Abstract
Objective:
To provide background information on methodologic factors that influence and add variance to endocrine outcome measurements. Our intent is to aid and improve the quality of exercise science and sports medicine research endeavors of investigators inexperienced in endocrinology.
Background:
Numerous methodologic factors influence human endocrine (hormonal) measurements and, consequently, can dramatically compromise the accuracy and validity of exercise and sports medicine research. These factors can be categorized into those that are biologic and those that are procedural-analytic in nature.
Recommendations:
Researchers should design their studies to monitor, control, and adjust for the biologic and procedural-analytic factors discussed within this paper. By doing so, they will find less variance in their hormonal outcomes and thereby will increase the validity of their physiologic data. These actions can assist the researcher in the interpretation and understanding of endocrine data and, in turn, make their research more scientifically sound.
Keywords: hormones, biomedical sciences, sport research design, study design
During the last 25 years, an increasing number of exercise science and sports medicine researchers have begun to incorporate endocrinologic measurements (eg, hormones, cytokines) into their designs.1,2 This approach has allowed for a heightened level of research examining the physiologic mechanisms associated with certain clinical and performance-related conditions found in athletes.
Unfortunately, some exercise science investigators have not always controlled certain factors (eg, time of day for blood sampling) that can influence many of the hormones within the human endocrine system. This lack of experimental control has often resulted in the emerging research being inconsistent, contradictory, and difficult to interpret. The inadequate accounting for experimental controls may be due to limited knowledge by exercise science and sports medicine researchers in the area of endocrine methodology. It is regrettable that this educational shortcoming occurs, but in reality, few laboratories throughout the world actively pursue exercise endocrinology as one of their primary areas of study or research foci.
The factors that influence hormonal measurements and contribute to variance in outcomes can be categorized as coming from 2 potential sources: factors affecting biologic variation (ie, affiliated with the physiologic function or status of the participant) and factors affecting procedural-analytic variation (ie, determined by the investigators conducting the research).1 Regardless of whether the source of variance is participant or investigator derived, if it is not controlled or accounted for appropriately, then hormonal measures can certainly be compromised. Such compromises can call into question the validity and scientific quality of a research study.
We developed this paper in order to serve as a primer for exercise science and sports medicine researchers on the methodologic factors involved in endocrine measurements, so that they can improve the quality of their research endeavors. This paper is by no means a complete and exhaustive treatise on this topic, and readers desiring more encompassing discussions are directed to more involved texts.1,3
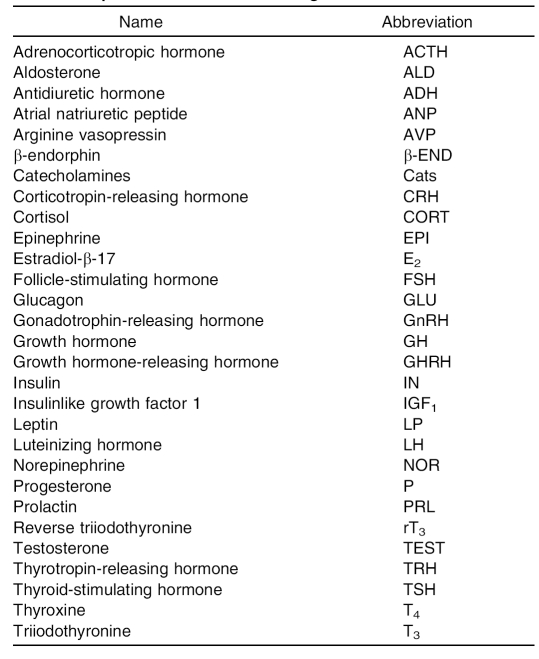
The field of endocrinology uses abbreviations for many hormones. These abbreviations can sometimes be confusing to novices in the field. To aid unfamiliar researchers, the Table lists the most common hormones associated with the area of exercise science and sports medicine and their typical abbreviations.4
Table. Hormone Abbreviations Commonly Used in Exercise Science and Sport Medicine Endocrinologic Research4
Biologic Factors
As noted in the opening text, factors that can influence hormonal measurements can be categorized into 2 broad areas: biologic and procedural-analytic. The biologic factors are those that are determined to be connected in some way to the physiologic function or status of the participant at the time of specimen collection. These factors can be viewed as endogenous in nature.
Sex
Until the onset of puberty, males and females exhibit little difference in their resting hormonal profiles. Once puberty is reached, however, males demonstrate increased androgen steroid hormone production and females show the characteristic menstrual cycle pulsatile release of gonadotrophin and sex steroid hormones.5–7 Additionally, at puberty, resting levels of leptin (an adipocyte cytokine, a low molecular-weight protein that has endocrinelike actions on select physiologic processes [eg, immune system]8) tend to become elevated in females as compared with those in males.9 The differences that manifest at puberty tend to persist through adulthood until women become postmenopausal.8,9
Some sex-specific differences in the hormonal responses to exercise also exist. These include an earlier and greater rise in testosterone in males and a greater pre-exercise growth hormone response in females. Additionally, the magnitude of the sex steroid hormonal response to exercise in females is influenced by the status and phase of the menstrual cycle.10,11 Interestingly, the menstrual cycle hormones can influence other hormones and their response to exercise (eg, increased estradiol-β-17 leads to increased growth hormone levels).10–12 The menstrual cycle is discussed further in a later section of this paper. It is also important to recognize that some hormones show little or no differences between the sexes in response to exercise (eg, water-balance hormones such as aldosterone and vasopressin).5,10,11
For the above reasons, researchers should be cautious when testing mixed-sex participant populations in their studies, depending upon desired outcomes. To avoid confounding results, the investigator needs to be certain that the hormonal outcomes being measured are not influenced by sex.
Age
In hormonal research, participants not matched for age and maturation level may demonstrate increased outcome variance. Prepubertal and postpubertal children of the same sex do not typically display the exact same hormonal responses or relationships.13,14 This is illustrated by the well-documented increase in insulin resistance observed as an adolescent goes through puberty.15
Such concerns also should be extended to the other end of the age spectrum. A postmenopausal woman or andropausal man could have drastically different hormonal responses than her or his prepausal counterparts. For example, growth hormone and testosterone typically decrease with age, whereas cortisol and insulin resistance increase.16–18
These types of age-related differences can exist at rest, in response to exercise, and even after an exercise training program. For this reason, in designing studies (and unless the researcher is studying age-related changes among populations3), it may be important to match participants by chronologic age or maturation level (or both) in order to increase the homogeneity of the responses and decrease interindividual variability.
Race
Many humoral constituents are known to vary among people of different races.1,3 Only a few hormonal differences, however, have been identified to exist. For example, resting parathyroid hormone levels tend to be higher in blacks than whites.19 White females tend to have higher levels of estrogens than Asian females.1,20 Reproductive hormone levels during gestational periods also may vary across races (whites, blacks, Hispanics, Asians, and Indians).20–23 Greater resting insulin levels and insulin resistance have been noted in certain Native American tribes (eg, Prima Indians); however, these differences may be related more to obesity issues than to race.24
Hormonal responses to exercise and exercise training related to race have not been well studied, and the limited data available do not suggest drastically different response outcomes. However, further research in this area is necessary.1,23,24
Body Composition
Varying levels of adiposity can greatly influence the cytokines released by adipose tissue.3,8,9 These cytokine substances in turn have autocrinelike, parcrinelike, and endocrinelike actions in the body and influence metabolic, reproductive, and inflammatory status.2,3,8,9 In addition, several cytokines have been linked directly to increased hormonal levels (eg, increased interleukin 6 is associated with increased cortisol).8 This situation is compounded as adiposity reaches the level of obesity and subsequently affects many hormones, potentially to a far greater extent (eg, insulin and leptin levels tend to be elevated at rest in many obese people).25–29
If a person's level of adiposity increases (toward obesity), the hormonal response to exercise and exercise training can change drastically from that of a normal-weight person. For example, in obese people, the catecholamine and growth hormone response to exercise is reduced.29 Cortisol responses to exercise have been elevated in some overweight-obese individuals, although reductions also have been reported.28,29 Exercise training often results in a loss of body mass, which helps to bring the responses of these hormones more in line with that observed in normal-weight people.29–33
To ensure that participants' various levels of body composition will not confound some hormonal outcomes, investigators need to match their volunteers for adiposity as closely as possible rather than simply matching body weights. Exactly how close a match is needed is not known, but grouping normal-weight, overweight (body mass index ≥ 25.0 ≤ 30.0 kg·m−2), and obese (body mass index >30.0 kg·m−2) individuals in the same participant pool can certainly complicate and add variance to some hormonal outcomes.1,29
Mental Health
Certain mental health conditions are associated with high anxiety levels (eg, posttraumatic stress disorder), which can lead to enhanced sympathetic nervous system and hypothalamic-pituitary-adrenal axis activity.34–36 Subsequently, resting levels of circulating catecholamines, adrenocorticotropic hormone, β-endorphin, and cortisol also may be elevated in these conditions. In contrast, persons who are depressed may have low arousal levels and suppressed levels of the aforementioned hormones. Furthermore, depression is sometimes accompanied by low activity levels in the hypothalamic-pituitary-thyroid axis (ie, low levels of thyrotropin-releasing hormone, thyroid-stimulating hormone, thyroxine, and triiodothyronine).34–36
These alterations in resting hormonal levels can, in turn, result in altered hormonal responses to exercise and exercise training in individuals who have high levels of anxiety or frustration.37–39 Responses may be heightened in some cases and diminished in others.37–39
Asking a participant to complete a mental health screening questionnaire can serve as an excellent tool to determine if a potential emotional or psychological problem exists that could confound hormonal measures. A variety of such screening tools are available, and the reader is directed to several references for discussions of the topic.40,41 However, any such screening should be performed by a trained, qualified individual.
Menstrual Cycle
The menstrual status (eumenorrheic versus amenorrheic) and cycle phase (follicular, ovulation, or luteal) in females can produce basal changes in key reproductive hormones such as estradiol-β-17, progesterone, luteinizing hormone, and follicle-stimulating hormone. These changes can be large and dramatic within select individuals. For example, the ovulatory and luteal phases result in increases in all of the aforementioned hormones above levels seen in the follicular phase (eg, 2-fold to 10-fold greater in eumenorrheic females).42 Furthermore, as noted earlier, certain reproductive hormones can, in turn, influence other nonreproductive hormones at rest.11,43,44
Menstrual status and cycle-phase hormonal influences can affect exercise and exercise training responses, too. Therefore, researchers may need to conduct exercise testing with females of similar menstrual status or in similar phases of their cycle (or both). This precaution also is applicable to females who are using oral contraceptives, which can mimic some hormonal fluctuations similar to cycle-phase changes.44,45
Circadian Rhythms
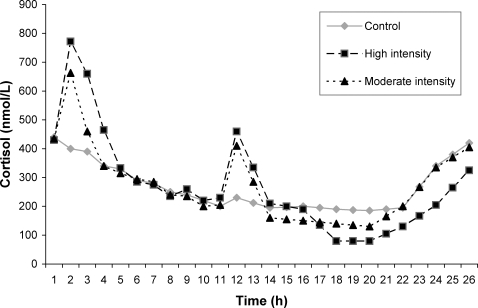
Many hormonal levels fluctuate and display circadian variations. In some cases, these variances are due to pulse generator aspects (ie, the spontaneous release of select hypothalamic hormone-releasing factors [hormones]46) within the endocrine regulatory axis. In other cases, variances are related to humoral stimuli changes brought on by participant behavior or environmental factors.47,48 Circadian hormones can display dramatic changes in levels due to their rhythm patterns, cortisol being a prime example. Morning cortisol levels are typically twice those found later in the day.49–51 The magnitude of this effect is illustrated in the Figure, which has been redrawn from the results of Hackney and Viru.49
Figure. The mean 24-hour cortisol responses of endurance-trained men (n = 17) on 3 separate days: a control baseline day with no exercise, a high-intensity exercise day (1 hour in the morning and 45 minutes in the afternoon of interval training at 100% to 110% maximal oxygen uptake [repeated bouts of 2 minutes of exercise and 2 minutes of recovery in each session]), and a moderate-intensity exercise day (1 hour in the morning and 45 minutes in the afternoon of continuous aerobic training at 60% to 65% of maximal oxygen uptake). The figure has been redrawn with permission.49.
When conducting exercise research, these fluctuations and circadian variations need to be addressed. The magnitude of exercise responses may not be similar at different times of the day, even if the exercise intensity and duration are held constant.1,49 Investigators should plan accordingly to carefully control and replicate the time of day in which research testing is conducted and the hormonal specimen collected.52,53
Procedural-Analytic Factors
The second category of factors influencing hormonal measurements is made up of those factors that are procedural or analytic in nature. That is, these factors are determined, selected, or in some way controlled (potentially) by the investigators conducting or the participant involved in the research.1 These factors can be viewed as exogenous in nature.
Environment
When conducting investigations, it is important to remember that excessive exposure to hot or cold ambient temperatures can stimulate various endocrine gland hormones: for example, those involved in water balance (aldosterone) or energy substrate mobilization (cortisol).37,54,55 Even elevated ambient relative humidity (water vapor) can induce this effect, primarily due to compromised heat dissipation through evaporation adding to the body core temperature.55 These effects can be further augmented if hypoxemia is induced, as with exposure to high altitudes.56–58
Many of the exercise and exercise training hormonal responses are affected tremendously by environmental factors. In particular, catecholamines, growth hormone, aldosterone, antidiuretic hormone (vasopressin), adrenocorticotropic hormone, and cortisol are susceptible to environmental conditions and show exacerbated responses in various conditions.1,37,54,55 To minimize these influences, it is critical to conduct exercise testing in controlled, standardized conditions, such as in a laboratory. However, if conducting field research (where standardization can be impossible), then it is important to measure and record environmental factors and convey them in any report of the data.
Nutrition
The prior nutritional status and practices of a research participant, including diet composition, caloric intake, and timing of meals, can greatly influence the hormones associated with energy substrate mobilization and use (eg, insulin, glucagon, epinephrine, growth hormone, insulinlike growth factor, cortisol).1,59,60 The exact nature of the effect (augmentation or attenuation) depends on the interaction of these nutritional factors and how severe the alterations are from the participant's normal nutritional regimes.1,29,1,29,34
The hormones listed above are critical during exercise to ensure that energy metabolism meets the demands of exercise. Thus, a participant's altered dietary practices and nutritional status can change energy substrate (glycogen) storage and availability.60–62 This, in turn, can cause the hormonal response to exercise to vary to some degree. For example, Galbo et al59 demonstrated that the glucagon, epinephrine, growth hormone, and cortisol responses to exercise were greater after 4 days of a low-carbohydrate, high-fat diet.
Typically, in clinical settings, participants should fast prior to (eg, for 8 hours before) blood hormonal evaluations. It is not always practical, however, for athletes to comply with such requests due to their high demand for caloric intake to maintain energy balance, anabolism, and muscle glycogen reserves. Therefore, a modified approach may be necessary for this special population. All the same, for a repeated-measures research design, exercise and sports medicine investigators should try to control and standardize the dietary practices of their participants as much as possible to mitigate the effects of different diets among and within volunteers.34,59
The eating disorder anorexia nervosa is a special concern relative to nutrition status due to its profound effect on the endocrine system.1,45,63 Anorexics tend to have lower levels of resting luteinizing hormone, follicle-stimulating hormone, and estradiol-β-17.63 Anorexia also affects the pituitary-thyroid gland axis. Specifically, the condition is associated with suppression of triiodothyronine, somewhat decreased thyroxine, elevation of reserve triiodothyronine, and occasionally decreases in thyroid-stimulating hormone.63 Such a thyroidal state is referred to as the euthyroid sick syndrome hormonal profile and can accompany severe body-weight loss.3,45,63 The adrenocortical axis also is affected, with higher levels of cortisol due to increased liberation of corticotrophin-releasing hormone.63 Growth hormone is increased, although insulinlike growth factor 1 (IGF-1) levels (which facilitate the physiologic actions of growth hormone) are suppressed in anorexia.63 Due to the psychological aspects of anorexia nervosa,64,65 this condition could be discussed organizationally with mental health issues. However, this condition is also biologic in nature and, consequently, has powerful effects on a multitude of endocrine measurements.
Stress and Sleep
Emotional stress and sleep deprivation are both known to affect certain hormones within the endocrine system. For example, emotionally distraught individuals typically have elevated basal catecholamine, growth hormone, cortisol, and prolactin levels.1,66–68 Those hormones with highly circadian patterns (eg, luteinizing hormone, follicle-stimulating hormone, adrenocorticotropic hormone, cortisol) can demonstrate shifts in their characteristic patterns when sleep cycles are disrupted.36,39,66–70
Factors such as stress and sleep deprivation also can influence the hormonal response to exercise and exercise training. Investigators must attempt to control these factors whenever possible. In fact, it is advisable to have a pre-exercise questionnaire completed by a participant to monitor and evaluate the level of these factors, and if a predetermined status is not attained, then hormonal measures and exercise testing should be rescheduled.
As a footnote to this issue, many investigators in the exercise and sports medicine area rely on college students as research volunteers. Such students can have high levels of emotional stress due to the demands of their education (eg, examination periods, projects being due, oral reports). Care should be taken to not study student participants in times of high emotional stress, because a multitude of hormones can display atypical values and responses.36
Physical Activity
Time between exercise sessions can affect the hormonal profiles of individuals.71,72 If inadequate amounts of time have elapsed (limited recovery), some hormonal responses at rest or in the subsequent exercise testing can be attenuated and others augmented. Furthermore, the magnitude of this effect can be influenced by the exertion required of the prior exercise (eg, high-intensity intervals require longer recovery).
Ideally, the researcher may require a 24-hour recovery period before a participant reports to the laboratory for testing. However, athletes may find it difficult to reduce their training or miss a workout session for experimental purposes. A modified approach may be necessary, such as limiting recovery to 8 or 12 hours, somewhat preventing stress and anxiety (which, as noted earlier, can themselves affect the endocrine system) as a result of missing less training time.1,72–74
A powerful influence on resting and exercise hormonal response of a participant is the exercise training status: trained versus sedentary. Better-trained participants typically display greater effects on the neuroendocrine system response. Many hormones usually show attenuated resting and submaximal exercise responses in trained individuals, although some can actually be augmented (eg, testosterone in resistance-trained individuals) in response to submaximal and maximal exercise.2,75–79 An extensive dialogue on the influence of exercise training on hormonal profiles at rest and in response to exercise is beyond the scope of this paper, but the reader is directed to additional references for more in-depth discussions.2,3
Participant Posture
As a person changes position, changes occur in the plasma volume component of the blood. Standing upright results in reduced plasma volume compared with the recumbent position.80 These shifts in plasma fluid are in response to gravitational effects as well as alterations in capillary filtration and osmotic pressures.80 Large molecular-size hormones and those bound to high-weight carrier proteins could be trapped in the vascular spaces; thus, a loss of plasma fluid increases the concentration of these hormones (hemoconcentration). Conversely, increasing plasma fluid decreases the concentration of these hormones (hemodilution).37,81 These adjustments in fluid volume to move in or out of the vascular space due to posture shifts typically require 10 to 30 minutes.80,81
When blood is drawn to assess hormone levels in exercise research, the participant's position during specimen collection should be controlled and should be reported in publications. This type of information is most certainly necessary if a postural change lasts 10 minutes or longer.51,81
Specimen Collection
Proper precautions must be taken in the collection and storage of blood specimens to ensure their viability for hormonal analysis. In clinical and exercise-related blood work, venous blood is the specimen typically used. If the specimen is being obtained by venipuncture, the tourniquet should remain on the volunteer's arm for 1 minute or less. Greater lengths of time can result in fluid movement from the vascular bed due to hydrostatic pressures.81 Once collected, the blood sample should be centrifuged at 4°C to separate the plasma (if the collection tube contains anticoagulant) or allowed to clot (if the collection tube is sterile) and then centrifuged for serum. If centrifugation cannot be done immediately, then the blood sample should be placed on ice, but centrifuging without delay is recommended. Once separated, the plasma or serum should be stored at a temperature of −20°C to −80°C until later analysis. The plasma or serum should be stored in airtight, cryofreeze tubes with screw caps, which allow for a longer storage period. It is also advisable to split specimens into several aliquots if multiple hormonal analyses are to be conducted. Once a sample is thawed, it has a relatively short shelf life in a refrigerator, and repeated unthawing and refreezing cycles can degrade certain hormonal constituents.82–84 Care should be taken to ensure that the assay procedures employed are specific for plasma or serum, because in some cases these procedures are not interchangeable (eg, adrenocorticotropic hormone is measured in plasma). Furthermore, examining the literature may be necessary to determine if one form of blood component is more popular or prevalently used in research.
In blood specimens, either plasma or serum is used for biochemical analysis, but some hormonal measures also can be assessed in urine and salivary samples. In general, plasma and serum provide very similar values in hormonal analysis and seldom is one considered better than the other in blood analysis.83 Be aware, however, that specific assay procedures do, in some situations, have a preferred bodily fluid for analysis. Thus, the researcher must know which substance each hormonal assay requires as the analyte and plan accordingly. This type of information is provided by the manufacturer of the analytical supplies and components used in the assay procedures.
Urine and saliva are attractive specimens to collect because they are noninvasive in nature. They do, however, have certain drawbacks. Urine analysis tends to be limited primarily to steroid-based hormones, and 24-hour urine samples must be collected, a tedious and demanding process for the participant. Urine measurements may not always reflect real-time hormonal status either, because urine can sit in the bladder for hours before being voided. Saliva allows for easier sampling and can reflect hormonal status in a more real-time fashion. However, saliva primarily allows only for steroid hormonal assessments (ie, constituents that can cross from the blood into the salivary gland).85 Furthermore, saliva is limited to free hormonal concentrations, because the protein-bound constituents typically cannot pass through the salivary gland. Research suggests that the blood and saliva levels can mirror each other in their changes but not perfectly, so associations (r values) of 0.7 to 0.8 are typically found.1,82,85 Investigators must determine if these limitations preclude the use of these biologic fluids in their studies.82,85,86
Analytical Assays
A variety of biochemical analytical methods (assays) exist for measuring hormones in biologic specimens. Chromatographic, receptor, and immunologic assays are all available. Perhaps the most prevalent contemporary technique in use is immunologic assays, which have variations such as chemoluminescence immunoassay (CLIA), radioimmunoassays (RIA), enzyme immunoassays (EIA), enzyme-linked immunoassays (ELISA), and electrochemoluminescence immunoassays (ECLIA).87–89 Each technique has its strengths and weaknesses, and the discussion of each is beyond the scope of this paper, but the reader is directed to additional references for more background and explanation.90–92
Exercise and sports medicine researchers need to know the particular aspects of the hormonal assay techniques they plan to use in their studies. Specifically, they should be aware of the precision of the assay (How accurate is it?), sensitivity of the assay (How small a change can it detect?), and the specificity of the assay (How much cross-reactivity is there with similar-looking chemical structures in the specimen?). Ideally, the researcher wants the most precise, highly sensitive, and specific assay obtainable, but cost considerations can affect decision making in these matters. It is advisable for the researcher to report precision, sensitivity, and cross-reactivity values in publications to allow readers to determine the quality of the analytical techniques and procedures of the assays used. Additionally, it is desirable to report the coefficient of variation (CV) within and between assays for each hormone measured. These data allow the reader to determine how well the analytical technical procedures were carried out.92,93 One step to mitigate the potential between-assays CV is to collect and analyze biologic samples in batches of specimens and not as isolated specimens on a day-by-day basis. However, caution is necessary here, because batches that are too large can influence the outcome by creating “end-of-run effects” within the assay. That is, running a large number of samples in a single batch may compromise the precision of the technician performing the assay (ie, procedural fatigue) or the kinetics of the specific assay may be influenced by the length of time it takes to pipette the various components in assay (ie, in adding the chemical reagents to the first sample tubes versus the last tubes, too much time has transpired, resulting in different lengths of time for chemical reactions to take place within the specimen tubes).92,93
Data Transformations
Before conducting statistical analysis of the collected hormonal data, it may be necessary to transform the data. Two of the most common transformations typically seen in the literature are (1) expressing the data as a percentage change from some precondition basal value and (2) conducting a logarithmic conversion of the data. The first is typically done to account for relative changes in hormonal concentrations when absolute magnitude of change may be misleading. For example, a cortisol change from 10.0 to 12.0 µg/dL is much different from a 2.0 to 4.0 µg/dL (20% versus 100%) change, even though the absolute magnitude is identical. A 100% increase in the hormonal concentration may have many more profound physiologic effects than the smaller percentage. In the second case, logarithmic transformation is normally performed due to a large degree of variance in the participant data, resulting in a nonnormal distribution. This can be due to sample size issues, variance in the analytical technique, or the physiologic nature of the hormone being studied. Regardless of the transformation used, it is vital that the researcher report in the publication if and how the data were manipulated before conducting the statistical analysis and the rationale for performing the transformation.87,94
A third data transformation that is used less frequently is the area under-the-curve (AUC) procedure. This is carried out when serial specimen samples from a participant are tested (repeated-measures design). These serial values are plotted, and then the area under the plotted responses is integrated, thus collapsing several data values into one response and potentially eliminating some of the variability associated with many hormonal measurements.95 Some investigators favor this approach, feeling that the overall response of the hormone and gland in question can be better quantified. Yet the procedure can be influenced by the number of serial samples collected and the circadian rhythm of the hormone. That is, highly variable (pulsatile) hormones require more frequent specimen sampling, and misleading results can occur if the sampling is too infrequent.96
Data Analysis
Of course, the statistical procedures applied to any research study are dictated by the design of that study. Most research in the exercise and sports medicine area tends to employ parametric analysis (eg, t test, analysis of variance, Pearson correlation). These analytical procedures work well with endocrine data, provided the underlying assumptions for their use are not violated.97 Furthermore, many North American journals prefer this form of analysis due to the robust nature of the technique and the reduced likelihood of making a type I error (indicating that findings are significant when they are, in fact, not significant). Nevertheless, nonparametric analysis (eg, Wilcoxon, Mann-Whitney U, Friedman tests) can be equally applicable for endocrine use when study designs are not excessively complex and sample sizes are relatively small.97 As above, it is vital that the researcher report for publication what the specific statistical analyses being used were and what the rationale was for their use.96–99
Once assays are performed and statistical results obtained, the researcher needs to try and understand the data in order to interpret the magnitude of treatment outcomes and other effects. In this interpretative process, many researchers focus intently on obtaining statistical significance. Although such significance is important, a key question that has to be addressed in the data is the issue of statistical significance versus practical (clinical) significance within the hormonal findings. To address that question, the researcher must take into account the smallest clinically important positive and negative response values or levels of the effect being researched: that is, the smallest change values or levels that matter. Studies can be statistically significant and yet largely insignificant clinically. It is important to note that large sample sizes can produce a statistically significant result, even though limited or no practical importance is associated with the finding.100
Effect sizes (ESs) are an increasingly important index used to quantify the degree of practical significance of study results.101 Once computed, the ES statistic can be a useful indicator of the practical or clinical importance of research results, because it can be operationally defined; it is possible to give the observed ES a rating such as negligible-trivial, moderate, or important-very large.102 From such ratings, the researcher can discern the form and quantity of significance obtained in the study. Furthermore, the ES statistic has 2 advantages over traditional statistical significance testing: (1) it is independent of the size of the sample, and (2) it is a scale-free index. Therefore, ES can be interpreted uniformly in different studies regardless of the sample size and the original scales of the variables being examined.101
Summary and Conclusions
Over the last 25 years, exercise science and sports medicine researchers have steadily increased the number of studies being published that examined hormones and the endocrine system. Regrettably, not all investigators working in this area of research are entirely aware of the factors that must be accounted for and controlled in order to ensure that data are valid and accurate. In this paper, we have reviewed some of the key biologic and procedural-analytic factors that can confound endocrine data and add variance to hormonal outcome measurements. If researchers inexperienced with endocrinology design their studies to monitor, control, and adjust for the factors mentioned here, they will find more consistency in their endocrine data and, thus, enhance the legitimacy of their research. Such actions can greatly aid investigators in interpreting and understanding endocrine data and, in turn, make their research more scientifically sound.
Acknowledgments
The main aspects of this paper were presented as part of a symposium presentation at the 2000 National Athletic Trainers' Association meeting in Nashville, Tennessee. This paper is dedicated to my coauthor, Dr Atko Viru, who was a great mentor, colleague, and friend. Regrettably, Dr Viru died while this paper was under revision.
Footnotes
Anthony C. Hackney, PhD, CPH, FACSM, and Atko Viru, PhD, DSc, contributed to conception and design; acquisition and analysis and interpretation of the data; and drafting, critical revision, and final approval of the article.
References
- 1.Trembly M.S, Chu S.Y, Mureika R. Methodological and statistical considerations for exercise-related hormone evaluations. Sports Med. 1990;20(2):90–108. doi: 10.2165/00007256-199520020-00004. [DOI] [PubMed] [Google Scholar]
- 2.Kraemer W.J, Ratamess N.A. Hormonal responses and adaptations to resistance exercise and training. Sports Med. 2005;35(4):339–361. doi: 10.2165/00007256-200535040-00004. [DOI] [PubMed] [Google Scholar]
- 3.McMurray R.G, Hackney A.C. The endocrine system and exercise. In: Garrett W, editor. Sports Medicine. New York, NY: Williams & Wilkins; 2000. pp. 135–162. [Google Scholar]
- 4.International Union of Pure and Applied Chemistry (International Union of Biochemistry and Molecular Biology) Recommendations on organic & biochemical nomenclature, symbols & terminology. http://www.chem.qmul.ac.uk/iupac/. Accessed August 19, 2008.
- 5.Warne G.L, Kanumakala S. Molecular endocrinology of sex differentiation. Sem Reprod Med. 2002;20(3):169–180. doi: 10.1055/s-2002-35381. [DOI] [PubMed] [Google Scholar]
- 6.Webb M.L, Wallace J.P, Hamill C, Hodgson J.L, Mashaly M.M. Serum testosterone concentration during two hours of moderate intensity treadmill running in trained men and women. Endocr Res. 1984;10(1):27–38. doi: 10.1080/07435808409046763. [DOI] [PubMed] [Google Scholar]
- 7.Bunt J.C, Bahr J.M, Bemben D.A. Comparison of estradiol and testosterone levels during and immediately following prolonged exercise in moderately active and trained males and females. Endocr Res. 1987;13(2):157–172. doi: 10.3109/07435808709023670. [DOI] [PubMed] [Google Scholar]
- 8.Pedersen B.K, Hoffman-Goetz L. Exercise and the immune system: regulation, integration, and adaptation. Physiol Rev. 2000;80(3):1055–1081. doi: 10.1152/physrev.2000.80.3.1055. [DOI] [PubMed] [Google Scholar]
- 9.Foster D.L, Nagatani S. Physiological perspectives on leptin as a regulator of reproduction: role in timing puberty. Biol Reprod. 1999;60(2):205–215. doi: 10.1095/biolreprod60.2.205. [DOI] [PubMed] [Google Scholar]
- 10.Ruby B.C, Robergs R.A. Gender differences in substrate utilisation during exercise. Sports Med. 1994;17(6):393–410. doi: 10.2165/00007256-199417060-00005. [DOI] [PubMed] [Google Scholar]
- 11.Bunt J.C. Metabolic actions of estradiol: significance for acute and chronic exercise responses. Med Sci Sports Exerc. 1990;22(3):286–290. [PubMed] [Google Scholar]
- 12.Hackney A.C, McCracken-Compton M.A, Ainsworth B.A. Substrate metabolism responses to submaximal exercise in the midfollicular and midluteal phases of the menstrual cycle. Int J Sport Nutr. 1994;4(3):299–308. doi: 10.1123/ijsn.4.3.299. [DOI] [PubMed] [Google Scholar]
- 13.Hackney A.C, McMurray R.G, Judelson D.A, Harrell J.S. Relationship between caloric intake, body composition, and physical activity to leptin, thyroid hormones, and cortisol in adolescents. Jpn J Physiol. 2003;53(6):475–479. doi: 10.2170/jjphysiol.53.475. [DOI] [PubMed] [Google Scholar]
- 14.Horswill C.A, Zipf W.B, Kien C.L, Kahle E.B. Insulin's contribution to growth in children and the potential for exercise to mediate insulin's action. Pediatr Exerc Sci. 1997;9(1):18–32. [Google Scholar]
- 15.Amile S.A, Caprio S, Sherwin R.S, Plewe G, Haymond M.W, Tamborlane W.V. Insulin resistance of puberty: a defect restricted to peripheral glucose metabolism. J Clin Endocr Metab. 1991;72(2):277–282. doi: 10.1210/jcem-72-2-277. [DOI] [PubMed] [Google Scholar]
- 16.Isurugi K, Fukutani K, Takayasu H, Wakabayashi K, Tamaoki B. Age-related changes in serum luteinizing hormone and follicle-stimulating hormone level in normal men. J Clin Endocr Metab. 1974;39(5):955–957. doi: 10.1210/jcem-39-5-955. [DOI] [PubMed] [Google Scholar]
- 17.Purifoy E.E, Koopmars L.H, Tatum R.W. Steroid hormones and aging: free testosterone, testosterone and androstenedione in normal females age 20–87 years. Hum Biol. 1980;52(2):181–191. [PubMed] [Google Scholar]
- 18.Orentreich N, Brind J.L, Rizer R.L, Vogelman J.H. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J Clin Endocr Metab. 1984;59(3):551–555. doi: 10.1210/jcem-59-3-551. [DOI] [PubMed] [Google Scholar]
- 19.Aloia J.F, Feuerman M, Yeh J.K. Reference range for serum parathyroid hormone. Endocr Pract. 2006;12(2):137–144. doi: 10.4158/EP.12.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adlercreutz H, Gorbach S.L, Goldin B.R, Woods M.N, Dwyer J.T, Hamalainen E. Estrogen metabolism and excretion in Oriental and Caucasian women. J Natl Cancer Inst. 1994;86(14):1076–1082. doi: 10.1093/jnci/86.14.1076. [DOI] [PubMed] [Google Scholar]
- 21.Benn P.A, Clive J.M, Collins R. Medians for second-trimester maternal serum alpha-fetoprotein, human chorionic gonadotropin, and unconjugated estriol: differences between race or ethnic groups. Clin Chem. 1997;43(2):333–337. [PubMed] [Google Scholar]
- 22.Mittelmark R.A. Hormonal responses to exercise in pregnancy. In: Mittelmark R.A, Wiswell R.A, Drinkwater B.L, editors. Exercise in Pregnancy. Baltimore, MD: Williams & Wilkins; 1991. pp. 175–184. [Google Scholar]
- 23.Wang C, Christenson P, Swerdloff R. Editorial: clinical relevance of racial and ethnic differences in sex steroids. J Clin Endocr Metab. 2007;92(7):2433–2435. doi: 10.1210/jc.2007-1085. [DOI] [PubMed] [Google Scholar]
- 24.Abbott W.G, Foley J.E. Comparison of body composition, adipocyte size, and glucose and insulin concentrations in Pima Indian and Caucasian children. Metabolism. 1987;36(6):576–579. doi: 10.1016/0026-0495(87)90170-3. [DOI] [PubMed] [Google Scholar]
- 25.Ivandic A, Prpic-Krizevac I, Sucic M, Iuric M. Hyperinsulinemia and sex hormones in healthy premenopausal women: relative contribution of obesity, obesity type, and duration of obesity. Metabolism. 1998;47(1):13–19. doi: 10.1016/s0026-0495(98)90186-x. [DOI] [PubMed] [Google Scholar]
- 26.Hansen B.C, Jen K.L, Belbez Pek S.B, Wolfe R.A. Rapid oscillations in plasma insulin, glucagon, and glucose in obese and normal weight humans. J Clin Endocrinol Metab. 1982;54(4):785–792. doi: 10.1210/jcem-54-4-785. [DOI] [PubMed] [Google Scholar]
- 27.Florkowski C.M, Collier G.R, Zimmet P.Z, Livesey J.H, Espiner E.A, Donald R.A. Low-dose growth hormone replacement lowers plasma leptin and fat stores without affecting body mass index in adults with growth hormone deficiency. Clin Endocrinol (Oxf) 1996;45(6):769–773. doi: 10.1046/j.1365-2265.1996.830895.x. [DOI] [PubMed] [Google Scholar]
- 28.Pasquali R, Vicennati V. Activity of the hypothalamic-pituitary-adrenal axis in different obesity phenotypes. Int J Obes Relat Metab Disorders. 2000;24(suppl 2):S47–S49. [PubMed] [Google Scholar]
- 29.McMurray R.G, Hackney A.C. Interactions of metabolic hormones, adipose tissue and exercise. Sports Med. 2005;35(5):393–412. doi: 10.2165/00007256-200535050-00003. [DOI] [PubMed] [Google Scholar]
- 30.Hurley B.F, Nemeth P.M, Martin W.H, III, Hagberg J.M, Dalsky G.P, Holloszy J.O. Muscle triglyceride utilization during exercise: effect of training. J Appl Physiol. 1986;60(2):562–567. doi: 10.1152/jappl.1986.60.2.562. [DOI] [PubMed] [Google Scholar]
- 31.Rahkila P, Soimajarvi J, Karvinrn E, Vihko V. Lipid metabolism during exercise, II: respiratory exchange ratio and muscle glycogen content during 4 h bicycle ergometry in two groups of healthy men. Eur J Appl Physiol Occup Physiol. 1980;44(3):246–254. doi: 10.1007/BF00421624. [DOI] [PubMed] [Google Scholar]
- 32.Pasman W.J, Westertrep-Plantenga M.S, Saris W.H.M. The effect of exercise training on leptin levels in obese males. Am J Physiol. 1998;274(2, pt 1):E280–E286. doi: 10.1152/ajpendo.1998.274.2.E280. [DOI] [PubMed] [Google Scholar]
- 33.Ryan A.S, Partley R.E, Elahi D, Goldberg A.P. Changes in leptin and insulin action with resistive training in postmenopausal women. Int J Obes Relat Metab Disord. 2000;24(1):27–32. doi: 10.1038/sj.ijo.0801080. [DOI] [PubMed] [Google Scholar]
- 34.Hackney A.C. Stress and the neuroendocrine system: the role of exercise as a stressor and modifier of stress. Expert Rev Endocrinol Metab. 2006;1(6):783–792. doi: 10.1586/17446651.1.6.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dorn L.D, Burgess E.S, Dichek H.L, Putman F.W, Chrousos G.P, Gold P.W. Thyroid hormone concentrations in depressed and nondepressed adolescents: group differences and behavioral relations. J Am Acad Child Adolesc Psychiatry. 1996;35(3):299–306. doi: 10.1097/00004583-199603000-00010. [DOI] [PubMed] [Google Scholar]
- 36.Vaernes R, Ursin H, Darragh A, Lambe R. Endocrine response patterns and psychological correlates. J Psychosom Res. 1982;26(2):123–131. doi: 10.1016/0022-3999(82)90030-7. [DOI] [PubMed] [Google Scholar]
- 37.Hackney A.C. Exercise as a stressor to the neuroendocrine system. Medicina (Kaunas) 2006;42(10):788–797. [PubMed] [Google Scholar]
- 38.Hamner M.B, Hitri A. Plasma beta-endorphin levels in post-traumatic stress disorder: a preliminary report on response to exercise-induced stress. J Neuropsychiatry Clin Neurosci. 1992;4(1):59–63. doi: 10.1176/jnp.4.1.59. [DOI] [PubMed] [Google Scholar]
- 39.Gerra G, Volpi R, Delsignore R, et al. ACTH and beta-endorphin responses to physical exercise in adolescent women tested for anxiety and frustration. Pyschiatry Res. 1992;41(2):179–186. doi: 10.1016/0165-1781(92)90109-g. [DOI] [PubMed] [Google Scholar]
- 40.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 41.Beck A.T, Epstein N, Brown G, Steer R.A. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56(6):893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 42.Landgren B, Aedo A, Diczfalusy E. Hormonal changes associated with ovulation and luteal function. In: Flamigni C, Givens J, editors. The Gonadotropins: Basic Science and Clinical Aspects in Females. London, England: Academic Press; 1982. pp. 200–212. [Google Scholar]
- 43.Hackney A.C, Cyren H.C, Brammeier M, Sharp R.L. Effects of the menstrual cycle on insulin-glucose at rest and in response to exercise. Biol Sport. 1993;10(2):73–81. [PMC free article] [PubMed] [Google Scholar]
- 44.Vanheest J.L, Mahoney C.E, Rodgers C.D. Oral contraceptive use and physical performance. In: Kramer W.J, Rogol A, editors. The Endocrine System in Sports and Exercise. Oxford, England: Blackwell Publishing; 2005. pp. 250–260. [Google Scholar]
- 45.Loucks A.B. Physical activity, fitness and female reproductive morbidity. In: Bouchard C, Shepard R.J, Stephens T, editors. Physical Activity, Fitness and Health: International Proceedings and Consensus Statement. Champaign, IL: Human Kinetics; 1994. pp. 943–954. [Google Scholar]
- 46.Matsumoto A.M, Bremner W.J. Modulation of pulsatile gonadotropin secretion by testosterone in man. J Clin Endocrinol Metab. 1984;58(4):609–614. doi: 10.1210/jcem-58-4-609. [DOI] [PubMed] [Google Scholar]
- 47.Rose R.M, Kreuz L.E, Holaday J.W, Sulak K.J, Johnson C.E. Diurnal variation of plasma testosterone and cortisol. J Endocrinol. 1972;54(1):177–178. doi: 10.1677/joe.0.0540177. [DOI] [PubMed] [Google Scholar]
- 48.Rose S.R, Nisula B.C. Circadian variation of thyrotropin in childhood. J Clin Endocrinol Metab. 1989;68(6):1086–1090. doi: 10.1210/jcem-68-6-1086. [DOI] [PubMed] [Google Scholar]
- 49.Hackney A.C, Viru A. Twenty-four-hour cortisol response to multiple daily exercise sessions of moderate and high intensity. Clin Physiol. 1999;19(2):178–182. doi: 10.1046/j.1365-2281.1999.00157.x. [DOI] [PubMed] [Google Scholar]
- 50.Weitzman E.D. Circadian rhythms and episodic hormone secretion. Annu Rev Med. 1976;27:225–243. doi: 10.1146/annurev.me.27.020176.001301. [DOI] [PubMed] [Google Scholar]
- 51.Goodman H.M. Endocrinology concepts for medical students. Adv Physiol Educ. 2005;25(1–4):213–224. doi: 10.1152/advances.2001.25.4.213. [DOI] [PubMed] [Google Scholar]
- 52.Hackney A.C, Zack E. Physiological day-to-day variability of select hormones at rest in exercise-trained men. J Endocrinol Invest. 2006;29(6):RC9–RC12. doi: 10.1007/BF03344136. [DOI] [PubMed] [Google Scholar]
- 53.Schulz P, Knabe R. Biological uniqueness and the definition of normality: part 2—the endocrine “fingerprint” of healthy adults. Med Hypotheses. 1994;42(1):63–68. doi: 10.1016/0306-9877(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 54.Finberg J.P, Berlyne G.M. Renin and aldosterone secretion following acute environmental heat exposure. Isr J Med Sci. 1976;12(6):844–847. [PubMed] [Google Scholar]
- 55.Galbo H, Houston M.E, Christensen N.J, et al. The effect of water temperature on the hormonal response to prolonged swimming. Acta Physiol Scand. 1979;105(3):326–337. doi: 10.1111/j.1748-1716.1979.tb06348.x. [DOI] [PubMed] [Google Scholar]
- 56.Mordes J.P, Blume F.D, Boyer S, Zheng M.R, Braverman L.E. High altitude pituitary-thyroid dysfunction on Mount Everest. New Engl J Med. 1983;308(19):1135–1138. doi: 10.1056/NEJM198305123081906. [DOI] [PubMed] [Google Scholar]
- 57.Rastogi G.K, Malhotra M.S, Srivastava M.C, et al. Study of the pituitary-thyroid functions at high altitude in man. J Clin Endocrinol Metab. 1977;44(3):447–452. doi: 10.1210/jcem-44-3-447. [DOI] [PubMed] [Google Scholar]
- 58.Hoyt R.W, Honig A. Body fluid and energy metabolism at high altitude. In: Fregley M.J, Blatteis C.M, editors. Handbook of Physiology, Section 4: Environmental Physiology. New York, NY: Oxford University Press; 1996. pp. 1277–1289. [Google Scholar]
- 59.Galbo H, Holst J.J, Christensen N.J. The effect of different diets and of insulin on the hormonal response to prolonged exercise. Acta Physiol Scand. 1979;107(1):19–32. doi: 10.1111/j.1748-1716.1979.tb06438.x. [DOI] [PubMed] [Google Scholar]
- 60.Phinney S.D, Horton E.S, Sims E.A, Hanson J.S, Danforth E, Jr, LaGrange B.M. Capacity for moderate exercise in obese subjects after adaptation to a hypocaloric, ketogenic diet. J Clin Invest. 1980;66(5):1152–1161. doi: 10.1172/JCI109945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jezova-Repcekova D, Vigas M, Klimes I. Decreased plasma cortisol response to pharmacological stimuli after glucose load in man. Endocrinol Exp. 1980;14(2):113–120. [PubMed] [Google Scholar]
- 62.Bonen A, Belcastro A.N, MacIntyre K, Gardner J. Hormonal responses during intense exercise preceded by glucose ingestion. Can J Appl Sport Sci. 1980;5(2):85–90. [PubMed] [Google Scholar]
- 63.Støving R.K, Hangaard J, Hansen-Nord M, Hagen C. A review of endocrine changes in anorexia nervosa. J Psychiatr Res. 1999;33(2):139–152. doi: 10.1016/s0022-3956(98)00049-1. [DOI] [PubMed] [Google Scholar]
- 64.Casper R.C. Recognizing eating disorders in women. Psychopharmacol Bull. 1998;34(3):267–269. [PubMed] [Google Scholar]
- 65.Södersten P, Bergh C, Zandian M. Psychoneuroendocrinology of anorexia nervosa. Psychoneuroendocrinology. 2006;31(10):1149–1153. doi: 10.1016/j.psyneuen.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 66.VanHelder T, Radomski M.W. Sleep deprivation and the effect on exercise performance. Sports Med. 1989;7(4):235–247. doi: 10.2165/00007256-198907040-00002. [DOI] [PubMed] [Google Scholar]
- 67.Aakvaag A, Bentdal O, Quigstad K, Walstad P, Ronningen H, Fonnum F. Testosterone and testosterone binding globulin (TeBG) in young men during prolonged stress. Int J Androl. 1978;1(6):22–31. [Google Scholar]
- 68.Aakvaag A, Sand T, Opstad P.O, Fonnum F. Hormonal changes in serum in young men during prolonged physical strain. Eur J Appl Physiol Occup Physiol. 1978;39(7):283–291. doi: 10.1007/BF00421452. [DOI] [PubMed] [Google Scholar]
- 69.Diamond P, Brisson G.R, Candas B, Peronnet F. Trait anxiety, submaximal physical exercise and blood androgens. Eur J Appl Physiol Occup Physiol. 1989;58(7):699–704. doi: 10.1007/BF00637379. [DOI] [PubMed] [Google Scholar]
- 70.Hackney A.C, Feith S, Pozos R, Seale J. Effects of high altitude and cold exposure on resting thyroid hormone concentrations. Aviat Space Environ Med. 1995;66(4):325–329. [PubMed] [Google Scholar]
- 71.Viru A.M, Hackney A.C, Valja E, Karelson K, Janson T, Viru M. Influence of prolonged continuous exercise on hormonal responses to subsequent exercise in humans. Eur J Appl Physiol. 2001;85(6):578–585. doi: 10.1007/s004210100498. [DOI] [PubMed] [Google Scholar]
- 72.Hackney A.C. The neuro-endocrine system, overload training, and regeneration. In: Lehmann M, editor. Ulm International Conference Proceeding: Performance, Overload Training and Regeneration. London, England: Plenum Press; 1999. pp. 173–186. [Google Scholar]
- 73.Viru A, Karelson K, Smirnova T. Stability and variability in hormonal responses to prolonged exercise. Int J Sports Med. 1992;13(3):230–235. doi: 10.1055/s-2007-1021259. [DOI] [PubMed] [Google Scholar]
- 74.Hartley L.H, Mason J.W, Hogan R.P, et al. Multiple hormonal responses to graded exercise in relation to physical training. J Appl Physiol. 1972;33(5):602–606. doi: 10.1152/jappl.1972.33.5.602. [DOI] [PubMed] [Google Scholar]
- 75.Richter E.A, Sutton J.R. Hormonal adaptation to physical activity. In: Bouchard C, Shephard R.J, Stephen T, editors. Physical Activity, Fitness and Health: International Proceedings and Consensus Statement. Champaign, IL: Human Kinetics; 1994. pp. 331–342. [Google Scholar]
- 76.Luger A, Deuster P.A, Kyle S.B, Gallucci W.T, Montgomery L.C, Gold P.W. Acute hypothalamic-pituitary-adrenal responses to the stress of treadmill exercise: physiologic adaptations to physical training. New Engl J Med. 1987;316(21):1309–1315. doi: 10.1056/NEJM198705213162105. [DOI] [PubMed] [Google Scholar]
- 77.Hackney A.C, Sinning W.E, Bruot B.C. Reproductive hormonal profiles in endurance-trained and untrained men. Med Sci Sports Exerc. 1988;20(1):60–65. doi: 10.1249/00005768-198802000-00009. [DOI] [PubMed] [Google Scholar]
- 78.Remes K, Kuoppasalmi K, Adlercreutz H. Effect of long-term physical training on plasma testosterone, androstenedione, luteinizing hormone and sex-hormone–binding globulin capacity. Scand J Clin Lab Invest. 1979;39(8):743–749. doi: 10.1080/00365517909108166. [DOI] [PubMed] [Google Scholar]
- 79.Häkkinen K, Pakarinen A. Acute hormonal responses to two different fatiguing heavy-resistance protocols in male athletes. J Appl Physiol. 1993;74(2):882–887. doi: 10.1152/jappl.1993.74.2.882. [DOI] [PubMed] [Google Scholar]
- 80.Westendorp R.G, Roos A.N, Riley L.C, Walma S, Frolich M, Mienders A.E. Chronic stimulation of atrial natriuretic peptide attenuates the secretory responses to postural changes. Am J Med Sci. 1993;306(6):371–375. doi: 10.1097/00000441-199312000-00003. [DOI] [PubMed] [Google Scholar]
- 81.Fawcett J.K, Wynn V. Effects of posture on plasma volume and some blood constituents. J Clin Path. 1960;13:304–313. doi: 10.1136/jcp.13.4.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen Y.M, Cintron N.M, Whitson P.A. Long-term storage of salivary cortisol samples at room temperature. Clin Chem. 1992;38(20):304. [PubMed] [Google Scholar]
- 83.Calam R.R. Reviewing the importance of specimen collection. J Am Med Technol. 1977;39(6):297–300. [Google Scholar]
- 84.Sonntag O. Hemolysis as an interference factor in clinical chemistry. J Clin Chem Biochem. 1986;24(2):127–139. [PubMed] [Google Scholar]
- 85.Obminski Z, Klusiewicz A, Stupnicki R. Changes in salivary and serum cortisol concentrations in junior athletes following exercises of different intensities. Biol Sport. 1994;11(1):49–57. [Google Scholar]
- 86.Caraway W.T. Chemical and diagnostic specificity of laboratory tests: effect of hemolysis, lipemia, anticoagulants, medications, contaminants, and other variables. Am J Clin Pathol. 1961;37(5):445–464. doi: 10.1093/ajcp/37.5.445. [DOI] [PubMed] [Google Scholar]
- 87.Kopchick J.J, Sackmann-Sala L, Ding J. Primer: molecular tools used for the understanding of endocrinology. Nat Clin Pract Endocrinol Metab. 2007;3(4):355–368. doi: 10.1038/ncpendmet0446. [DOI] [PubMed] [Google Scholar]
- 88.Bowers L.D. Analytical advances in detection of performance-enhancing compounds. Clin Chem. 1997;43(7):1299–1304. [PubMed] [Google Scholar]
- 89.Dudley R.F. Chemiluminescence immunoassay: an alternative to RIA. Lab Med. 1990;21(4):216–222. [Google Scholar]
- 90.Shah V.P, Midha K.K, Findlay J.W.A, et al. Bioanalytic method validation: a revisit with a decade of progress. Pharm Res. 2000;17(12):1551–1557. doi: 10.1023/a:1007669411738. [DOI] [PubMed] [Google Scholar]
- 91.De Ronde W, van der Schouw Y.T, Pols H.A.P, et al. Calculation of bioavailable and free testosterone in men: a comparison of 5 published algorithms. Clin Chem. 2006;52(9):1777–1784. doi: 10.1373/clinchem.2005.063354. [DOI] [PubMed] [Google Scholar]
- 92.Rosner W, Auchus R.J, Azziz R, Sluss P.M, Raff H. Position statement: utility, limitations, and pitfalls in measuring testosterone: an Endocrine Society position statement. J Clin Endocrinol Metab. 2007;92(2):405–413. doi: 10.1210/jc.2006-1864. [DOI] [PubMed] [Google Scholar]
- 93.Rodbard D. Statistical quality control and routine data processing for radioimmunoassays and immunoradiometric assays. Clin Chem. 1974;20(10):1255–1270. [PubMed] [Google Scholar]
- 94.Fraser C.G, Harris E.K. Generation and application of data on biological variation in clinical chemistry. Crit Rev Clin Lab Sci. 1989;27(5):409–437. doi: 10.3109/10408368909106595. [DOI] [PubMed] [Google Scholar]
- 95.Hackney A.C, Premo M.C, McMurray R.G. Influence of aerobic versus anaerobic exercise on the relationship between reproductive hormones in men. J Sports Sci. 1995;13(4):305–311. doi: 10.1080/02640419508732244. [DOI] [PubMed] [Google Scholar]
- 96.Veldhuis J.D, Johnson M.L. Deconvolution analysis of hormone data. Methods Enzymol. 1992;210:539–575. doi: 10.1016/0076-6879(92)10028-c. [DOI] [PubMed] [Google Scholar]
- 97.Kingle R.D, Johnson G.F. Statistical procedures. In: Tietz N.W, editor. Textbook of Clinical Chemistry. Philadelphia, PA: WB Saunders; 1986. pp. 287–355. [Google Scholar]
- 98.Pincus S.M, Hartman M.L, Roelfsema F, Thorner M.O, Veldhuis J.D. Hormone pulsatility discrimination via coarse and short time sampling. Am J Physiol Endocrinol Metab. 1999;277(5, pt 1):E948–E957. doi: 10.1152/ajpendo.1999.277.5.E948. [DOI] [PubMed] [Google Scholar]
- 99.Matthews D.R. Time series analysis in endocrinology. Acta Paediatr Scand Suppl. 1988;347:55–62. [PubMed] [Google Scholar]
- 100.Hopkins W.G. Measures of reliability in sports medicine and science. Sports Med. 2000;30(1):1–15. doi: 10.2165/00007256-200030010-00001. [DOI] [PubMed] [Google Scholar]
- 101.Hojat M, Xu G. A visitor's guide to effect sizes: statistical significance versus practical (clinical) importance of research findings. Adv Health Sci Educ Theory Pract. 2004;9(3):241–249. doi: 10.1023/B:AHSE.0000038173.00909.f6. [DOI] [PubMed] [Google Scholar]
- 102.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Englewood, NJ: Lawrence Erlbaum Associates; 1988. pp. 116–173. [Google Scholar]