Abstract
Context: Acylated ghrelin is the putatively bioactive GH secretagogue.
Hypothesis: Estradiol (E2) stimulates the synthesis rather than inhibits the metabolic clearance of acylated ghrelin.
Setting: The study took place at an academic medical center.
Subjects: Healthy postmenopausal women participated.
Interventions: Interventions included prospectively randomized, double-blind separate-day iv infusions of saline or five graded doses of ghrelin in estrogen-deficient (n = 12) and E2-supplemented (n = 8) women.
Outcomes: Metabolic clearance rate (MCR), volume of distribution, half-life, and secretion rate of acylated ghrelin were assessed.
Results: In pilot iv bolus ghrelin infusions, the median half-lives of acylated and total ghrelin were 21 and 36 min (P < 0.01), MCRs 58 and 8.1 liters/kg·d (P < 0.01), and volumes of distribution of 1.0 and 0.32 liters/kg (P < 0.01), respectively. Transdermal E2 supplementation for 3 wk increased peak nighttime acylated ghrelin concentrations from 99 ± 12 to 141 ± 34 pg/ml (P = 0.039). Exposure to E2 did not alter the linear relationships between 1) plasma acylated ghrelin concentration and ghrelin infusion rate (638 ± 12 slope units), 2) MCR of acylated ghrelin and ghrelin infusion rate (10 ± 2.5 slope units), and 3) MCR and plasma concentration of acylated ghrelin (0.017 ± 0.004 slope units). These data predict peak nighttime production rates of acylated ghrelin of 3.8 ± 0.9 (E2) and 1.9 ± 0.2 (no E2) ng/kg·min (P = 0.039).
Conclusion: Acylated ghrelin has a multifold larger distribution volume and MCR than total ghrelin. An estrogenic milieu augments synthesis and/or acylation of ghrelin peptide without altering its MCR.
Estradiol stimulates ghrelin acylation in postmenopausal women without affecting its metabolic clearance rate.
Ghrelin is a 28-amino-acid Ser3-octanoylated GH-releasing peptide of gastro-pancreatico-hypothalamo-pituitary origin (1). Ghrelin and synthetic analogs [GH secretagogues (GHS)] stimulate GH secretion by 2- to 3-fold in vitro and 5- to 20-fold in vivo (2,3,4,5,6,7). Transgenic silencing of the central nervous system ghrelin receptor in the female mouse reduces GH secretion and IGF-I concentrations (3). In humans, mutations of the GHS receptor are associated with short stature (8). In healthy adults, bolus infusion of ghrelin or GHS evokes a prompt burst-like release of GH (2,3,4,5,6,7,9,10,11,12,13,14). Continuous iv infusion of ghrelin in graded increments stimulates GH secretion dose dependently (6). Thus, ghrelin or GHS may have utility in the treatment of short stature and selected GH-deficiency states. Because ghrelin also inhibits fat oxidation, suppresses insulin secretion, reduces diastolic blood pressure, and increases cardiac output (1,15), other exploratory therapeutic applications of ghrelin include congestive heart failure (16), cachectic states such as AIDS (1), chronic obstructive pulmonary disease (17) and cancer (18), eating disorders, osteoporosis, aging, and postoperative catabolism (1,19,20).
Despite the potential for diverse therapeutic applications, the physiological regulation of ghrelin production remains poorly understood (21,22). Even less is known about the secretion of acylated (putatively bioactive) ghrelin. Recent cloning of the acyl transferase that octanoylates ghrelin and the deacylase that removes n-octanoic acid from ghrelin in the stomach and intestine highlights the need to elucidate endocrine factors that control the systemic availability of the acylated form (23,24).
Available data suggest that sex steroids increase total ghrelin concentrations. This hypothesis is supported by the following observations: 1) in hypogonadal states, such as severe undernutrition associated with anorexia nervosa, 6 months of estrogen supplementation increased total ghrelin concentrations (25); 2) in postmenopausal women, oral estrogen replacement for 6 months elevated active ghrelin concentrations (26); 3) combined estrogen-progestin therapy in postmenopausal women for 2 yr was associated with higher total ghrelin concentrations (27); and 4) testosterone administration to hypogonadal men for 6 months increased total ghrelin concentrations by 60% (28). However, in a fifth study, total ghrelin concentrations were no different in eu- and hypoestrogenemic women (29). Limitations of these investigations include lack of measurements of acylated ghrelin except in one study, absence of information on short-term estrogen effects, and the need to clarify whether estrogen stimulates ghrelin secretion or inhibits ghrelin metabolism (1,30).
The present study tests the hypothesis that exposure to estradiol (E2) increases the secretion without altering the metabolic clearance of acylated ghrelin.
Subjects and Methods
Subjects
Participants signed witnessed, voluntary informed consent approved by the Mayo Institutional Review Board and reviewed by the U.S. Food and Drug Administration under an investigator-initiated new drug number. Exclusion criteria were premenopausal status (screening FSH concentration <30 IU/liter and E2 >50 pg/ml); concurrent use of neuroactive medications or sex hormones; systemic illness; diabetes mellitus; untreated endocrinopathy; systemic disease defined by medical history, physical examination, or biochemical screening data; acute or chronic organ (including inflammatory) disease; drug or alcohol abuse; hemoglobin less than 11.6 gm/dl; history of thrombotic arterial disease (stroke, transient ischemic attack, myocardial infarction, or angina) or thrombophlebitis; history or suspicion of breast cancer; and unwillingness to provide written informed consent. Postmenopausal status was defined by the absence of spontaneous menses for at least one year, FSH more than 30 IU/liter, and E2 less than 15 pg/ml. Each subject had an unremarkable medical history and physical examination and normal screening laboratory tests of hepatic, renal, endocrine, metabolic, and hematological function.
Estrogen administration
A transdermal E2 repletion schedule was used to mimic E2 concentrations attained in the young-adult late follicular or preovulatory phase (31). The modified schedule comprised daily E2 patches at a starting dose of 0.05 mg (d 1), escalated on d 6 to 0.1 mg and on d 12 to 0.15 mg. The last dose was continued until d 21. The two infusion studies were performed at least 48 h apart during the time window d 18–21 inclusive. Medroxyprogesterone was given orally (10 mg daily) for 12 d beginning on d 22 for women with an intact uterus.
Pilot study
To test feasibility, eight healthy subjects (five men and three women; age range, 20–65 yr; body mass index 19–25 kg/m2) were given a ghrelin 1 or 3 μg/kg iv bolus. Blood was withdrawn every 15 min for 165 min to measure plasma total and acylated ghrelin concentrations.
Protocol
The study design was parallel-cohort, double-masked and prospectively randomized. Each woman underwent two separate study sessions at least 48 h apart (Fig. 1). Subjects were admitted to the Clinical Research Unit (CRU) before 1700 h and stayed overnight. To limit nutritional confounds, volunteers received a constant meal (vegetarian or nonvegetarian) in the CRU at 1800 h the night before study comprising 8 kcal/kg distributed as 50% carbohydrate, 20% protein, and 30% fat. Participants then remained fasting, alcohol-abstinent, and caffeine-free overnight until the end of the infusion the next day.
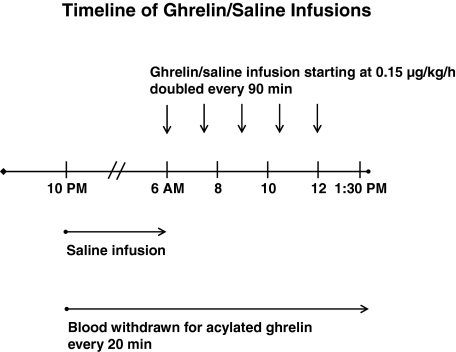
Figure 1.
Timeline of protocol of continuous iv infusion of ghrelin starting at a dose of 0.15 μg/kg·h, which was doubled every 90 min to a maximal rate of 2.4 μg/kg·h.
In the CRU, iv catheters were placed in contralateral forearm veins at 1700 h to allow simultaneous infusion of ghrelin or saline and blood sampling. Blood was withdrawn at baseline (1700 h) for later assay of E2, LH, FSH, IGF-I, IGF-binding protein (IGFBP)-1, IGFBP-3, and SHBG concentrations. Serum was sampled every 10 min overnight for 8 h (2200–0600 h) to obtain a baseline and then during the 7.5-h infusion interval (0600–1330 h) the following morning for GH measurements. Intravenous infusions comprised saline (10 ml/h) and graded ghrelin doses at a starting rate of 0.15 μg/kg·h. The rate was doubled every 90 min four times in succession (total of five doses) to reach a final rate of 2.4 μg/kg·h, analogous to the protocol described by Wren et al. (6). Concomitantly, plasma was sampled every 20 min starting at 2200 h for later measurement of acylated ghrelin concentrations.
Hormone assays
Plasma active (octanoylated) ghrelin concentrations were measured by competitive RIA (Linco/Millipore Research, St. Charles, MO). Intraassay coefficients of variation (CV) were 7.3 and 7.8% at 78 and 538 pg/ml, respectively. Interassay CV were 9 and 12% at 40 and 356 pg/ml. Assay sensitivity is 7.8 pg/ml when using a 0.1-ml sample size. Assay specificity is as follows: human ghrelin, 100%; ghrelin 1–10, 100%; des-octanoylghrelin, less than 0.1%; ghrelin 14–28, not detectable; and motilin-related peptide, not detectable.
E2 concentrations were quantified by tandem liquid chromatography-ion spray mass spectrometry (ThermoFisher Scientific, Franklin, MA, and Applied Biosystems-MDS Sciex, Foster City, CA). Intraassay CV were 3.1, 5.0, and 3.5% at 29, 109, and 325 pg/ml, respectively (multiply by 3.67 to convert to pmol/liter). Interassay CV were 8.6, 9.0, 6.6, and 4.8% at 24, 61, 125, and 360 pg/ml, respectively.
IGFBP-1, IGFBP-3, and total IGF-I concentrations were measured by immunoradiometric assay (Diagnostic Systems Laboratories, Webster, TX) (12,32). Interassay CV for IGF-I were 9% at 64 μg/liter and 6.2% at 157 μg/liter. Intraassay CV were 3.4% at 9.4 μg/liter, 3% at 55 μg/liter and 1.5% at 264 μg/liter.
LH and FSH were assayed using the DxI automated two-site immunoenzymatic system (Beckman Instruments, Chaska, MN). For LH, intraassay CVs were 4.3 and 4.0% at 1.2 and 38.5 IU/liter, and interassay CV were 9.3, 6.0, and 6.0% at 1.4, 15.6, and 48.8 IU/liter, respectively. For FSH, intraassay CV were 3.2 and 2.8% at 8.6 and 47.1 mIU/ml, and interassay CV were 3.6, 3.2, and 4.7% at 6.5, 16.7, and 58.0 mIU/ml, respectively.
Statistical analysis
An unpaired Student’s t test was used to compare outpatient and preinfusion hormone measurements in the parallel cohorts. Linear regression analysis was applied to examine the relationships between the acylated ghrelin concentration and the ghrelin infusion rate, between the metabolic clearance rate (MCR) and the ghrelin infusion rate, and between the MCR and the incremental acylated ghrelin concentrations (33). Slopes were compared by 95% statistical confidence intervals. Data are presented as the mean ± sem. P < 0.05 was construed as statistically significant.
Kinetic analyses
Ghrelin kinetics were analyzed as individual decay curves (pilot data) in eight subjects, as steady-state estimates (for each of 20 subjects), and as a population model of all 20 subjects together using nonlinear mixed-effects modeling (NONMEM) (34).
Ghrelin (total and acylated) decay curves were fit to an exponential function (35). For the bolus injections, a monoexponential fit was justified, yielding a single half-life (t1/2). One then calculates the MCR as MCR = k × Vd, where k = ln 2/t1/2, and Vd = volume of distribution, and Vd = (injected ghrelin dose)/(peak minus nadir ghrelin concentration).
Individual estimates of the MCR were made for each of the five infusion rates in each subject. The mean baseline endogenous acylated ghrelin concentration was calculated from the infusion time window on the saline day (0600–1330 h) in the same individual. This value was subtracted from the acylated ghrelin concentration measured at the end of each 90-min infusion. The resultant incremental concentration was divided by the ghrelin infusion rate to give the MCR.
The ghrelin production rate from 2200–0200 h was calculated as follows: production rate (nanograms per kilogram per day) = MCR (liters per kilogram per day) × ghrelin concentration (nanograms per liter).
In NONMEM, the entire cohort constitutes the experimental unit, rather than each individual (34). Ghrelin data from the 20 subjects were simultaneously fit to a model using NONMEM VI (ICONUS, Ellicott City, MD) (36). The MCR of acylated ghrelin is estimated as a regression parameter along with its variance. MCR values were assumed to be log-normally distributed in the population. Between-subject variability was expressed as a CV. Residual unexplained variability was estimated via a combined error model with proportional (expressed as a CV) and additive error (expressed as a sd in concentration units) components. The covariate of interest was estrogen exposure or nonexposure compared by a likelihood ratio test. Confidence intervals for the MCR estimate were computed from 1000 bootstrapped data sets.
Results
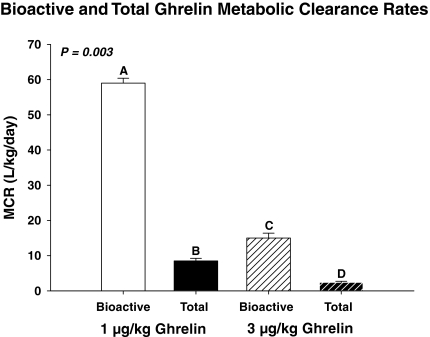
As a pilot estimate of ghrelin kinetics, four volunteers each received a single iv bolus injection of 1 μg/kg ghrelin and four others 3 μg/kg. Table 1 summarizes kinetic data for both doses. The mean half-life of acylated ghrelin after a bolus dose of 1 μg/kg iv was shorter than that of total ghrelin (21 ± 3.0 vs. 36 ± 2.4 min, respectively, P < 0.01). The estimated Vd was 3.1-fold larger for acylated than total ghrelin (P < 0.01). The mean MCR was 7.2-fold higher for acylated than total ghrelin (P < 0.01; Fig. 2). After bolus injection of 3 μg/kg ghrelin, peak bioactive and total ghrelin concentrations were higher (P < 0.001), and corresponding Vd and MCR values lower (both P < 0.001). The half-life of bioactive ghrelin after injection of 3 vs. 1 μg/kg was higher (median 45 vs. 21 min, P = 0.003), indicating saturability of acylated ghrelin’s disappearance.
Table 1.
Bolus iv ghrelin kinetics
| Kinetic parameters | 1 μg/kg iv ghrelin bolus
|
3 μg/kg iv ghrelin bolus
|
|||
|---|---|---|---|---|---|
| Acylated ghrelin | Total ghrelin | Acylated ghrelin | Total ghrelin | P value | |
| Peak (pg/ml) | 1012 ± 114a | 4438 ± 407b | 1671 ± 191c | 8176 ± 273d | <0.001 |
| K (min−1) | 0.034 ± 0.005aa | 0.019 ± 0.0012a,ba | 0.017 ± 0.003b | 0.012 ± 0.002b | 0.003 |
| Baseline (pg/ml) | 82 ± 24a | 1120 ± 320b | 140 ± 17a | 1060 ± 38b | <0.001 |
| t1/2 (min) | 21 ± 3.0aa | 36 ± 2.4a,ba | 47 ± 4.8b | 64 ± 7.8b | 0.003 |
| Vd (liters/kg) | 1.1 ± 0.12a | 0.31 ± 0.024b | 0.70 ± 0.11C | 0.14 ± 0.006d | <0.001 |
P values reflect one-way ANOVA. Data are the mean ± sem. K, Fractional rate constant.
a–d Different (unshared) superscripts denote significantly different means by ANOVA and Tukey’s post hoc test for multiple comparisons.
P < 0.01 by paired Student’s t test for a priori hypothesis.
Figure 2.
Mean (± sem) MCRs of acylated ghrelin and total ghrelin estimated after bolus injection of 1 or 3 μg/kg iv. Data were subjected to ANOVA. Different letters denote significantly different means by Tukey’s post hoc test.
Screening baseline (pretreatment) hormone concentrations in the 20 other women given continuous saline vs. ghrelin infusions studied are summarized in Table 2. Baseline concentrations of IGF-I, IGFBP-3, IGFBP-1, LH, FSH, prolactin, SHBG, and E2 did not differ in the randomized cohorts. After 3 wk of estrogen compared with placebo supplementation, concentrations of E2 were 13.4-fold higher (P = 0.002), LH 1.6-fold lower (P = 0.02), FSH 3.3-fold lower (P = 0.002), and prolactin 1.8-fold higher (P = 0.006). E2 exposure did not affect IGF-I, IGFBP-1, IGFBP-3, or SHBG concentrations.
Table 2.
Screening outpatient and preinfusion hormone measurements
| Hormone | Outpatient screeninga
|
Preinfusion datab
|
||||
|---|---|---|---|---|---|---|
| No E2(n = 12) | E2(n = 8) | P value | No E2(n = 12) | E2(n = 8) | P value | |
| IGF-I (μg/liter) | 131 ± 14 | 128 ± 44 | 0.8 | 107 ± 8.6 | 87 ± 14 | 0.25 |
| IGFBP-3 (mg/liter) | 4.7 ± 0.2 | 4.7 ± 0.3 | 0.9 | 3.9 ± 0.2 | 4.7 ± 0.3 | 0.06 |
| IGFBP-1 (μg/liter) | 39 ± 4.9 | 62 ± 2.3 | 0.1 | 4.7 ± 0.22 | 4.7 ± 0.3 | 0.9 |
| LH (IU/liter) | 102 ± 12 | 91 ± 12 | 0.5 | 27 ± 3.4 | 16 ± 2.1 | 0.02 |
| FSH (IU/liter) | 32 ± 4 | 36 ± 5 | 0.6 | 73 ± 8a | 22 ± 7a | 0.0002 |
| Prolactin (μg/liter) | 7.4 ± 0.8 | 9.2 ± 0.9 | 0.2 | 7 ± 1.2 | 13 ± 1.5 | 0.008 |
| SHBG (nmol/liter) | 54 ± 7.2 | 50 ± 9.1 | 0.7 | 49 ± 6.7 | 83 ± 15 | 0.07 |
| E2(pg/ml) | <10b | <10b | 1.0 | 16 ± 1.8c | 215 ± 60c | 0.01 |
Data are the mean ± sem. Outpatient screening was before sex-steroid administration. Preinfusion data was from after sex-steroid administration but before ghrelin/saline infusion.
One sample missing.
Measured by RIA.
Measured by liquid chromatography-tandem mass spectrometry.
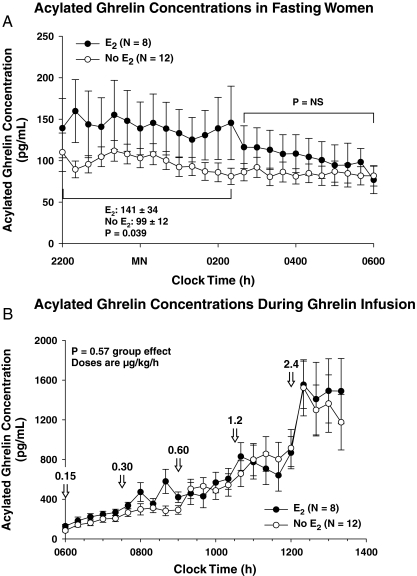
Two-way ANOVA of overnight acylated ghrelin concentrations revealed 1.42-fold higher values in E2-treated than E2-untreated women during the interval 2200–0210 h (P = 0.039). Ghrelin levels then declined to a similar mean (0220–0600 h; (Fig. 3A). Acylated ghrelin concentrations did not vary during saline infusion but rose stepwise during ghrelin infusion (Fig. 3B). There were no statistically significant differences in acylated ghrelin concentrations between untreated and E2-treated cohorts at any infusion rate.
Figure 3.
A, Fasting plasma acylated ghrelin concentrations monitored overnight every 20 min. The indicated difference was determined by two-way ANOVA and Tukey’s test. B, Plasma acylated ghrelin concentrations monitored every 20 min beginning at 0600 h after initiation of ghrelin infusion of 0.15 μg/kg·h, which was doubled every 90 min to a maximal rate of 2.4 μg/kg·h. Data are the mean ± sem (n = 12, no-E2 group; n = 8, E2 group). MN, Midnight; NS, not significant.
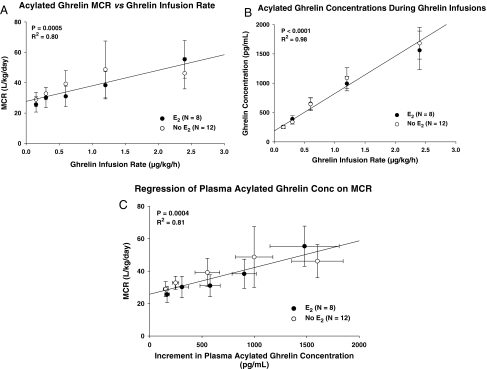
Figure 4 (top) shows the mean linear relationship between ghrelin infusion rates and plasma acylated ghrelin concentrations measured at the end of each 90 min. There was a strong correlation in both the E2 and no-E2 cohort. Because the slopes and y-intercepts did not differ at multiple-comparison P < 0.01 (Table 3), linear regression was applied to the combined groups (n = 20 subjects).
Figure 4.
Top, Regression of the MCR of acylated ghrelin on the ghrelin infusion rate in the combined groups (n = 20 volunteers). Numerical values are the square of the correlation coefficient and associated P value. Middle, Regression of acylated ghrelin concentration on ghrelin infusion rate (see Fig. 3 legend). Bottom, Regression of the MCR on the increment in plasma acylated ghrelin concentration above the fasting baseline (see Fig. 4 legend).
Table 3.
Weighted linear regression analysis: no-E2 vs. E2 comparisons
| P value | r2 | slope | y-intercept | |
|---|---|---|---|---|
| Ghrelin concentration vs. ghrelin infusion | ||||
| No E2 (n = 12) | 0.0012 | 0.98 | 676 ± 18 | 166 ± 13 |
| E2 (n = 8) | 0.0013 | 0.98 | 602 ± 17 | 205 ± 14 |
| Combined (n = 20) | <0.0001 | 0.98 | 638 ± 12 | 186 ± 9.5 |
| MCR vs. ghrelin infusion rate | ||||
| No E2 (n = 12) | 0.084 | 0.68 | 7.9 ± 3.5 | 31 ± 3.9 |
| E2 (n = 8) | 0.0011 | 0.98 | 13 ± 3.6 | 24 ± 3.7 |
| Combined (n = 20) | 0.0005 | 0.80 | 10 ± 2.5 | 28 ± 2.7 |
| MCR vs. ghrelin concentration increment | ||||
| No E2(n = 12) | 0.043 | 0.79 | 0.013 ± 0.005 | 30 ± 4.3 |
| E2 (n = 8) | 0.0054 | 0.95 | 0.021 ± 0.006 | 22 ± 4.4 |
| Combined (n = 20) | 0.0004 | 0.81 | 0.017 ± 0.004 | 26 ± 3.0 |
Data are the mean ± sem with n as indicated.
Estimated MCRs of acylated ghrelin were linearly dependent on the ghrelin infusion rate (Fig. 4, middle). As summarized in Table 3, slopes and intercepts were not affected by E2 supplementation (P > 0.20). Hence, a single regression was estimated for all 20 subjects. A linear concentration-dependent relationship was also observed when MCR values were regressed on incremental plasma acylated ghrelin concentrations. The E2 and no-E2 cohorts did not differ with respect to regression parameters (Table 3), allowing a combined slope estimate (Fig. 4, bottom).
NONMEM estimated a mean MCR of 43 liters/kg·d during the 8-h ghrelin infusion with no effect of the E2 regression parameter, thus corroborating the foregoing inference.
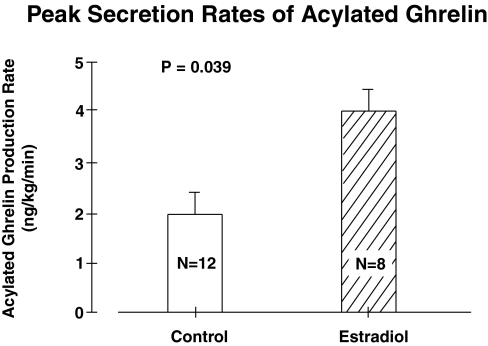
Production rate estimates were made using the mean plasma acylated ghrelin concentrations at 2200–0210 h, when the E2 elevated acylated ghrelin levels by 1.42-fold. The secretion rate is the product of MCR and the concentration of acylated ghrelin (141 ± 34 pg/ml for E2 and 99 ± 12 pg/ml for no E2). The MCRs of acylated ghrelin extrapolated to these concentrations are 27 and 26 liters/kg·d. Resultant calculated secretion rates were 2-fold higher in the presence than in the absence of E2 (P = 0.039; Fig. 5).
Figure 5.
Acylated ghrelin secretion rates overnight during peak endogenous ghrelin concentrations in 12 women given E2 and 10 others not given E2 supplementation. Data are the mean ± sem. The comparison is by unpaired t test.
Discussion
The present prospectively randomized, placebo-controlled, double-blind investigation used a five-step graded ghrelin infusion regimen to estimate the MCR of acylated ghrelin in estrogen-deficient and estrogen-supplemented postmenopausal women. Analyses demonstrated that E2 does not affect the MCR of bioactive ghrelin. Accordingly, the 42% higher nighttime concentrations of acylated ghrelin attained during E2 supplementation must reflect increased production of acylated peptide. The demonstration of comparable MCRs with and without E2 administration was corroborated by an independent population model of ghrelin kinetics. The mechanisms mediating increased secretion of acylated ghrelin are unknown. Because genes encoding the ghrelin peptide and ghrelin octanoyl-acyltransferase enzyme are expressed predominantly in the stomach and proximal intestine (1,23), we speculate that E2 induces gastroduodenal ghrelin and/or ghrelin acyltransferase.
Overnight acylated ghrelin concentrations peaked at 2200–0210 h in the E2-exposed cohort. This observation might reflect transient stimulation by higher initial E2 concentrations produced by the E2 patch applied at bedtime (26). The inference would be consistent with the postulate that estrogen stimulates gastroduodenal ghrelin production. Three other studies in younger adults also noted that either total or acylated ghrelin concentrations peak during the time window 2200–0300 h (37). The present data indicate that E2 enhances this nighttime rhythm in postmenopausal women. The basis for the nighttime rhythmicity is not established. However, acylation proceeds in the gastrointestinal tract and deacylation in the stomach via lysophospholipase I (24) and plasma via butyrylcholinesterase (38). Although GH, free fatty acids, and cortisol exhibit diurnal rhythmicity, GH does not affect whereas free fatty acids and cortisol suppress (total) ghrelin concentrations (39,40,41). Thus, low cortisol concentrations at and before midnight may contribute to up-regulation of ghrelin secretion.
Several studies have evaluated ghrelin gene expression in the stomach. The results are discrepant. Gualillo et al. (42) observed no effect of ovariectomy or orchiectomy on gastric ghrelin mRNA in adult rats. However, Sakata et al. (43) reported that estrogen produced locally in the stomach stimulates ghrelin gene expression even after gonadectomy. A third study inferred suppressive effects of ovarian E2 on stomach ghrelin gene expression as well on the plasma ghrelin concentrations (44). Coexpression of ghrelin and estrogen receptor-α in gastric epithelial cells would be consistent with a possible role of estrogen in directing ghrelin expression (44). Further studies are required in the human to address this point, given that ghrelin can also arise from other sources, including brain, pituitary gland, pancreas, intestine, gonad, and kidneys (2).
Bolus and steady-state infusions of ghrelin yielded concentration-dependent (median) estimates of the MCR of acylated ghrelin of 16–58 liters/kg·d. By way of comparison, the MCR of GH in women is about 3.8 liters/kg·d (45), testosterone 7 liters/kg·d (46), and dehydroepiandrosterone and androstenedione 27 liters/kg·d (based on 70-kg body weight) (46). Thus, the MCR of acylated ghrelin is quite high. The MCR of acylated ghrelin (1 μg/kg) in the bolus study was 7.2-fold greater than that of total ghrelin in the same subjects, in part reflecting a 3.1-fold larger distribution volume. The corresponding half-life of acylated ghrelin was 42% shorter than that of total ghrelin. These data establish that acylated peptide is removed from plasma and/or is inactivated in plasma much more rapidly than total peptide.
Only one other study has evaluated the disappearance of acylated ghrelin (22). Seven clinical studies have estimated the disappearance of total ghrelin in adults (11,18,21,22,47,48,49). In these analyses, bolus ghrelin doses ranged from 1–10 μg/kg, and continuous ghrelin infusion rates from 3.4–16.9 ng/kg·min. Mean terminal half-life estimates for total ghrelin varied from as low as 10 min to as high as 31 min after bolus injection and 146 min after constant infusion. Analogous disparities exist for estimated MCRs of total ghrelin, extending from 2–59 liters/kg·d. Factors that account for such variable published estimates of total ghrelin kinetics are not known. The one assessment of acylated ghrelin kinetics reported a terminal half-life of 9.6 min and MCR of 42 liters/kg·d (22). The use of graded ghrelin infusions in the current investigation revealed a positive linear correlation between ghrelin infusion rate (or plasma acylated ghrelin concentration) and the MCR of acylated peptide (Fig. 4). Thus, the concentration dependence of ghrelin kinetics may contribute to earlier disparities in the ghrelin literature.
In conclusion, analyses of bolus and graded continuous iv infusions of ghrelin indicate that the MCR of acylated ghrelin is dose and concentration dependent. Moreover, the MCR of the acylated form is greater than that of total ghrelin. Inasmuch as estrogen supplementation elevates acylated ghrelin concentrations without altering the MCR, a plausible postulate is that estrogen stimulates gastroduodenal synthesis and/or acylation of this peptide.
Acknowledgments
We thank Kay Nevinger for support of manuscript preparation, Ashley Bryant for data analysis and graphics, the Mayo Immunochemical Laboratory for assay assistance, and the Mayo research nursing staff for implementing the protocol.
Footnotes
This work was supported in part via the Center for Translational Science Activities (CTSA) Grant Number 1 UL 1 RR024150 to the Mayo Clinic and Foundation from the National Center for Research Resources (Rockville, MD) and R01 NIA AG29362 and AG19695 from the National Institutes of Health (Bethesda, MD).
Disclosure Statement: The authors have nothing to disclose.
First Published Online August 12, 2008
Abbreviations: CRU, Clinical Research Unit; E2, estradiol; GHS, GH secretagogue; IGFBP, IGF-binding protein; MCR, metabolic clearance rate; NONMEM, nonlinear mixed-effects modeling; Vd, volume of distribution.
References
- Kojima M, Kangawa K 2005 Ghrelin: structure and function. Physiol Rev 85:495–522 [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Roemmich JN, Richmond EJ, Bowers CY 2006 Somatotropic and gonadotropic axes linkages in infancy, childhood, and the puberty-adult transition. Endocr Rev 27:101–140 [DOI] [PubMed] [Google Scholar]
- Shuto Y, Shibasaki T, Otagiri A, Kuriyama H, Ohata H, Tamura H, Kamegai J, Sugihara H, Oikawa S, Wakabayashi I 2002 Hypothalamic growth hormone secretagogue receptor regulates growth hormone secretion, feeding, and adiposity. J Clin Invest 109:1429–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaya K, Ariyasu H, Kanamoto N, Iwakura H, Yoshimoto A, Harada M, Mori K, Komatsu Y, Usui T, Shimatsu A, Ogawa Y, Hosoda K, Akamizu T, Kojima M, Kangawa K, Nakao K 2000 Ghrelin strongly stimulates growth hormone release in humans. J Clin Endocrinol Metab 85:4908–4911 [DOI] [PubMed] [Google Scholar]
- Arvat E, Maccario M, Di Vito L, Broglio F, Benso A, Gottero C, Papotti M, Muccioli G, Dieguez C, Casanueva FF, Deghenghi R, Camanni F, Ghigo E 2001 Endocrine activities of ghrelin, a natural growth hormone secretagogue (GHS), in humans: comparison and interactions with hexarelin, a nonnatural peptidyl GHS, and GH-releasing hormone. J Clin Endocrinol Metab 86:1169–1174 [DOI] [PubMed] [Google Scholar]
- Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, Dhillo WS, Ghatei MA, Bloom SR 2001 Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab 86:5992 [DOI] [PubMed] [Google Scholar]
- Di Vito L, Broglio F, Benso A, Gottero C, Prodam F, Papotti M, Muccioli G, Dieguez C, Casanueva FF, Deghenghi R, Ghigo E, Arvat E 2002 The GH-releasing effect of ghrelin, a natural GH secretagogue, is only blunted by the infusion of exogenous somatostatin in humans. Clin Endocrinol (Oxf) 56:643–648 [DOI] [PubMed] [Google Scholar]
- Pantel J, Legendre M, Cabrol S, Hilal L, Hajaji Y, Morisset S, Nivot S, Vie-Luton MP, Grouselle D, de Kerdanet M, Kadiri A, Epelbaum J, Le Bouc Y, Amselem S 2006 Loss of constitutive activity of the growth hormone secretagogue receptor in familial short stature. J Clin Invest 116:760–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giustina A, Veldhuis JD 1998 Pathophysiology of the neuroregulation of growth hormone secretion in experimental animals and the human. Endocr Rev 19:717–797 [DOI] [PubMed] [Google Scholar]
- Weikel JC, Wichniak A, Ising M, Brunner H, Friess E, Held K, Mathias S, Schmid DA, Uhr M, Steiger A 2003 Ghrelin promotes slow-wave sleep in humans. Am J Physiol 284:E407–E415 [DOI] [PubMed] [Google Scholar]
- Nagaya N, Kojima M, Uematsu M, Yamagishi M, Hosoda H, Oya H, Hayashi Y, Kangawa K 2001 Hemodynamic and hormonal effects of human ghrelin in healthy volunteers. Am J Physiol 280:R1483–R1487 [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Evans WS, Bowers CY 2003 Estradiol supplementation enhances submaximal feedforward drive of growth hormone (GH) secretion by recombinant human GH-releasing hormone-1,44-amide in a putatively somatostatin-withdrawn milieu. J Clin Endocrinol Metab 88:5484–5489 [DOI] [PubMed] [Google Scholar]
- Anderson SM, Shah N, Evans WS, Patrie JT, Bowers CY, Veldhuis JD 2001 Short-term estradiol supplementation augments growth hormone (GH) secretory responsiveness to dose-varying GH-releasing peptide infusions in healthy postmenopausal women. J Clin Endocrinol Metab 86:551–560 [DOI] [PubMed] [Google Scholar]
- Anderson SM, Wideman L, Patrie JT, Weltman A, Bowers CY, Veldhuis JD 2001 Estradiol supplementation selectively relieves GH’s autonegative feedback on GH-releasing peptide-2-stimulated GH secretion. J Clin Endocrinol Metab 86:5904–5911 [DOI] [PubMed] [Google Scholar]
- Bowers CY, Laferrere B, Hurley DL, Veldhuis JD 2008 The role of growth hormone and ghrelin in feeding and body composition. In Donahoe PA, ed. Energy metabolism and obesity: research and clinical applications. Totowa, NJ: Humana;125–154 [Google Scholar]
- Nagaya N, Miyatake K, Uematsu M, Oya H, Shimizu W, Hosoda H, Kojima M, Nakanishi N, Mori H, Kangawa K 2001 Hemodynamic, renal, and hormonal effects of ghrelin infusion in patients with chronic heart failure. J Clin Endocrinol Metab 86:5854–5859 [DOI] [PubMed] [Google Scholar]
- Nagaya N, Kojima M, Kangawa K 2006 Ghrelin, a novel growth hormone-releasing peptide, in the treatment of cardiopulmonary-associated cachexia. Intern Med 45:127–134 [DOI] [PubMed] [Google Scholar]
- Neary NM, Small CJ, Wren AM, Lee JL, Druce MR, Palmieri C, Frost GS, Ghatei MA, Coombes RC, Bloom SR 2004 Ghrelin increases energy intake in cancer patients with impaired appetite: acute, randomized, placebo-controlled trial. J Clin Endocrinol Metab 89:2832–2836 [DOI] [PubMed] [Google Scholar]
- Pihoker C, Badger TM, Reynolds GA, Bowers CY 1997 Treatment effects of intranasal growth hormone releasing peptide-2 in children with short stature. J Endocrinol 155:79–86 [DOI] [PubMed] [Google Scholar]
- Svensson J, Monson JP, Vetter T, Hansen TK, Savine R, Kann P, Bex M, Reincke M, Hagen C, Beckers A, Ilondo MM, Zdravkovic M, Bengtsson BA, Korbonits M 2003 Oral administration of the growth hormone secretagogue NN703 in adult patients with growth hormone deficiency. Clin Endocrinol (Oxf) 58:572–580 [DOI] [PubMed] [Google Scholar]
- Vestergaard ET, Hansen TK, Gormsen LC, Jakobsen P, Moller N, Christiansen JS, Jorgensen JO 2007 Constant intravenous ghrelin infusion in healthy young men: clinical pharmacokinetics and metabolic effects. Am J Physiol Endocrinol Metab 292:E1829–E1836 [DOI] [PubMed] [Google Scholar]
- Akamizu T, Takaya K, Irako T, Hosoda H, Teramukai S, Matsuyama A, Tada H, Miura K, Shimizu A, Fukushima M, Yokode M, Tanaka K, Kangawa K 2004 Pharmacokinetics, safety, and endocrine and appetite effects of ghrelin administration in young healthy subjects. Eur J Endocrinol 150:447–455 [DOI] [PubMed] [Google Scholar]
- Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL 2008 Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell 132:387–396 [DOI] [PubMed] [Google Scholar]
- Shanado Y, Kometani M, Uchiyama H, Koizumi S, Teno N 2004 Lysophospholipase I identified as a ghrelin deacylation enzyme in rat stomach. Biochem Biophys Res Commun 325:1487–1494 [DOI] [PubMed] [Google Scholar]
- Grinspoon S, Miller KK, Herzog DB, Grieco KA, Klibanski A 2004 Effects of estrogen and recombinant human insulin-like growth factor-I on ghrelin secretion in severe undernutrition. J Clin Endocrinol Metab 89:3988–3993 [DOI] [PubMed] [Google Scholar]
- Kellokoski E, Poykko SM, Karjalainen AH, Ukkola O, Heikkinen J, Kesaniemi YA, Horkko S 2005 Estrogen replacement therapy increases plasma ghrelin levels. J Clin Endocrinol Metab 90:2954–2963 [DOI] [PubMed] [Google Scholar]
- Di CC, Tommaselli GA, Gargano V, Sammartino A, Bifulco G, Tauchmanova L, Colao A, Nappi C 2007 Effects of estrogen-progestin therapy on serum levels of RANKL, osteoprotegerin, osteocalcin, leptin, and ghrelin in postmenopausal women. Menopause 14:38–44 [DOI] [PubMed] [Google Scholar]
- Pagotto U, Gambineri A, Pelusi C, Genghini S, Cacciari M, Otto B, Castaneda T, Tschop M, Pasquali R 2003 Testosterone replacement therapy restores normal ghrelin in hypogonadal men. J Clin Endocrinol Metab 88:4139–4143 [DOI] [PubMed] [Google Scholar]
- Purnell JQ, Weigle DS, Breen P, Cummings DE 2003 Ghrelin levels correlate with insulin levels, insulin resistance, and high-density lipoprotein cholesterol, but not with gender, menopausal status, or cortisol levels in humans. J Clin Endocrinol Metab 88:5747–5752 [DOI] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K 2000 Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402:656–660 [DOI] [PubMed] [Google Scholar]
- Erickson D, Keenan DM, Mielke K, Bradford K, Bowers CY, Miles JM, Veldhuis JD 2004 Dual secretagogue drive of burst-like growth hormone secretion in postmenopausal compared with premenopausal women studied under an experimental estradiol clamp. J Clin Endocrinol Metab 89:4746–4754 [DOI] [PubMed] [Google Scholar]
- Van den Berghe G, Wouters P, Weekers F, Mohan S, Baxter RC, Veldhuis JD, Bowers CY, Bouillon R 1999 Reactivation of pituitary hormone release and metabolic improvement by infusion of growth hormone-releasing peptide and thyrotropin-releasing hormone in patients with protracted critical illness. J Clin Endocrinol Metab 84:1311–1323 [DOI] [PubMed] [Google Scholar]
- Fisher LD, van Belle G 1996 Descriptive statistics. Biostatistics: a methodology for the health sciences. New York: John Wiley, Sons; 58–74 [Google Scholar]
- Sheiner LB, Rosenberg B, Marathe VV 1977 Estimation of population characteristics of pharmacokinetic parameters from routine clinical data. J Pharmacokinet Biopharm 5:445–479 [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Fraioli F, Rogol AD, Dufau ML 1986 Metabolic clearance of biologically active luteinizing hormone in man. J Clin Invest 77:1122–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal SL, Sheiner LB, Boeckmann AJ, eds 1989 NONMEM users guides (1989–2006). Ellicott City, MD: Icon Development Solutions [Google Scholar]
- Nass R, Farhy L, Liu J, Prudom C, Johnson ML, Veldhuis P, Pezzoli SS, Oliveri MC, Gaylinn BD, Geysen HM, Thorner MO 2008 Evidence for acyl-ghrelin modulation of growth hormone release in the fed state. J Clin Endocrinol Metab 93:1988–1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vriese C, Hacquebard M, Gregoire F, Carpentier Y, Delporte C 2007 Ghrelin interacts with human plasma lipoproteins. Endocrinology 148:2355–2362 [DOI] [PubMed] [Google Scholar]
- Gormsen LC, Nielsen C, Gjedsted J, Gjedde S, Vestergaard ET, Christiansen JS, Jorgensen JO, Moller N 2007 Effects of free fatty acids, growth hormone and growth hormone receptor blockade on serum ghrelin levels in humans. Clin Endocrinol (Oxf) 66:641–645 [DOI] [PubMed] [Google Scholar]
- Otto B, Tschop M, Heldwein W, Pfeiffer AF, Diederich S 2004 Endogenous and exogenous glucocorticoids decrease plasma ghrelin in humans. Eur J Endocrinol 151:113–117 [DOI] [PubMed] [Google Scholar]
- Espelund U, Hansen TK, Hojlund K, Beck-Nielsen H, Clausen JT, Hansen BS, Orskov H, Jorgensen JO, Frystyk J 2005 Fasting unmasks a strong inverse association between ghrelin and cortisol in serum: studies in obese and normal-weight subjects. J Clin Endocrinol Metab 90:741–746 [DOI] [PubMed] [Google Scholar]
- Gualillo O, Caminos JE, Kojima M, Kangawa K, Arvat E, Ghigo E, Casanueva FF, Dieguez C 2001 Gender and gonadal influences on ghrelin mRNA levels in rat stomach. Eur J Endocrinol 144:687–690 [DOI] [PubMed] [Google Scholar]
- Sakata I, Tanaka T, Yamazaki M, Tanizaki T, Zheng Z, Sakai T 2006 Gastric estrogen directly induces ghrelin expression and production in the rat stomach. J Endocrinol 190:749–757 [DOI] [PubMed] [Google Scholar]
- Matsubara M, Sakata I, Wada R, Yamazaki M, Inoue K, Sakai T 2004 Estrogen modulates ghrelin expression in the female rat stomach. Peptides 25:289–297 [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Bidlingmaier M, Anderson SM, Evans WS, Wu Z, Strassburger CJ 2002 Impact of experimental blockade of peripheral growth hormone (GH) receptors on the kinetics of endogenous and exogenous GH removal in healthy women and men. J Clin Endocrinol Metab 87:5737–5745 [DOI] [PubMed] [Google Scholar]
- Longcope C 1998 Androgen metabolism and the menopause. Semin Reprod Endocrinol 16:111–115 [DOI] [PubMed] [Google Scholar]
- Damjanovic SS, Lalic NM, Pesko PM, Petakov MS, Jotic A, Miljic D, Lalic KS, Lukic L, Djurovic M, Djukic VB 2006 Acute effects of ghrelin on insulin secretion and glucose disposal rate in gastrectomized patients. J Clin Endocrinol Metab 91:2574–2581 [DOI] [PubMed] [Google Scholar]
- Murray CD, Martin NM, Patterson M, Taylor SA, Ghatei MA, Kamm MA, Johnston C, Bloom SR, Emmanuel AV 2005 Ghrelin enhances gastric emptying in diabetic gastroparesis: a double blind, placebo controlled, crossover study. Gut 54:1693–1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druce MR, Wren AM, Park AJ, Milton JE, Patterson M, Frost G, Ghatei MA, Small C, Bloom SR 2005 Ghrelin increases food intake in obese as well as lean subjects. Int J Obes (Lond) 29:1130–1136 [DOI] [PubMed] [Google Scholar]