Abstract
Introduction: The prevalence of obesity is higher in Blacks with racial divergence in adiposity in girls starting during adolescence. Our hypothesis is that in Black children, puberty associated increase in fat oxidation is diminished and could play a role in predisposing to fat accretion triggered during puberty. Thus, we examined the relationships between race, pubertal development, and postabsorptive fat oxidation in youth.
Subjects and Methods: This was a cross-sectional design of healthy Black (n = 50) and white (n = 51) youth. Resting metabolic rate (RMR) and substrate oxidation rate were measured after an overnight fast with indirect calorimetry. Body composition was measured by dual-energy x-ray absorptiometry.
Results and Discussion: Within each race, RMR (kcal/kg fat free mass · min) was lower (puberty effect; P < 0.05) in the pubertal vs. prepubertal group independent of gender. In girls, RMR was lower (race effect; P < 0.05) in Blacks vs. whites. In girls but not boys, Blacks had lower (race effect; P = 0.033) fat oxidation (μmol/kg fat free mass · min) compared with whites independent of pubertal status. Furthermore, the difference in fat oxidation between the prepubertal vs. pubertal groups tended to be greater (puberty × race interaction; P = 0.089) in white girls (3.7 ± 0.5 vs. 6.5 ± 0.5) than in Black girls (3.4 ± 0.6 vs. 4.5 ± 0.5). These data suggest that the lower fat oxidation and RMR during puberty in Black girls could be a risk factor predisposing to obesity. This metabolic phenotype could potentially explain the divergence in adiposity in Black girls during adolescence against the backdrop of an obesogenic environment.
Resting metabolic rate and postabsorptive fat oxidation are substantially lower in black versus white girls. This racial difference is more pronounced in the pubertal group.
The most recent National Health and Nutrition Examination Survey reports that between 1999 and 2004, the prevalence of at risk of overweight [body mass index (BMI) ≥ 85th percentile or overweight BMI ≥ 95th percentile in youth (12–19 yr)] has continuously increased from 30–34.4% and 14.8–17.4%, respectively (1). Similar to the previous National Health and Nutrition Examination Survey report (2), racial disparities persist, particularly in girls, such that 25% of Black girls (12–19 yr) are now considered overweight compared with 15% of white girls of similar age (1). The National Heart, Lung, and Blood Institute Growth and Health Study shows that the racial divergence in adiposity starts during adolescence in girls (3).
Although the mechanisms explaining the racial differential in the rates of childhood obesity are not fully understood, high-energy intake (4), low-physical activity level (5,6), and sedentary behavior (e.g. increased television watching) (6) have been reported in Black vs. white girls. However, in adult women the racial differences in the prevalence of obesity remain after accounting for these environmental factors (7), suggesting inherent metabolic/physiological differences between the two racial groups.
Reduced postabsorptive fat oxidation is one of the contributing factors leading to positive energy balance and, therefore, future weight gain (8). It has been reported that in adults, Black lean women have significantly lower fat oxidation both at rest and during physical activity compared with their matched white peers (9). In a longitudinal study of adult Pima Indians, individuals with low fat oxidation were at 2.5 times greater risk for future weight gain (≥5 kg) compared with those with high-fat oxidation independent of 24-h resting metabolic rate (RMR) (8).
In cross-sectional and longitudinal studies, we have previously demonstrated that puberty is characterized by higher rates of fat oxidation compared with prepuberty and adulthood (10,11,12). These higher rates of fat oxidation correlate with IGF-I, which increases during puberty (10,12). It is currently unknown whether there are racial differences in postabsorptive fat oxidation in youth, particularly related to the pubertal period. Thus, we examined the relationships between race and puberty on postabsorptive fat oxidation in normal weight boys and girls. We hypothesized that in Black girls, puberty associated increase in fat oxidation is diminished compared with their white peers, and could play a role in predisposing to fat accumulation triggered during puberty.
Subjects and Methods
Subjects consisted of healthy nonoverweight (BMI < 95th percentile) Black (n = 50) and white (n = 51) prepubertal [mean, 9.9 ± 0.2; range, 8.0–11.9 yr) and pubertal [mean, 12.8 ± 0.2; range: 9.9–15.3 yr) youth, some of whom were reported previously (13). Study participants were recruited through newspaper advertisements in the greater Pittsburgh area, flyers posted in the city public transportation, and posters placed on campus. Racial background was verified by self-identification in three generations. The investigation was approved by the institutional review board and performed in the Pediatric Clinical and Translational Research Center at Children’s Hospital of Pittsburgh. Parental informed consent and child assent were obtained from all participants. Pubertal development was assessed by physical examination according to Tanner criteria (breast development in females, genital development in males, and pubic hair in both), and was confirmed by measurement of plasma testosterone in males, estradiol in females, and dehydroepiandrosterone sulfate in both.
All subjects were admitted to the Pediatric Clinical and Translational Research Center on the previous day, for testing on the following morning after a 10- to 12-h overnight fast. RMRs were measured for 30 min using an open-circuit indirect calorimetry (DeltaTrac, Anaheim, CA), and substrate oxidation was calculated according to Frayn formulas (11). Total body fat was assessed by dual-energy x-ray absorptiometry.
Cardiorespiratory fitness (peak oxygen consumption) was performed in 35 Blacks and 40 whites as shown by us previously (14). A 24-h weekday food recall was administered by a trained nutritionist in 37 Blacks and 42 whites as reported by us previously (15). Statistical procedures were performed using SPSS (Version 15; SPSS, Inc., Chicago, IL). Race and pubertal group [prepuberty (Tanner I) vs. puberty (Tanner II-V)] differences (statistical significance, P < 0.05) in the anthropometric/metabolic variables were assessed using 2 × 2 ANOVA. Race and puberty group were entered as categorical variables, and dependent variables (e.g. RMR, fat oxidation, etc.) were entered as continuous variables. Differences in fat oxidation between the pubertal and prepubertal groups were compared using independent t tests.
Results
Within each gender, Blacks and whites did not differ (P > 0.05) with respect to age and total adiposity (Table 1). Within each race, the pubertal group had higher (P < 0.05) fat mass (FM) in girls alone, and higher (P < 0.05) fat free mass (FFM) in both boys and girls. Independent of gender, cardiorespiratory fitness was significantly (P < 0.05) lower in Blacks vs. whites. The 24-hr diet recall revealed no racial effect on dietary composition in girls (data not shown). However, in boys, carbohydrate intake was lower in Black vs. white prepubertal (49.8 ± 4.2% vs. 58.3 ± 2.6%) and pubertal (49.4 ± 3.5% vs. 54.5 ± 2.3%) groups [race effect, P = 0.047; puberty effect, P = not significant (NS)].
Table 1.
Subject characteristics
| Blacks
|
Whites
|
Groupa or interaction effectb | |||
|---|---|---|---|---|---|
| Prepuberty (Tanner I) | Puberty (Tanner II-V) | Prepuberty (Tanner I) | Puberty (Tanner II-V) | ||
| Boys (n) | 8 | 15 | 10 | 15 | |
| Age (yr) | 9.7 ± 0.2 | 13.4 ± 0.3 | 10.3 ± 0.3 | 13.3 ± 0.3 | Puberty |
| Tanner stage (I–V) | 1 | 3.1 ± 0.2 | 1 | 3.1 ± 0.2 | Puberty |
| BMI (kg/m2) | 16.7 ± 0.4 | 20.9 ± 0.6 | 18.0 ± 0.6 | 18.8 ± 0.6 | Puberty, race × puberty |
| FM (kg) | 4.1 ± 0.7 | 7.2 ± 1.0 | 6.9 ± 1.0 | 7.6 ± 1.1 | |
| FFM (kg) | 25.4 ± 1.1 | 44.7 ± 2.1 | 27.8 ± 1.3 | 37.4 ± 1.7 | Puberty, race × puberty |
| Cardiorespiratory fitness (ml/kg · min) | 33.3 ± 2.5 | 39.5 ± 1.4 (n = 11) | 37.7 ± 2.6 (n = 9) | 51.1 ± 2.4 (n = 10) | Race, puberty |
| RMR (kcal/24 h) | 1237.5 ± 39.0 | 1706.0 ± 55.6 | 1334.0 ± 63.5 | 1635.3 ± 48.8 | Puberty |
| Fat oxi | |||||
| (μmol/min) | 92.3 ± 16.8 | 197.1 ± 21.7 | 109.3 ± 17.5 | 182.2 ± 20.8 | Puberty |
| (μmol/kg · min) | 3.0 ± 0.5 | 3.5 ± 0.3 | 2.9 ± 0.5 | 3.8 ± 0.4 | |
| (μmol/kg FM · min) | 25.1 ± 6.0 | 34.9 ± 5.3 | 17.9 ± 3.2 | 28.3 ± 4.0 | Puberty |
| (μmol/kg FFM · min) | 3.6 ± 0.6 | 4.4 ± 0.4 | 3.9 ± 0.6 | 4.9 ± 0.6 | |
| C oxi | |||||
| (μmol/min) | 490.7 ± 44.3 | 493.7 ± 46.9 | 458.6 ± 43.9 | 506.6 ± 45.5 | |
| (μmol/kg · min) | 15.9 ± 1.4 | 9.1 ± 0.9 | 12.8 ± 1.5 | 10.8 ± 1.1 | Puberty, race × puberty |
| (μmol/kg FM · min) | 135.2 ± 19.0 | 81.5 ± 11.5 | 79.0 ± 14.5 | 83.4 ± 12.5 | Race × puberty |
| (μmol/kg FFM · min) | 19.5 ± 1.7 | 11.5 ± 1.3 | 17.1 ± 2.2 | 13.8 ± 1.3 | Puberty |
| F oxi/C oxi ratio | 0.22 ± 0.06 | 0.53 ± 0.12 | 0.29 ± 0.06 | 0.47 ± 0.11 | Puberty |
| RQ | 0.89 ± 0.01 | 0.84 ± 0.01 | 0.87 ± 0.01 | 0.85 ± 0.01 | Puberty |
| Girls (n) | 10 | 17 | 12 | 14 | |
| Age (yr) | 10.3 ± 0.4 | 12.3 ± 0.4 | 9.4 ± 0.3 | 12.2 ± 0.4 | Puberty |
| Tanner stage | 1 | 3.2 ± 0.3 | 1 | 3.0 ± 0.3 | Puberty |
| BMI (kg/m2) | 17.4 ± 0.6 | 20.7 ± 0.7 | 17.7 ± 0.5 | 19.6 ± 0.8 | Puberty |
| FM (kg) | 7.3 ± 1.3 | 12.6 ± 1.1 | 7.9 ± 1.0 | 10.9 ± 1.0 | Puberty |
| FFM (kg) | 24.9 ± 1.0 | 34.0 ± 1.5 | 23.3 ± 0.9 | 30.0 ± 1.0 | Race, puberty |
| Cardiorespiratory fitness (ml/kg · min) | 25.1 ± 1.4 (n = 9) | 27.4 ± 3.0 (n = 7) | 32.9 ± 1.3 (n = 11) | 34.8 ± 1.9 (n = 10) | Race |
| RMR (kcal/24 h) | 1161.0 ± 33.4 | 1369.4 ± 37.4 | 1241.7 ± 25.5 | 1462.9 ± 39.6 | Race, puberty |
| Fat oxi | |||||
| (μmol/min) | 87.1 ± 15.7 | 147.8 ± 13.1 | 88.2 ± 14.2 | 190.9 ± 12.0 | Puberty |
| (μmol/kg · min) | 2.5 ± 0.4 | 3.2 ± 0.3 | 2.6 ± 0.3 | 4.4 ± 0.3 | Race, puberty |
| (μmol/kg FM · min) | 13.3 ± 2.9 | 13.8 ± 2.0 | 11.3 ± 1.7 | 19.9 ± 2.4 | Puberty |
| (μmol/kg FFM · min) | 3.4 ± 0.6 | 4.5 ± 0.5 | 3.7 ± 0.5 | 6.5 ± 0.5 | Race, puberty |
| C oxi | |||||
| (μmol/min) | 468.1 ± 21.7 | 438.6 ± 33.8 | 504.5 ± 34.8 | 406.7 ± 29.4 | |
| (μmol/kg · min) | 14.0 ± 1.0 | 9.2 ± 0.6 | 16.1 ± 1.8 | 9.4 ± 0.6 | Puberty |
| (μmol/kg FM · min) | 77.1 ± 10.0 | 39.5 ± 4.0 | 76.6 ± 12.0 | 44.7 ± 8.1 | Puberty |
| (μmol/kg FFM · min) | 19.1 ± 1.1 | 13.5 ± 1.0 | 22.3 ± 2.1 | 13.5 ± 0.8 | Puberty |
| F oxi/C oxi ratio | 0.20 ± 0.04 | 0.40 ± 0.06 | 0.20 ± 0.04 | 0.52 ± 0.07 | Puberty |
| RQ | 0.89 ± 0.01 | 0.85 ± 0.01 | 0.89 ± 0.01 | 0.83 ± 0.01 | Puberty |
Mean ± se. C, Carbohydrate; F, fat; oxi, oxidation; RQ, respiratory quotent.
Group denotes racial (Black, white) or pubertal status (prepuberty, puberty group).
P ≤ 0.05.
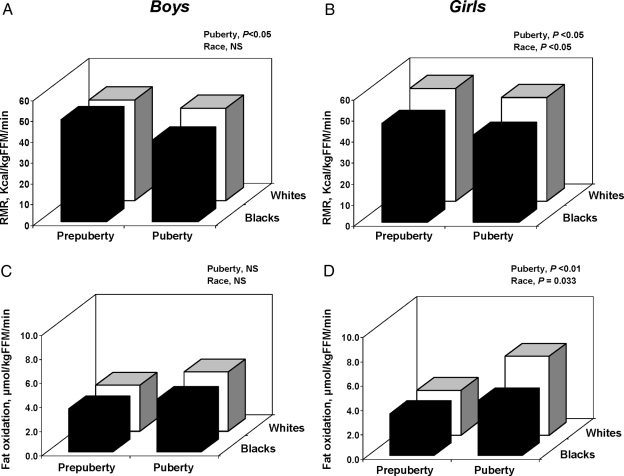
Within each race, RMR adjusted for FFM (kcal/kg FFM · min) was lower in the pubertal vs. prepubertal group independent of gender (puberty effect, P < 0.05) (Fig. 1, A and B). In girls but not boys, RMR was lower in Blacks vs. whites (race effect, P < 0.05) (Fig. 1B). Within each race, fat oxidation, whether it is expressed as absolute (μmol/min) or relative to FM (μmol/kg FM · min), was higher in pubertal vs. prepubertal boys, with a similar tendency when data were expressed relative to FFM (puberty effect, P = 0.085) (Fig. 1C). In pubertal girls, Blacks had lower fat oxidation compared with whites when the data were expressed per body weight (μmol/kg · min) or FFM (μmol/kg FFM · min) (race effect, P = 0.033; Fig. 1D). The differences in fat oxidation (relative to body weight, FM and FFM) between the pubertal and prepubertal groups were significant in white girls (Δ1.87 ± 0.46, Δ8.64 ± 3.04, and Δ2.80 ± 0.68, respectively; P < 0.01 for all), but not in Black girls (Δ0.74 ± 0.53, Δ0.56 ± 3.35, and Δ 1.06 ± 0.73, respectively; P > 0.1 for all). Our observation that in girls, but not boys, Blacks have significantly lower fat oxidation than their white peers remained unchanged when the data (μmol/min) were adjusted either for body weight, FM, or FFM using analysis of covariance (data not shown).
Figure 1.
Postabsorptive RMR and fat oxidation in boys and girls (▪: Blacks; □: whites).
Discussion
We observe that in girls but not boys, Blacks have significantly lower RMR than their white peers independent of pubertal status. Consistent with our hypothesis, the puberty associated increase in fat oxidation is diminished in Black girls compared with their white peers. These findings suggest that lower RMR and reduced fat oxidation during puberty in Black girls may be a metabolic risk phenotype predisposing them to future weight gain and obesity in an obesogenic environment.
The lower RMR in Blacks compared with whites has been shown in adults (16,17) and children (18,19,20). In addition, during low and moderate-intensity exercise, Black women have lower rates of fat oxidation, 30 and 50%, respectively, than their white peers (9). Similarly, using the doubly labeled water method, Wong et al. (19) demonstrated that total energy expenditure and energy expended for physical activity under free-living conditions were approximately 410 and 462 kcal/d, respectively, lower in pubertal Black girls compared with white girls.
Several factors influence resting substrate oxidation, such as genetic factors, the amount of adipose tissue, level of feeding (positive vs. negative energy balance), and composition of the diet (21). However, the underlying mechanism(s) of racial differences in fat oxidation is still unclear. Our present finding of reduced fat oxidation in Black pubertal girls is not explained by the differences in adiposity because these values are similar between races. In addition, it is unlikely that the lower fat oxidation in Black girls is due to differences in food composition because diet composition is similar between Black vs. white girls.
Gallagher et al. (22) recently demonstrated that in adults, the mass of metabolically active organs (e.g. brain, liver, heart, kidney, and spleen) was significantly smaller in Blacks than in whites, and that the racial differences in RMR observed in this study disappeared once the mass of these organs was accounted. It is currently unknown whether this holds true in children. Although speculative, greater skeletal muscle mass (explaining only 20–30% of resting energy expenditure) and lower metabolically active organ mass (explaining 60–70% of resting energy expenditure) (23) with puberty in Black than in white youth may explain the racial difference in resting substrate utilization.
It is also plausible that our previously observed lower cardiorespiratory fitness and higher inactivity levels in Black vs. white normal weight children (24) may contribute to the lower RMR and decreased fat oxidation. It remains to be determined if both the smaller mass of metabolically active organs, lower cardiorespiratory fitness, and lower fat oxidation rates are biologically/genetically driven or environmentally determined. In favor of a potential biological mechanism in the literature on mitochondrial dysfunction and risk of obesity and type 2 diabetes, skeletal muscles of obese or type 2 diabetic individuals are characterized by a smaller skeletal muscle mitochondria size and reduced mitochondrial oxidative capacity (25). It is currently unknown whether racial differences in mitochondrial dysfunction exist. In a small study of young adult Black (n = 9) and white (n = 9) men, we demonstrated race-related variations in skeletal muscle oxidative metabolism, using 31phosphorous nuclear magnetic resonance spectroscopy, which potentially explained the lower peak oxygen consumption in Blacks (26). In this study (26), Black men had lower im pH and higher phosphorous/phosphocreatine during exercise, suggestive of a lower proportion of type I oxidative fibers and a higher proportion of type II glycolytic fibers. These observations are in agreement with Hunter et al. (27) who report a lower muscle oxidative capacity of the calf muscle and lower hemoglobin in Black lean women compared with their white peers. Currently, it remains to be determined if similar Black vs. white differences in skeletal muscle oxidative metabolism are present in childhood.
Limitations of this study warrant mention. Our study is a cross-sectional observation. Clearly, there is a need for longitudinal studies to investigate if the observed racial/gender differences in fat oxidation in the present study occur during the physiological transition from prepuberty to puberty. Patient-oriented research in pediatrics, especially in healthy normal weight children, with repeated measures over time, has several significant barriers making such investigations more difficult than in any adult population.
In summary, we observed that RMR and postabsorptive fat oxidation are substantially lower in Black vs. white girls, and this racial difference is more pronounced in the pubertal group. Such a metabolic phenotype could explain the racial divergence in adiposity in girls during adolescence. The underlying mechanism(s) for these observed racial differences is yet unclear and warrants further investigation.
Acknowledgments
We thank the study participants and their parents, the previous pediatric endocrine fellows who contributed to this research during their training, and the Pediatric Clinical and Translational Research Center staff for their invaluable assistance.
Footnotes
This research was funded by Grants R01-HD-27503, K24-HD-01357, and UL1 RR024153 CTSA (previously M01-RR-00084) (to S.A.A.). S.L. is supported by a Junior Faculty Award from the American Diabetes Association.
Disclosure Statement: The authors have nothing to declare.
First Published Online September 9, 2008
Abbreviations: BMI, Body mass index; FFM, fat free mass; FM, fat mass; NS, not significant; RMR, resting metabolic rate.
References
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM 2006 Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 295:1549–1555 [DOI] [PubMed] [Google Scholar]
- Ogden CL, Flegal KM, Carroll MD, Johnson CL 2002 Prevalence and trends in overweight among US children and adolescents, 1999–2000. JAMA 288:1728–1732 [DOI] [PubMed] [Google Scholar]
- Kimm SY, Barton BA, Obarzanek E, McMahon RP, Sabry ZI, Waclawiw MA, Schreiber GB, Morrison JA, Similo S, Daniels SR 2001 Racial divergence in adiposity during adolescence: the NHLBI Growth and Health Study. Pediatrics 107:E34 [DOI] [PubMed] [Google Scholar]
- Troiano RP, Briefel RR, Carroll MD, Bialostosky K 2000 Energy and fat intakes of children and adolescents in the united states: data from the national health and nutrition examination surveys. Am J Clin Nutr 72(Suppl):1343S–1353S [DOI] [PubMed] [Google Scholar]
- Kimm SYS, Glynn NW, Kriska AM, Barton BA, Kronsberg SS, Daniels SR, Crawford PB, Sabry ZI, Liu K 2002 Decline in physical activity in black girls and white girls during adolescence. N Engl J Med 347:709–715 [DOI] [PubMed] [Google Scholar]
- Eisenmann JC, Bartee RT, Wang MQ 2002 Physical activity, TV viewing, and weight in U.S. youth: 1999 Youth Risk Behavior Survey. Obes Res 10:379–385 [DOI] [PubMed] [Google Scholar]
- Burke GL, Bild DE, Hilner JE, Folsom AR, Wagenknecht LE, Sidney S 1996 Differences in weight gain in relation to race, gender, age and education in young adults: the CARDIA Study. Coronary Artery Risk Development in Young Adults. Ethn Health 1:327–335 [DOI] [PubMed] [Google Scholar]
- Zurlo F, Lillioja S, Esposito-Del Puente A, Nyomba BL, Raz I, Saad MF, Swinburn BA, Knowler WC, Bogardus C, Ravussin E 1990 Low ratio of fat to carbohydrate oxidation as predictor of weight gain: study of 24-h RQ. Am J Physiol 259(5 Pt 1):E650–E657 [DOI] [PubMed] [Google Scholar]
- Hickner RC, Privette J, McIver K, Barakat H 2001 Fatty acid oxidation in African-American and Caucasian women during physical activity. J Appl Physiol 90:2319–2324 [DOI] [PubMed] [Google Scholar]
- Arslanian SA, Kalhan SC 1994 Correlations between fatty acid and glucose metabolism. Potential explanation of insulin resistance of puberty. Diabetes 43:908–914 [DOI] [PubMed] [Google Scholar]
- Arslanian S, Suprasongsin C 1997 Glucose-fatty acid interactions in prepubertal and pubertal children: effects of lipid infusion. Am J Physiol 272(4 Pt 1):E523–E529 [DOI] [PubMed] [Google Scholar]
- Hannon TS, Janosky J, Arslanian SA 2006 Longitudinal study of physiologic insulin resistance and metabolic changes of puberty. Pediatr Res 60:759–763 [DOI] [PubMed] [Google Scholar]
- Lee S, Gungor N, Bacha F, Arslanian S 2007 Insulin resistance: link to the components of the metabolic syndrome and biomarkers of endothelial dysfunction in youth. Diabetes Care 30:2091–2097 [DOI] [PubMed] [Google Scholar]
- Lee S, Bacha F, Gungor N, Arslanian SA 2006 Cardiorespiratory fitness in youth: relationship to insulin sensitivity and β-cell function. Obesity (Silver Spring) 14:1579–1585 [DOI] [PubMed] [Google Scholar]
- Arslanian SA, Saad R, Lewy V, Danadian K, Janosky J 2002 Hyperinsulinemia in African-American children: decreased insulin clearance and increased insulin secretion and its relationship to insulin sensitivity. Diabetes 51:3014–3019 [DOI] [PubMed] [Google Scholar]
- Foster GD, Wadden TA, Vogt RA 1997 Resting energy expenditure in obese African American and Caucasian women. Obes Res 5:1–8 [DOI] [PubMed] [Google Scholar]
- Sharp TA, Bell ML, Grunwald GK, Schmitz KH, Sidney S, Lewis CE, Tolan K, Hill JO 2002 Differences in resting metabolic rate between white and African-American young adults. Obes Res 10:726–732 [DOI] [PubMed] [Google Scholar]
- Morrison JA, Alfaro MP, Khoury P, Thornton BB, Daniels SR 1996 Determinants of resting energy expenditure in young black girls and young white girls. J Pediatr 129:637–642 [DOI] [PubMed] [Google Scholar]
- Wong WW, Butte NF, Ellis KJ, Hergenroeder AC, Hill RB, Stuff JE, Smith EO 1999 Pubertal African-American girls expend less energy at rest and during physical activity than Caucasian girls. J Clin Endocrinol Metab 84:906–911 [DOI] [PubMed] [Google Scholar]
- Yanovski JA 2001 Resting energy expenditure in African American and white children. Am J Clin Nutr 73:149–150 [DOI] [PubMed] [Google Scholar]
- Schutz Y 1995 Abnormalities of fuel utilization as predisposing to the development of obesity in humans. Obes Res 3(Suppl 2):173S–178S [DOI] [PubMed] [Google Scholar]
- Gallagher D, Albu J, He Q, Heshka S, Boxt L, Krasnow N, Elia M 2006 Small organs with a high metabolic rate explain lower resting energy expenditure in African American than in white adults. Am J Clin Nutr 83:1062–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher D, Belmonte D, Deurenberg P, Wang Z, Krasnow N, Pi-Sunyer FX, Heymsfield SB 1998 Organ-tissue mass measurement allows modeling of REE and metabolically active tissue mass. Am J Physiol 275(2 Pt 1):E249–E258 [DOI] [PubMed] [Google Scholar]
- Andreacci JL, Robertson RJ, Dube JJ, Aaron DJ, Balasekaran G, Arslanian SA 2004 Comparison of maximal oxygen consumption between black and white prepubertal and pubertal children. Pediatr Res 56:706–713 [DOI] [PubMed] [Google Scholar]
- Kelley DE, He J, Menshikova EV, Ritov VB 2002 Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 51:2944–2950 [DOI] [PubMed] [Google Scholar]
- Suminski RR, Robertson RJ, Goss FL, Arslanian S 2000 Peak oxygen consumption and skeletal muscle bioenergetics in African-American and Caucasian men. Med Sci Sports Exerc 32:2059–2066 [DOI] [PubMed] [Google Scholar]
- Hunter GR, Weinsier RL, McCarthy JP, Enette Larson-Meyer D, Newcomer BR 2001 Hemoglobin, muscle oxidative capacity, and VO2max in African-American and Caucasian women. Med Sci Sports Exerc 33:1739–1743 [DOI] [PubMed] [Google Scholar]