for estrogen regulation of vascular Mef2a expression (Fig. 6B), with loss of estrogen regulation of Mef2a in ERα-KO aortas and increased estrogen-regulated expression in ERβ-KO aortas, although the observed increase in the absence of ERβ was not statistically significant (P = 0.1). We have previously observed a reciprocal effect of the two ER isoforms on gene expression in the blood vessel with ERα playing a predominant role in the up-regulation of vascular estrogen target genes and ERβ playing a predominant role in the down-regulation of vascular estrogen target genes (14).
Figure 6.
Vascular Regulation of the TF ELK3 and MEF2A by Estrogen Requires Vascular ERα
Wild-type, ERα-KO, and ERβ-KO aortas were treated ex vivo with estrogen or vehicle for 4 h. qRT-PCR was used to quantify ELK3 (A) or MEF2A (B) expression normalized to β2-microglobulin expression. Bars indicate mRNA fold change compared with the average of the vehicle control: gray, vehicle; black, estrogen treated. *, P < 0.05 vs. vehicle; #, P < 0.05 vs. estrogen-treated wild type.
DISCUSSION
Estrogen Directly Regulates Vascular Gene Expression
In this study, we present a comprehensive analysis of estrogen’s immediate recruitment of vascular gene programs in whole blood vessels and set the stage for delineating the molecular upstream regulatory events that lead to estrogen’s long-term effects on vascular function. Our analysis of early estrogen-regulated genes in the blood vessel demonstrates that 1) at early time points, estrogen-mediated vascular gene repression predominates; 2) estrogen regulates temporally distinct cascades of vascular gene expression; 3) these temporally distinct cascades of estrogen-regulated genes have distinct vascular functions; 4) the earliest estrogen-induced vascular genes are enriched in direct ER binding sites in their promoters; and 5) the earliest estrogen-recruited gene program is enriched in vascular TF that can interact with binding sites present in subsequent estrogen-regulated vascular genes.
Using a short-term, ex vivo, estrogen treatment approach, the observed gene expression changes are expected to be due to direct effects of estrogen on the vascular cells rather than to vascular events caused secondarily to estrogen effects in other tissues, which is common and has confounded interpretations of estrogen effects on the vasculature. For example, in vivo estrogen administration has been shown to alter serum lipid concentrations, the balance between coagulation and fibrinolysis, and the level of oxidative stress to which the vessels are exposed (26), each of which are likely to modify vascular gene expression. We specifically chose to study murine aortic gene expression because the aorta is an important site of atherosclerotic lesion development in humans and in mouse models and provides a sufficient source of vascular tissue for these experiments. The transcriptional response of other vascular beds to estrogen, specifically the resistance vessels that play an important role in the pathogenesis of hypertension, may differ and remains to be investigated. It is worth noting that the gene expression changes that we have identified are likely to occur predominantly in VSMC because these cells constitute the vast majority of the cell population in the tissue studied, the intact aorta. Comparing our results with other gene expression profiling data revealed that fewer than 10% of the estrogen-induced vascular genes and fewer than 5% of the estrogen-repressed vascular genes overlap with estrogen-regulated genes in MCF7 breast cancer cells treated with a similar ex vivo estrogen protocol supporting distinct cell- and tissue-specific effects of estrogen on gene expression.
Estrogen Regulates Temporally Distinct Vascular Gene Sets with Distinct Vascular Functions
Cluster analysis uncovered temporally coregulated sets of genes with estrogen-mediated gene repression occurring in three distinct, successive cascades and estrogen-mediated gene induction occurring in two waves, one that spans 2–4 h and a distinct group that is regulated after 4–8 h (Fig. 2), and the fraction of estrogen-induced genes increasing over time (Fig. 1). In our previous 8-d in vivo estrogen treatment study, we found twice as many vascular genes induced vs. repressed by estrogen (14). From the group of genes induced after 8 h estrogen treatment in the present study (Cluster D, Fig. 3) we found 13 genes that are also estrogen induced (fold change ≥ 1.5; P ≤ 0.01) in our 8-d study (data not shown), suggesting that some of these early gene expression changes in response to estrogen persist in the vessel and may effect long-term vascular function. Although the comparison with our long-term data set is intriguing, it is important to note that the significant differences in the experimental approaches such as systemic vs. direct estrogen application are a limitation of the comparison.
Molecular and biological functions of estrogen-responsive vascular genes differed significantly between the investigated time points with physiologically important functions predominating. Lipid metabolism was the second most overrepresented biological function in the 2-h estrogen-regulated gene set. It is well established that lipid abnormalities are a major risk factor for the progression of atherosclerotic vascular disease and for adverse cardiovascular outcomes (27,28,29), and treatment of hypercholesterolemia is an established therapy for primary and secondary prevention of cardiovascular events (30). Estrogen replacement therapy has been demonstrated to have predominantly beneficial effects on lipid profiles, specifically decreasing serum total cholesterol and low-density lipoprotein-cholesterol levels while increasing serum high-density lipoprotein cholesterol and triglyceride concentrations in postmenopausal women (10). These effects have been attributed to estrogen effects on hepatic lipid metabolism (1,31,32,33). It is also interesting to note that microarray analysis of white adipose tissue of mice lacking the estrogen-related receptor-α (ERRα, an associated receptor that shares targets with ERα) revealed fat metabolism as an important differentially regulated function compared with wild type mice with cholesterol 25-hydroxylase (one of our vascular estrogen-regulated genes) identified as an estrogen-related receptor-regulated gene in white fat (34,35). However, the finding that estrogen directly and rapidly regulates genes involved in lipid metabolism in the vessel wall suggests a completely novel mechanism by which estrogen may modulate vascular function and disease.
The highest overrepresented function in genes regulated in response to estrogen after 8 h treatment is cellular growth and proliferation. Endothelial cell proliferation (reendothelialization) is a protective vascular response to injury, whereas VSMC proliferation can lead to vascular stenosis. Estrogen promotes EC growth and proliferation (7,36) and inhibits VSMC proliferation both in vitro and in vivo (5,6,9,37) by mechanisms that are incompletely understood. Our results are consistent with the role of vascular ER in mediating estrogen’s effects on vascular cell growth and proliferation and suggest a rapid estrogen-responsive gene-regulatory component of these processes. The estrogen-regulated gene thrombospondin, a member of the functional group of cellular growth and proliferation, has also been described to be up-regulated in response to estrogen in MCF7 breast cancer cells (38). This may suggest that estrogen regulation of this pathway and of this gene in particular could be of importance not only in the vascular cell proliferation but also in other tissues as well.
Predicted ERE in Early Estrogen-Regulated Vascular Target Genes
To address the mechanism of the estrogen-regulated transcriptional changes, we searched for ER binding sites (ERE) in the promoters of estrogen-regulated vascular genes and found a large number of genes containing ERE (74% at 2 h) with a reduction of the fraction of genes with ERE over the investigated period of time. The high prevalence of predicted promoter ERE (>85%) was specific for the rapidly estrogen-induced genes (Fig. 5A), supporting the hypothesis that direct, ERE-dependent mechanisms may mediate early vascular gene induction with other transcriptional mechanisms mediating early gene repression and later estrogen-regulated vascular genes. Estrogen has rapid (5–30 min) so called nongenomic actions that activate signaling cascades via posttranslational modifications resulting in rapid activation of non-ER TF by estrogen that may subsequently regulate (up or down) vascular genes at 2 h or later. Additional possible mechanisms for the repression of genes by estrogen may be operative, including direct, ER-mediated gene repression or repression of other TF by ER via binding of ER to the TF on DNA or squelching of limiting factors necessary for activation of specific genes.
TF binding sites have been most extensively studied within 1000 bases of TSS, and these sequences have therefore been widely used to study gene regulation (39,40). We have extended our search to 2000 bases for the ERE and TF overrepresentation studies because regulatory elements have been discovered at greater distances from the TSS. In fact, functional ER binding sites have recently been identified more than 50 kb from TSS in breast cancer cells (15,16), and whether this is true for ER in the vasculature remains to be investigated. In addition, ERE were identified computationally, and further experiments will be necessary to determine which of these binding sites support estrogen-dependent ER binding in vivo in blood vessels. All these possibilities constitute important mechanisms that warrant further analysis by ChIP-on-Chip and/or other related approaches.
Estrogen-Regulated Vascular TF
The functional category of greatest statistical overrepresentation within the early estrogen-regulated genes (2 and 4 h) is gene expression, in which we identified nine vascular estrogen-regulated DNA-binding TF (Table 3), seven of which (excluding Mef2a and Rreb1) have predicted estrogen binding sites in their promoters (data not shown). Together with their early estrogen response, this suggests that these TF may be direct ER target genes. This observation is consistent with a study in uterine tissue demonstrating that estrogen rapidly regulates TF expression in this tissue as well (41). For Mef2A and Elk3, we demonstrated that transcriptional regulation by estrogen specifically requires vascular ERα. Several of the estrogen-regulated TF identified in the aorta have important roles in vascular function. The group of early estrogen-repressed TF includes three members of the proline- and acidic-rich region b-ZIP family of TF (Dbp, Hlf, and Tef), all clock output genes. Vascular clock function has been studied most extensively in aortic SMC, and diurnal variations in Dbp expression have been demonstrated in aortic SMC (42,43). Mef2a is a member of the Mef2 family of TF, important regulators of muscle cell differentiation (18,44) and is the major isoform expressed in VSMC (19). Human genetic mutations in MEF2A have been identified as the cause of familial coronary artery disease (45,46); however, few vascular Mef2a target genes have been identified, and the mechanism for its role in vascular function is not understood (47). Here we report for the first time that Mef2a is an estrogen target gene, regulated by ligand-bound ERα in the aorta.
Although Mef2a mRNA was induced after 4 h hormone treatment, one of the gene sets that showed Mef2a binding site enrichment was the group of genes down-regulated after 2 h estrogen treatment. Mef2a function and stability are known to be regulated by phosphorylation events (48), which may have occurred in response to rapid estrogen effects on signaling cascades and could therefore account for Mef2a binding site enrichment before Mef2a message was detectably induced by estrogen in this study. There is precedent for this paradigm of regulation in vascular EC in which endothelial nitric oxide synthase (eNOS) activity is rapidly increased via phosphorylation by estrogen-activated Akt, and subsequently, eNOS gene transcription is regulated by estrogen via genomic mechanisms (26,49,50). It has also been reported that Mef2 TF can function both as a transcriptional activator and repressor by recruitment of distinct coregulators in skeletal muscle (51,52), and this may explain our finding that MEF2a binding sites were enriched both in estrogen-induced and -repressed gene sets.
Rapid Estrogen Regulation of a Transcriptional Gene Program in the Vasculature
Five of the vascular estrogen-regulated TF (Mef2a, Dbp, Hlf, Rreb1, and NfIb) have enriched binding sites in the vascular estrogen-regulated genes (Table 3). For estrogen-induced genes, as the percentage of ERE-containing gene promoters declines, the number of genes containing binding sites for estrogen-regulated TF rises (three, eight, and 49 binding sites at 2, 4, and 8 h, respectively). For estrogen-repressed vascular genes, we find an increase in the presence of estrogen-regulated TF binding sites by 4 h (nine, 48, and 36 binding sites at 2, 4, and 8 h, respectively). At 2 h, the estrogen-repressed genes contain fewer ERE and few ER-regulated TF binding sites; hence, the earliest estrogen-mediated vascular gene repression may be mediated by different mechanisms including posttranslational modification of other TF via nongenomic estrogen actions or physiological squelching of limiting factors as has been proposed by others to explain similar findings (15). Further analysis of our steady-state estrogen data set reveals that three of these rapidly estrogen-regulated TF (Mef2a, Idb4, and Mga) also have enriched binding sites in estrogen-regulated genes after 8 d in vivo hormone treatment. Together this indicates that the observed early estrogen recruitment of specific vascular transcriptional regulators may play an important role in regulating downstream events that mediate estrogen effects on vascular function and disease.
Overall, this study demonstrates that estrogen causes rapid and direct changes in vascular gene expression and suggest multiple novel mechanistic hypotheses for the vascular protective effects of estrogen. Future testing of these new hypotheses will begin to elucidate the molecular mechanisms of estrogen’s role in vascular function and may generate new, more specific drug targets to treat or prevent cardiovascular disease in men and women.
MATERIALS AND METHODS
Animal Studies
Female C57BL/6J (wild type) mice were obtained from The Jackson Laboratory (Bar Harbor, ME). ERα−/− (ERα-KO) (53) and ERβ−/− (ERβ-KO) (54) female mice were obtained from propagation of heterozygous mouse colonies. All animals were handled in accordance with National Institutes of Health standards and procedures approved by the Tufts Medical Center Institutional Animal Care and Use Committee.
For gene expression profiling, wild-type female mice, age 10–12 wk, were ovariectomized, and 1 wk later, aortas were harvested. Six groups of nine aortas were similarly treated with 10−8 m 17β-estradiol or ethanol vehicle for 2, 4, or 8 h at 37 C in DMEM (GIBCO, Carlsbad, CA) and then immediately frozen in liquid nitrogen. For each time point and treatment, RNA from three aortas was pooled for one array and three pooled, biological replicates were performed. For qRT-PCR experiments, aortas from ovariectomized female wild-type, ERβ-KO, or ERα-KO mice (11–15 wk old) were treated as described above.
Uteri harvested from the mice used for the gene expression analyses were treated identically, and qRT-PCR using primers specific for MAD2 and adrenomedullin (two previously identified estrogen-regulated uterine genes), demonstrated estrogen-mediated gene expression changes consistent with previous reports (41,55), confirming the validity of our ex vivo treatment approach (data not shown).
RNA Isolation, Microarray Hybridization, and Data Analysis
Total aortic RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA), followed by column purification (RNeasy; QIAGEN, Valencia, CA). RNA was reverse transcribed into cDNA, labeled, and hybridized to Mouse Genome 430A 2.0 microarrays (Affymetrix, Santa Clara, CA) at the Dana Farber Cancer Institute Microarray Core Facility (http://chip.dfci.harvard.edu/lab/services.php). The BioConductor suite of programs was used to perform the microarray data analysis (56). A uniform and high quality of all arrays was confirmed by a quality control check as implemented in Simpleaffy (57). All calculated parameters such as the scale factor, average background ratio, percent present calls, and 3′/5′ ratios were within the recommended value ranges; further 3′/5′ RNA degradation plots excluded that any of the arrays had been affected by RNA degradation. Background correction, normalization, and summarization of the raw probe intensities were carried out using the GC content Robust Multichip Analysis (GCRMA) protocol as implemented in BioConductor (58). The following filter was used to select only significantly expressed genes for further analysis. All probe sets that did not show an expression value of 100 U in at least 25% of the chips and an interquartile range of expression values of 0.5 (in log2 units) were removed from the rest of the calculation. Gene expression values for each sample were estimated using the linear model of the data as implemented in the LIMMA package (59), and the differential expression of genes between estrogen and vehicle treatment at each time point was calculated. The eBayes package that allows borrowing information across genes for each of these contrasts was used to calculate the moderated statistics for these contrasts (59). Subsequently, the topTable function was applied to generate a list of differentially expressed genes ranked by the P values of differential expression (59). Only genes with estrogen-regulated expression change that was both highly reproducible (P ≤ 0.01) and varied by at least 1.5-fold were considered as significantly differentially expressed and were used in all further analyses.
qRT-PCR Analysis
Total aortic RNA was isolated from treated mouse aortas using TRIzol reagent (Invitrogen). RNA was reverse transcribed and qRT-PCR carried out using SybrGreen (QIAGEN) and the Stratagene (La Jolla, CA) real-time PCR machine and MxPro-Stratagene software as previously described (60). Each PCR was performed in triplicate. The final products in each case were subjected to thermal denaturation to ensure that the product denatured as a uniform peak at the appropriate temperature. For each gene, the RNA level was normalized to that of β2-microglobulin and is expressed as fold change in estrogen-treated samples compared with vehicle-treated samples. The primers are specified in supplemental Table S8.
Bioinformatics Analyses of Gene Clustering and Functional Categorization
K-Means-clustering was performed on all vascular estrogen-regulated genes using the Genomic Research MultiExperiment Viewer (17) using Euclidian distance to separate the genes into four groups based on the fold changes at the three time points. The analysis for enrichment of biological and molecular functions was performed using the Ingenuity Pathway Analysis Tool (Ingenuity Systems, www.ingenuity.com).
TF Binding Site Analysis
Genomic regions 2 kb upstream of the TSS and including 200 bp of the 5′-untranslated region for each gene in the regulated and background gene sets were extracted from the ENSMART mouse genome database (assembly version NCBI 36). A set of 1000 randomly selected genes that are not present in any of the six investigated categories was used as a background gene set for this analysis. The TF binding site analysis was carried out using the Comprehensive Regulatory Element Analysis and Discovery (CREAD) suite of programs (61) and the entire set of vertebrate TF binding site matrices (motifs) in the TRANSFAC Professional database (Biobase Corp., Beverley, MA, version 10.3). The Motifclass program was used to test the overrepresentation of the TRANSFAC motifs in the regulated, compared with the background, gene sets. For candidate TF binding site scanning approaches, we carried out a more focused analysis of overrepresentation of motifs using a binomial P value to determine the significance of the overrepresentation of each motif. The occurrences of ERE were determined by searching the sequences for sites that match ERE motifs represented as position weight matrices (62). Only those matches that had a functional depth of at least 0.9 were considered as significant. The functional depth is defined as F = (S − Smin)/(Smax − Smin), where S is the score of occurrence for a motif in a squence and Smin and Smax are the minimum and maximum that a motif match can score. A functional depth of 1.0 signifies the best match of a motif to the sequence. To estimate the significance of the number of EREs present at each of the six conditions, a background set of genes containing the same number of promoter sequences were permuted 1000 times and the number of EREs calculated. The mean and sd values of the resulting distribution of number of EREs were calculated for these background conditions.
Supplementary Material
Footnotes
This work was supported in part by a grant from the National Institutes of Health, NIH R01 HL50569 (to M.E.M.).
Disclosure Statement: There are no conflicts of interest to declare.
First Published Online September 11, 2008
Abbreviations: EC, Endothelial cell; ER, estrogen receptor; ERE, estrogen-responsive element; KO, knockout; qRT-PCR, quantitative RT-PCR; SMC, smooth muscle cell; TF, transcription factor; TSS, transcription start site; VSMC, vascular SMC.
References
- Mendelsohn ME, Karas RH 2005 Molecular and cellular basis of cardiovascular gender differences. Science 308:1583–1587 [DOI] [PubMed] [Google Scholar]
- Manson JE, Allison MA, Rossouw JE, Carr JJ, Langer RD, Hsia J, Kuller LH, Cochrane BB, Hunt JR, Ludlam SE, Pettinger MB, Gass M, Margolis KL, Nathan L, Ockene JK, Prentice RL, Robbins J, Stefanick ML, WHI and WHI-CACS Investigators 2007 Estrogen therapy and coronary-artery calcification. N Engl J Med 356:2591–2602 [DOI] [PubMed] [Google Scholar]
- Mendelsohn ME, Karas RH 2007 HRT and the young at heart. N Engl J Med 356:2639–2641 [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Barnabei VM, Ko M, LaCroix AZ, Margolis KL, Stefanick ML 2007 Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 297:1465–1477 [DOI] [PubMed] [Google Scholar]
- Bhalla RC, Toth KF, Bhatty RA, Thompson LP, Sharma RV 1997 Estrogen reduces proliferation and agonist-induced calcium increase in coronary artery smooth muscle cells. Am J Physiol 272:H1996–H2003 [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Suzuki T, Miki Y, Tazawa C, Senzaki K, Moriya T, Saito H, Ishibashi T, Takahashi S, Yamada S, Sasano H 2004 Estrogen receptors in atherosclerotic human aorta: inhibition of human vascular smooth muscle cell proliferation by estrogens. Mol Cell Endocrinol 219:17–26 [DOI] [PubMed] [Google Scholar]
- Morales DE, McGowan KA, Grant DS, Maheshwari S, Bhartiya D, Cid MC, Kleinman HK, Schnaper HW 1995 Estrogen promotes angiogenic activity in human umbilical vein endothelial cells in vitro and in a murine model. Circulation 91:755–763 [DOI] [PubMed] [Google Scholar]
- Hodgin JB, Krege JH, Reddick RL, Korach KS, Smithies O, Maeda N 2001 Estrogen receptor α is a major mediator of 17β-estradiol’s atheroprotective effects on lesion size in Apoe−/− mice. J Clin Invest 107:333–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare G, Krust A, Karas RH, Dupont S, Aronovitz M, Chambon P, Mendelsohn ME 2002 Estrogen receptor-α mediates the protective effects of estrogen against vascular injury. Circ Res 90:1087–1092 [DOI] [PubMed] [Google Scholar]
- 1995 Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. The Writing Group for the PEPI Trial. JAMA [Erratum (1995) 274:1676] 273:199–208 [PubMed] [Google Scholar]
- Zhu Y, Bian Z, Lu P, Karas RH, Bao L, Cox D, Hodgin J, Shaul PW, Thoren P, Smithies O, Gustafsson JA, Mendelsohn ME 2002 Abnormal vascular function and hypertension in mice deficient in estrogen receptor β. Science 295:505–508 [DOI] [PubMed] [Google Scholar]
- Bjornstrom L, Sjoberg M 2005 Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol 19:833–842 [DOI] [PubMed] [Google Scholar]
- Mendelsohn ME 2000 Nongenomic, ER-mediated activation of endothelial nitric oxide synthase: how does it work? What does it mean? Circ Res 87:956–960 [DOI] [PubMed] [Google Scholar]
- O'Lone R, Knorr K, Jaffe IZ, Schaffer ME, Martini PG, Karas RH, Bienkowska J, Mendelsohn ME, Hansen U 2007 Estrogen receptors α and β mediate distinct pathways of vascular gene expression, including genes involved in mitochondrial electron transport and generation of reactive oxygen species. Mol Endocrinol 21:1281–1296 [DOI] [PubMed] [Google Scholar]
- Carroll JS, Meyer CA, Song J, Li W, Geistlinger TR, Eeckhoute J, Brodsky AS, Keeton EK, Fertuck KC, Hall GF, Wang Q, Bekiranov S, Sementchenko V, Fox EA, Silver PA, Gingeras TR, Liu XS, Brown M 2006 Genome-wide analysis of estrogen receptor binding sites. Nat Genet 38:1289–1297 [DOI] [PubMed] [Google Scholar]
- Carroll JS, Liu XS, Brodsky AS, Li W, Meyer CA, Szary AJ, Eeckhoute J, Shao W, Hestermann EV, Geistlinger TR, Fox EA, Silver PA, Brown M 2005 Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell 122:33–43 [DOI] [PubMed] [Google Scholar]
- Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M, Sturn A, Snuffin M, Rezantsev A, Popov D, Ryltsov A, Kostukovich E, Borisovsky I, Liu Z, Vinsavich A, Trush V, Quackenbush J 2003 TM4: a free, open-source system for microarray data management and analysis. Biotechniques 34:374–378 [DOI] [PubMed] [Google Scholar]
- Lin Q, Lu J, Yanagisawa H, Webb R, Lyons GE, Richardson JA, Olson EN 1998 Requirement of the MADS-box transcription factor MEF2C for vascular development. Development 125:4565–4574 [DOI] [PubMed] [Google Scholar]
- Suzuki E, Guo K, Kolman M, Yu YT, Walsh K 1995 Serum induction of MEF2/RSRF expression in vascular myocytes is mediated at the level of translation. Mol Cell Biol 15:3415–3423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader BL, Rayburn H, Crowley D, Hynes RO 1998 Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all αv integrins. Cell 95:507–519 [DOI] [PubMed] [Google Scholar]
- Eliceiri BP, Cheresh DA 1999 The role of αv integrins during angiogenesis: insights into potential mechanisms of action and clinical development. J Clin Invest 103:1227–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halayko AJ, Solway J 2001 Molecular mechanisms of phenotypic plasticity in smooth muscle cells. J Appl Physiol 90:358–368 [DOI] [PubMed] [Google Scholar]
- Chatila K, Ren G, Xia Y, Huebener P, Bujak M, Frangogiannis NG 2007 The role of the thrombospondins in healing myocardial infarcts. Cardiovasc Hematol Agents Med Chem 5:21–27 [DOI] [PubMed] [Google Scholar]
- Galis ZS, Khatri JJ 2002 Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res 90:251–262 [PubMed] [Google Scholar]
- Schoenhagen P, Vince DG, Ziada KM, Kapadia SR, Lauer MA, Crowe TD, Nissen SE, Tuzcu EM 2002 Relation of matrix-metalloproteinase 3 found in coronary lesion samples retrieved by directional coronary atherectomy to intravascular ultrasound observations on coronary remodeling. Am J Cardiol 89:1354–1359 [DOI] [PubMed] [Google Scholar]
- Mendelsohn ME, Karas RH 1999 The protective effects of estrogen on the cardiovascular system. N Engl J Med 340:1801–1811 [DOI] [PubMed] [Google Scholar]
- Berliner JA, Navab M, Fogelman AM, Frank JS, Demer LL, Edwards PA, Watson AD, Lusis AJ 1995 Atherosclerosis: basic mechanisms. Oxidation, inflammation, and genetics. Circulation 91:2488–2496 [DOI] [PubMed] [Google Scholar]
- Carmena R, Duriez P, Fruchart JC 2004 Atherogenic lipoprotein particles in atherosclerosis. Circulation 109:III2–III7 [DOI] [PubMed] [Google Scholar]
- Barter PJ, Nicholls S, Rye KA, Anantharamaiah GM, Navab M, Fogelman AM 2004 Antiinflammatory properties of HDL. Circ Res 95:764–772 [DOI] [PubMed] [Google Scholar]
- Kostis JB 2007 The importance of managing hypertension and dyslipidemia to decrease cardiovascular disease. Cardiovasc Drugs Ther 21:297–309 [DOI] [PubMed] [Google Scholar]
- Gorodeski GI 2002 Update on cardiovascular disease in post-menopausal women. Best Pract Res Clin Obstet Gynaecol 16:329–355 [DOI] [PubMed] [Google Scholar]
- Mendelsohn ME 2002 Protective effects of estrogen on the cardiovascular system. Am J Cardiol 89:12E–17E [DOI] [PubMed] [Google Scholar]
- Seed M, Knopp RH 2004 Estrogens, lipoproteins, and cardiovascular risk factors: an update following the randomized placebo-controlled trials of hormone-replacement therapy. Curr Opin Lipidol 15:459–467 [DOI] [PubMed] [Google Scholar]
- Luo J, Sladek R, Carrier J, Bader JA, Richard D, Giguere V 2003 Reduced fat mass in mice lacking orphan nuclear receptor estrogen-related receptor α. Mol Cell Biol 23:7947–7956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanacker JM, Pettersson K, Gustafsson JA, Laudet V 1999 Transcriptional targets shared by estrogen receptor-related receptors (ERRs) and estrogen receptor (ER)α, but not by ERβ. EMBO J 18:4270–4279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasinski K, Spyridopoulos I, Asahara T, van der ZR, Isner JM, Losordo DW 1997 Estradiol accelerates functional endothelial recovery after arterial injury. Circulation 95:1768–1772 [DOI] [PubMed] [Google Scholar]
- Sullivan Jr TR, Karas RH, Aronovitz M, Faller GT, Ziar JP, Smith JJ, O'Donnell Jr TF, Mendelsohn ME 1995 Estrogen inhibits the response-to-injury in a mouse carotid artery model. J Clin Invest 96:2482–2488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh MG, Thompson DA, Weigel RJ 2000 PDZK1 and GREB1 are estrogen-regulated genes expressed in hormone-responsive breast cancer. Cancer Res 60:6367–6375 [PubMed] [Google Scholar]
- Odom DT, Zizlsperger N, Gordon DB, Bell GW, Rinaldi NJ, Murray HL, Volkert TL, Schreiber J, Rolfe PA, Gifford DK, Fraenkel E, Bell GI, Young RA 2004 Control of pancreas and liver gene expression by HNF transcription factors. Science 303:1378–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren B, Cam H, Takahashi Y, Volkert T, Terragni J, Young RA, Dynlacht BD 2002 E2F integrates cell cycle progression with DNA repair, replication, and G2/M checkpoints. Genes Dev 16:245–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Suzuki A, Kobayashi M, Takahashi E, Itamoto M, Lubahn DB, Handa H, Iguchi T 2003 Analysis of temporal changes in the expression of estrogen-regulated genes in the uterus. J Mol Endocrinol 30:347–358 [DOI] [PubMed] [Google Scholar]
- Reilly DF, Westgate EJ, FitzGerald GA 2007 Peripheral circadian clocks in the vasculature. Arterioscler Thromb Vasc Biol 27:1694–1705 [DOI] [PubMed] [Google Scholar]
- Young ME, Razeghi P, Taegtmeyer H 2001 Clock genes in the heart: characterization and attenuation with hypertrophy. Circ Res 88:1142–1150 [DOI] [PubMed] [Google Scholar]
- Kaushal S, Schneider JW, Nadal-Ginard B, Mahdavi V 1994 Activation of the myogenic lineage by MEF2A, a factor that induces and cooperates with MyoD. Science 266:1236–1240 [DOI] [PubMed] [Google Scholar]
- Wang L, Fan C, Topol SE, Topol EJ, Wang Q 2003 Mutation of MEF2A in an inherited disorder with features of coronary artery disease. Science 302:1578–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagavatula MR, Fan C, Shen GQ, Cassano J, Plow EF, Topol EJ, Wang Q 2004 Transcription factor MEF2A mutations in patients with coronary artery disease. Hum Mol Genet 13:3181–3188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JP, Dodou E, Heidt AB, De Val SJ, Jaehnig EJ, Greene SB, Olson EN, Black BL 2004 HRC is a direct transcriptional target of MEF2 during cardiac, skeletal, and arterial smooth muscle development in vivo. Mol Cell Biol 2:3757–3768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DM, Du M, Marback M, Yang EC, Chan J, Siu KW, McDermott JC 2003 Phosphorylation motifs regulating the stability and function of myocyte enhancer factor 2A. J Biol Chem 278:15297–15303 [DOI] [PubMed] [Google Scholar]
- Lu Q, Pallas DC, Surks HK, Baur WE, Mendelsohn ME, Karas RH 2004 Striatin assembles a membrane signaling complex necessary for rapid, nongenomic activation of endothelial NO synthase by estrogen receptor α. Proc Natl Acad Sci USA 101:17126–17131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn ME 2002 Genomic and nongenomic effects of estrogen in the vasculature. Am J Cardiol 90:3F–6F [DOI] [PubMed] [Google Scholar]
- McKinsey TA, Zhang CL, Olson EN 2001 Control of muscle development by dueling HATs and HDACs. Curr Opin Genet Dev 11:497–504 [DOI] [PubMed] [Google Scholar]
- McKinsey TA, Zhang CL, Lu J, Olson EN 2000 Signal-dependent nuclear export of a histone deacetylase regulates muscle differentiation. Nature 408:106–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M 2000 Effect of single and compound knockouts of estrogen receptors β (ERα) and β (ERβ) on mouse reproductive phenotypes. Development 127:4277–4291 [DOI] [PubMed] [Google Scholar]
- Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O 1998 Generation and reproductive phenotypes of mice lacking estrogen receptor β. Proc Natl Acad Sci USA 95:15677–15682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Takahashi E, Kobayashi M, Goto M, Krust A, Chambon P, Iguchi T 2006 The estrogen-responsive adrenomedullin and receptor-modifying protein 3 gene identified by DNA microarray analysis are directly regulated by estrogen receptor. J Mol Endocrinol 36:81–89 [DOI] [PubMed] [Google Scholar]
- Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, Hornik K, Hothorn T, Huber W, Iacus S, Irizarry R, Leisch F, Li C, Maechler M, Rossini AJ, Sawitzki G, Smith C, Smyth G, Tierney L, Yang JY, Zhang J 2004 Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 5:R80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CL, Miller CJ 2005 Simpleaffy: a BioConductor package for Affymetrix Quality Control and data analysis. Bioinformatics 21:3683–3685 [DOI] [PubMed] [Google Scholar]
- Wu Z, Irizarry RA 2004 Preprocessing of oligonucleotide array data. Nat Biotechnol 22:656–658 [DOI] [PubMed] [Google Scholar]
- Smyth GK 2004 Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 3:3 [DOI] [PubMed] [Google Scholar]
- Jaffe IZ, Mendelsohn ME 2005 Angiotensin II and aldosterone regulate gene transcription via functional mineralocorticoid receptors in human coronary artery smooth muscle cells. Circ Res 96:643–650 [DOI] [PubMed] [Google Scholar]
- Smith AD, Sumazin P, Xuan Z, Zhang MQ 2006 DNA motifs in human and mouse proximal promoters predict tissue-specific expression. Proc Natl Acad Sci USA 103:6275–6280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schones DE, Smith AD, Zhang MQ 2007 Statistical significance of cis-regulatory modules. BMC Bioinformatics 8:19 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.