Abstract
Context: Suppression of cortisol secretion with a low-dose dexamethasone (Dex) followed by the administration of ovine CRH (Dex-oCRH) is used in the evaluation of adults with a pseudo-Cushing syndrome state (PCSS) vs. Cushing syndrome (CS).
Objective: The aim of the study was to determine the value of Dex-oCRH testing in the investigation of childhood CS.
Design: We conducted a retrospective analysis of data from children evaluated for CS vs. PCSS from 1998–2006; body mass index Z (BMIZ) and height-for-age Z (HAZ) scores were estimated.
Setting: A clinical research center was the setting for the study.
Main Outcome Measures: The main outcomes were confirmation of the diagnosis of CS by histology and response to Dex-oCRH.
Results: Thirty-two children (ages 3–17 yr) were studied: 11 had CS and 21 had PCSS; of the latter, 11 had a BMIZ score greater than 2. Children with CS had a mean HAZ score of −1.3 ± 0.51 vs. 0.31 ± 0.38 in nonobese and 0.71 ± 0.39 in obese children (P < 0.001). The previously established criterion of a cortisol of 1.4 μg/dl (38 nmol/liter) after Dex-oCRH identified all 10 normal children who were not very obese and those with CS; 5 of 11 normal children with more severe obesity had cortisol values greater than 1.4 μg/dl (38 nmol/liter) after Dex-oCRH, lowering the test specificity to 55%. Without consideration for obesity, an increase of the cutoff cortisol value after Dex-oCRH to 3.2 μg/dl (88 nmol/liter) will have 91% sensitivity and 95% specificity; the corresponding values for a cutoff of 2.2 μg/dl (61 nmol/liter) were 100 and 90.5%, respectively.
Conclusion: Our study showed that height gain is a simple way of distinguishing children with PCCS from those with CS; the interpretation of Dex-oCRH in children is confounded by severe obesity, which limits the utility of this test.
The combined administration of low dose dexamethasone and ovine CRH testing is not as good a test for the differential diagnosis of Cushing’s versus pseudo-Cushing’s syndrome in pediatric patients.
Endogenous Cushing syndrome (CS) is a rare disorder in children, and it is most commonly caused by ACTH-producing pituitary tumors, a disorder also known as “Cushing disease” (CD), or ACTH-independent, cortisol-producing adrenocortical tumors (1,2,3,4,5,6,7,8). CD is the most frequent cause of endogenous CS in all ages, including older children and adolescents (1,2,3,4,5,7). The diagnosis of CS involves the demonstration of excessive production of cortisol and its metabolites in plasma and urine (1,2,3,4,5,6,7,8). Currently available screening tests for the diagnosis of CS include urinary excretion of free cortisol (UFC), late-night salivary cortisol, 1 mg overnight dexamethasone (Dex), and low-dose Dex suppression testing (LDDST), plasma cortisol circadian rhythm, and the combination of LDDST and ovine CRH (oCRH) stimulation (Dex-oCRH) test (1,2,3,4,5,6,7,8,9,10,11,12).
Of all these tests, the Dex-oCRH was reported to be the most useful test for the exclusion of pseudo-Cushing syndrome states (PCSS) in adults (11,12). The concept behind the combined use of LDDST and oCRH is that normal corticotrophs are suppressed by Dex and the administration of oCRH after LDDST will only stimulate abnormal corticotroph cells; by combining two well-known tests (the LDDST and the oCRH stimulation test) the accuracy of the differential diagnosis of CS and PCSS appears to be increased (11,12).
In the original series of the patients studied by Yanovski et al. (12), Dex-oCRH testing was performed prospectively during an inpatient evaluation. The sample included 58 patients. Of those, 35 had CD, two had ectopic ACTH production, two had primary adrenal disease, and 19 had PCSS. The PCSS group was composed mostly of patients with an affective disorder based on the revised 3rd edition of the Psychiatric Diagnostic and Statistical Manual of Mental Disorders. All patients had documented mild hypercortisolemia, and most of them were obese. For this sample, the diagnostic accuracy of the Dex-oCRH stimulation test was 100% in terms of differentiating patients with CS from those with PCSS (11,12). This suggests that the 100% accuracy of the Dex-oCRH as reported by Yanovski et al. (12) may apply to a subset of patients with PCSS, i.e. patients with mostly affective disorders and with documented hypercortisolemia. In addition, the diagnostic accuracy of the Dex-oCRH was 100% when administered to normal volunteers (12).
Recently, studies showed that the performance of the Dex-oCRH stimulation test decreased when the test was administered on an outpatient basis and to those with or without any evidence of hypercortisolemia (13,14,15,16). In addition, the performance of the test decreased when the sample included a diverse range of patients with PCSS, such as those with alcohol dependence or excess, polycystic ovary syndrome (PCOS), depression or depression symptoms and/or anxiety disorders; patients on antidepressant or chronic narcotic pain medication; and adults with obstructive sleep apnea, headaches, binge eating disorder, neurological disorder, and others (13,14,15,16).
Although the Dex-oCRH has been widely used in adult patients, the role of this test in evaluating pediatric patients with possible CS is unknown. We conducted a retrospective study of children referred to us for the investigation of possible CS who underwent Dex-oCRH testing in the same controlled setting as the one used by Yanovski et al. (11,12). The data indicate that Dex-oCRH using the original cutoff values underperforms in an unselected series of pediatric patients as a diagnostic test for PCSS; the main reason for that appears to be pediatric obesity. On the other hand, these patients can safely be diagnosed with PCSS by their characteristically unabated height advancement that is concurrent with their weight gain.
Subjects and Methods
Subjects
Thirty-two patients were admitted consecutively to the National Institutes of Health Clinical Center from January 1998 to August 2006, under investigational protocol 97-CH-0076. Their data were reviewed retrospectively. The National Institute of Child Health & Human Development (NICHD) Institutional Review Board approved these studies for all our patients; informed consent from the patients’ parents (and assent from older children) was obtained.
Pediatric endocrinologists primarily from the United States referred all 32 children to our center because of clinical suspicion of CS that always included weight gain. Additional reasons for referral included at least one abnormal screening test: abnormal 1 mg Dex test or at least one 24-h UFC above the normal range.
Eleven of the 32 children were diagnosed with CS by standard methods (UFC, plasma cortisol circadian rhythm variation, high-dose Dex suppression test, oCRH stimulation test, and bilateral inferior petrosal venous sampling when medically indicated). All 11 children with CS underwent transsphenoidal surgery for identification and excision of a pituitary adenoma. Histopathological examination of the surgical specimen and postoperative biochemical evidence of remission of disease, as evidenced by hypocortisolism, confirmed the diagnosis of all ACTH-secreting adenomas identified in this cohort.
The remaining 21 children did not have the disease after follow-up for up to 8 yr. We classified these children as experiencing a PCSS as defined by the presence of some stigmata of CS and at least one abnormal screening test but lack of consistent hypercortisolemia or progression of the disease, subsequent normalization of any biochemical abnormalities, and generally normal growth and pubertal development. Of these 21 children, 11 were obese, nine were overweight or at risk of becoming overweight, and one had normal weight throughout the observation period. Other morbidity in these children included anxiety (n = 1), depression (n = 1), and PCOS (n = 1).
Interestingly, mild hypercortisolemia was confirmed initially in five of the referred children (three obese children, one overweight, and one with depression). For these five children, median and mean UFC was 83 μg/m2·24 h or 229 nmol/24 h (mean, 83 ± 10 μg/m2·24 h or 229 ± 27.5 nmol/24 h; range, 72.5–96 μg/m2·24 h or 200–265 nmol/24 h; normal, <70 μg/m2·24 h or <193 nmol/24 h). However, none of these children ever developed CS, and subsequently their data normalized in all cases.
Additional testing during the follow-up time in all 21 children included biochemical testing such as measurements of corticotropin in plasma, midnight cortisol levels in serum, and serial UFC. These studies remained normal in all these children, including the five mentioned above; the last visit median UFC was 33 μg/m2·24 h or 91 nmol/24 h (mean, 36 ± 14 μg/m2·24 h or 99 ± 39 nmol/24 h; range, 18–67 μg/m2·24 h or 50–185 nmol/24 h; normal, <70 μg/m2·24 h or <193 nmol/24 h).
To rule out cyclical CS, serial UFC were collected over 3 consecutive months. Only one child out of the 11 children with CS indeed had cyclical CS.
Study protocol
Anthropometric measurements and UFC corrections
Data are presented as height-for-age and sex Z (HAZ) score and body mass index (BMI)-for-age and sex Z (BMIZ) score based on the National Center for Health Statistics (NCHS) data (17,18). BMI was calculated using the common formula (weight in kilograms divided by the square of height in meters). Obesity was defined as a BMIZ score above 2.0. Severe obesity was defined as a BMIZ score above 2.5 (19,20). Twenty-four-hour UFCs were expressed per square meter of body surface area (μg/m2·24 h); all 24-h urine collections were obtained for at least 3 consecutive days (21).
Dex-oCRH test
LDDST was performed and was followed by an oCRH test. Dex was given orally every 6 h for eight doses (the dose was adjusted for body weight, 30 μg/kg, not to exceed 500 μg per dose) (3,4,22). Both urinary (UFC) and serum cortisol values were studied after LDDST. The oCRH test was performed at 0800 h (2 h after the last dose of Dex). Plasma levels for cortisol and ACTH were taken before (−15, −5, and 0 min) and after (+15 min) iv injection of 1 μg/kg of oCRH. A plasma sample for a Dex level was taken before oCRH administration (at −15 min) (11,12).
Hormone assays
Plasma ACTH, UFC, and serum cortisol were measured as previously described (23,24,25). For serum cortisol functional sensitivity and minimum detectable limit [0.8–1.0 μg/dl (22–28 nmol/liter)], the intraassay coefficient of variation was 3%, and the interassay coefficient of variation was 5.2%. Plasma samples were assayed for Dex levels (Endocrine Sciences, Calabasas Hills, CA). The intra- and interassay variability values for the plasma Dex assay were 3.4 and 8.4%, respectively.
Statistical analysis
All data are expressed as mean ± sd for descriptive statistics and mean ± sem for comparing groups. Using previously published criteria, a cortisol value greater than 1.4 μg/dl (38 nmol/liter) pre-oCRH (−15 min) and post-oCRH (+15 min) was used as the cutoff value for the estimation of sensitivity and specificity of the Dex-oCRH (11,12). The 95% confidence intervals (CI95) were determined for sensitivity and specificity values. Friedman’s repeated measures’ ANOVA was used initially within each group to assess any differences in cortisol levels in response to oCRH. Wilcoxon matched-pair test within and the Mann-Whitney U test followed this between groups. Receiver operating characteristic (ROC) curves were constructed as previously described (23,25,26) and were used to assess the utility of each measure for differential diagnosis. To compare interval variables (mean age, mean BMIZ scores, mean HAZ scores, mean UFC, etc.) among study groups, measurements of ANOVA were performed where appropriate; for nominal variables (sex, severe obesity), a nonparametric test was done (2×2 table Fisher exact test statistics). For all statistical comparisons, P < 0.05 was considered significant. Data were analyzed using the STATA 10.0 statistical software (STATA Corp., College Station, TX).
Results
Demographics
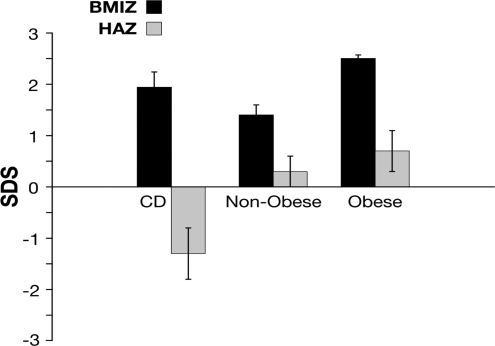
The sample study was divided into three study groups: children with CS (or CD), nonobese PCSS (normal weight and overweight), and obese children with PCSS. There were no differences in the mean age between children with CS and those with PCSS. The median age for children with CS was 12 yr (range, 8–17; mean, 13 ± 3 yr). For the nonobese children, median age was 14 yr (range, 3–16; mean, 13 ± 4 yr); in the obese group, median age was 13 yr (range, 11–16; mean, 13.8 ± 2 yr). HAZ score was key in separating these children. Children with CS mean HAZ score was below the mean at −1.3 ± 0.51 vs. 0.31 ± 0.38 in nonobese and 0.71 ± 0.39 in obese children (Table 1, P < 0.001). In contrast, mean BMIZ score was above the mean for all groups (Fig. 1 and Tables 1 and 2).
Table 1.
Baseline characteristics of our patients
| Variables | CS | Nonobese | Obese |
|---|---|---|---|
| Age (yr) | 13 (3) | 13 (4) | 13.8 (2) |
| Sex (females/males) | 7/4 | 2/8 | 6/5 |
| HAZ score | −1.3 (1.7) | 0.31 (1.2) | 0.7 (1.3) |
| BMIZ score | 1.9 (0.82) | 1.4 (0.43) | 2.5 (0.22) |
| LDDST | |||
| UFC (μg/m2·24 h) | 164 (75) | 20 (7.0) | 24 (7.2) |
| ACTH (pg/ml) | 10 (14) | 4 (1.5) | 4 (2.0) |
| Cortisol (μg/dl) | <1 to 18 | <1 | <1 to 7.3 |
Data represent the mean (sd). To convert UFC (micrograms per 24 h) to Systeme International (SI) units (nanomoles per 24 h), multiply by 2.759; to convert values for corticotropin (picograms per milliliter) to SI units (picomoles per liter), multiply by 0.2202; to convert serum cortisol (micrograms per deciliter) to SI units (nanomoles per liter), multiply by 27.59.
Figure 1.
Mean BMIZ scores and HAZ scores for patients in the study. Overall mean BMIZ score for pediatric patients with CD is usually above the mean, but the mean HAZ score is not. Data are presented as mean ± sem.SDS, sd score.
Table 2.
Results of BMIZ score, UFC, LDDST, Dex-oCRH, and Dex levels in patients of the study
| Patient ID | BMIZ score | UFC | F (−15 min) LDDST | F (+15 min) oCRH | Dex (ng/dl) |
|---|---|---|---|---|---|
| Children with CS | |||||
| 1 | 0.2 | 170 | 3.0 | 13 | 969 |
| 2 | 0.8 | 294 | 4.0 | 10 | 818 |
| 3 | 1.6 | 99 | 8.0 | 7.0 | 316 |
| 4 | 1.8 | 173 | 7.1 | 7.6 | 378 |
| 5 | 2.0 | 203 | <1 | 8.5 | 461 |
| 6 | 2.3 | 87 | 2.5 | 2.3 | 852 |
| 7 | 2.3 | 101 | 9 | 17 | 547 |
| 8 | 2.4 | 175 | 5 | 17 | 870 |
| 9 | 2.5 | 120 | 17 | 26 | 984 |
| 10 | 2.5 | 90 | 3 | 16 | 310 |
| 11 | 3.0 | 291 | 18 | 19 | NA |
| Nonobese children, non-CS | |||||
| 1 | 0.26 | 11 | <1 | <1 | NA |
| 2 | 1.20 | 20 | <1 | <1 | 296 |
| 3 | 1.30 | 33 | <1 | <1 | 394 |
| 4 | 1.34 | 15 | <1 | <1 | 411 |
| 5 | 1.35 | 23 | <1 | <1 | 344 |
| 6 | 1.42 | 22 | <1 | <1 | 67 |
| 7 | 1.50 | 10 | <1 | <1 | 550 |
| 8 | 1.60 | 23 | <1 | <1 | 334 |
| 9 | 1.65 | 26 | <1 | <1 | 410 |
| 10 | 1.90 | 21 | <1 | <1 | 220 |
| Obese children, non-CS | |||||
| 1 | 2.1 | 26 | 7.3 | 21 | 480 |
| 2 | 2.4 | 15 | <1 | <1 | 161 |
| 3 | 2.5 | 30 | <1 | <1 | 475 |
| 4 | 2.5 | 33 | <1 | <1 | 482 |
| 5 | 2.5 | 21 | <1 | <1 | 365 |
| 6 | 2.5 | 23 | <1 | 3.2 | 800 |
| 7 | 2.6 | 14 | 1.1 | 1.5 | 910 |
| 8 | 2.6 | 18 | <1 | 2.2 | 440 |
| 9 | 2.6 | 31 | 1.2 | 2.2 | 746 |
| 10 | 2.7 | 20 | 1.1 | <1 | 275 |
| 11 | 3.0 | 34 | <1 | <1 | 210 |
NA, Non available.
Urinary excretion of cortisol after LDDST
Mean urinary excretion of cortisol was significantly higher in children with CS on the second day after LDDST than in children with PCSS (obese and nonobese) (P < 0.001) (Table 1). Using the previously established criterion of UFC above 36 μg/m2·24 h or 99 nmol/24 h (11,12) after 2 d of LDDST, all children with CS were identified, and all children who did not have the disease, regardless of weight status, were excluded. This gave the test 100% sensitivity and 100% specificity (Table 2). UFCs during the LDDST phase that preceded oCRH administration had no false-positive or false-negative results.
Plasma ACTH and cortisol
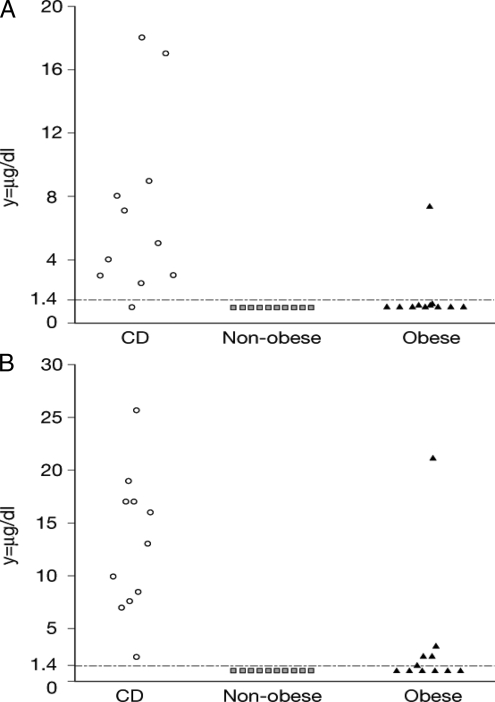
The second day after LDDST, mean basal serum ACTH was significantly higher in children with CS than in patients with PCSS (P < 0.001) (Table 1, Fig. 2). Mean basal serum cortisol was also significantly higher in children with CS the second day after LDDST than in patients with PCSS (Tables 1 and 2). Using the criterion of serum cortisol above 1.4 μg/dl (38 nmol/liter) (13,14) after the LDDST that preceded oCRH administration, all patients with CS, except for one, were identified; the test sensitivity was 91% [10 of 11 (CI95, 57–100)]. All nonobese children had a serum cortisol below 1.4 μg/dl (38 nmol/liter); the test specificity for the nonobese group was 100% [10 of 10 (CI95, 66–100)]. In contrast, one child in the obese group had a positive test, giving the serum cortisol measurement after the LDDST but before the oCRH administration a specificity of 91% [10 of 11 (CI95, 57–100)] for obese children. Serum cortisol after LDDST had low false-positive and false-negative results. No false-positive results were reported for nonobese children; however, 9% of the obese children [1 of 11 (CI95, 0.48–43)] had false-positive results. One child with CS had a negative test, giving the test a false-negative rate of 9% [1 of 11 (CI95, 0.48–43)] (Table 2).
Figure 2.
Basal (−15 min) (A) and stimulated (+15 min) (B) plasma cortisol levels in response to the Dex-oCRH test for the patients of the study [the y-axis represents plasma cortisol levels (μg/dl), and the x-axis represents the different study groups].
If one does not separate very obese children from the analysis of all serum cortisol-after-LDDST data, a cutoff value of 1.2 μg/dl (33 nmol/liter) has the best balance between sensitivity and specificity (91% sensitivity and 95% specificity) in diagnosing CS (Table 3).
Table 3.
LDDST-serum cortisol
| Criterion | Sensitivity | CI95 | Specificity | CI95 | +LR | −LR | +PV | −PV |
|---|---|---|---|---|---|---|---|---|
| ≥1 | 100.00 | 71.3–100.0 | 0.00 | 0.0–16.3 | 1.00 | 34.4 | ||
| >1 | 90.91 | 58.7–98.5 | 80.95 | 58.1–94.4 | 4.77 | 0.11 | 71.4 | 94.4 |
| >1.1 | 90.91 | 58.7–98.5 | 90.48 | 69.6–98.5 | 9.55 | 0.10 | 83.3 | 95.0 |
| >1.2a | 90.91 | 58.7–98.5 | 95.24 | 76.1–99.2 | 19.09 | 0.095 | 90.9 | 95.2 |
| >2.5 | 81.82 | 48.2–97.2 | 95.24 | 76.1–99.2 | 17.18 | 0.19 | 90.0 | 90.9 |
| >3 | 63.64 | 30.9–88.8 | 95.24 | 76.1–99.2 | 13.36 | 0.38 | 87.5 | 83.3 |
| >4 | 54.55 | 23.5–83.1 | 95.24 | 76.1–99.2 | 11.45 | 0.48 | 85.7 | 80.0 |
| >5 | 45.45 | 16.9–76.5 | 95.24 | 76.1–99.2 | 9.55 | 0.57 | 83.3 | 76.9 |
| >7.1 | 36.36 | 11.2–69.1 | 95.24 | 76.1–99.2 | 7.64 | 0.67 | 80.0 | 74.1 |
| >7.3 | 36.36 | 11.2–69.1 | 100.00 | 83.7–100.0 | 0.64 | 100.0 | 75.0 | |
| >8 | 27.27 | 6.3–60.9 | 100.00 | 83.7–100.0 | 0.73 | 100.0 | 72.4 | |
| >9 | 18.18 | 2.8–51.8 | 100.00 | 83.7–100.0 | 0.82 | 100.0 | 70.0 | |
| >17 | 9.09 | 1.5–41.3 | 100.00 | 83.7–100.0 | 0.91 | 100.0 | 67.7 | |
| >18 | 0.00 | 0.0–28.7 | 100.00 | 83.7–100.0 | 1.00 | 65.6 |
A criterion of 1.2 μg/dl (33 nmol/liter) for serum cortisol, before oCRH administration, has the highest possible sensitivity and specificity value for the diagnosis of CS (and exclusion of PCSS) for all patients regardless of BMI; 100% sensitivity is achieved with a criterion of 1.0 μg/dl (28 nmol/liter). LR, Likelihood ratio; PV, predictive value; +, positive; −, negative.
Cortisol levels after oCRH administration
Mean cortisol levels were significantly higher in patients with CS than in patients with PCSS during Dex-oCRH (P = 0.001) (Table 2, Fig. 2). A cortisol value above 1.4 μg/dl (38 nmol/liter) after the administration of oCRH was observed in all children with CS: sensitivity was 100% [11 of 11 (CI95, 68–100)]. All children with BMIZ scores below 2 had a serum cortisol less than 1.4 μg/dl (38 nmol/liter) in response to oCRH; for this group, the test specificity was 100% [10 of 10 (CI95, 66–100)]. Five obese children responded to oCRH with cortisol values above 1.4 μg/dl (38 nmol/liter); the specificity of the test for this group was 55% [6 of 11 (CI95, 25–82)], and the false-positive rate was 31% [5 of 16 (CI95, 12–59)] with no false-negative results. Obese children (especially those with severe obesity) were indeed significantly more likely to have a false-positive result (P = 0.001) than children with BMIZ scores below 2. If one does not separate very obese children from the analysis of the Dex-oCRH data, an increase of the cutoff cortisol value to 3.2 μg/dl (88 nmol/liter) has the best balance between sensitivity and specificity (91% sensitivity and 95% specificity) in diagnosing CS (Table 4). In addition, as shown in Table 4 also, 100% sensitivity was achieved with a cutoff value for serum cortisol above 2.2 μg/dl (61 nmol/liter) after oCRH administration with an estimated specificity of 90.5%.
Table 4.
Dex-oCRH stimulation test
| Criterion | Sensitivity | CI95 | Specificity | CI95 | +LR | −LR | +PV | −PV |
|---|---|---|---|---|---|---|---|---|
| ≥1 | 100.00 | 71.3–100.0 | 0.00 | 0.0–16.3 | 1.00 | 34.4 | ||
| >1 | 100.00 | 71.3–100.0 | 76.19 | 52.8–91.7 | 4.20 | 0.00 | 68.7 | 100.0 |
| >1.5 | 100.00 | 71.3–100.0 | 80.95 | 58.1–94.4 | 5.25 | 0.00 | 73.3 | 100.0 |
| >2.2 | 100.00 | 71.3–100.0 | 90.48 | 69.6–98.5 | 10.50 | 0.00 | 84.6 | 100.0 |
| >2.3 | 90.91 | 58.7–98.5 | 90.48 | 69.6–98.5 | 9.55 | 0.10 | 83.3 | 95.0 |
| >3.2a | 90.91 | 58.7–98.5 | 95.24 | 76.1–99.2 | 19.09 | 0.095 | 90.9 | 95.2 |
| >7 | 81.82 | 48.2–97.2 | 95.24 | 76.1–99.2 | 17.18 | 0.19 | 90.0 | 90.9 |
| >7.6 | 72.73 | 39.1–93.7 | 95.24 | 76.1–99.2 | 15.27 | 0.29 | 88.9 | 87.0 |
| >8.5 | 63.64 | 30.9–88.8 | 95.24 | 76.1–99.2 | 13.36 | 0.38 | 87.5 | 83.3 |
| >10 | 54.55 | 23.5–83.1 | 95.24 | 76.1–99.2 | 11.45 | 0.48 | 85.7 | 80.0 |
| >13 | 45.45 | 16.9–76.5 | 95.24 | 76.1–99.2 | 9.55 | 0.57 | 83.3 | 76.9 |
| >16 | 36.36 | 11.2–69.1 | 95.24 | 76.1–99.2 | 7.64 | 0.67 | 80.0 | 74.1 |
| >17 | 18.18 | 2.8–51.8 | 95.24 | 76.1–99.2 | 3.82 | 0.86 | 66.7 | 69.0 |
| >19 | 9.09 | 1.5–41.3 | 95.24 | 76.1–99.2 | 1.91 | 0.95 | 50.0 | 66.7 |
| >21 | 9.09 | 1.5–41.3 | 100.00 | 83.7–100.0 | 0.91 | 100.0 | 67.7 | |
| >26 | 0.00 | 0.0–28.7 | 100.00 | 83.7–100.0 | 1.00 | 65.6 |
A criterion of 3.2 μg/dl (88 nmol/liter) for serum cortisol, after oCRH administration, has the highest possible sensitivity and specificity value for the diagnosis of CS (and exclusion of PCSS) for all patients, regardless of BMI; 100% sensitivity is achieved with a criterion of 2.2 μg/dl (61 nmol/liter). LR, Likelihood ratio; PV, predictive value; +, positive; −, negative.
Diagnostic utility of each test (ROC calculations)
The area under the ROC curve calculations showed that UFC collection after LDDST had a high value (1.00) and was the most accurate test in diagnosing CS, regardless of weight status. The diagnostic power of Dex-oCRH (1.00) was superior to that of LDDST-serum cortisol (0.96) for the differential diagnosis of CS when the sample population included only nonobese children. On the other hand, when the sample population included the very obese children, the diagnostic accuracy of the Dex-oCRH (0.91) and the LDDST-serum cortisol (0.89) for the differential diagnosis of CS vs. PCSS decreased. If one does not separate very obese children from the analysis, the overall diagnostic accuracy is 0.95 for the Dex-oCRH and 0.92 for the LDDST-serum cortisol.
Dex levels
A serum Dex level was obtained before oCRH testing. As shown in Table 2, Dex levels were considered adequate in most children, except for one. This child with PCSS had a suppressed response to Dex-oCRH, despite low levels of Dex, and the test was not repeated; in follow-up, this child has continued to grow well and remains free of any evidence of CS.
Discussion
The purpose of the present study was to evaluate the usefulness of the Dex-oCRH in assessing pediatric patients referred for possible CS in the absence of consistent hypercortisolemia or bona fide stigmata of the syndrome; on the other hand, all of these patients had at least some clinical finding suspicious for CS (atypical stretch marks, on- and off- hypertension, obesity of varying degrees, facial plethora) and at least one test as an outpatient that was abnormal (usually a 24-h UFC above the normal range and occasionally a 1 mg overnight Dex test). These patients were thus different from those reported by Yanovski et al. (11,12), not only because of their age, but also because of the relative absence of factors that are characteristically causes of PCSS in adults (mental disease, alcoholism and other forms of dependence, various drugs). At the conclusion of the study, only three patients from this cohort could be diagnosed with an adult-like PCSS—two patients with psychiatric disease and one with PCOS.
We have indicated in a recent report of a large cohort of pediatric patients with CS (25) that the disease, albeit rare, should be considered in any child with weight gain and growth retardation. Indeed, as shown in Fig. 1, overall mean BMIZ score for pediatric patients with CS is usually above the mean, but the mean HAZ score is not. This contrasts with children who are overweight whose BMIZ scores and HAZ scores are above the mean.
The results of our present study showed that, in children, a cutoff value above 1.4 μg/dl (38 nmol/liter) for serum cortisol during Dex-oCRH provided 100% sensitivity and 100% specificity for the differential diagnosis of CS only when the comparison group included nonobese children who were either normal or overweight. In contrast, when the sample population included obese children, the specificity of the test decreased. As in the case of the Dex-oCRH test, the specificity of the serum cortisol after LDDST for children was highly dependent on their weight status. When the sample population was composed of nonobese children, serum cortisol after LDDST had 91% sensitivity and 100% specificity. But when the sample population included obese children, the specificity of serum cortisol after LDDST also decreased. However, the diagnostic power of Dex-oCRH was only slightly superior to the serum cortisol after LDDST: diagnostic accuracy of the Dex-oCRH test was 95%, whereas for cortisol after LDDST the value was 92% (again, if one does not separate very obese children from the analysis).
In contrast, UFC measurements during LDDST appear to be superior to both the serum cortisol and the combined Dex-oCRH test; excretion of UFC after 2 d of LDDST was more sensitive and specific as a screening tool for CS than serum cortisol in the same test and before the administration of oCRH. We used a cutoff value for UFC after the LDDST of 36 μg/m2·24 h (99 nmol/24 h) (11,12). This cutoff value gave the test 100% sensitivity and 100% specificity for the differential diagnosis of CS. The area under the ROC curve showed that UFC excretion after 2 d of LDDST was the most accurate test to diagnose CS, regardless of weight status. The problem with urine collections is that they cannot be obtained reliably in the outpatient setting, especially in the pediatric population. In this study, urine collections were obtained in a controlled environment. When UFCs are obtained in a less controlled setting, the performance of this measurement for the diagnosis of CS decreases, whether as a screening test or as part of LDDST (25).
Our study showed the limitations of establishing a universal screening threshold in tests that are designed to diagnose CS. If the goal of the Dex-oCRH is to achieve 100% sensitivity, then the best cutoff value is a cortisol above 2.2 μg/dl (61 nmol/liter) after oCRH administration, with an estimated specificity of 90.5%. A cutoff value above 3.2 μg/dl (88 nmol/liter) provides the best specificity and sensitivity based on our ROC curve analysis (91% sensitivity, 95% specificity). This was also the case for the serum cortisol after LDDST; the best sensitivity and specificity was achieved with a cutoff value of 1.2 μg/dl (33 nmol/liter), 91% sensitivity and 95% specificity, whereas a 100% sensitivity was achieved with a cutoff value of 1.0 μg/dl (28 nmol/liter), which gave the test a 0% specificity.
It is important that, to interpret the LDDST and the Dex-oCRH accurately, Dex levels must be adequate; inadequate levels might be due to noncompliance with medication, rapid drug clearance or metabolism, in addition to interactions with other compounds such as oral contraceptives (11,12). In our study, as shown in Table 2, the levels of Dex were adequate in all, except for one patient; the Dex level was 67 ng/dl for this nonobese child, and cortisol levels were suppressed during the Dex-oCRH. In addition, one patient (excluded from the study) whose oral contraceptives were discontinued 6 wk before testing had a positive result (serum cortisol = 1.7 μg/dl, +15 min after Dex-oCRH). For this patient, cortisol-binding globulin (CBG) levels were elevated (20 mg/dl). These data emphasize the importance of obtaining serum CBG levels in patients with prior history of oral contraceptive use. Of particular interest was a child with cyclical CS whose Dex-oCRH was positive only when this child was cycling (patient 6).
In the case of obese patients with adequate Dex levels, the lack of suppression might be multifactorial in origin. Studies suggest that obesity is associated with an altered hypothalamic-pituitary-adrenal axis (27). Indeed, the set point for glucocorticoid suppression may be genetically determined, and there is individual glucocorticoid suppression variation that may be affected by obesity and its hypothalamic effects (28). Meals may also influence the hypothalamic pituitary axis, increasing cortisol secretion (27); our patients received oCRH in the fasting state, but their diet on the days before the test was not monitored. Finally, obesity is associated with decreased levels of SHBG that may result in an increase in free steroid hormone levels. Excess aromatization in obesity and decreased SHBG may lead to increased estrogens and a consequent rise in CBG (28). However, CBG, adrenal androgen, and estrogen levels in our patients were not statistically different in the children with false-positive results (data not shown); besides, CBG levels are usually not increased in obese patients (29).
The strength of our study lies in the fact that children were tested because of clinical suspicion of CS. They entered the study sequentially, preventing selection bias; and the testing occurred in a controlled environment with adherence to the timing of the administration of Dex and oCRH because Yanovski et al. (11,12) suggested that may be important for the proper interpretation of the test. All children had Dex levels withdrawn, ensuring that adequate levels were present to interpret the test, and all children were followed longitudinally. Limitations of our study include the fact that this was a single-center study with a small number of children. Serum cortisol assays vary, and the assay used for this test in our center may not be the same at the local hospital or clinic. An assay with a high coefficient of variation lacks reproducibility, and a higher detection limit may hinder the interpretation of low cutoff values. Lastly, the results of our study do not apply to the general population of children but do apply to those with a clinical suspicion of CS.
A question that is now raised is where Dex-oCRH stands in comparison with other screening tests available for the diagnosis of pediatric CS (9,10,25,30,31,32,33). As shown in Table 5, Dex-oCRH performance is comparable to other screening tests for CS in children (9,10,25,30,31,32,33), but of course it is much more cumbersome than most. But overall, some of these tests are more suitable and convenient for children, and when available these tests should be performed instead of a Dex-oCRH test; midnight salivary cortisol is one such test (9,10).
Table 5.
Screening tests for CS in children
| Screening test (Ref.) | Sensitivity (%) | Specificity (%) | Setting |
|---|---|---|---|
| UFC (25)a | 88 | 80 | Outpatient |
| Midnight cortisol (25) | 99 | 100 | Inpatient |
| Midnight salivary cortisol (9) | 93 | 100 | Outpatient |
| Salivary cortisol (23 h) (10) | 100 | 95.2 | Outpatient |
| Salivary cortisol after Dex (10) | 100 | 95.2 | Outpatient |
| Salivary cortisol (23 h) and after Dex (10) | 100 | 100 | Outpatient |
| LDDST-UFC (present study) | 100 | 100 | Outpatient |
| LDDST-serum cortisol (present study) | Outpatient | ||
| CD vs. nonobese children (1.4 μg/dl or 38 nmol/liter) | 91 | 100 | |
| CD vs. obese children (1.4 μg/dl or 38 nmol/liter) | 91 | 91 | |
| CD vs. obese and nonobese | |||
| 1.2 μg/dl or 33 nmol/liter | 91 | 95 | |
| 1.0 μg/dl or 28nmol/liter | 100 | 0 | |
| 1 mg ODST (10,30,31,32,33)b | 82.5–100 | 87.5–95.2 | Outpatient |
| Dex-oCRH test (present study) | Outpatient | ||
| CD vs. nonobese children (1.4 μg/dl or 38 nmol/liter) | 100 | 100 | |
| CD vs. obese children (1.4 μg/dl or 38 nmol/liter) | 100 | 55 | |
| CD vs. obese and nonobese | |||
| 3.2 μg/dl or 88 nmol/liter | 91 | 95 | |
| 2.2 μg/dl or 61 nmol/liter | 100 | 90.5 |
Setting indicates which tests can be performed as outpatient or inpatient. ODST, Overnight Dex suppression.
UFC collection sensitivity and specificity varies with the number of days of collection.
Results are mostly in adult patients; reported values are for serum or salivary cortisol after 1 mg ODST.
We conclude that the Dex-oCRH has a limited place in the work-up of a pediatric patient with CS. As shown in Table 5, there are other tests with similar performance to the Dex-oCRH that are more suitable and convenient for children; when available, these tests should be performed first and one should not rush to perform a Dex-oCRH test. Obesity, especially severe obesity, appears to confound the results, but when BMI is considered as a factor the test can be useful; a higher cutoff value may be used especially in severely obese children for whom obtaining accurate growth points over the course of more than a few years is of paramount importance in making the right diagnosis and avoiding unnecessary medical interventions.
Footnotes
This work was supported by the National Institutes of Health, National Institute of Child Health and Human Development intramural project Z01-HD-000642-04 (Principal Investigator, C. A. Stratakis).
Disclosure Statement: All authors have no conflict of interest to report.
First Published Online August 26, 2008
Abbreviations: BMI, Body mass index; BMIZ, BMI-for-age and sex Z (score); CBG, cortisol-binding globulin; CD, Cushing disease; CI95, 95% confidence interval; CS, Cushing syndrome; Dex, dexamethasone; Dex-oCRH, LDDST followed by oCRH stimulation; HAZ, height-for-age and sex Z (score); LDDST, low-dose Dex suppression test; oCRH, ovine CRH; PCOS, polycystic ovary syndrome; PCSS, pseudo-Cushing syndrome state; ROC, receiver operating characteristic; UFC, urinary free cortisol.
References
- Lindholm JF, Juul S, Jorgensen JO, Astrup J, Bjerre P, Feldt-Rasmussen U, Hagen C, Jorgensen J, Kosteljanetz M, Kristensen L, Launberg P, Schmidt K, Weeke J 2001 Incidence and late prognosis of Cushing’s syndrome: a population-based study. J Clin Endocrinol Metab 86:117–123 [DOI] [PubMed] [Google Scholar]
- Newell-Price JF, Trainer PF, Besser MF, Grossman AB 1998 The diagnosis and differential diagnosis of Cushing’s syndrome and pseudo-Cushing’s states. Endocr Rev 19:647–672 [DOI] [PubMed] [Google Scholar]
- Magiakou MA, Mastorakos G, Oldfield EH, Gomez MT, Doppman JL, Cutler Jr GB, Nieman LK, Chrousos GP 1994 Cushing’s syndrome in children and adolescents. Presentation, diagnosis, and therapy. N Engl J Med 331:629–636 [DOI] [PubMed] [Google Scholar]
- Savage MO, Lienhardt AF, Lebrethon MC, Johnston LB, Huebner A, Grossman AB, Afsher F, Plowman PN, Besser GM 2001 Cushing’s disease in childhood: presentation, investigation, treatment and long-term outcome. Horm Res 55:24–30 [DOI] [PubMed] [Google Scholar]
- Arnaldi G, Angeli A, Atkinson AB, Bertagna X, Cavagnini F, Chrousos GP, Fava GA, Findling JW, Gaillard RC, Grossman AB, Kola B, Lacroix A, Mancini T, Mantero F, Newell-Price J, Nieman LK, Sonino N, Vance ML, Giustina A, Boscaro M 2003 Diagnosis and complications of Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab 88:5593–5602 [DOI] [PubMed] [Google Scholar]
- Nieman LK 2002 Diagnostic tests for Cushing’s syndrome. Ann NY Acad Sci 970:112–118 [DOI] [PubMed] [Google Scholar]
- Boscaro MF, Barzon L, Fallo F, Sonino N 2001 Cushing’s syndrome. Lancet 357:783–791 [DOI] [PubMed] [Google Scholar]
- Findling JW, Raff H 2005 Screening and diagnosis of Cushing’s syndrome. Endocrinol Metab Clin North Am 34:385–402 [DOI] [PubMed] [Google Scholar]
- Gafni RI, Papanicolau DA, Nieman LK 2000 Nighttime salivary cortisol measurement as a simple, noninvasive, outpatient screening test for Cushing’s syndrome in children and adolescents. J Pediatr 137:30–35 [DOI] [PubMed] [Google Scholar]
- Martinelli Jr CE, Sade SL, Oliveira EB, Daneluzzi JC, Moreira AC 1999 Salivary cortisol for screening of Cushing’s syndrome in children. Clin Endocrinol 51:67–71 [DOI] [PubMed] [Google Scholar]
- Yanovski JA, Cutler Jr GB, Chrousos GP, Nieman LK 1993 Corticotropin-releasing hormone stimulation following low-dose dexamethasone administration. A new test to distinguish Cushing’s syndrome from pseudo-Cushing’s states. JAMA 269:2232–2238 [PubMed] [Google Scholar]
- Yanovski JA, Cutler Jr GB, Chrousos GP, Nieman LK 1998 The dexamethasone-suppressed corticotropin-releasing hormone stimulation test differentiates mild Cushing’s disease from normal physiology. J Clin Endocrinol Metab 83:348–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecori Giraldi F, Pivonello R, Ambrogio AG, De Martino MC, De Martin M, Scacchi M, Colao A, Toja PM, Lombardi G, Cavagnini F 2007 The dexamethasone-suppressed corticotropin-releasing hormone stimulation test and the desmopressin test to distinguish Cushing’s syndrome from pseudo-Cushing’s states. Clin Endocrinol (Oxf) 66:251–257 [DOI] [PubMed] [Google Scholar]
- Martin N, Dhillo W, Banerjee A, Abdulali A, Jayasena CN, Donaldson M, Todd JF, Meeran K 2006 Comparison of the dexamethasone-suppressed corticotropin-releasing hormone test and low dose dexamethasone suppression test in the diagnosis of Cushing’s syndrome. J Clin Endocrinol Metab 91:2582–2586 [DOI] [PubMed] [Google Scholar]
- Erickson D, Natt N, Nippoldt N, Young Jr WF, Carpenter PC, Petterson T, Christianson T 2007 Dexamethasone-suppressed corticotropin-releasing hormone stimulation test for diagnosis of mild hypercortisolism. J Clin Endocrinol Metab 92:2972–2976 [DOI] [PubMed] [Google Scholar]
- Gatta B, Chabre O, Cortet C, Martinie M, Corcuff JB, Roger P, Tabarin A 2007 Reevaluation of the combined dexamethasone suppression-corticotropin-releasing hormone test for differentiation of mild Cushing’s disease from pseudo-Cushing’s syndrome. J Clin Endocrinol Metab 92:4290–4293 [DOI] [PubMed] [Google Scholar]
- Hammer LD, Kraener HC, Wilson DM, Ritter PL, Dornbusch SM 1991 Standardized percentile curves for body-mass index for children and adolescents. Am J Dis Childhood 145:259–263 [DOI] [PubMed] [Google Scholar]
- Pietrobelli A, Faith MS, Allison DB, Gallagher D, Chiurnello G, Heymsfield SB 1998 Body mass index as a measure of adiposity among children and adolescents: a validation study. J Pediatr 132:204–210 [DOI] [PubMed] [Google Scholar]
- Dhuper S, Cohen HW, Daniel J, Gumidyala P, Agarwalla V, St Victor R, Dhuper S 2007 Utility of the modified ATP III defined metabolic syndrome and severe obesity as predictors of insulin resistance in overweight children and adolescents: a cross-sectional study. Cardiovasc Diabetol 6:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprio S 2005 Definitions and pathophysiology of the metabolic syndrome in obese children and adolescents. Int J Obes 29(Suppl 2):S24—S25 [DOI] [PubMed] [Google Scholar]
- Gomez MT, Malozowski S, Winterer J, Vamvakopoulos NC, Chrousos GP 1991 Urinary-free cortisol values in normal children and adolescents. J Pediatr 118:256–258 [DOI] [PubMed] [Google Scholar]
- Streeten DH, Faas FH, Elders MJ, Dalakos TG, Voorhess M 1975 Hypercortisolism in childhood: shortcomings of conventional diagnostic criteria. Pediatrics 56:797–803 [PubMed] [Google Scholar]
- Papanicolaou DA, Yanovski JA, Cutler Jr GB, Chrousos GP, Nieman LK 1998 A single midnight serum cortisol measurement distinguishes Cushing’s syndrome from pseudo-Cushing states. J Clin Endocrinol Metab 83:1163–1167 [DOI] [PubMed] [Google Scholar]
- Nieman LK, Oldfield EH, Wesley R, Chrousos GP, Loriaux DL, Cutler Jr GB 1993 A simplified morning ovine corticotropin-releasing hormone stimulation test for the differential diagnosis of adrenocorticotropin-dependent Cushing’s syndrome. J Clin Endocrinol Metab 77:1308–1312 [DOI] [PubMed] [Google Scholar]
- Batista DL, Riar R, Keil MF, Stratakis CA 2007 Diagnostic tests for children who are referred for the investigation of Cushing syndrome. Pediatrics 120:e575–e86 [DOI] [PubMed] [Google Scholar]
- Dichek HL, Nieman LK, Oldfield EH, Pass HI, Malley JD, Cutler Jr GB 1994 A comparison of the standard high-dose dexamethasone suppression test and the overnight 8-mg dexamethasone suppression test for the differential diagnosis of adrenocorticotropin-dependent Cushing’s syndrome. J Clin Endocrinol Metab 78:418–422 [DOI] [PubMed] [Google Scholar]
- Pasquali R, Vicennati V, Cacciaru M, Pagotto U 2006 The hypothalamic-pituitary-adrenal axis activity in obesity and the metabolic syndrome. Ann NY Acad Sci 1083:111–128 [DOI] [PubMed] [Google Scholar]
- Ljung T, Andersson B, Bengtsson BA, Bjorntorp P, Marin P 1996 Inhibition of cortisol secretion by dexamethasone in relation to body fat distribution: a dose-response study. Obes Res 4:277–282 [DOI] [PubMed] [Google Scholar]
- Fernandez-Real JM, Grasa M, Casamitjana R, Pugeat M, Barret C, Ricart W 2002 Corticosteroid binding globulin: a new target for cortisol-driven obesity. J Clin Endocrinol Metab 87:4686–4690 [Google Scholar]
- Gorges R, Knappe G, Gerl H, Ventz M, Stahl F 1999 Diagnosis of Cushing’s syndrome: re-evaluation of midnight plasma cortisol vs urinary free cortisol and low-dose dexamethasone suppression test in a large patient group. J Endocrinol Invest 22:241–249 [DOI] [PubMed] [Google Scholar]
- Barrou Z, Guiban D, Maroufi A, Fournier C, Dugue M-A, Luton J-P, Thomopoulos P 1996 Overnight dexamethasone suppression test: comparison of plasma and salivary cortisol measurement for the screening of Cushing’s syndrome. Eur J Endocrinol 134:93–96 [DOI] [PubMed] [Google Scholar]
- Cronin C, Igoe D, Duffy MJ, Cunningham SK, McKenna TJ 1990 The overnight dexamethasone test is a worthwhile screening procedure. Clin Endocrinol (Oxf) 33:27–33 [DOI] [PubMed] [Google Scholar]
- Findling JM, Raff H, Aron DC 2004 The low-dose dexamethasone suppression test: a reevaluation in patients with Cushing’s syndrome. J Clin Endocrinol Metab 289:1222–1226 [DOI] [PubMed] [Google Scholar]