Abstract
Context: Because androgens are obligatory precursors of estrogens, it is reasonable to assume that their serum concentrations would exhibit positive correlations. If so, then epidemiologic studies that examine the association between androgens and pathological processes should adjust the results for the independent effect of estrogens.
Objective: The objective of the study was to examine the interrelationships among testosterone (T), androstenedione, estradiol (E2), estrone, and SHBG in postmenopausal women.
Design: This was a cross-sectional study of women participating in the National Heart, Blood, and Lung Institute-sponsored Women’s Ischemia Syndrome Evaluation study.
Setting: The study was conducted at four academic medical centers.
Patients: A total of 284 postmenopausal women with chest pain symptoms or suspected myocardial ischemia.
Main Outcome Measures: Post hoc analysis of the relationships among sex steroid hormones with insulin resistance, body mass index (BMI), and presence or absence of coronary artery disease as determined by coronary angiography.
Results: BMI was significantly associated with insulin resistance, total E2, free E2, bioavailable E2, and free T. Highly significant correlations were found for total T, free T, and androstenedione with total E2, free E2, bioavailable E2, and estrone and persisted after adjustment for BMI and insulin resistance. A significant relationship was present between total and free T and the presence of coronary artery disease after adjustment for the effect of E2.
Conclusions: Serum levels of androgens and estrogens track closely in postmenopausal women referred for coronary angiography for suspected myocardial ischemia. Epidemiological studies that relate sex steroid hormones to physiological or pathological processes need to control for the independent effect of both estrogens and androgens.
Epidemiologic studies that relate sex steroid hormones to pathological processes should control for the independent effect of both estrogens and androgens as they track together.
Several epidemiologic studies have examined the relationship between serum testosterone (T) levels in women and the presence of coronary artery disease (CAD), breast cancer, and uterine cancer (reviewed in Ref. 1). In some of these studies, an increase in odds ratio for disease occurrence was found with increasing quartiles of serum T. However, not all of the studies adjusted the correlations for the independent effect of estrogens and other factors, such as age and insulin resistance. Because T is a prohormone for estradiol (E2) production and androstenedione (A) for estrone (E1) production, it is reasonable to expect that the serum levels of the androgens will correlate with those of estrogen. If this hypothesis is correct, then epidemiological studies of the relationship between endogenous androgens and pathological conditions should be adjusted for the independent effect of estrogen.
Indeed, a number of prior studies have examined the relationship between androgens and estrogens and found correlation coefficients in the range of 0.1–0.5 (2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21). Unfortunately, the T assays used in many of these studies were not optimized for the levels found in women, which are one 10th to one 20th of those found in men and are even lower in postmenopausal women (22). To examine these relationships, we evaluated in a cross-sectional manner androgens and estrogens measured with well-validated assays in sera from postmenopausal women not using exogenous hormones who were participating in the National Heart, Blood, and Lung Institute-sponsored Women’s Ischemia Syndrome Evaluation (WISE) a prospective, observational cohort study of women undergoing coronary angiography for suspected ischemic heart disease.
Studies have shown that body mass index (BMI) is positively associated with E1, E2, free E2, and free T and negatively associated with SHBG in postmenopausal women (23). Also, insulin resistance has been shown to lower SHBG concentrations, which would increase free testosterone levels (24), and insulin stimulates ovarian aromatase activity (25,26). Therefore, for each association examined in our study, correlations were performed unadjusted and after adjustments for BMI and insulin resistance.
Patients and Methods
Patients
The WISE was a four-center study whose purpose was to improve the diagnostic precision and pathophysiological understanding of ischemic heart disease for women. Women with chest pain symptoms or suspected ischemia underwent a full history, clinical and biochemical evaluation, and coronary angiography. CAD was defined as 50% or greater stenosis in one or more coronary arteries. Menopausal status was determined by a previously published reproductive status algorithm developed by the WISE investigators (27). Of the 936 women entered into the study, 284 satisfied the criteria of being postmenopausal and not taking any estrogens, androgens, or glucocorticoids and having sufficient data for analysis. The protocol was approved by the institutional review boards at each of the centers.
Hormone and SHBG assays
T, A, E2, bioavailable E2, E1, and SHBG were measured by validated immunoassays that were optimized for measurements in women. Specific details are described below.
Testosterone
Serum was extracted with hexane-ethyl acetate (3:2) and purified by Celite column partition chromatography before RIA, using ethylene glycol as the stationary phase. Elution off the column was carried out with 40% toluene in isooctane. Sensitivity is 1.5 ng/dl. The intraassay coefficient of variation (CV) is 7.0% at 14.3 ng/dl and interassay CV is 10.4% at 6.1 ng/dl (28). Normal postmenopausal range is 5–50 ng/dl. (To convert nanograms per deciliter to picomoles per liter, multiply by 34.67).
Androstenedione
Serum was extracted as described for T (above) but eluted off the column with isooctane without toluene. Sensitivity is 30 pg/ml. Intraassay CV is 6% at 400 pg/ml and interassay CV is 7.8% at 130 pg/ml (29). Normal postmenopausal range is 160-1200 pg/ml. (To convert nanograms per deciliter to picomoles per liter, multiply by 3.492).
Estradiol
Serum was extracted as described above and subjected to Celite column chromatography with elution by 40% ethyl acetate in isooctane before RIA. Sensitivity is 4 pg/ml. Intraassay CV is 8.9% at 14 pg/ml and interassay CV is 14% at 14 pg/ml (30). Normal postmenopausal levels are less than 25 pg/ml. (To convert picograms per milliliter to picomoles per liter, multiply by 3.67).
Bioavailable E2 (non-SHBG bound)
Bioavailable E2 (non-SHBG bound) was determined by a modified ammonium-sulfate precipitation method with a sensitivity of 1.5 pg/ml, intraassay CV of 6.1%, and interassay CV of 7.9% (31). Normal postmenopausal range is 0.19–14 pg/ml (28). (To convert picograms per milliliter to picomoles per liter, multiply by 3.67).
Estrone
Serum was extracted as described for E2 but eluted off of the column with 15% ethyl acetate in isooctane, with a sensitivity of 5 pg/ml, intraassay CV of 7.9% at 26 pg/ml and interassay CV of 12% at 26 pg/ml (30). Normal postmenopausal range is 15–60 pg/ml (32). (To convert picograms per milliliter to picomoles per liter, multiply by 3.7).
SHBG
Solid-phase, two-site chemiluminescent immunoassay using the Immulite analyzer (Siemens Diagnostic Products Corp., Los Angeles, CA) with a sensitivity of 0.2 nmol/liter, intraassay and interassay CVs of 4.1–7.7 and 5.8–13%, respectively was used. Normal range is 20–100 nmol/liter. (To convert nanomoles per liter to micrograms per deciliter, divide by 34.67).
Calculated free T and free E2
Free (nonprotein bound) T and E2 as well as bioavailable E2 were calculated using a validated algorithm, based on equations derived by Sodergard et al. (33) and Vermeulen et al. (34). The algorithm uses the measured concentration of total T or E2 and SHBG, an assumed average concentration of albumin as well as the appropriate affinity constants of SHBG and albumin for T or E2. This method has been shown to have high validity (35). Normal postmenopausal range for free T is 0.6–6.7 pg/ml and for free E2 is 0.06–0.75 pg/ml. Using the regression equation derived from the data in this paper [calculated bioavailable E2 = 1.08 + (measured bioavailable E2)], the normal range for calculated bioavailable E2 is 1.26–14.5 pg/ml. The correlation coefficient between the calculated and measured bioavailable E2 was 0.93, and therefore, only the measured bioavailable E2 are reported for this study.
Statistical analysis
Because the distributions of the hormones were skewed, Spearman correlations were performed to examine the relationships between androgens, estrogens, SHBG, and BMI. Using Bonferroni adjustment for multiple testing, P = 0.002 was considered statistically significant for the Spearman correlations. Because of missing insulin or glucose data in 81 women, insulin resistance was defined in one of three ways: 1) homeostasis model assessment (HOMA) [fasting plasma glucose (mmol/liter) times fasting plasma insulin (microunits per liter) divided by 22.5] of 2.6 or greater; 2) insulin 15 μU/ml or greater; or 3) fasting blood glucose greater than 110 mg/dl and triglycerides greater than 150 mg/dl.
The relationship between androgen and the presence or absence of CAD was examined with logistic regression, unadjusted and adjusted for E2. P < 0.05 was considered statistically significant. The relationship between insulin resistance and BMI was assessed by the nonparametric Wilcoxon rank sums method. Serum hormone levels between naturally and surgically menopausal women were compared using the Kruskal-Wallis test to derive P values.
Results
The 284 women ranged in age from 40 to 86 yr (mean ± sd, 65 ± 10) (Table 1). There was a high prevalence of CAD risk factors including diabetes, hypertension, dyslipidemia, smoking, family history of CAD, obesity, and insulin resistance, whereas only about one third were using preventive prescription medications. The mean baseline hormone values were within the normal ranges for postmenopausal women that had been established for the assays. Several of the women in the present study had values that exceeded the upper limits of the 95% confidence intervals that had been established in a healthy population of postmenopausal women, possibly reflecting the high average BMI of the present population, the selection of women with possible CAD, and the increased probability of a chance occurrence due to the multiple hormone measurements. The average age of the women with surgical menopause was 60.8 ± 11.4 yr, which was significantly younger than those who underwent natural menopause (65.5 ± 9.3 yr) (P = 0.009). The surgically menopausal women had somewhat lower total testosterone levels than the naturally menopausal women (22.1 ± 10.7 vs. 27.0 ± 16.8 ng/dl), which approached statistical significance (P = 0.08). No differences were found for any other hormone, including dehydroepiandrosterone sulfate (data not shown).
Table 1.
Study population characteristics (n = 284)
| Characteristic | Mean ± sd or % | Range |
|---|---|---|
| Age | 64.8 ± 9.9 | 39.9–86.1 |
| White race (%) | 81 | |
| High school graduate (%) | 73 | |
| CAD (%) | 48 | |
| Self-reported CAD risk factors | ||
| Diabetes mellitus (%) | 30 | |
| Hx hypertension (%) | 61 | |
| Hx dyslipidemia (%) | 61 | |
| Current smoker (%) | 18 | |
| Ever smoked (%) | 53 | |
| Family hx of CAD (%) | 64 | |
| Clinical and laboratory measures | ||
| BMI | 30.0 ± 6.7 | 17.0–57.2 |
| Waist to height ratio | 0.87 ± 0.12 | 0.68–1.62 |
| Systolic blood pressure (mm Hg) | 140 ± 21 | 82–221 |
| Diastolic blood pressure (mm Hg) | 76 ± 10 | 50–104 |
| Total cholesterol (mg/dl) | 196 ± 47 | 82–404 |
| HDL (mg/dl) | 52 ± 11 | 29–97 |
| LDL (mg/dl) | 115 ± 41 | 11–300 |
| Triglycerides (mg/dl) | 149 ± 85 | 29–482 |
| Fasting glucose (mg/dl) | 122 ± 61 | 63–427 |
| Hemoglobin (g/dl) | 12.9 ± 1.4 | 7.7–16.1 |
| Creatinine (mg/dl) | 0.9 ± 0.3 | 0.4–2.4 |
| C-reactive protein (mg/liter) | 8.0 ± 16.4 | 0.2–170 |
| Insulin (μU/ml) | 13.8 ± 15.9 | 1.5–108.0 |
| HOMA | 5.0 ± 8.2 | 0.3–61.1 |
| High insulin resistance (%) | 41 | |
| Current medication use | ||
| Aspirin (%) | 69 | |
| Statins (%) | 31 | |
| ACE inhibitors (%) | 29 | |
| β-Blockers (%) | 40 | |
| Hysterectomy (%) | 51 | |
| Bilateral oophorectomy (%) | 27 | |
| Past use of HRT (%) | 28 | |
| Past use of OC (%) | 29 | |
| Hormone values | ||
| Estradiol (pg/ml) | 12.9 ± 8.1 | 2.0–65.0 |
| Free estradiol (pg/ml) | 0.35 ± 0.22 | 0.5–1.66 |
| Bioavailable estradiol (pg/ml) | 8.0 ± 5.6 | 0.5–42.0 |
| Estrone (pg/ml) | 48 ± 33.9 | 3.0–244.0 |
| Testosterone (ng/dl) | 26.2 ± 16.2 | 4.7–139.1 |
| Free testosterone (pg/ml) | 4.5 ± 3.0 | 0–21.4 |
| Androstenedione (pg/ml) | 573 ± 313 | 87–1886 |
| SHBG (nmol/liter) | 41.3 ± 24.1 | 5.2–211.0 |
CAD was 50% or greater stenosis in one or more epicardial vessels; HOMA score was as follows: [fasting plasma glucose (millimoles per liter)(fasting plasma insulin (microunits per milliliter)/22.5], available for only 200 women; high insulin resistance was as follows: HOMA 2.6 or greater or (fasting plasma glucose >110 and triglycerides >150) or insulin greater than 15. Hx, History of; HDL, high-density lipoproteins; LDL, low-density lipoproteins calculated by Friedewald formula (not calculated if triglycerides ≥150 mg/dl); ACE, angiotensin-converting enzyme; HRT, hormone replacement therapy; OC, oral contraceptives.
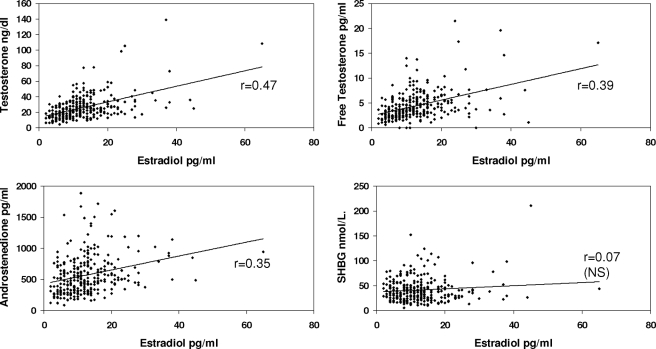
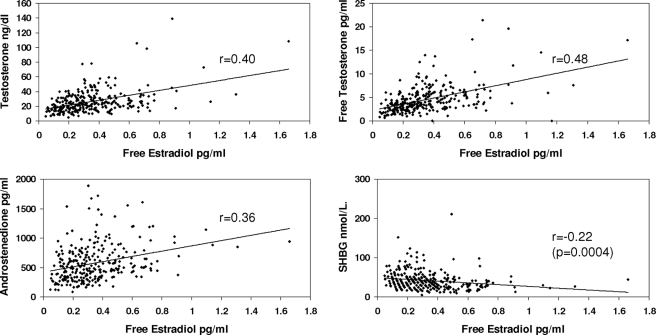
BMI was significantly associated with insulin resistance (P < 0.0001), total E2 (r = 0.29, P < 0.0001), free E2 (r = 0.34, P < 0.0001), and bioavailable E2 (r = 0.37, P < 0.0001), as well as free T (r = 0.17, P < 0.005) but not total T or A and inversely related to SHBG levels (r = −0.28, P < 0.0001).
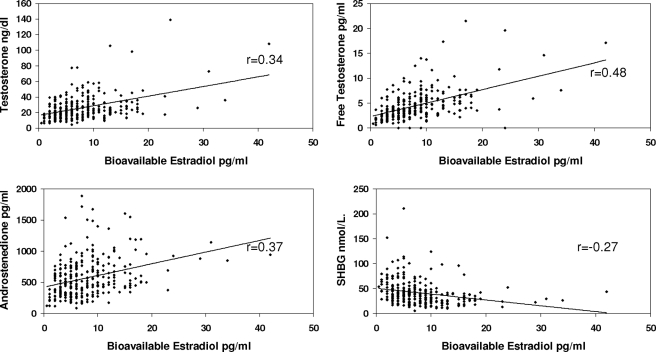
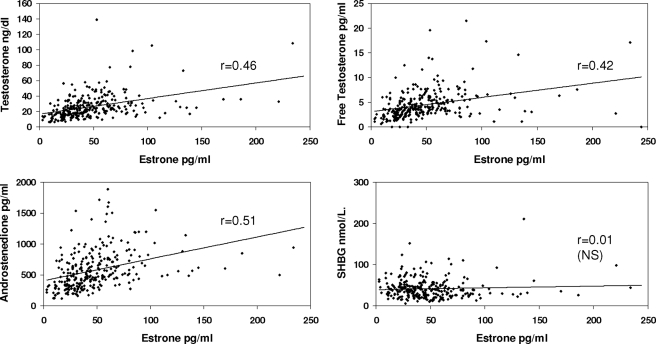
Table 2 provides the correlations between the various hormones with each other and with SHBG, adjusted for BMI and insulin resistance. As can be seen in the table and Figs. 1–4, correlations between T, free T, and A, with E2, free E2, Bio E2 and E1, were highly significant. Not unexpectedly, the strongest pairs of correlations were between E2 and T, and between E1 and A, reflecting the precursor-product relationship of these steroids. Restricting the analysis to the subgroup of women without insulin resistance slightly improved the degree of correlation between the estrogens and androgens (Table 3). Adjusting, in addition, for age and race did not alter the correlations.
Table 2.
Hormone correlations adjusted for insulin resistance and BMI
| Testosterone (n = 266) | Free testosterone (n = 270) | Androstenedione (n = 266) | SHBG (n = 266) | |
|---|---|---|---|---|
| Estradiol | 0.47 | 0.39 | 0.35 | 0.07 (NS) |
| Free estradiol | 0.40 | 0.48 | 0.36 | −0.22 (<0.0004) |
| Bioestradiol | 0.34 | 0.48 | 0.37 | −0.31 |
| Estrone | 0.46 | 0.42 | 0.51 | 0.01 (NS) |
Unless otherwise stated, all correlations (r statistic) are significant at less than 0.0001. NS, Nonsignificant (i.e. P > 0.002, considering the Bonferonni adjustment for multiple analyses).
Figure 1.
Correlations between serum levels of total E2 and T, free T, A, and SHBG concentrations. Correlations have been adjusted for insulin resistance and BMI. NS, Nonsignificant.
Figure 2.
Correlations between serum levels of free E2 and T, free T, A, and SHBG concentrations. Correlations have been adjusted for insulin resistance and BMI.
Figure 3.
Correlations between serum levels of bioavailable E2 and T, free T, A, and SHBG concentrations. Correlations have been adjusted for insulin resistance and BMI.
Figure 4.
Correlations between serum levels of E1 and T, free T, A, and SHBG concentrations. Correlations have been adjusted for insulin resistance and BMI. NS, Nonsignificant.
Table 3.
Hormone correlations in subgroup of women without insulin resistance
| Testosterone (n = 162) | Free testosterone (n = 164) | Androstenedione (n = 162) | SHBG (n = 162) | |
|---|---|---|---|---|
| Estradiol | 0.50 | 0.50 | 0.41 | −0.05 (NS) |
| Free estradiol | 0.43 | 0.55 | 0.39 | −0.20 (NS) |
| Bioestradiol | 0.38 | 0.58 | 0.39 | −0.40 |
| Estrone | 0.49 | 0.54 | 0.54 | −0.14 (NS) |
Unless otherwise stated, all correlations (r statistic) are significant at less than 0.0001. NS, Nonsignificant (i.e. P > 0.002, considering the Bonferonni adjustment for multiple analyses).
The relationships between the androgens and SHBG and the presence or absence of CAD, both unadjusted and adjusted for the various moieties of E2, are shown in Table 4. When unadjusted, there was no significant relationship between the androgens or SHBG and CAD. However, when adjusted for total E2, a weak, but statistically significant relationship between total and free T and the presence of CAD was found. Neither A nor SHBG levels were significantly associated with CAD, either with or without adjustment for E2. Similar results were found for adjustment for free E2 or bioavailable E2. Thus, adjustment for E2 in our cohort improved the relationship between T and free T and CAD.
Table 4.
Risk of CAD (yes/no) associated with androgens and SHBG using logistic regression, unadjusted and adjusted for E2, free E2, or bioavailable E2 (odds ratios, 95% confidence intervals, P values)
| Unadjusted | Adjusted for
|
|||
|---|---|---|---|---|
| Total E2 | Free E2 | Bioavailable E2 | ||
| Testosterone | 1.01 (0.999, 1.03) P = 0.07 | 1.02 (1.01, 1.04) P = 0.009 | 1.03 (1.00, 1.04) P = 0.022 | 1.02 (1.002, 1.04) P = 0.025 |
| Free testosterone | 1.08 (0.998, 1.18) P = 0.055 | 1.13 (1.03, 1.24) P = 0.012 | 1.12 (1.01, 1.23) P = 0.027 | 1.13 (1.20, 1.25) P = 0.016 |
| Androstenedione | 1.0 (0.999, 1.001) NS | 1.00 (1.00, 1.001) NS | 1.00 (1.00, 1.001) NS | 1.00 (1.00, 1.001) NS |
| SHBG | 0.99 (0.98, 1.004) NS | 0.99 (0.98, 1.004) NS | 0.99 (0.98, 1.003) NS | 0.99 (0.98, 1.003) NS |
NS, Nonsignificant.
The estrogen-adjusted models in Table 4 were evaluated for linear relationships among predictors in the model (collinearity) that have the potential for rendering significance testing unreliable. Using standard diagnostic techniques (36), these models were found to be highly reliable (tolerance = 0.75–0.99; condition index = 1.01–1.33), suggesting no collinearity between the androgens and estrogens.
Discussion
Our study demonstrates that the serum concentrations of T and A are highly correlated with the concentrations of E2 and E1, respectively, both in women with and without insulin resistance and independent of any modulating effect of BMI on hormone levels. Although our correlations are among the highest reported, several other investigators also have demonstrated significant relationships between E2 and/or E1 and T (2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21). A strength of our study was the use of androgen and estrogen assays that were optimized for the low levels found in both premenopausal and postmenopausal women, an advantage over some of the direct immunoassays used in some of the studies (4,5,7,8,12,14,15,17,20,22). Together these studies indicate that the serum levels of androgens and estrogens track together, not surprising in view of the fact that androgens are obligatory precursors of estrogens. These findings have important implications for interpreting the epidemiological and pathophysiological studies that have found associations between serum androgen levels and the risk of endometrial cancer, breast cancer, and cardiovascular disease in women because failure to adjust the association for the effect of estrogens may lead to a spurious conclusion.
For example, in a nested case-control study of the association of endometrial cancer risk with quartile of serum estrogen or androgen levels, Lukanova et al. (14) found that circulating T and A levels were directly related to endometrial cancer, although not as strongly as the estrogen concentrations. However, when they adjusted the T and A models for E2 or E1 concentrations, there was a loss of statistical significance.
Similar findings have been reported for some studies that examined the relationship between androgen levels and breast cancer. Various investigators demonstrated a significant association between increasing serum T levels and breast cancer risk (5,7,8,9,12,17,37,38,39,40,41), although not all agree (9,19,20,42,43,44,45). Some of the studies demonstrating an association have not examined whether the association persisted after the independent effects of estrogens were statistically accounted for through multivariate analysis (17,37,41). In many of the studies carrying out multivariate analysis and adjusting for the independent effect of E2 or E1, the apparent trend of increasing breast cancer risk with increasing T levels lost statistical significance (7,8,9,12,13,38); in others it remained, although frequently with a reduction in the calculated odds ratio (5,39,40).
Although less well studied, the epidemiological relationship between androgen levels and CAD disease or risk factors in women has been fraught with the same issues. Some studies did not find an association (46,47,48), whereas several others found a significant inverse association with CAD, carotid intimal thickness, or biochemical cardiovascular risk factors (49,50,51,52,53). A positive association between T, free T index, and free E2 index and CAD risk factors was found by Lambrinoudaki et al. (54) after adjusting for age, BMI, cigarette smoking, alcohol intake, and exercise. However, they did not examine the independent effect of T levels after adjusting for the effects of E2. Phillips et al. (55) examined serum sex steroid hormone levels in 60 postmenopausal women undergoing coronary angiography. They found that free T was significantly related to CAD and that this relationship remained after E2 was incorporated into the multivariate analysis. In the WISE study, we found that the odds ratio for T and free T levels with the presence of CAD was nonsignificant before adjustment for E2 or bioavailable E2 but did achieve statistical significance with a slight increase in odds ratio when such adjustments were made. Thus, at present, there are too many discrepancies between the different studies and inadequate control for the independent effect of estrogen on CAD in most of the studies to draw a conclusion about the effect of T on CAD risk from the epidemiological investigations.
One potential problem with the current study is that the results were obtained in a highly selected group of women undergoing coronary angiography for suspected ischemia and who had a high CAD risk factor burden, raising the possibility that these findings may not be relevant to broader groups of women. Nevertheless, the associations found with many of the studies that we cited provide some reassurance that the concepts are generalizable.
In summary, our results and those of others demonstrate that serum levels of androgens and estrogens track closely in postmenopausal women undergoing coronary angiography for suspected myocardial ischemia. Therefore, epidemiological studies that attempt to examine the relationship between sex steroid levels, and physical or pathological parameters, need to control for the independent effect of both estrogens and androgens.
Footnotes
This work was supported by Contracts N01-HV-68161, N01-HV-68162, N01-HV-68163, and N01-HV-68164 and Grants U01644829, U01 HL649141, and U01 HL649241 from the National Heart, Lung, and Blood Institutes; a General Clinical Research Center Grant M01-RR00425 from the National Center for Research Resources; and grants from the Gustavus and Luis Pfeiffer Research Foundation (Denville, NJ), The Women’s Guild of Cedars-Sinai Medical Center (Los Angeles, CA), The Ladies Hospital Aid Society of Western Pennsylvania (Pittsburgh, PA), QMED, Inc. (Laurence Harbor, NJ), and The Edythe L. Broad Endowment, Cedars-Sinai Medical Center (Los Angeles, CA).
Disclosure Summary: None of the authors have any potential conflicts of interests with entities directly related to the material being published in this manuscript.
First Published Online August 26, 2008
Abbreviations: A, Androstenedione; BMI, body mass index; CAD, coronary artery disease; CV, coefficient of variation; E1, estrone; E2, estradiol; HOMA, homeostasis model assessment; T, testosterone; WISE, Women’s Ischemia Syndrome Evaluation.
References
- Braunstein GD 2007 Safety of testosterone treatment in postmenopausal women. Fertil Steril 88:1–17 [DOI] [PubMed] [Google Scholar]
- Rannevik G, Jeppsson S, Johnell O, Bjerre B, Laurell-Borulf Y, Svanberg L 1995 A longitudinal study of the perimenopausal transition: altered profiles of steroid and pituitary hormones, SHBG and bone mineral density. Maturitas 21:103–113 [DOI] [PubMed] [Google Scholar]
- Burger HG, Dudley EC, Hopper JL, Shelley JM, Green A, Smith A, Dennerstein L, Morse C 1995 The endocrinology of the menopausal transition: a cross-sectional study of a population-based sample. J Clin Endocrinol Metab 80:3537–3545 [DOI] [PubMed] [Google Scholar]
- Bancroft J, Cawood EHH 1996 Androgens and the menopause: a study of 40–60 year-old women. Clin Endocrinol (Oxf) 45:577–587 [DOI] [PubMed] [Google Scholar]
- Berrino F, Muti P, Micheli A, Bolelli G, Krogh V, Sciajno R, Pisani P, Banico S, Secreto G 1996 Serum sex hormone levels after menopause and subsequent breast cancer. J Natl Cancer Inst 88:291–296 [DOI] [PubMed] [Google Scholar]
- Slemenda C, Longcope C, Peacock M, Hui S, Johnston CC 1996 Sex steroids, bone mass, and bone loss. A prospective study of pre-, peri-, and postmenopausal women. J Clin Invest 97:14–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas HV, Key TJ, Allen DS, Moore JW, Dowsett M, Fentiman IS, Wang DY 1997 A prospective study of endogenous serum hormone concentrations and breast cancer risk in postmenopausal women on the island of Guernsey. Br J Cancer 76:401–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeleniuch-Jacquotte A, Bruning PF, Bonfrer JM, Koenig KL, Shore RE, Kim MY, Pasternack BS, Toniolo P 1997 Relation of serum levels of testosterone and dehydroepiandrosterone sulfate to risk of breast cancer in postmenopausal women. Am J Epidemiol 145:1030–1038 [DOI] [PubMed] [Google Scholar]
- Hankinson SE, Willett WC, Manson JE, Colditz GA, Hunter DJ, Spiegelman D, Barbieri RL, Speizer FE 1998 Plasma sex steroid hormone levels and risk of breast cancer in postmenopausal women. J Natl Cancer Inst 90:1292–1299 [DOI] [PubMed] [Google Scholar]
- The Endogenous Hormones and Breast Cancer Collaborative Group 2002 Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst 94:606–616 [DOI] [PubMed] [Google Scholar]
- Kalish GM, Barrett-Connor E, Laughlin GA, Gulanski BI 2003 Association of endogenous sex hormones and insulin resistance among postmenopausal women: results from the Postmenopausal Estrogen/Progestin Intervention Trial. J Clin Endocrinol Metab 88:1646–1652 [DOI] [PubMed] [Google Scholar]
- Zeleniuch-Jacquotte A, Shore RE, Koenig KL, Akhmedkhanov A, Afanasyeva Y, Kato I, Kim MY, Rinaldi S, Kaaks R, Toniolo P 2004 Postmenopausal levels of oestrogen, androgen, and SHBG and breast cancer: long-term results of a prospective study. Br J Cancer 90:153–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missmer SA, Eliassen H, Barbieri RL, Hankinson SE 2004 Endogenous estrogen, androgen, and progesterone concentrations and breast cancer risk among postmenopausal women. J Natl Cancer Inst 96:1856–1865 [DOI] [PubMed] [Google Scholar]
- Lukanova A, Lundin E, Micheli A, Arslan A, Ferrari P, Rinaldi S, Krogh V, Lenner P, Shore RE, Biessy C, Muti P, Riboli E, Koenig KL, Levitz M, Stattin P, Berrino F, Hallmans G, Kaaks R, Toniolo P, Zeleniuch-Jacquotte A 2004 Circulating levels of sex steroid hormones and risk of endometrial cancer in postmenopausal women. Int J Cancer 108:425–432 [DOI] [PubMed] [Google Scholar]
- Santoro N, Torrens J, Crawford S, Allsworth JE, Finkelstein JS, Gold EB, Korenman S, Lasley WL, Luborsky JL, Mc Connell D, Sowers MF, Weiss G 2005 Correlates of circulating androgens in mid-life women: the Study of Women’s Health Across the Nation. J Clin Endocrinol Metab 90:4836–4845 [DOI] [PubMed] [Google Scholar]
- Schairer C, Hill D, Sturgeon SR, Fears T, Mies C, Ziegler RG, Hoover RN, Sherman ME 2005 Serum concentrations of estrogens, sex hormone binding globulin, and androgens and risk of breast hyperplasia in postmenopausal women. Cancer Epidemiol Biomarkers Prev 14:1660–1665 [DOI] [PubMed] [Google Scholar]
- Kaaks R, Berrino F, Key T, Rinaldi S, Dossus L, Biessy C, Secreto G, Amiano P, Bingham S, Boeing H, Bueno de Mesquita HB, Chang-Claude J, Clavel-Chapelon F, Fournier A, van Gils CH, Gonzalez CA, Gurrea AB, Critselis E, Khaw KT, Krogh V, Lahmann PH, Nagel G, Olsen A, Onland-Moret NC, Overvad K, Palli D, Panico S, Peeters P, Quiros JR, Roddam A, Thiebaut A, Tjonneland A, Chirlaque MD, Trichopoulou A, Trichopoulos D, Tumino R, Vineis P, Norat T, Ferrari P, Slimani N, Riboli E 2005 Serum sex steroids in premenopausal women and breast cancer risk within the European Prospective Investigation into Cancer and Nutrition (EPIC). J Natl Cancer Inst 97:755–765 [DOI] [PubMed] [Google Scholar]
- Greendale GA, Palla SL, Ursin G, Laughlin GA, Crandall C, Pike MC, Reboussin BA 2005 The association of endogenous sex steroids and sex steroid binding proteins with mammographic density: results from the Postmenopausal Estrogen/Progestin Interventions mammographic density study. Am J Epidemiol 162:826–834 [DOI] [PubMed] [Google Scholar]
- Adly L, Hill D, Sherman ME, Sturgeon SR, Fears T, Mies C, Ziegler RG, Hoover RN, Schairer C 2006 Serum concentrations of estrogens, sex hormone-binding globulin, and androgens and risk of breast cancer in postmenopausal women. Int J Cancer 119:2402–2407 [DOI] [PubMed] [Google Scholar]
- Beattie MS, Costantino JP, Cummings SR, Wickerham DL, Vogel VG, Dowsett M, Folkerd EJ, Willett WC, Wolmark N, Hankinson SE 2006 Endogenous sex hormones, breast cancer risk, and tamoxifen response: an ancillary study in the NSABP Breast Cancer Prevention Trial (P-1). J Natl Cancer Inst 98:110–115 [DOI] [PubMed] [Google Scholar]
- Missmer SA, Spiegelman D, Bertone-Johnson ER, Barbieri RL, Pollak MN, Hankinson SE 2006 Reproductibility of plasma steroid hormones, prolactin, and insulin-like growth factor levels among premenopausal women over a 2- to 3-year period. Cancer Epidemiol Biomarkers Prev 15:972–978 [DOI] [PubMed] [Google Scholar]
- Rosner W, Auchus RJ, Azziz R, Sluss PM, Raff H 2007 Position Statement: utility, limitations, and pitfalls in measuring testosterone: an Endocrine Society position statement. J Clin Endocrinol Metab 92:405–413 [DOI] [PubMed] [Google Scholar]
- McTiernan A, Wu L, Chen C, Chlebowski R, Mossavar-Rahmani Y, Modugno F, Perri MG, Stanczyk FZ, Van Horn L, Wang CY 2006 Women’s Health Initiative Investigators. Relation of BMI and physical activity to sex hormones in postmenopausal women. Obesity 14:1662–1677 [DOI] [PubMed] [Google Scholar]
- Akin F, Bastemir M, Alkis E 2007 Effect of insulin sensitivity on SHBG levels in premenopausal versus postmenopausal obese women. Adv Ther 24:1210–1220 [DOI] [PubMed] [Google Scholar]
- Garzo VG, Dorrington JH 1984 Aromatase activity in human granulosa cells during follicular development and the modulation by follicle-stimulating hormone and insulin. Am J Obstet Gynecol 148:657–662 [DOI] [PubMed] [Google Scholar]
- la Marca A, Morgante G, Palumbo M, Cianci A, Petraglia F, De Leo V 2002 Insulin-lowering treatment reduces aromatase activity in response to follicle-stimulating hormone in women with polycystic ovary syndrome. Fertil Steril 78:1234–1239 [DOI] [PubMed] [Google Scholar]
- Johnson BD, Merz CN, Braunstein GD, Berga SL, Bittner V, Hodgson TK, Gierach GL, Reis SE, Vido DA, Sharaf BL, Smith KM, Sopko G, Kelsey SF 2004 Determination of menopausal status in women: the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) study. J Womens Health 13:872–887 [DOI] [PubMed] [Google Scholar]
- Goebelsmann U, Arce JJ, Thorneycroft IH, Mishell Jr DR 1974 Serum testosterone concentrations in women throughout the menstrual cycle and following HCG administration. Am J Obstet Gynecol 119: 445–52 [DOI] [PubMed] [Google Scholar]
- Goebelsmann U, Horton R, Mestman JH, Arce JJ, Nagata Y, Nakamura RM, Thorneycroft IH, Mishell Jr DR 1973 Male pseudohermaphroditism due to testicular 17β-hydroxysteroid dehydrogenase deficiency. J Clin Endocrinol Metab 36:867–879 [DOI] [PubMed] [Google Scholar]
- Anderson DC, Hopper BR, Lasley BL, Yen SS 1976 A simple method for the assay of eight steroids in small volumes of plasma. Steroids 28:179–196 [DOI] [PubMed] [Google Scholar]
- Tremblay RR, Dube JY 1974 Plasma concentrations of free and non-TeBG bound testosterone in women on oral contraceptives. Contraception 10:599–605 [DOI] [PubMed] [Google Scholar]
- Oh JY, Barrett-Connor E, Wedick NM, Wingard DL 2002 Endogenous sex hormones and the development of type 2 diabetes in older men and women: the Rancho Bernardo study. Diabetes Care 25:55–60 [DOI] [PubMed] [Google Scholar]
- Sodergard R, Backstrom T, Shanbhag V, Carstensen H 1982 Calculation of free and bound fractions of testosterone and estradiol-17β to human plasma protein at body temperature. J Steroid Biochem 26:801–810 [DOI] [PubMed] [Google Scholar]
- Vermeulen A, Verdonck L, Kaufman JM 1999 A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 84:3666–3672 [DOI] [PubMed] [Google Scholar]
- Rinaldi S, Geay A, Déchaud H, Biessy C, Zeleniuch-Jacquotte A, Akhmedkhanov A, Shore RE, Riboli E, Toniolo P, Kaaks R 2002 Validity of free testosterone and free estradiol determinations in serum samples from postmenopausal women by theoretical calculations. Cancer Epidemiol Biomark Prev 11:1065–1071 [PubMed] [Google Scholar]
- Belsey A, Kuh E, Welsch RE 1980 Regression diagnostics: identifying influential data and sources of collinearity. New York: John Wiley & Sons [Google Scholar]
- Secreto G, Toniolo P, Berrino F, Recchione C, Cavalleri A, Pisani P, Totis A, Fariselli G, DiPietro S 1991 Serum and urinary androgens and risk of breast cancer in postmenopausal women. Cancer Res 51:2572–2576 [PubMed] [Google Scholar]
- Cauley JA, Lucas FL, Kuller LH, Stone K, Browner W, Cummings SR 1999 Elevated serum estradiol and testosterone concentrations are associated with a high risk for breast cancer. Ann Intern Med 130:270–277 [DOI] [PubMed] [Google Scholar]
- Yu H, Shu XO, Shi R, Dai Q, Jin F, Gao YT, Li BDL, Zheng W 2003 Plasma sex steroid hormones and breast cancer risk in Chinese women. Int J Cancer 105:92–97 [DOI] [PubMed] [Google Scholar]
- Manjer J, Johansson R, Berglund G, Janzon L, Kaaks R, Agren A, Lenner P 2003 Postmenopausal breast cancer risk in relation to sex steroid hormones, prolactin and SHBG (Sweden). Cancer Causes Control 14:599–607 [DOI] [PubMed] [Google Scholar]
- Micheli A, Muti P, Secreto G, Krogh V, Meneghini E, Venturelli E, Sieri S, Pala V, Berrino F 2004 Endogenous sex hormones and subsequent breast cancer in premenopausal women. In J Cancer 112:312–318 [DOI] [PubMed] [Google Scholar]
- Wysowski DK, Comstock GW, Helsing KJ, Lau HL 1987 Sex hormone levels in serum in relation to the development of breast cancer. Am J Epidemiol 125:791–799 [DOI] [PubMed] [Google Scholar]
- Garland CF, Friedlander NJ, Barrett-Conner E, Khaw KT 1992 Sex hormones and postmenopausal breast cancer: a prospective study in an adult community. Am J Epidemiol 135:1220–1230 [DOI] [PubMed] [Google Scholar]
- Sturgeon SR, Potischman N, Malone KE, Dorgan JF, Daling J, Schairer C, Brinton LA 2004 Serum levels of sex hormones and breast cancer risk in premenopausal women: a case-control study (USA). Cancer Causes and Control 15:45–53 [DOI] [PubMed] [Google Scholar]
- Eliassen AH, Missmer SA, Tworoger SS, Spiegelman D, Barbieri RL, Dowsett M, Hankinson SE 2006 Endogenous steroid hormone concentrations and risk of breast cancer among premenopausal women. J Natl Cancer Inst 98:1406–1415 [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, Goodman-Gruen D 1995 Prospective study of endogenous sex hormones and fatal cardiovascular disease in postmenopausal women. BMJ 311:1193–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rexrode KM, Manson JA, Lee IM, Ridker PM, Sluss PM, Cook NR, Buring JE 2003 Sex hormone levels and risk of cardiovascular events in postmenopausal women. Circulation 108:1688–1693 [DOI] [PubMed] [Google Scholar]
- Bell RJ, Davison SL, Papalia MA, McKenzie DP, Davis SR 2007 Endogenous androgen levels and cardiovascular risk profile in women across the adult life span. Menopause 14:630–638 [DOI] [PubMed] [Google Scholar]
- Haffner SM, Moss SE, Klein BEK, Klein R 1996 Sex hormones and DHEA-SO4 in relation to ischemic heart disease mortality in diabetic subjects: the Wisconsin Epidemiologic Study of Diabetic Retinopathy. Diabetes Care 19:1045–1050 [DOI] [PubMed] [Google Scholar]
- Bernini GP, Sgro M, Moretti A, Argenio GF, Barlascini CO, Cristofani R, Salvetti A 1999 Endogenous androgens and carotid intimal-medial thickness in women. J Clin Endocrinol Metab 84:2008–2012 [DOI] [PubMed] [Google Scholar]
- Joffee HV, Ridker PM, Manson JE, Cook NR, Buring JE, Rexrode KM 2006 Sex hormone-binding globulin and serum testosterone are inversely associated with C-reactive protein levels in postmenopausal women at high risk for cardiovascular disease. Ann Epidemiol 16:105–112 [DOI] [PubMed] [Google Scholar]
- Kaczmarek A, Reczuch K, Majda J, Banasiak W, Ponikowski P 2003 The association of lower testosterone level with coronary artery disease in postmenopausal women. Int J Card 87:53–57 [DOI] [PubMed] [Google Scholar]
- Golden SH, Maguire A, Ding J, Crouse JR, Cauley JA, Zacur H, Szklo M 2002 Endogenous postmenopausal hormones and carotid atherosclerosis: a case-control study of the Atherosclerosis Risk in Communities Cohort. Am J Epidemiol 155:437–445 [DOI] [PubMed] [Google Scholar]
- Lambrinoudaki I, Christodoulakos G, Rizos D, Economou E, Argeitis J, Vlachou S, Creatsa M, Kouskouni E, Botsis D 2006 Endogenous sex hormones and risk factors for atherosclerosis in healthy Greek postmenopausal women. Eur J Endocrinol 154:907–916 [DOI] [PubMed] [Google Scholar]
- Phillips GB, Pinkernell BH, Jing TY 1997 Relationship between serum sex hormones and coronary artery disease in postmenopausal women. Arterioscler Thromb Vasc Biol 17:695–701 [DOI] [PubMed] [Google Scholar]