Thrombotic thrombocytopenic purpura (TTP) is a potentially fatal thrombotic microangiopathy (1). The mechanism underlying the initial onset and/or burst of TTP episode remains poorly understood. We hypothesize that certain inflammatory cytokines released during acute inflammation and/or infection may suppress biosynthesis of ADAMTS13, a metalloprotease that cleaves von Willebrand factor (vWF), which results in an acquired deficiency of plasma ADAMTS13 activity and TTP burst. To test this hypothesis, we determined the biological effects of several inflammatory cytokines including interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α) and interleukin-4 (IL-4), which are released during systemic inflammation and markedly elevated in patients with acute episode of TTP (2–5), on biosynthesis and secretion of ADAMTS13 metalloprotease in rat primary hepatic stellate cells (HSCs) and human primary umbilical vein endothelial cells (HUVECs), two major types of cells producing ADAMTS13 in humans (6, 7).
ADAMTS13 mRNA and/or proteolytic activity was determined in rat primary HSCs and HUVECs or medium after incubation with IFN-γ, TNF-α and IL-4. In addition, vWF antigen was also determined in the conditioned medium of HUVECs cells with or without cytokine treatment. ADAMTS13 mRNA and 18S ribosomal RNA (18S rRNA) were determined by a real-time PCR with an internal standard curve for quantification as described previously (6, 8). ADAMTS13 activity was determined by FRETS-vWF73 assay (9). The vWF antigen was determined by an enzyme-linked immunoassay using two polyclonal antibodies against human vWF for capturing and detection (10).
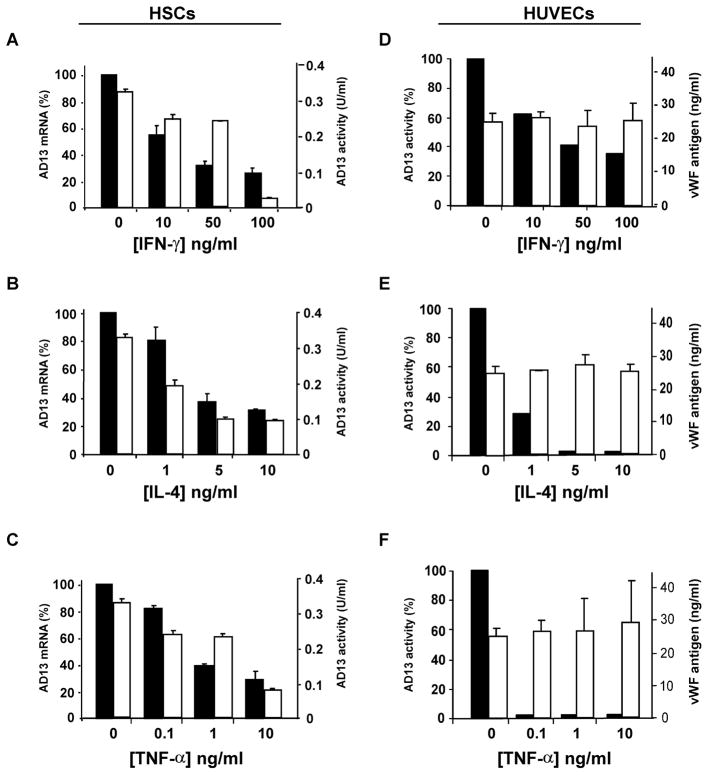
We showed that after 24 hours of treatment with IFN-γ (0, 10, 50 and 100 ng/ml), IL-4 (0, 1, 5 and 10 ng/ml) and TNF-α (0, 0.1, 1 and 10 ng/ml) in culture medium containing 10% fetal bovine serum, the levels of ADAMTS13 mRNA relative to 18S rRNA (Fig. 1A, B, and C, black bars) and proteolytic activity in primary rat HSCs (Fig. 1, A, B and C, white bars) and HUVECs (Fig. 1D, E and F, black bars) were significantly reduced in a dose-dependent manner. In contrast, the levels of vWF antigen in the conditioned medium of HUVECs were not significantly altered after similar treatment (Fig. 1D, E and F, white bars), suggesting that certain inflammatory cytokines selectively inhibit ADAMTS13 synthesis without triggering release of its known substrate, vWF.
Figure 1. Inhibition of ADAMTS13 expression by inflammatory cytokines in primary hepatic stellate cells and human endothelial cells.
Rat primary HSCs at 7 days after isolation (Panels A-C) and HUVECs at passage between 4 and 5 (Panels D-F) were treated with various concentrations of interferon-γ (INF-γ) (Panels A and D), interleukin-4 (IL-4) (panels B and E) and tumor necrosis factor-α (TNF-α) (Panels C and F) as indicated for 24 hours. The cells and conditioned media were collected. The relative levels of ADAMTS13 (AD13) mRNA to 18S rRNA quantified by a real-time PCR were shown in the y-axis (Panels A, B and C, black bars). The values in untreated cells were defined as 100%. ADAMTS13 activity in the conditioned medium of rat HSCs (Panels A, B and C, white bars) and HUVECs (Panels D, E and F, black bars) was determined by a FRETS-VWF73 assay using pooled normal human plasma as a reference (defined as 1 unit per ml). The vWF antigen in the conditioned medium of HUVECs (Panels D, E and F, white bars) was determined by a Sandwich ELISA assay. The entries represent the Mean ± SD of three independent experiments except for ADAMTS13 activity in HUVECs medium (Mean, n=2).
Moreover, incubation of IFN-γ (100 ng/ml), IL-4 (10 ng/ml) and TNF-α (10 ng/ml) with purified recombinant ADAMTS13 did not inhibit proteolytic cleavage of FRETS-vWF73 by ADAMTS13 in vitro (data not shown). These results are consistent with that reported by others (2). However, we could not exclude the possibility that the cytokines used inhibit ADAMTS13 activity under flow or in vivo. At least, a previous study has shown that interleukin-6 (IL-6) blocks the cleavage of newly synthesized “unusually large” (UL)-vWF multimers anchored on endothelial cell surface by ADAMTS13 under flow conditions (2).
The dramatic inhibition of biosynthesis and secretion of ADAMTS13 protease in rat primary hepatic stellate cells and human endothelial cells IFN-γ, IL-4 and TNF-α may offer a coherent explanation how systemic inflammation and/or infection might trigger onset and/or burst of TTP syndrome in patients with pre-existing low (threshold) levels of plasma ADAMTS13 activity, either owing to severely impaired secretion or proteolytic activity of ADAMTS13 protease as a result of mutations on ADAMTS13 gene (11) or because of autoantibodies that inhibit proteolytic activity of ADAMTS13 protease (12).
The specific activity of inflammatory cytokines on release of vWF or suppression of ADAMTS13 synthesis may vary from cytokine to cytokine under different conditions. For instance, IL-6 and TNF-α were shown to trigger the release of UL-vWF multimers from cultured endothelial cells, which could be visualized under microscope by their ability to bind flowing platelets (2). The data from this study, however, showed that IFN-γ, IL-4 and TNF-α inhibited ADAMTS13 synthesis without affecting vWF secretion (Fig. 1, D, E and F).
In conclusion, we demonstrate that several inflammatory cytokines significantly inhibit ADAMTS13 synthesis and secretion. These results may not only shed some light on the mechanism underlying cytokine-induced TTP burst, but also support a notion to routinely use corticosteroids as an adjunctive therapy to suppress systemic inflammation during acute TTP burst.
Acknowledgments
This study was supported in part by grants from the National Institutes of Health (HL079027) to X.L.Z. and Chinese Council Scholarship to W.J.C.
Authors thank Dr. Douglas Cines for providing us with human umbilical cords for isolation of vascular endothelial cells and Rebecca Wells for providing rat HSCs.
Reference List
- 1.Zheng XL, Sadler JE. Pathogenesis of Thrombotic Microangiopathies. Annual Rev Path Mech Dis. 2007;3:249–77. doi: 10.1146/annurev.pathmechdis.3.121806.154311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernardo A, Ball C, Nolasco L, Moake JF, Dong JF. Effects of inflammatory cytokines on the release and cleavage of the endothelial cell-derived ultralarge von Willebrand factor multimers under flow. Blood. 2004;104:100–6. doi: 10.1182/blood-2004-01-0107. [DOI] [PubMed] [Google Scholar]
- 3.Furlan M, Lammle B. Aetiology and pathogenesis of thrombotic thrombocytopenic purpura and haemolytic uraemic syndrome: the role of von Willebrand factor-cleaving protease. Best Pract Res Clin Haematol. 2001;14:437–54. doi: 10.1053/beha.2001.0142. [DOI] [PubMed] [Google Scholar]
- 4.Stefanescu R, Bassett D, Modarresi R, Santiago F, Fakruddin M, Laurence J. Synergistic interactions between interferon-γ and TRAIL modulate c-FLIP in endothelial cells, mediating their lineage-specific sensitivity to thrombotic thrombocytopenic purpura plasma-associated apoptosis. Blood. 2008 doi: 10.1182/blood-2007-10-119552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wada H, Kaneko T, Ohiwa M, Tanigawa M, Tamaki S, Minami N, Takahashi H, Deguchi K, Nakano T, Shirakawa S. Plasma cytokine levels in thrombotic thrombocytopenic purpura. Am J Hematol. 1992;40:167–70. doi: 10.1002/ajh.2830400303. [DOI] [PubMed] [Google Scholar]
- 6.Shang D, Zheng XW, Niiya M, Zheng XL. Apical sorting of ADAMTS13 in vascular endothelial cells and Madin-Darby canine kidney cells depends on the CUB domains and their association with lipid rafts. Blood. 2006;108:2207–15. doi: 10.1182/blood-2006-02-002139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uemura M, Tatsumi K, Matsumoto M, Fujimoto M, Matsuyama T, Ishikawa M, Iwamoto TA, Mori T, Wanaka A, Fukui H, Fujimura Y. Localization of ADAMTS13 to the stellate cells of human liver. Blood. 2005;106:922–4. doi: 10.1182/blood-2005-01-0152. [DOI] [PubMed] [Google Scholar]
- 8.Niiya M, Uemura M, XWZ, Pollak E, Dockal M, Scheiflinger F, Wells RGXZ. Increased ADAMTS13 proteolytic activity in rat hepatic stellate cells upon activation in vitro and in vivo. J Thromb Haemost. 2006;4:1063–70. doi: 10.1111/j.1538-7836.2006.01893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kokame K, Nobe Y, Kokubo Y, Okayama A, Miyata T. FRETS-VWF73, a first fluorogenic substrate for ADAMTS13 assay. Br J Haematol. 2005;129:93–100. doi: 10.1111/j.1365-2141.2005.05420.x. [DOI] [PubMed] [Google Scholar]
- 10.Tousoulis D, Tentolouris C, Bosinakou E, Apostolopoulos T, Kyriakides M, Toutouzas P. Von Willebrand factor in patients evolving Q-wave versus non-Q-wave acute myocardial infarction. Int J Cardiol. 1996;56:259–62. doi: 10.1016/0167-5273(96)02735-0. [DOI] [PubMed] [Google Scholar]
- 11.Kokame K, Matsumoto M, Soejima K, Yagi H, Ishizashi H, Funato M, Tamai H, Konno M, Kamide K, Kawano Y, Miyata T, Fujimura Y. Mutations and common polymorphisms in ADAMTS13 gene responsible for von Willebrand factor-cleaving protease activity. Proc Natl Acad Sci U S A. 2002;99:11902–7. doi: 10.1073/pnas.172277399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsai HM, Lian EC. Antibodies to von Willebrand factor-cleaving protease in acute thrombotic thrombocytopenic purpura. N Engl J Med. 1998;339:1585–94. doi: 10.1056/NEJM199811263392203. [DOI] [PMC free article] [PubMed] [Google Scholar]