Abstract
Context: The relative contribution of central adiposity vs. weight on GH response to stimulation testing in obesity is not known.
Objective: We aimed to assess the contribution of weight and specific measures of central and peripheral adiposity to GH response to GHRH-arginine testing in lean, overweight, and obese men.
Design: A total of 75 men [mean age, 44.3 ± 1.1 yr; body mass index (BMI), 28.8 ± 0.7 kg/m2] were investigated. Subjects were classified as lean (BMI < 25 kg/m2; n = 23), overweight (BMI ≥ 25 and <30 kg/m2; n = 28), or obese (BMI ≥ 30 kg/m2; n = 24). Subjects were also stratified by waist circumference (WC) (<102 cm, n = 47; ≥102 cm, n = 28). Body composition and regional adiposity were assessed by anthropometrics, dual-energy x-ray absorptiometry (DEXA), and abdominal computed tomography (CT) scans.
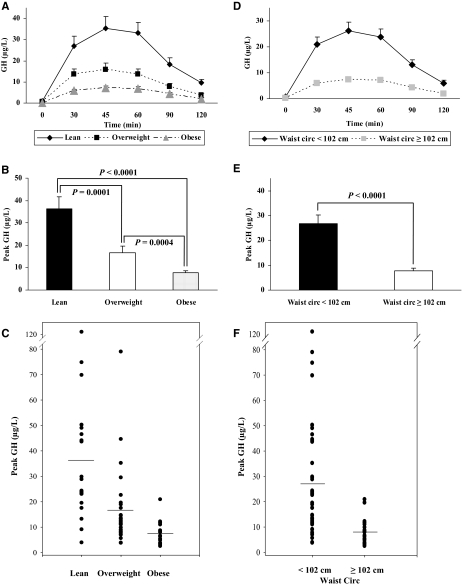
Results: Peak stimulated GH was 36.4 ± 5.4, 16.6 ± 2.9, and 7.6 ± 0.9 μg/liter among lean, overweight, and obese subjects, respectively (P < 0.001 for all comparisons). Peak stimulated GH was 26.9 ± 3.4 μg/liter among subjects with WC less than 102 cm compared to 7.9 ± 0.9 μg/liter among subjects with WC of 102 cm or greater (P < 0.0001). Separate multivariate models using anthropometric, DEXA, and CT-derived measures of central adiposity demonstrated strong associations between peak stimulated GH and measures of central adiposity including WC, trunk fat by DEXA, and visceral adiposity by CT, controlling for age, BMI, and more general measures of adiposity. WC was independently associated with peak GH response to GHRH-arginine in a model including age, BMI, and hip circumference. In this model, BMI was no longer significant, and peak GH was reduced 1.02 μg/liter for each 1 cm increase in WC (P = 0.02).
Conclusions: GH response to GHRH-arginine testing is reduced in both overweight and obese subjects and negatively associated with indices of central abdominal obesity including WC, trunk fat, and visceral adipose tissue. The use of waist circumference, as a surrogate for central adiposity, adds predictive information to the determination of GH response, independent of BMI.
This study demonstrates an independent association between peak GH response to GHRH arginine and waist circumference, controlling for body mass index. Waist circumference should be considered in addition to body mass index in interpreting results of the GHRH-arginine stimulation test.
Obesity is associated with decreased basal and pulsatile release of GH (1,2,3) as well as decreased stimulated GH release (4,5,6,7,8,9). This GH deficiency associated with obesity is relative or functional, because weight loss restores 24-h GH pulse characteristics (10). Previous reports have suggested a potential relationship between relative GH deficiency of obesity and visceral adiposity. In a small study (n = 16) of obese premenopausal women, Pijl et al. (11) demonstrated decreased 24-h GH secretion in women with large visceral fat area compared with body mass index (BMI)-matched women with small visceral fat area, as determined by magnetic resonance imaging. In a study of 20 postmenopausal women, Franco et al. (12) demonstrated a negative association between basal and pulsatile GH secretion and visceral adiposity as determined by abdominal computed tomography (CT). In addition, trunk fat as assessed by dual-energy x-ray absorptiometry (DEXA) was inversely associated with mean 24-h GH pulse in a small study (n = 15) of nonobese women (13). Furthermore, Vahl et al. also demonstrated a negative association between mean 24-h GH levels and intraabdominal fat as measured by CT in 42 healthy men and women (14).
However, the contribution of central adiposity vs. body weight to the GH response to GHRH-arginine testing, a stimulation test commonly used in clinical practice, remains unknown in men and has not been assessed in relationship to specific measures of regional adiposity. More recently, the GH deficiency of obesity has been associated with increased carotid intima-media thickness (15). Taken together, these data suggest that reduced GH secretion in obesity may have cardiovascular consequences and may be mediated in part by central adiposity. These data also suggest the importance of determining the relative contribution of overall weight and central adiposity to GH response to standardized stimulation algorithms. We hypothesized that peak stimulated GH levels on GHRH-arginine test would be specifically associated with measures of central adiposity in men.
Subjects and Methods
Study subjects
Seventy-five men from the Boston community were recruited between October 1999 and January 2008 using local print advertisement. Data from a subset (n = 43) were previously published in a study comparing GH secretion in non-HIV men to HIV-infected men (16,17). Male subjects with a range of BMI, between the ages of 20 and 60 yr, and who were otherwise healthy without known pituitary dysfunction including dysfunction of the adrenal, GH, thyroid or gonadal axes were selected. Subjects receiving GH, anabolic hormones, glucocorticoids, testosterone, or any medication known to affect GH were excluded. Subjects with known diabetes mellitus, hemoglobin level less than 11 g/dl, creatinine above 1.5 mg/dl, aspartate aminotransferase above 2.5-fold upper limit of normal, and chronic illness such as HIV were also excluded. Subjects with a history and physical exam suggestive of pituitary dysfunction were also excluded. Written informed consent was obtained from each subject before testing, in accordance with the Committee on the use of Humans as Experimental Subjects of the Massachusetts Institute of Technology and the Subcommittee on Human Studies at the Massachusetts General Hospital.
Biochemical assessment
GH stimulation testing was performed using standard GHRH-arginine stimulation. After an overnight fast, sermorelin acetate (GHRH 1–29) (Geref; Serono Laboratories, Inc., Rockland, MA) was administered iv at a dose of 1 μg/kg. Subsequently, arginine hydrochloride (30 g/300 ml) was administered at a dose of 0.5 g/kg (maximum 30 g) via iv pump at 600 ml/h over 30 min. GH levels were assessed at 0, 30, 45, 60, 90, and 120 min after sermorelin administration. The 30-min time point coincides with the completion of the arginine infusion. Serum GH was measured by an immunoradiometric assay using kits from Nichols Institute (San Juan Capistrano, CA) [n = 43; intraassay coefficient of variation (CV), 4.4%; interassay CV, 6.6%] and Diagnostic Systems Laboratories (Webster, TX) (n = 32; intraassay CV ranging from 3.1 to 5.4%; interassay CV ranging from 5.9 to 11.5%). The World Health Organization First International Standard code 80/505 was used as GH standard with both kits. Results were recapitulated in separate analyses using data from each assay (data not shown) and combined to increase power because no obvious differences were seen by assay.
Anthropometric assessment
Height and body weight were obtained after an overnight fast. Measurement of waist circumference was performed in triplicate at the iliac crest in a standardized fashion with the subject in an upright position. Measurement of hip circumference was performed at the widest point, also with the subject in an upright position. Fat and fat-free mass were determined by DEXA testing using a Hologic-4500 densitometer (Hologic, Inc., Waltham, MA). Measurements of regional adiposity using DEXA were standardized (1995 User’s Guide Hologic Inc). Lower extremity fat represents the arithmetic sum of fat mass in each leg. The technique has a precision error (1 sd) of 3% for fat and 1.5% for lean body mass (18). In addition, 1-cm cross-sectional abdominal CT scans were performed at the level of L4 to assess the distribution of total abdominal adipose tissue (TAT), abdominal sc adipose tissue (SAT), and abdominal visceral adipose tissue (VAT) as previously described (16).
Statistical analysis
Continuous variables were tested for normality of distribution with the use of the Wilk-Shapiro test and examination of the histogram distribution. Variables that were normally distributed were compared using the Student’s t test, and variables that were not normally distributed were compared using the nonparametric Wilcoxon rank sum test. Nominal variables were compared using the χ2 test. GH area under the curve (AUC) response to GHRH-arginine testing was determined using the trapezoid method. For comparison between groups, subjects were stratified by BMI into lean (BMI < 25 kg/m2), overweight (BMI ≥ 25 and < 30 kg/m2), and obese (BMI ≥ 30 kg/m2), as well as by waist circumference using 102 cm as a cutoff as per the National Cholesterol Education Program (NCEP) guidelines of the Adult Treatment Panel III (19). Univariate regression analysis was performed comparing peak stimulated GH levels with measures of overall and regional adiposity using the Pearson correlation coefficient. Multivariate regression analysis with standard least squares modeling was also performed using age, BMI, and various measures of regional adiposity as covariates and peak stimulated GH as the dependent variable. Specific anthropometric, DEXA, and CT models were constructed. Combined models were not constructed due to the colinearity of measures of central adiposity by the various techniques and so that clear information would be provided on the specific utility of measures using each technique independently. Statistical analysis was performed using JMP Statistical Database Software (SAS Institute, Inc., Cary, NC). Statistical significance was determined as P < 0.05.
Results
Clinical characteristics of study subjects
The subjects ranged in age from 20 to 60 yr. The BMI ranged from 20.0 to 47.3 kg/m2. Of the 75 subjects, 23 were lean (BMI < 25 kg/m2), 28 were overweight (BMI between 25 and 29.9 kg/m2), and 24 were obese (BMI ≥ 30 kg/m2) (Table 1). The subjects in each group were similar in age and ethnicity as well as height. Body composition variables including measures of both total and regional adiposity differed significantly across the strata. In addition, both systolic and diastolic blood pressure differed across BMI categories (Table 1).
Table 1.
Baseline characteristics of study subjects stratified by BMI (n = 75)
| Lean | Overweight | Obese | P | |
|---|---|---|---|---|
| n | 23 | 28 | 24 | |
| Age (yr) | 44.0 ± 1.9 | 44.1 ± 1.7 | 44.9 ± 2.0 | 0.82 |
| Race, n (%) | 0.37 | |||
| Caucasian | 20 (87.0) | 20 (71.4) | 15 (62.5) | |
| African-American | 0 | 4 (14.3) | 5 (20.8) | |
| Asian | 3 (13.0) | 2 (7.1) | 0 | |
| Hispanic | 0 | 2 (7.1) | 3 (12.5) | |
| Other | 0 | 0 | 1 (4.2) | |
| Blood pressure | ||||
| Systolic (mm Hg) | 117 ± 3 | 120 ± 3 | 129 ± 3 | 0.04 |
| Diastolic (mm Hg) | 72 ± 2 | 76 ± 2 | 82 ± 2 | 0.01 |
| Anthropometrics | ||||
| Height (cm) | 176.3 ± 1.4 | 175.1 ± 1.6 | 175.9 ± 1.4 | 0.84 |
| Body weight (kg) | 70.3 ± 1.4 | 84.5 ± 1.8 | 111.7 ± 2.6 | <0.0001 |
| BMI (kg/m2) | 22.6 ± 0.3 | 27.5 ± 0.3 | 36.2 ± 1.0 | <0.0001 |
| Waist (cm) | 84.4 ± 1.3 | 97.0 ± 1.2 | 117.7 ± 2.2 | <0.0001 |
| Hip (cm) | 97.7 ± 1.0 | 104.3 ± 0.8 | 117.1 ± 1.5 | <0.0001 |
| Waist:hip | 0.86 ± 0.01 | 0.93 ± 0.009 | 1.0 ± 0.01 | <0.0001 |
| Abdominal CT scan | ||||
| TAT (cm2) | 252.8 ± 28.6 | 477.7 ± 32.7 | 726.9 ± 33.1 | <0.0001 |
| SAT (cm2) | 126.4 ± 8.8 | 243.8 ± 13.1 | 435.3 ± 26.3 | <0.0001 |
| VAT (cm2) | 77.7 ± 9.1 | 133.7 ± 11.7 | 235.0 ± 16.6 | <0.0001 |
| DEXA | ||||
| Total fat (kg) | 13.7 ± 0.8 | 21.8 ± 1.1 | 36.5 ± 2.2 | <0.0001 |
| Lower extremity fat (kg) | 4.9 ± 0.3 | 7.1 ± 0.4 | 11.5 ± 0.8 | <0.0001 |
| Trunk fat (kg) | 6.2 ± 0.4 | 11.1 ± 0.7 | 19.4 ± 1.1 | <0.0001 |
| % Fat mass | 18.8 ± 0.9 | 24.7 ± 0.9 | 32.1 ± 1.4 | <0.0001 |
| % Lean mass | 77.6 ± 0.9 | 69.8 ± 2.6 | 65.4 ± 1.3 | 0.0002 |
Results are presented as mean ± sem. Lean was defined as BMI < 25 kg/m2, overweight as BMI from 25 to <30 kg/m2, and obese as BMI ≥ 30 kg/m2. For determination of significance for race, a χ2 test was performed for Caucasian or not.
In a separate analysis, subjects were stratified according to waist circumference using 102 cm as the cutoff value as per NCEP guidelines (19). Forty-seven subjects had waist circumference less than 102 cm, whereas 28 subjects had waist circumference of at least 102 cm. The two groups had similar age, ethnicity, and height. BMI and measures of both total and regional adiposity differed significantly between groups stratified by waist circumference (Table 2).
Table 2.
Baseline characteristics of study subjects stratified by waist circumference (n = 75)
| Waist circumference
|
P | ||
|---|---|---|---|
| <102 cm | ≥102 cm | ||
| n | 47 | 28 | |
| Age (yr) | 43.1 ± 1.3 | 46.4 ± 1.7 | 0.07 |
| Race, n (%) | 0.71 | ||
| Caucasian | 36 (76.7) | 19 (67.9) | |
| African-American | 4 (8.5) | 5 (17.9) | |
| Asian | 5 (10.6) | 0 | |
| Hispanic | 2 (4.2) | 3 (10.7) | |
| Other | 0 | 1 (3.6) | |
| Blood pressure | |||
| Systolic (mm Hg) | 119 ± 2 | 127 ± 3 | 0.01 |
| Diastolic (mm Hg) | 74 ± 1 | 81 ± 2 | 0.003 |
| Anthropometrics | |||
| Height (cm) | 175.2 ± 1.1 | 176.7 ± 1.3 | 0.41 |
| Body weight (kg) | 77.1 ± 1.6 | 108.6 ± 2.7 | <0.0001 |
| BMI (kg/m2) | 25.1 ± 0.4 | 34.9 ± 1 | <0.0001 |
| Waist (cm) | 89.8 ± 1.1 | 116.5 ± 2.0 | <0.0001 |
| Hip (cm) | 100.8 ± 0.8 | 115.7 ± 1.4 | <0.0001 |
| Waist:hip | 0.89 ± 0.007 | 1.01 ± 0.01 | <0.0001 |
| Abdominal CT scan | |||
| TAT (cm2) | 365.5 ± 28.7 | 699.0 ± 31.2 | <0.0001 |
| SAT (cm2) | 182.8 ± 11.5 | 416.0 ± 24.7 | <0.0001 |
| VAT (cm2) | 97.7 ± 6.6 | 235.7 ± 14.9 | <0.0001 |
| DEXA | |||
| Total fat (kg) | 17.2 ± 0.8 | 36.0 ± 1.9 | <0.0001 |
| Lower extremity fat (kg) | 5.9 ± 0.3 | 11.3 ± 0.7 | <0.0001 |
| Trunk fat (kg) | 8.3 ± 0.5 | 19.3 ± 1.0 | <0.0001 |
| % Fat mass | 21.2 ± 0.7 | 32.3 ± 1.1 | <0.0001 |
| % Lean mass | 74.0 ± 1.7 | 65.2 ± 1.1 | <0.0001 |
Waist circumference ≥102 cm was used as the cutoff as per the NCEP guidelines. Results are presented as mean ± sem. For determination of significance for race, a χ2 test was performed for Caucasian or not.
GHRH-arginine stimulation testing
The response to GHRH-arginine stimulation clearly segregated by BMI status (Fig. 1A). The peak stimulated GH occurred at 45 min and progressively declined over the remainder of the test. The peak stimulated GH levels were lower in the overweight subjects and lower still in the obese subjects (Fig. 1, B and C). The lean subjects had peak stimulated GH of 36.4 ± 5.4 μg/liter. In comparison, the overweight subjects had a peak stimulated GH level of 16.6 ± 2.9 μg/liter (P = 0.0001 in comparison with lean), and the obese subjects had a peak stimulated GH level of 7.6 ± 0.9 μg/liter (P < 0.0001 in comparison with lean; P = 0.0004 in comparison with overweight). Similarly, the AUC for GH during the GHRH-arginine stimulation test also differed by BMI (data not shown).
Figure 1.
A, GHRH-arginine stimulation tests in lean (n = 23), overweight (n = 28), and obese (n = 24) subjects. The results are presented as mean ± sem. P < 0.01 in all time points in lean vs. overweight subjects; P < 0.001 in all time points in lean vs. obese subjects; P < 0.05 in all time points except 90 min where P = 0.08 in overweight vs. obese subjects. B, Peak stimulated GH levels from GHRH-arginine stimulation tests in lean (n = 23), overweight (n = 28), and obese (n = 24) subjects. The results are presented as mean ± sem. P = 0.0001 in lean vs. overweight subjects; P < 0.0001 in lean vs. obese subjects; P = 0.0004 in overweight vs. obese subjects. C, Scatter plot for peak stimulated GH levels from the GHRH-arginine stimulation tests in lean (n = 23), overweight (n = 28), and obese (n = 24) subjects. D, GHRH-arginine stimulation tests in subjects with waist circumference less than 102 cm (n = 47) and subjects with waist circumference of 102 cm or greater (n = 28). The results are presented as mean ± sem. All time points are significant by P < 0.01. E, Peak stimulated GH levels from GHRH-arginine stimulation tests in subjects with waist circumference less than 102 cm (n = 47) and subjects with waist circumference of at least 102 cm (n = 28). The results are presented as mean ± sem. P < 0.0001. F, Scatter plot for peak stimulated GH levels from the GHRH-arginine stimulation tests in subjects with waist circumference less than 102 cm (n = 47) and subjects with waist circumference of at least 102 cm (n = 28).
The response to GHRH-arginine stimulation also segregated by waist circumference (Fig. 1D). Subjects with waist circumference less than 102 cm had higher peak stimulated GH levels compared with subjects with waist circumference of at least 102 cm (26.9 ± 3.4 vs. 7.9 ± 0.9 μg/liter; P < 0.0001) (Fig. 1, E and F). Similarly, the GH AUC was also significantly higher in subjects with waist circumference less than 102 cm compared with subjects with waist circumference of 102 cm or more (data not shown).
Relationship of peak stimulated GH to measures of total and regional adiposity
Univariate analysis was performed among all subjects (including lean, overweight, and obese subjects; n = 75). Peak stimulated GH was negatively related to body weight, BMI, and various anthropometric measurements including waist circumference, hip circumference, waist-to-hip ratio, and measures of adiposity including TAT, VAT, SAT, lower extremity fat, trunk fat, total fat mass, and percentage fat mass. The peak stimulated GH levels were positively related to percentage lean body mass (Table 3). Similar results were seen for GH AUC (data not shown).
Table 3.
Univariate analysis of peak stimulated GH levels to various parameters in all subjects (n = 75)
| Parameter | r | P |
|---|---|---|
| Body weight (kg) | −0.49 | <0.0001 |
| BMI (kg/m2) | −0.50 | <0.0001 |
| Waist (cm) | −0.56 | <0.0001 |
| Hip (cm) | −0.48 | <0.0001 |
| Waist:hip | −0.55 | <0.0001 |
| TAT (cm2) | −0.44 | <0.0001 |
| VAT (cm2) | −0.54 | <0.0001 |
| SAT (cm2) | −0.51 | <0.0001 |
| Lower extremity fat (kg) | −0.39 | 0.0009 |
| Trunk fat (kg) | −0.56 | <0.0001 |
| Total fat (kg) | −0.52 | <0.0001 |
| % Fat | −0.54 | <0.0001 |
| % Lean | +0.36 | 0.002 |
Multivariate regression analysis was performed using standard least squares modeling among all subjects (n = 75). In a simple model consisting solely of age and BMI, BMI was significantly and inversely associated with peak stimulated GH levels (P < 0.0001; r2 = 0.26) (Table 4A). Subsequently, three distinct models were created to assess the significance of generalized and regional adiposity using: 1) anthropometric, 2) DEXA, and 3) abdominal CT scan measurements of overall adiposity, central, and regional fat depots.
Table 4.
Multivariate analyses of peak stimulated GH level and parameters of regional fat distribution (n = 75)
| Variables | Estimate | se | P value |
|---|---|---|---|
| A. Basic modela | |||
| Age | −0.23 | 0.23 | 0.32 |
| BMI (kg/m2) | −1.67 | 0.34 | <0.0001 |
| B. Anthropometric modelb | |||
| Age | −0.06 | 0.24 | 0.80 |
| BMI (kg/m2) | 0.31 | 1.10 | 0.78 |
| Hip (cm) | 0.31 | 0.58 | 0.60 |
| Waist (cm) | −1.02 | 0.44 | 0.02 |
| C. DEXA modelc | |||
| Age | −0.14 | 0.23 | 0.54 |
| BMI (kg/m2) | 0.05 | 0.89 | 0.96 |
| Lower extremity fat (kg) | 1.86 | 1.06 | 0.08 |
| Trunk fat (kg) | −2.66 | 0.93 | 0.006 |
| D. Abdominal CT modeld | |||
| Age | 0.03 | 0.27 | 0.90 |
| BMI (kg/m2) | 0.49 | 1.02 | 0.64 |
| TAT (cm2) | 0.01 | 0.02 | 0.57 |
| SAT (cm2) | −0.06 | 0.04 | 0.16 |
| VAT (cm2) | −0.10 | 0.04 | 0.02 |
Total r2 for the model was 0.26. P < 0.0001.
Total r2 for the model was 0.32. P < 0.0001.
Total r2 for the model was 0.35. P < 0.0001.
Total r2 for the model was 0.34. P < 0.0001.
In the anthropometric model, waist circumference was significantly and inversely related to peak stimulated GH levels controlling for age, BMI, and hip circumference (P = 0.02; r2 = 0.32) (Table 4B). With the inclusion of waist circumference, BMI was no longer significantly associated with peak stimulated GH in the model. The effect size was −1.02, suggesting a 1 μg/liter decrease in peak stimulated GH for each 1 cm increase in waist circumference, controlling for age and BMI.
In the DEXA model, trunk fat was significantly inversely related to peak stimulated GH levels controlling for age, BMI, and lower extremity fat (P = 0.006; r2 = 0.35). With the inclusion of trunk fat, BMI and lower extremity fat were no longer significantly associated with peak stimulated GH in the model (Table 4C). The significant relationship between trunk fat and peak stimulated GH held with the addition of total lean mass to the model (P = 0.006; r2 = 0.36).
In the third model using CT measurements of specific depots of abdominal fat, a significant inverse relationship between VAT and peak stimulated GH was identified controlling for age, BMI, TAT, and SAT (P = 0.02; r2 = 0.34). With the inclusion of VAT, neither BMI, TAT, nor SAT was significantly associated with peak stimulated GH (Table 4D). The multivariate regression model was reanalyzed without TAT because this covariate is colinear with VAT; however, no significant changes were noted in the relationship between VAT and peak stimulated GH while controlling for age, BMI, and SAT (P = 0.02; r2 = 0.33).
Similar results were obtained for GH AUC in all three models (data not shown).
Correlation between anthropometric measurements, DEXA, and VAT
Both waist circumference (r = +0.84; P < 0.0001) and trunk fat (r = +0.86; P < 0.0001) were significantly and strongly associated with VAT in univariate regression analysis.
Discussion
Previous studies have demonstrated an association between peak stimulated GH levels and BMI (9,20). Furthermore, Pijl et al. (11) demonstrated an association between 24-h GH secretion and visceral fat as assessed by magnetic resonance imaging in obese premenopausal women, whereas Franco et al. (12) demonstrated an association between basal and pulsatile GH secretion on 24-h frequent sampling and visceral fat as assessed by CT in postmenopausal subjects. In addition, Miller et al. (13) demonstrated an association between 24-h GH secretion and truncal adiposity in nonobese women. However, prior studies have not investigated the relationship between GH response to GHRH-arginine and specific measures of regional adiposity in men. Our data demonstrate that peak stimulated GH levels on GHRH-arginine testing are most strongly associated with measures of central adiposity such as waist circumference, trunk fat, and VAT when controlling for age, BMI, and peripheral fat. These data highlight the importance of central adiposity, independent of weight per se, as a strong predictor of GH response to standardized testing.
Visceral adiposity is thought to confer increased risk for metabolic complications of obesity including dyslipidemia, insulin resistance, and increased cardiovascular disease risk (21,22,23). The data in this study suggest that increased visceral adiposity may contribute to reduced GH secretion, which may be a further mechanism for increased cardiovascular disease risk in men with central adiposity. Among women, Utz et al. (15) recently demonstrated that relative GH deficiency of obesity is associated with increased carotid intima-media thickness, analogous to the cardiovascular disease risk seen with GH deficiency of hypopituitarism (24). Similar studies investigating the independent effects of reduced GH secretion on cardiovascular disease risk among obese but otherwise healthy men are currently under way.
Although VAT was significantly associated with GH response, abdominal CT scanning to assess specific fat depot remains an investigational tool. It is therefore of clinical interest to note that in this study, a simple surrogate of central adiposity, waist circumference, was also significantly associated with peak stimulated GH independent of BMI. Given the strong association between VAT and waist circumference that we demonstrate, waist circumference may be a simple but effective surrogate for visceral adiposity among overweight and obese patients. Moreover, the use of waist circumference may be useful to predict GH response to standardized GH testing and may provide information beyond that of BMI in assessing the anticipated reduction in GH response with increasing weight. Indeed, the effect size suggests that a 1-cm increase in waist circumference is associated with a decrease in peak stimulated GH of 1 μg/liter. The importance of measuring waist circumference for assessment of metabolic and cardiovascular consequences was recently highlighted in the position statement of the North American Society for Obesity (25).
There are several limitations to our study. First of all, causality cannot be determined in a cross-sectional study, and therefore low GH could also be contributing to increased central adiposity. However, this possibility does not negate our findings that measures of central adiposity provide useful information in predicting GH response to stimulation testing. Moreover, the finding that weight loss can restore the GH pulsatility associated with obesity (10) suggests that weight gain and central adiposity are the proximal causes in this relationship. Nonetheless, exogenous GH clearly reduces VAT (26,27), so this relationship is dynamic and may be bidirectional. Interventions to block the feedback loop at either point may interrupt a vicious cycle and result in functional improvement of both central adiposity and GH levels. This study was limited to men, but significant gender differences exist with respect to GH secretion (28) and body fat distribution (29) in men and women, and gender-specific analyses are therefore necessary. Physical activity data were not available in our study, but all subjects were healthy and ambulatory. Our data complement smaller studies demonstrating similar relationships of central fat to endogenous GH secretion in women by DEXA (13) and use highly specific body composition methods to assess this relationship, including CT scanning.
In summary, our study confirms a significant inverse relationship between BMI and GH response to standard stimulation testing (20). In addition, data from this study demonstrate a strong independent relationship between measures of central adiposity and peak stimulated GH levels, controlling for BMI. These data therefore suggest that measures of central adiposity, in addition to BMI, should be considered in the determination of appropriate cutoffs to define GH deficiency. Consideration should be given to adjusting cutoffs on GH stimulation testing for waist circumference, a standardized and easy to obtain measure of central adiposity. Furthermore, our data suggest the potential utility of simple anthropometric measurements as an aid in the determination of normal GH response in overweight and obese men. Further research is needed to develop optimal cutoffs for GH deficiency using waist circumference in men and women.
Footnotes
This work was supported by National Institutes of Health Grant 1 R01 HL085268-01A1 (to S.G.). The studies were conducted at the General Clinical Research Center at the Massachusetts Institute of Technology and funded by a grant (M01 RR-01066) from the National Center for Research Resources, National Institutes of Health.
Disclosure Statement: The authors have nothing to disclose.
First Published Online September 2, 2008
For editorial see page 4221
Abbreviations: AUC, Area under the curve; BMI, body mass index; CT, computed tomography; CV, coefficient of variation; DEXA, dual-energy x-ray absorptiometry; SAT, sc adipose tissue; TAT, total abdominal adipose tissue; VAT, visceral adipose tissue.
References
- Iranmanesh A, Lizarralde G, Veldhuis JD 1991 Age and relative adiposity are specific negative determinants of the frequency and amplitude of growth hormone (GH) secretory bursts and the half-life of endogenous GH in healthy men. J Clin Endocrinol Metab 73:1081–1088 [DOI] [PubMed] [Google Scholar]
- Riedel M, Hoeft B, Blum WF, von zur Muhlen A, Brabant G 1995 Pulsatile growth hormone secretion in normal-weight and obese men: differential metabolic regulation during energy restriction. Metabolism 44:605–610 [DOI] [PubMed] [Google Scholar]
- Van Dam EW, Roelfsema F, Helmerhorst FH, Frolich M, Meinders AE, Veldhuis JD, Pijl H 2002 Low amplitude and disorderly spontaneous growth hormone release in obese women with or without polycystic ovary syndrome. J Clin Endocrinol Metab 87:4225–4230 [DOI] [PubMed] [Google Scholar]
- Vizner B, Reiner Z, Sekso M 1983 Effect of l-dopa on growth hormone, glucose, insulin, and cortisol response in obese subjects. Exp Clin Endocrinol 81:41–48 [DOI] [PubMed] [Google Scholar]
- Williams T, Berelowitz M, Joffe SN, Thorner MO, Rivier J, Vale W, Frohman LA 1984 Impaired growth hormone responses to growth hormone-releasing factor in obesity. A pituitary defect reversed with weight reduction. N Engl J Med 311:1403–1407 [DOI] [PubMed] [Google Scholar]
- Kopelman PG, Noonan K, Goulton R, Forrest AJ 1985 Impaired growth hormone response to growth hormone releasing factor and insulin-hypoglycaemia in obesity. Clin Endocrinol (Oxf) 23:87–94 [DOI] [PubMed] [Google Scholar]
- Ghigo E, Procopio M, Boffano GM, Arvat E, Valente F, Maccario M, Mazza E, Camanni F 1992 Arginine potentiates but does not restore the blunted growth hormone response to growth hormone-releasing hormone in obesity. Metabolism 41:560–563 [DOI] [PubMed] [Google Scholar]
- Cordido F, Alvarez-Castro P, Isidro ML, Casanueva FF, Dieguez C 2003 Comparison between insulin tolerance test, growth hormone (GH)-releasing hormone (GHRH), GHRH plus acipimox and GHRH plus GH-releasing peptide-6 for the diagnosis of adult GH deficiency in normal subjects, obese and hypopituitary patients. Eur J Endocrinol 149:117–122 [DOI] [PubMed] [Google Scholar]
- Bonert VS, Elashoff JD, Barnett P, Melmed S 2004 Body mass index determines evoked growth hormone (GH) responsiveness in normal healthy male subjects: diagnostic caveat for adult GH deficiency. J Clin Endocrinol Metab 89:3397–3401 [DOI] [PubMed] [Google Scholar]
- Rasmussen MH, Hvidberg A, Juul A, Main KM, Gotfredsen A, Skakkebaek NE, Histed J, Skakkebae NE 1995 Massive weight loss restores 24-hour growth hormone release profiles and serum insulin-like growth factor-I levels in obese subjects. J Clin Endocrinol Metab 80:1407–1415 [DOI] [PubMed] [Google Scholar]
- Pijl H, Langendonk JG, Burggraaf J, Frolich M, Cohen AF, Veldhuis JD, Meinders AE 2001 Altered neuroregulation of GH secretion in viscerally obese premenopausal women. J Clin Endocrinol Metab 86:5509–5515 [DOI] [PubMed] [Google Scholar]
- Franco C, Veldhuis JD, Iranmanesh A, Brandberg J, Lonn L, Andersson B, Bengtsson BA, Svensson J, Johannsson G 2006 Thigh intermuscular fat is inversely associated with spontaneous GH release in post-menopausal women with abdominal obesity. Eur J Endocrinol 155:261–268 [DOI] [PubMed] [Google Scholar]
- Miller KK, Biller BM, Lipman JG, Bradwin G, Rifai N, Klibanski A 2005 Truncal adiposity, relative growth hormone deficiency, and cardiovascular risk. J Clin Endocrinol Metab 90:768–774 [DOI] [PubMed] [Google Scholar]
- Vahl N, Jorgensen JO, Skjaerbaek C, Veldhuis JD, Orskov H, Christiansen JS 1997 Abdominal adiposity rather than age and sex predicts mass and regularity of GH secretion in healthy adults. Am J Physiol 272:E1108—E1116 [DOI] [PubMed] [Google Scholar]
- Utz AL, Yamamoto A, Hemphill L, Miller KK 2008 Growth hormone deficiency by growth hormone releasing hormone-arginine testing criteria predicts increased cardiovascular risk markers in normal young overweight and obese women. J Clin Endocrinol Metab 93:2507–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietschel P, Hadigan C, Corcoran C, Stanley T, Neubauer G, Gertner J, Grinspoon S 2001 Assessment of growth hormone dynamics in human immunodeficiency virus-related lipodystrophy. J Clin Endocrinol Metab 86:504–510 [DOI] [PubMed] [Google Scholar]
- Koutkia P, Eaton K, You SM, Breu J, Grinspoon S 2006 Growth hormone secretion among HIV infected patients: effects of gender, race and fat distribution. AIDS 20:855–862 [DOI] [PubMed] [Google Scholar]
- Mazess RB, Barden HS, Bisek JP, Hanson J 1990 Dual-energy x-ray absorptiometry for total-body and regional bone-mineral and soft-tissue composition. Am J Clin Nutr 51:1106–1112 [DOI] [PubMed] [Google Scholar]
- 2001 Executive summary of the third report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 285:2486–2497 [DOI] [PubMed] [Google Scholar]
- Corneli G, Di Somma C, Baldelli R, Rovere S, Gasco V, Croce CG, Grottoli S, Maccario M, Colao A, Lombardi G, Ghigo E, Camanni F, Aimaretti G 2005 The cut-off limits of the GH response to GH-releasing hormone-arginine test related to body mass index. Eur J Endocrinol 153:257–264 [DOI] [PubMed] [Google Scholar]
- Johannsson G, Bengtsson BA 1999 Growth hormone and the metabolic syndrome. J Endocrinol Invest 22:41–46 [PubMed] [Google Scholar]
- Hayashi T, Boyko EJ, McNeely MJ, Leonetti DL, Kahn SE, Fujimoto WY 2008 Visceral adiposity, not abdominal subcutaneous fat area, is associated with an increase in future insulin resistance in Japanese Americans. Diabetes 57:1269–1275 [DOI] [PubMed] [Google Scholar]
- Lear SA, Humphries KH, Kohli S, Frohlich JJ, Birmingham CL, Mancini GB 2007 Visceral adipose tissue, a potential risk factor for carotid atherosclerosis: results of the Multicultural Community Health Assessment Trial (M-CHAT). Stroke 38:2422–2429 [DOI] [PubMed] [Google Scholar]
- Colao A, Di Somma C, Savanelli MC, De Leo M, Lombardi G 2006 Beginning to end: cardiovascular implications of growth hormone (GH) deficiency and GH therapy. Growth Horm IGF Res 16(Suppl A):S41—S48 [DOI] [PubMed] [Google Scholar]
- Klein S, Allison DB, Heymsfield SB, Kelley DE, Leibel RL, Nonas C, Kahn R 2007 Waist circumference and cardiometabolic risk: a consensus statement from Shaping America’s Health: Association for Weight Management and Obesity Prevention; NAASO, The Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Am J Clin Nutr 85:1197–1202 [DOI] [PubMed] [Google Scholar]
- Johannsson G, Marin P, Lonn L, Ottosson M, Stenlof K, Bjorntorp P, Sjostrom L, Bengtsson BA 1997 Growth hormone treatment of abdominally obese men reduces abdominal fat mass, improves glucose and lipoprotein metabolism, and reduces diastolic blood pressure. J Clin Endocrinol Metab 82:727–734 [DOI] [PubMed] [Google Scholar]
- Franco C, Brandberg J, Lonn L, BA Bengtsson B, Johansson G 2005 Growth hormone treatment reduces abdominal visceral fat in postmenopausal women with abdominal obesity: a 12-month placebo-controlled trial. J Clin Endocrinol Metab 90:1466–1474 [DOI] [PubMed] [Google Scholar]
- Jessup SK, Dimaraki EV, Symons KV, Barkan AL 2003 Sexual dimorphism of growth hormone (GH) regulation in humans: endogenous GH-releasing hormone maintains basal GH in women but not in men. J Clin Endocrinol Metab 88:4776–4780 [DOI] [PubMed] [Google Scholar]
- Ley CJ, Lees B, Stevenson JC 1992 Sex- and menopause-associated changes in body-fat distribution. Am J Clin Nutr 55:950–954 [DOI] [PubMed] [Google Scholar]