Abstract
Profound thrombocytopenia and microangiopathic hemolytic anemia characterize thrombotic microangiopathy, which includes two major disorders: thrombotic thrombocytopenic purpura (TTP) and hemolytic uremic syndrome (HUS). TTP has at least three types: congenital or familial, idiopathic, and nonidiopathic. The congenital and idiopathic TTP syndromes are caused primarily by deficiency of ADAMTS13, owing to mutations in the ADAMTS13 gene or autoantibodies that inhibit ADAMTS13 activity. HUS is similar to TTP, but is associated with acute renal failure. Diarrhea-associated HUS accounts for more than 90% of cases and is usually caused by infection with Shiga-toxin-producing Escherichia coli (O157:H7). Diarrhea-negative HUS is associated with complement dysregulation in up to 50% of cases, caused by mutations in complement factor H, membrane cofactor protein, factor I or factor B, or by autoanti-bodies against factor H. The incomplete penetrance of mutations in either ADAMTS13 or complement regulatory genes suggests that precipitating events or triggers may be required to cause thrombotic microangiopathy in many patients.
Keywords: thrombotic thrombocytopenic purpura, hemolytic uremic syndrome, von Willebrand factor-cleaving metalloprotease, ADAMTS13, complement dysregulation
INTRODUCTION
Thrombotic microangiopathy (TMA) is characterized pathologically by occlusive microvascular thrombosis and clinically by profound thrombocytopenia, microangiopathic hemolytic anemia, and variable signs and symptoms of organ ischemia. TMA includes primarily two syndromes, thrombotic thrombocytopenic purpura (TTP) and hemolytic uremic syndrome (HUS), and each of these syndromes appears to be caused by several distinct pathophysiologic mechanisms.
TTP was first described by Moschcowitz in 1924 (1), who reported of a 16-year-old girl admitted into a hospital in New York city because of acute fever, severe anemia, heart failure, and stroke. Unfortunately, the patient died within two weeks of illness. A limited autopsy showed extensive hyaline thrombi in terminal arterioles and capillaries of the heart and kidney. In a review of 271 cases, Amorosi & Ultmann (2) in 1966 established a diagnostic pentad for TTP: thrombocytopenia, microangiopathic hemolytic anemia, neurologic symptoms and signs, various degrees of renal functional abnormalities, and fever without other explanation. In the 1970s, plasma infusion and plasma exchange were shown to be effective treatments for TTP, and since that time the diagnostic criteria have evolved to be less stringent (2, 3). Currently, a patient presenting with thrombocytopenia and microangiopathic hemolytic anemia, with no comorbidities that could explain these findings, is given a presumptive diagnosis of TTP, and plasma exchange therapy is initiated as soon as possible (3, 4) because the mortality of untreated TTP is as high as 85% to 100% (5), whereas plasma exchange has reduced the mortality to between 10% and 30%.
HUS is another type of TMA syndrome, reported first by Gasser et al. in 1955 (6), and distinguished from TTP by the presence of acute renal failure in addition to the thrombocytopenia and microangiopathic hemolytic anemia common to both syndromes. HUS can be classified into two types: diarrhea-associated (D+) or typical HUS, and diarrhea-negative (D−) or atypical HUS. D+HUS is associated with a prodromal diarrheal illness usually caused by infection with Escherichia coli O157 or another Shiga-toxin-producing strain of bacteria. D+HUS constitutes approximately 95% of the HUS cases in children. It does occur in adults, but is less common. D-HUS is relatively rare and very heterogeneous as to its etiology and its age of onset and clinical presentations.
The clinical distinction between TTP and HUS does not always correlate perfectly with the characteristic pathophysiologic mechanisms associated with these syndromes. For example, some patients with TTP caused by ADAMTS13 deficiency do develop significant renal insufficiency (4, 7, 8). Conversely, some patients with Shiga-toxin-associated D+HUS develop neurologic symptoms (4, 7, 8). Furthermore, some patients diagnosed with HUS have been reported to respond to plasma exchange therapy (4, 7, 8). For this reason, plasma exchange therapy is usually offered to all adults who meet the criteria of thrombocytopenia and microangiopathic hemolytic anemia, with or without neurologic symptoms or renal dysfunction. In this review, such patients are discussed in the section on Thrombotic Thrombocytopenic Purpura, which is roughly equivalent to the term TTP-HUS (3, 4), or TMA (9, 10) as used by others. HUS refers to a distinct group of disorders, usually occurring in children and associated with severe renal failure and typically caused by infection with Shiga-toxin-producing E. coli (11, 12), by complement dysregulation (13–15), or by other unknown mechanisms. This review focuses on our current understanding of the molecular pathogenesis of TTP and HUS, which may provide some guidance for the diagnosis and treatment of these potentially fatal illnesses.
THROMBOTIC THROMBOCYTOPENIC PURPURA
TTP can be classified into at least three distinct entities: congenital TTP (also named Upshaw-Schülman syndrome), idiopathic TTP, and nonidiopathic TTP (8). Patients with congenital TTP have severe deficiency of ADAMTS13, a plasma metalloprotease that cleaves von Willebrand factor (VWF) (16–19). Idiopathic TTP is usually caused by acquired deficiency of the same metalloprotease owing to autoantibodies that inhibit ADAMTS13 activity or induce its clearance from the circulation (17, 20, 21). Nonidiopathic TTP is associated with conditions or comorbidities, including hematopoietic progenitor cell transplantation (HPCT) (22–25), certain drugs (25–27), malignancy (28, 29), and pregnancy (30–32). These various conditions may directly injure endothelial cells, resulting in the deposition of platelets and fibrin and the formation of microvascular thrombi independent of VWF or ADAMTS13. Further investigation of the molecular mechanisms that cause nonidiopathic TTP may eventually provide some guidance for the diagnosis, classification, and treatment of this heterogeneous group of patients.
Incidence and Risk Factors
TTP is relatively rare, but its incidence appears to be rising, probably because of increased awareness of the diagnosis and the availability of plasma exchange as an effective treatment. In the United States, several thousand new cases of idiopathic TTP are diagnosed annually, with an estimated incidence of 3 to 10 per one million residents per year (33, 34). The incidence of nonidiopathic TTP appears to be much higher, but difficult to determine accurately. For instance, approximately 5% of patients with disseminated malignancy are reported to have TTP (35). However, the signs of concurrent disseminated intravascular coagulation often are present and may invalidate a diagnosis of TTP. Various malignancies including adenocarcinomas, breast cancer, small cell lung cancer, squamous cell carcinomas, thymoma, Hodgkin disease, and non-Hodgkin lymphoma have been shown to be associated with TTP. The incidence of TTP following HPCT varies considerably, ranging from 0% to 74% with a median incidence of 7.9% (36). The wide range of reported incidences probably reflects the use of different diagnostic criteria as well as other confounding complications associated with HPCT. In particular, underlying infection or sepsis after HPCT can mimic the hematologic features of TTP (36). Human immunodeficiency virus (HIV) infection can be associated with TTP (7, 37). In a recent study, the prevalence of TMA in HIV-positive patients was 0.3%, occurring mainly in patients with advanced HIV disease (38). Women who are pregnant or in the postpartum period make up 12% to 31% of TTP patients in some series (32, 39, 40). The estimated incidence of TTP in women with pregnancy is reported to be approximately 1 in 25,000 births (41), with about three-fourths of these patients present with symptoms in their third trimester or peripartum. The decrease in plasma ADAMTS13 activity (42) and increase in procoagulants such as VWF (42) and factor VIII in the second and third trimester may result in a prothrombotic status in pregnant women.
Many drugs including quinine, mitomycin, cyclosporine, FK506, ticlopidine, and clopidogrel may cause TTP. The estimated incidence of TTP in patients who take ticlopidine, a potent antiplatelet agent used to maintain patency after coronary artery stenting, is approximately 1 in 1600 to 5000 patients (27, 43). Clopidogrel, another related antiplatelet drug, has replaced ticlopidine as the agent of choice to combine with aspirin for patients undergoing arterial stenting. The incidence rate of TTP is lower with clopidogrel than with ticlopidine and is estimated to be 1.2 to 27.8 per million (26, 27, 44–46).
Histopathology
Tissue biopsy is neither necessary nor sensitive for diagnosing TTP. If performed, the characteristic pathological features for TTP include hyaline thrombi in terminal arterioles and capillaries. The thrombotic lesions are accompanied by localized endothelial cell proliferation and detachment. In patients who died of TTP, microthrombi may be found in all tissue organs, most strikingly in the myocardium, with variable involvement of the kidney, pancreas, adrenal glands, and brain (47). The pulmonary and hepatic vessels are usually spared (47). Immunohistochemistry may show that the platelet thrombi are rich in VWF, but contain little fibrinogen or fibrin deposition in the lesions (48, 49). Immunoglobulins and complement components are often found in the thrombi as well, which is consistent with the autoimmune nature of acquired TTP in most cases. The organ involvement and immunohistochemical feature of the microthrombi found in patients with TTP are quite different from those in patients with HUS, which will be discussed further in the Histopathology of Hemolytic Uremic Syndrome section.
von Willebrand Factor
VWF is synthesized in all vascular endothelial cells and megakaryocytes. A monomer of proVWF (275 kDa) is initially synthesized in the endoplasmic reticulum and forms dimers there via disulfide bonds at the carboxyl terminus. After transport to the Golgi apparatus, proVWF dimers form homopolymers or multimers through an additional disulfide bond near the amino terminus of the mature subunit, and the propeptide is then cleaved from proVWF by furin. The multimeric VWF is stored in the Weibel-Palade bodies of endothelial cells and α-granules of platelets. A small fraction of VWF multimers is constitutively secreted from endothelial cells. Upon stimulation, VWF is secreted from endothelial cells as unusually large VWF (UL-VWF) multimers that form string-like structures attached to the endothelial cell surface, perhaps through interaction with P-selectin (50). Under fluid shear stress, the UL-VWF strings are cleaved by ADAMTS13 at the Tyr1605-Met1606 bond in the A2 domain (51) to generate the range of VWF multimer sizes that normally circulate in the blood, from approximately 500 kDa to 20 million Daltons (52). The proteolytic cleavage of VWF multimers by plasma ADAMTS13 appears to be critical to preventing thrombosis in the microvasculature.
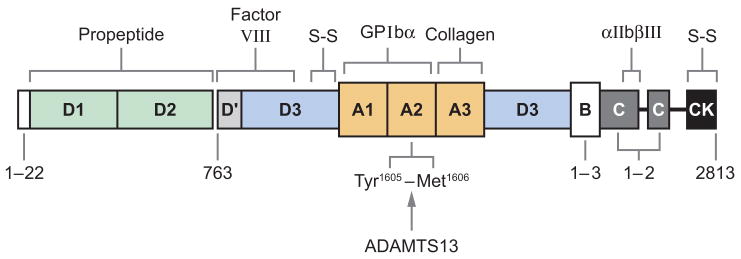
VWF stabilizes factor VIII in the circulation and mediates platelet adhesion to sites of vascular injury. These hemostatic functions depend upon the ability of VWF to bind circulating factor VIII, subendothelial collagens, platelet glycoprotein Ibα (GPIbα), and integrin αIIbβIII, but the feedback inhibition of platelet adhesion depends upon cleavage by ADAMTS13 (Figure 1) (52). However, VWF in plasma adopts a compact conformation that does not bind tightly to platelet GPIbα and is not cleaved by ADAMTS13. Fluid shear stress (53), or binding to certain surfaces, changes the conformation of VWF so that it binds platelet GPIbα tightly and can be recognized by ADAMTS13. A similar modulating effect in vitro is achieved by including antibiotic ristocetin or by denaturing reagents such as urea and guanidine-HCl (51, 54). In addition, certain mutations in the VWF protein increase the proteolysis of VWF and reduce the size of VWF multimers, leading to the compromised hemostatic function in patients with von Willebrand disease (52, 55). Conversely, inability to cleave the newly released UL-VWF multimers (18, 21, 56) owing to hereditary or acquired deficiency of plasma ADAMTS13 activity may induce spontaneous VWF-dependent platelet adhesion and aggregation (57), leading to disseminated microvascular thrombosis as seen in patients with TTP.
Figure 1.
Structure-function relationships of von Willebrand factor. Binding sites are indicated for collagen, factor VIII, platelet glycoprotein Ibα (GPIbα), and integrin αIIbβ III. The cleavage site (Tyr1605-Met1606) for ADAMTS13 is located at the central A2 domain of von Willebrand factor. The locations of intersubunit disulfide bonds (S-S) are shown.
ADAMTS13 Protease
Molecular cloning of ADAMTS13
Moake et al. (56) first proposed a link between TTP and a defect in VWF proteolysis in 1982, on the basis of the occurrence of potentially thrombogenic UL-VWF multimers in the plasma of patients with chronic relapsing TTP. In 1996, Tsai (51) and Furlan et al. (54) reported a metalloprotease that specifically cleaves VWF. This plasma protease cleaved the Tyr1605-Met1606 bond in domain A2 when VWF multimers were incubated in a buffer containing low-concentration guanidine-HCl (51) or urea (54). The proteolytic activity required divalent cations such as Ba2+, Zn2+, Ca2+, or Co2+ (51). In 2001 the protease was purified to homogeneity and its partial amino terminal sequence was determined (58, 59). This led to its identification as a new member of the ADAMTS (a disintegrin and metalloprotease with thrombospondin-type repeats) family, designated ADAMTS13 (60–62). Simultaneously, the ADAMTS13 gene was identified and cloned by a genome-wide linkage analysis in families with congenital TTP (63). The ADAMTS13 gene is located on chromosome 9q34, spanning 37 kb in length and containing 29 exons (62, 63). Several alternatively spliced mRNA variants have been characterized; their significance remains unknown (62, 63).
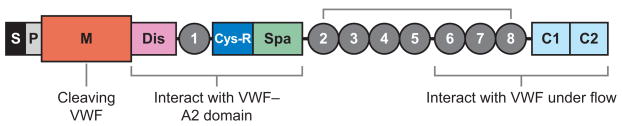
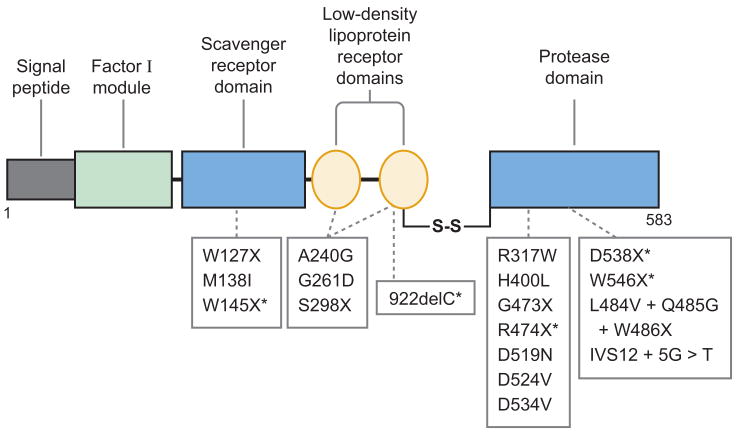
The ADAMTS family of zinc metallo-proteases contains 19 members that share the common structure of a hydrophobic signal sequence, a propeptide, a metallo-protease domain, a thrombospondin type 1 (TSP1) repeat, a disintegrin-like domain, a cysteine-rich domain, and a spacer domain (Figure 2 ) (62, 63). The carboxyl terminus of ADAMTS13 contains seven more TSP1 repeats and two CUB domains (Figure 2), which are named after motifs first identified in complement components C1r and C1s, sea urchin protein UEGF (or fibropellin), and bone morphogenetic protein (64).
Figure 2.
The structure of ADAMTS13. The structural domains are indicated: signal peptide (S), propeptide (P), metalloprotease (M), disintegrin domain (Dis), first thrombospondin type 1 (TSP1) repeat, cysteine-rich domain (Cys-R), spacer domain (Spa), and two CUB domains (C1 and C2). The metalloprotease domain is the catalytic center that cleaves von Willebrand factor (VWF). The proximal carboxyl-terminal domains from Dis to Spa interact with the A2 domain of VWF. More distal carboxyl-terminal domains (TSP1 2–8) interact with VWF under fluid shear stress.
Biosynthesis of ADAMTS13
ADAMTS13 is synthesized in the liver (60–63), endothelial cells (65, 66), and megakaryocytes or platelets (67, 68) and secreted into plasma as an active enzyme. The ADAMTS13 primary translation product consists of 1427 amino acid residues. It is extensively glycosylated and has a mass of ~170–195 kDa. Mutations in the ADAMTS13 gene (65) or inflammatory cytokines (69) may result in a reduced or an aberrant secretion of ADAMTS13 protein into the circulation. Various truncated forms of ADAMTS13 are detectable in plasma (70), perhaps owing to alternative splicing of ADAMTS13 mRNA or proteolysis of ADAMTS13 by serine proteases such as thrombin (71) and leukocyte elastase (72). Human placenta and skeletal muscle synthesize a 2.4-kb ADAMTS13 mRNA (62).
In human and mouse liver, the ADAMTS13 mRNA and protein are localized to the hepatic stellate cells (73, 74) that lie between the hepatocytes and endothelial cells. ADAMTS13 mRNA or activity is not detectable in mouse hepatocytes, sinusoidal endothelial cells, or Küppfer cells in vivo or in culture (73). Cell lines derived from normal hepatocytes (THLE-3) or hepatocellular carcinoma (HuH-7) do not express detectable ADAMTS13 activity, but those derived from hepatic stellate cells, such as human LX-2 cells (75), human hTERT-HSC, rat primary hepatic stellate cells (73), and rat CFSC-3H and -8B (73), do express ADAMTS13, supporting the conclusion that hepatic stellate cells produce ADAMTS13 in liver.
However, the contribution of hepatic stellate cells to plasma levels of ADAMTS13 remains to be determined. Patients with severe cirrhosis, who show significant activation and proliferation of hepatic stellate cells and liver fibrosis, have decreased or variable levels of plasma ADAMTS13 activity (42). In a rat model, plasma ADAMTS13 activity is not significantly increased after injection of carbon tetrachloride, even though this treatment causes hepatic stellate cell activation, extensive liver fibrosis, and dramatic local increases of ADAMTS13 mRNA and protease activity (75). The data suggest that ADAMTS13 may function locally to modulate hepatic fibrosis or cirrhosis, possibly by preventing microvascular thrombosis after injury. Considering the large surface area of vascular endothelial beds, plasma ADAMTS13 might be derived mainly from endothelial cells even though each endothelial cell produces little ADAMTS13 compared to hepatic stellate cells (65, 66).
The ADAMTS13 synthesized in endothelial cells appears to be delivered directly to the apical domain of polarized cells (i.e., toward the lumen of the vessels) (65). Deletion of the CUB domains, unique to ADAMTS13, abolishes the polarized (apical) secretion of ADAMTS13 in transfected Madin-Darby canine kidney cells (65), suggesting that the CUB domains may interact with intracellular sorting receptors that direct apical targeting. In addition, the CUB domains appear to associate with detergent-resistant glycosphingolipid and cholesterol-enriched membrane microdomains, termed rafts, which correlates with the apical secretion of ADAMTS13 in polarized cells (65). The role of the other carboxyl-terminal domains of ADAMTS13 in its biosynthesis and secretion is yet to be determined.
Proteolytic cleavage of von Willebrand factor by ADAMTS13
The catalytic domain (metalloprotease) of ADAMTS13 contains a conserved active site motif (H224EXXHXXGXXHD235) with three His residues that coordinate the essential Zn2+ ion, and conserved residues Glu83, Asp173, Cys281, and Asp284 that are predicted to coordinate a structural Ca2+ ion (62). The presence of binding sites for both Ca2+ and Zn2+ is consistent with data showing that enzymatic activity is inhibited by chelation of either cation (76, 77). ADAMTS13 activity in citrate-anticoagulated plasma was enhanced approximately twofold by zinc ions, approximately threefold by calcium ions, and approximately sixfold by both ions (78), suggesting a cooperative activation of ADAMTS13 by divalent cations. At optimal zinc and calcium concentrations, ADAMTS13 cleaves VWF with a Kmapp of 3.7 ± 1.4 μg ml−1 (~15 nM for VWF subunits) (78). ADAMTS13 cleaves FRETS-VWF73 with a Kmapp of 3.3 ± 1.1 μM, consistent with an ~200-fold decrease in affinity compared with VWF (78), suggesting that the substrate recognition depends upon structural features or exosites on multimeric VWF that are missing from FRETS-VWF73.
Consistent with this hypothesis, we found that the metalloprotease domain alone is not sufficient to cleave multimeric VWF (76, 77, 79) or smaller substrates such as GST-VWF73-H (79, 80), which contains 73 amino acid residues from the central A2 domain of VWF, flanked by a glutathione S-transferase (GST) at the amino terminus and a 6xHis tag at the carboxyl terminus (81, 82). The addition of one or several more proximal carboxyl-terminal domains to the metalloprotease domain of ADAMTS13 gradually restores its proteolytic activity toward VWF (79, 80). The spacer domain was recently shown to bind a complementary site that includes residues Glu1660-Arg1668 at the carboxyl terminus of the A2 domain of VWF (80). Deleting the spacer domain from ADAMTS13 or deleting the carboxyl-terminal end of the A2 domain reduced the rate of cleavage by ~20-fold (80). In addition, the carboxyl-terminal cleavage product of VWF73 inhibited proteolytic cleavage of VWF multimers or of model peptide substrates, but only when the ADAMTS13 enzyme contained the spacer domain (80, 83). The disintegrin, TSP1, and Cys-rich domains between the metalloprotease and the spacer domain are also critical for substrate recognition and cleavage because the mutants lacking one or more of these domains do not cleave multimeric VWF (79). The addition of the remaining TSP1 domains (TSP1 2–8) and two CUB domains does not further increase the rate of proteolytic cleavage of VWF (76, 77) or GST-VWF73 (79, 80), suggesting that these more distal carboxyl-terminal domains may be dispensable for substrate recognition, at least under static conditions.
Substrate recognition under fluid shear stress appears to be more complex. For example, ADAMTS13 truncated after the spacer domain was reported to be hyperactive in cleaving UL-VWF strings on endothelial cell surfaces in a flow chamber assay (84), which suggests the distal TSP1 2–8 and CUB domains may inhibit the cleavage of UL-VWF. However, mutations in these distal domains of ADAMTS13 are reported in congenital TTP (85, 86), and autoantibodies directed against them occur in acquired idiopathic TTP (87). Furthermore, synthetic peptides or recombinant fragments derived from one of the TSP1 2–8 repeats (88) and the CUB domains block the cleavage of the UL-VWF multimers bound to endothelial cell surfaces (89). More recently, Zhang et al. (90) have shown, using a simple vortex-induced flow assay, that the ADAMTS13 variant truncated after the spacer domain exhibited significantly impaired proteolytic activity toward plasma-derived VWF. Together, these data support a hypothesis that the middle TSP1 repeats and distal CUB domains may be critical for productive recognition of VWF under flow shear stress.
ADAMTS13–von Willebrand factor binding
ADAMTS13 does not bind VWF detectably in solution in the absence of fluid shear stress or denaturing agents such as urea or guanidine hydrochloride. However, ADAMTS13 does bind VWF that is adsorbed onto the surface of a plastic microtiter plate (91), indicating that surface binding alters the structure of the ADAMTS13 binding site on VWF. The estimated Kd for ADAMTS13 binding to immobilized VWF is approximately 14 nM, comparable to the Km of 16 nM determined independently for the cleavage of VWF pretreated with guanidine-HCl (91). Deletion of the middle and distal carboxyl-terminal domains of ADAMTS13 has little effect: The truncated enzyme binds immobilized VWF with a Kd of ~24 nM. Further deletion of the spacer domain markedly impairs binding to VWF (91), suggesting that the spacer domain contributes substantially to the ADAMTS13-VWF interaction. The metalloprotease domain alone at the concentration tested (~220 nM) does not bind VWF detectably (91).
The binding between ADAMTS13 and VWF under flow has recently been determined by surface plasmon resonance on a BI-Acore system (90). The data show that full-length ADAMTS13 binds flowing VWF with high affinity (KD = 50 nM). A removal of the CUB domain (construct delCUB) results in a fivefold reduction in affinity (KD = 274 nM), but further removal of the TSP1 2–8 repeats (construct MDTCS, which contains metalloprotease domain, disintegrin, first TSP1 repeat, Cys-rich, and spacer domains) significantly impairs the binding to flowing VWF. Moreover, the isolated CUB domains are neither sufficient to bind VWF detectably nor capable of inhibiting proteolytic cleavage of VWF by ADAMTS13 under flow. Addition of the TSP1 5–8 (construct T5–8CUB) or TSP1 2–8 repeats (construct T2–8CUB) to the CUB domains restores the binding affinity toward VWF and the inhibitory effect on cleavage of VWF by ADAMTS13 under flow. These data demonstrate directly and quantitatively that the cooperative activity between the middle carboxyl-terminal TSP1 repeats and the distal carboxyl-terminal CUB domains may be crucial for the recognition and cleavage of VWF under flow.
The domains of VWF that interact with ADAMTS13 remain to be determined. Binding studies using surface plasmon resonance have suggested that the A1, A2, and A3 domains of VWF all bind ADAMTS13 under flow conditions (92). More detailed studies have been conducted on the interaction between ADAMTS13 and VWF73, a minimal fragment from the central A2 domain (Asp1659–Arg1668) of VWF that is recognized by ADAMTS13. Full-length ADAMTS13 and the variant truncated after the spacer domain bind VWF73 with values for Kd of ~4.5 nM and ~7.8 nM, respectively (79). In addition, each of the proximal carboxyl-terminal domains appears to bind VWF independently and may act together to produce high-affinity binding. For instance, the isolated disintegrin domain, first TSP1 repeat, Cys-rich domain, or spacer domain binds VWF73 with Kd values of ~489 nM, ~136 nM, ~121 nM, and ~108 nM, respectively (79). When these domains are expressed as a single fragment, it binds VWF73 with a much higher affinity, with a Kd value of 13 nM (79), suggesting the proximal carboxyl-terminal domains of ADAMTS13 bind cooperatively to the central A2 domain of VWF. In these studies, ADAMTS13 binds VWF73 ~3–5 times more tightly than multimeric VWF (79, 91), suggesting that domains surrounding the central A2 domain of VWF can inhibit the interaction of ADAMTS13 with VWF multimers. It is interesting that the Kd values of 4–14 nM for binding VWF73 are substantially lower than the Km values of 1.7 μM for cleaving VWF73 (80) or 0.25 μM for cleaving a similar VWF78 construct (83), determined by different methods. The cause of this discrepancy is not known at the present time.
Regulation of ADAMTS13
VWF is not cleaved in solution without flow shear stress owing to structural inaccessibility of the binding or cleavage site on the VWF molecule by ADAMTS13. Modeling of the A2 domain of VWF suggests that the Tyr1605-Met1606 bond lies buried within the core β-sheet of the native structure (93), and that fluid shear stress (53, 94) and denaturing reagents (51, 54) unfold the A2 domain and expose this bond to ADAMTS13. In addition, the cleavage of VWF may be modulated by the other structural elements of VWF (95), heparin sulfate, platelet GPIbα, sodium chloride (96), and inflammatory cytokines (97). For example, a recombinant VWF construct comprising A2–A3 domains is cleaved ~10-fold faster than a similar substrate comprising A1–A3 domains (95), suggesting that the A1 domain may block the access of ADAMTS13 to the cleavage site. In addition, this blockage can be relieved by certain ligands that bind to A1 domain, such as platelet GPIbα or heparin (95).
Other factors in the milieu may influence ADAMTS13 and VWF interactions, such as ionic strength (96) and hemolysis (98). The cleavage of VWF in the absence of sodium chloride at pH 8.0 occurs with apparent kcat/km of 3.4 × 104 M−1s−1, but this value decreases approximately 10-fold in the presence of 0.15 M sodium chloride (96). The potency of the inhibitory effect varies with different anions and decreases in the order ClO−4 > Cl− > F− (96). The inflammatory cytokine interleukin-6, but not interleukin-8 or tumor necrosis factor α (TNFα), inhibits the cleavage of UL-VWF multimers under flow (97). The findings suggest a potential linkage between inflammation and thrombosis, although the concentration of interleukin-6 required for inhibiting ADAMTS13 activity is relatively high compared with the levels that have been documented in vivo.
Thrombin, factor Xa, leukocyte elastase, and plasmin can cleave and inactivate ADAMTS13, and therefore might regulate ADAMTS13 activity at the site of thrombus formation. ADAMTS13 is proteolytically cleaved during blood coagulation in human plasma in vitro (71), and thrombin (0.9–90 nM) degrades ADAMTS13 in a time- and concentration-dependent fashion (71). This cleavage is prevented when thrombin binds soluble thrombomodulin but not heparin, suggesting the involvement of thrombin exosite I but not exosite II in ADAMTS13 recognition. Plasmin cleaves ADAMTS13 more rapidly than thrombin or factor Xa (71), indicating that plasmin might enhance VWF-dependent platelet adhesion by degrading ADAMTS13 at sites of tissue repair. Leukocyte elastase activity is increased in the plasma of patients with disseminated intravascular coagulation owing to severe sepsis, suggesting that cleavage of ADAMTS13 by leukocyte elastase might contribute to the relatively severe deficiency of ADAMTS13 activity reported in some of these patients (72).
Mutations of the ADAMTS13 gene
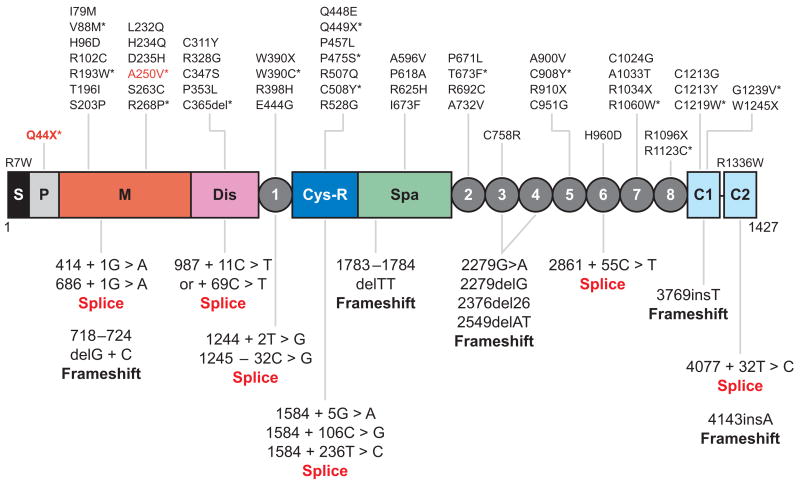
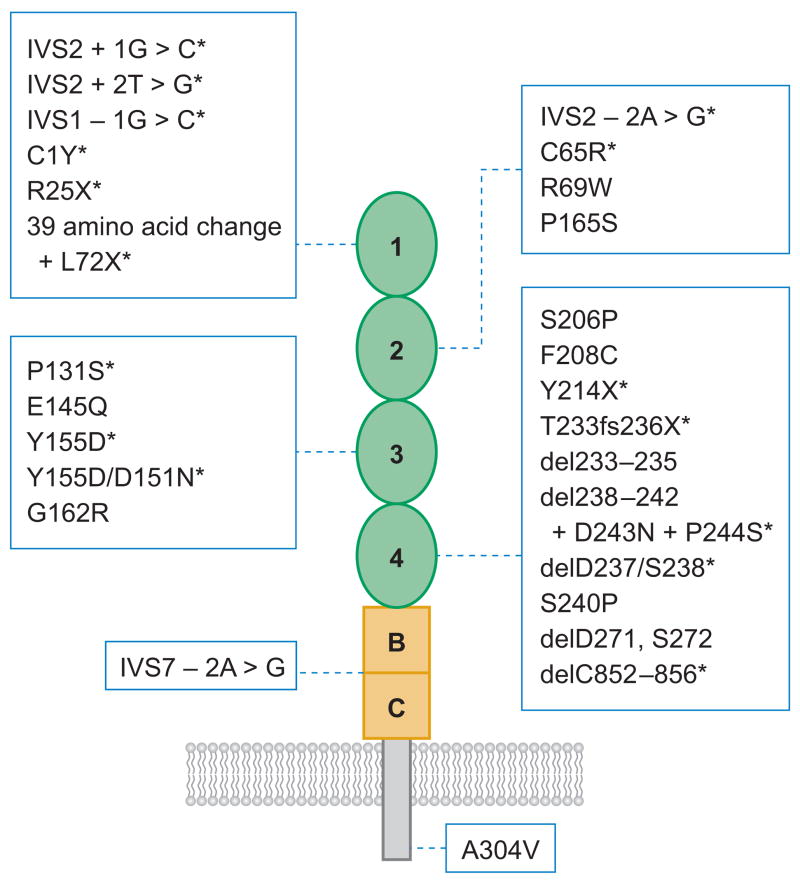
More than 50 mutations on the ADAMTS13 gene have been described in patients with congenital or familial TTP and severe deficiency of plasma ADAMTS13 activity (Figure 3) (63, 85, 99–107). The majority are missense mutations (~50%), followed by splice site, nonsense, and frameshift mutations (Figure 3) (63, 85, 99–107). The mutations are distributed throughout the ADAMTS13 gene without an apparent cluster or hot spot. Homozygous mutations have been found in some families, but compound heterozygous mutations are more common (63, 85, 99–107). Sixteen missense mutations involving cysteine residues have been identified (Figure 3), indicating the importance of proper formation of disulfide structure for the function of ADAMTS13 protease.
Figure 3.
Location of the mutations found in patients with congenital thrombotic thrombocytopenic purpura. The point mutations that cause single amino acid substitutions and premature stop codons (X) are shown above the domain structure of ADAMTS13. The mutations that result in alternative splicing of ADAMTS13 mRNA or frameshifts are listed under the domain structure of ADAMTS13. Those mutants expressed as recombinant proteins in cell culture (*) cause defects in ADAMTS13 secretion (black) or catalytic activity (red ). S, signal peptide; P, propeptide; M, metalloprotease; Dis, disintegrin domain; 1, the first thrombospondin type 1 (TSP1) repeat; Cys-R, cysteine-rich domain; Spa, spacer domain; 2 through 8, the second to eighth TSP1 repeats; C1 and C2, the CUB domains 1 and 2.
Approximately one-half of the patients with familial TTP experience their first acute episodes of disease during childhood, but the remaining are present in adulthood (106, 108). It is not often clear why some patients have prolonged intervals without symptoms, but acute thrombotic microangiopathy may first occur during pregnancy, infection, or other stressful conditions that could confer an increased risk of microvascular thrombosis (106, 108).
Most of the missense mutations that have been investigated appear to cause ADAMTS13 deficiency by impairing protein secretion (Figure 3) (102, 106, 109). The mutant P475S is an exception, which is secreted normally by transfected cells, but shows low proteolytic activity toward VWF (99). Interestingly, approximately 9.6% of Japanese may be heterozygous for this mutation, which may be responsible for reduced protease activity (99). It is rare in Caucasians (0.5%) (110) and in Chinese (1.7%) (111). Similarly, the ADAMTS13 protease with the nonsense mutation Q449X is also secreted normally in transfected cells, but has very low proteolytic activity (99), consistent with reports that the spacer domain is necessary for ADAMTS13 activity (76, 77, 80).
Another recombinant missense mutation, Q448E, which is secreted normally and retains full activity in transfected cells, has been reported as a single nucleotide polymorphism (SNP) (63, 99). Many other SNPs have also been reported (Figure 3), but none has yet shown a clear association with TTP (109) or with other conditions such as coronary artery disease, where ADAMTS13 activity may be slightly decreased (112). Interestingly, some SNPs in the ADAMTS13 gene can interact to modulate the secretion or specific activity of ADAMTS13 in patients with certain missense mutations, depending upon the sequence context. For example, the congenital TTP mutations P618A and R1336W and the A732V/P618 combination strongly reduce ADAMTS13 specific activity and secretion. The common polymorphisms R7W and Q448E have no effect on the ADAMTS13 expression by themselves, but rescue the secretion defect of mutation A732V/P618A and exacerbate the detrimental effect of mutation R1336W (113).
Several mutations that cause premature termination have been shown to impair secretion or reduce ADAMTS13 proteolytic activity. For example, the 4143–4144insA frameshift mutation deletes the second CUB domain and impairs ADAMTS13 secretion (65, 85), without significantly affecting its specific activity (85). A number of ADAMTS13 gene mutations have been identified at splice sites, which disrupt the conventional GT-AG consensus splice sequences and prevent the production of normally spliced transcripts (63, 102). Whether alternatively spliced transcripts contribute to ADAMTS13 heterogeneity in human plasma (59) is not known.
Expression studies in vitro indicate that ADAMTS13 mutations can have heterogeneous effects on biosynthesis, secretion, and protease activity. Most congenital TTP patients harbor compound heterozygous mutations. The parents of hereditary TTP patients (asymptomatic carriers) usually have approximately a 50% level of ADAMTS13 activity, although asymptomatic carriers who are compound heterozygous with one P475S allele can have very low levels of ADAMTS13 activity in vitro (less than 10% of normal) (114). As expected, most of the described homozygous mutations such as R692C (63), Q449X (99), 414 + 1G > A splice (102), L232Q (102, 104), and 1783delTT (102, 115) occur in consanguineous pedigrees.
Autoantibodies against ADAMTS13
Autoantibody inhibitors were initially reported in 65% to 96% of patients with idiopathic TTP and severe ADAMTS13 deficiency (< 5% to 10% of normal human plasma) (20, 21). Some subsequent studies have found a lower prevalence of inhibitors of 31% and 44% (7, 116, 117), perhaps because the patient populations were less highly selected. By an enzyme-linked immunosorbent assay, IgG antibody is detected in almost all patients with idiopathic TTP and severe ADAMTS13 deficiency (86). Additional immunoglobulin isotypes such as IgM were also detected in 11% of patients (118, 119). Although noninhibitory IgG autoantibodies to ADAMTS13 have been found in rare patients with non-idiopathic TTP, inhibitory IgG is otherwise detected almost exclusively in patients with idiopathic TTP (7, 21, 116–118). TTP associated with ticlopidine and (less commonly) clopidogrel represent interesting exceptions consistent with a drug-induced autoimmune disorder. ADAMTS13 inhibitors were detected in six of seven patients with ticlopidine-associated TTP (43, 120), and in two patients with clopidogrel-induced TTP (44). The detection of high-titer anti-ADAMTS13 autoantibodies in patients with acquired TTP is correlated with relapsing disease and poor prognosis (117).
The ADAMTS13 epitopes recognized by autoantibodies have been mapped for a total of 35 TTP patients, and so far all plasmas contain at least some antibodies directed against the Cys-rich/spacer domain (76, 87, 121). Fine mapping by mutagenesis suggests that the amino acid residues Thr572-Asn579 and Val657-Gly666 comprise a common core region in the spacer domain that is recognized by anti-ADAMTS13 antibodies (121). Autoantibodies reactive with other domains of ADAMTS13 have been detected in some patient plasma samples, including antibodies against the CUB domains in approximately 64%, against the first TSP1 repeat (or a larger fragment containing the metalloprotease, disintegrin, and the TSP1 repeat) in 56%, against TSP1 repeats 2–8 in 28%, and against the ADAMTS13 propeptide in 20% (87).
Screening of the phage display libraries of individuals of acquired TTP has shown that most anti-ADAMTS13 autoantibodies have a heavy chain encoded by VH1–69 paired with a VL3 family lambda light chain (122, 123). The remaining autoantibodies often have a heavy chain from the VH1, VH3, or VH4 families, usually paired with kappa light chains (123). When cloned and prepared as monoclonal antibodies, many of these autoantibodies inhibit ADAMTS13 activity in vitro and in vivo in mice (123).
Other Potential Mechanisms in Idiopathic Thrombotic Thrombocytopenic Purpura
The thrombophilic factor V Leiden mutation is reported in one study to be associated with acquired TTP among patients with normal ADAMTS13 levels, with an odds ratio of 17:1 (124). This association was not confirmed in a subsequent study (125). Other genetic risk factors for arterial and venous thrombosis do not appear to be associated with TTP (124). One study has reported that the HLA class II antigen DR53 protects against the development of TTP and confers a relative risk of 0.09 compared with the absence of DR53 (126). Other mechanisms have been proposed to contribute to the pathogenesis of TTP, but their roles have not been conclusively established. These include endothelial cell activation (127) and cell death (128, 129), caused by antibodies against endothelial cells or against GP IV (CD36) on the platelet and endothelial cell surface (130, 131). The activation and death of microvascular endothelial cells are associated with markedly increased plasma microparticles rich in tissue factor and procoagulant phospholipids (128, 129, 132). These events could result in further endothelial activation, blood coagulation, platelet aggregation, and microvascular thrombosis.
HEMOLYTIC UREMIC SYNDROME
HUS is classified into D+HUS and D-HUS. D+HUS accounts for more than 90% of cases and is caused by an enteric infection with Shiga-toxin-producing organisms, most frequently E. coli O157:H7 (133, 134). D-HUS is less common and quite heterogeneous: Some cases are familial, some have a recurring course, some have a neonatal onset, and some are triggered by infection with neuraminidase-producing microbes (135). Many causes of D-HUS do not have effective treatments, and the overall mortality rate is approaching 26% (136). Recent studies have suggested that a subset of D-HUS patients (30% to 50%) can be attributed to dysregulation of the alternative complement pathway.
Incidence and Risk Factors
D+HUS occurs most commonly in children, but affects all ages. E. coli O157:H7 accounts for at least 60% of D+HUS associated with Shiga-toxin-producing bacterial infection (137). The annual incidence of human infection with E. coli O157 has been reported to be at least 8 per 100,000 people in North America (134). The percentage of cases with bloody diarrhea that progresses to HUS ranged from 3% to 7% in a series of sporadic cases (138), to between 20% and 30% in some outbreaks (11, 133). HUS usually occurs 4 to 6 days after the onset of diarrhea. Antibiotic treatment may increase the incidence of HUS in children (139).
Five to ten percent of patients with HUS patients have so-called D-HUS, without antecedent diarrhea or an identifiable gastrointestinal infection with Shiga-toxin-producing organisms. D-HUS has a relatively poor prognosis, with a death rate of up to 25% in the acute phase and 50% of survivors requiring at least temporary dialysis therapy (107). Depending upon the underlying cause, D-HUS may occur in adults or in very young children.
Histopathology
HUS preferentially involves the kidneys. The vascular lesions typically spare the myocardium and occur infrequently in the pancreas, brain, and adrenal glands (47). The thrombi of D+HUS appear to be composed predominantly of fibrin, with few platelets and little VWF (47, 140, 141). These pathological changes are quite distinct from those seen in idiopathic TTP, in which platelets and VWF are major constituents of the lesions (47, 48). Endothelial cell damage is a hallmark of D+HUS in children, and endothelial cell swelling may be so extreme as to occlude the capillary lumen (127, 142). Dramatic destruction of the renal cortex may occur, and glomeruli show a range of changes that varies according to the age of the process and whether arterial changes are present. Glomerular thrombosis is characteristic, with an enlarged appearance that suggests capillary congestion rather than ischemia. Extension of thrombosis into the afferent arteriole is common. Mesangial changes appear to be uncommon. The tubules are often atrophic, may show necrosis, and frequently contain hyaline casts and red blood cells (142).
Fewer cases of D-HUS have been studied histologically, so information about this heterogeneous disorder is relatively incomplete and generalization is difficult. However, the lesions appear to be distinct from those of D+HUS or idiopathic TTP, with substantial mesangial involvement and less glomerular thrombosis in D-HUS (142, 143). The extent of arteriolar involvement has been reported to correlate with an adverse outcome.
Escherichia coli Infection and Shiga Toxin
As described above, E. coli O157:H7 accounts for at least 60% of Shiga-toxin-producing bacteria associated with D+HUS in children (137) and occasionally with adults by ingestion of contaminated food or water. Most food-borne outbreaks have been traced to foods derived from cattle, especially ground meat and raw milk. Unpasteurized fruits and juices account for a growing number of recognized outbreaks. Water-borne outbreaks of E. coli O157 infection have occurred as a result of drinking and swimming in unchlorinated water. Person to person transmission has occurred in day-care and chronic-care facilities.
E. coli O157 produces Shiga toxins, including Shiga toxin 1 (Stx1) and Shiga toxin 2 (Stx2). The Shiga toxins are composed of one A subunit (~33 kDa) and five B subunits (~7.7 kDa). The B subunits mediate Shiga toxin binding to the membrane glycosphingolipid globotriaosyl ceramide (144) on microvascular endothelial cells in the brain and kidney, and on monocytes and platelets. The A subunit is subsequently internalized and inhibits protein synthesis by removing a specific adenine base from the 28S ribosomal RNA subunit, leading to cell death. Stx1 also stimulates the release of TNFα, interleukin-1, interleukin-6, and interleukin-10 from monocytes and epithelial cells of renal glomeruli and tubules. These cytokines can increase the expression of globotriaosyl ceramide on renal endothelial cells and increase the binding of Shiga toxins. The Shiga toxins can also induce endothelial cells to secrete UL-VWF multimers (144), impair ADAMTS13 cleavage of UL-VWF multimers (145), and increase the expression of vitronectin (αvβ 3 integrin) receptors, P-selectin, and platelet endothelial-cell adhesion molecule 1. These events may directly or indirectly damage endothelial cells, which may potentiate renal microvascular thrombosis by promoting the activation of the blood coagulation cascade.
Complement Dysregulation
Complement is a central immune surveillance system in humans. This complex system is designed to recognize antigen-antibody complexes and combat invading microbes. The human complement system can be activated through three different pathways: the classical and lectin pathways and the alternative pathway that converges to generate either classical or alternative C3 convertase enzymes (C3b) and to activate factor B enzyme by factor D (giving rise to the Ba and Bb fragments). The dissociation of the Ba fragment from the complex leaves the Bb fragment attached to C3b to form in the presence of Mg2+ the active C3 convertase enzyme C3bBb. Factor B is essential for the opsonization and cell-killing functions of the alternative pathway. Factor H and membrane cofactor protein (MCP) serve as cofactors to the C3b-inactivating enzyme complement factor I convertase.
Defects in complement regulation can lead to cellular damage and death, which in turn can activate the blood coagulation cascade and cause thrombosis. Low serum concentrations of complement C3 and factor B have been described in patients with D-HUS, which is consistent with the activation of the complement system. Approximately 50% of patients with D-HUS/atypical HUS have mutations in one of the complement regulatory proteins factor H (107, 146, 147), factor I (148, 149), MCP (149–151), or factor B (152–154). A few patients have also been described with autoantibodies against factor H. The patients with MCP mutation had a better prognosis than patients with factor H mutations or no mutation. In patients with MCP mutation, plasma treatment did not impact the outcome significantly. Remission was achieved in approximately 90% of both plasma-treated and plasma-untreated acute episodes (148).
The outcome of kidney transplantation appears to correlate with cause of complement dysregulation. Disease has not recurred in transplanted kidneys for patients with MCP mutations, presumably because the new kidney has protective levels of endogenous MCP. In contrast, kidney transplantation alone does not correct the deficiency of plasma factors made in the liver, and the outcome was poor in patients with factor H or factor I mutations owing to disease recurrence (146, 148, 150, 155). However, a combined liver and kidney transplantation may lead to a favorable long-term outcome for recurrent HUS associated with a factor H mutation (156).
Complement factor H
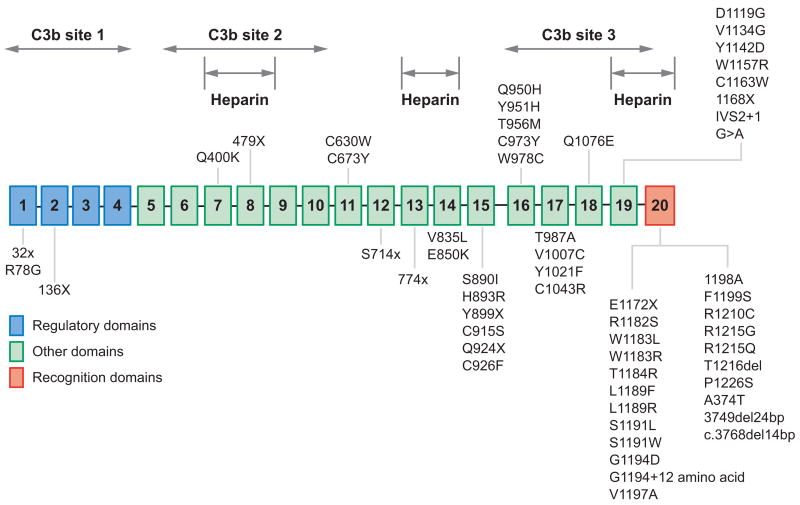
Factor H, a 150-kDa plasma protein, is predominantly synthesized in the liver. Small amounts are also produced or localized in endothelial cells, platelets, and fibroblasts. The plasma concentration of factor H is approximately 0.5 mg ml−1. Factor H is composed of 20 homologous units of approximately 60 amino acids, named short consensus repeats (SCRs). Factor H regulates the alternative pathway of complement by functioning as a cofactor for factor I–mediated proteolytic inactivation of C3b through interaction with SCR1–4, SCR6–10, and SCR16–20 (Figure 4). Factor H also competes with factor B for C3b binding and accelerates the decay of the C3 convertase (C3bBb) into its components. The terminal SCR20 domain contains polyanionic or heparin binding sites that may enable factor H to bind endothelial cells and protect them against complement attack.
Figure 4.
Structure-function relationship of factor H and mutations found in patients with diarrhea-negative hemolytic uremic syndrome (D-HUS). The plasma protein factor H is composed of 20 consecutive short consensus repeat domains. The complement C3b and the heparin binding domains are indicated. Most of the mutations associated with D-HUS or familial HUS are clustered in the twentieth short consensus repeat domain.
Thompson & Winterborn (157) first suggested an association between factor H and D-HUS and subsequently confirmed it by demonstrating linkage to the factor H locus on chromosome 1q32 and identifying factor H mutations in families with D-HUS. Approximately 10% to 30% of patients who have either familial or sporadic D-HUS may have factor H deficiency caused by mutations in the factor H gene (155, 158, 159). The mutations are found throughout the gene, but tend to cluster near the carboxyl terminus of factor H (SCR20), an area important for both binding to anionic molecules and to complement C3b (Figure 4). Approximately 94% of the reported cases are heterozygous and may have normal, reduced, or even increased serum levels of factor H, depending upon the location of the factor H mutation. In a few cases, factor H mutations are identified on both alleles, which may result in extremely reduced serum levels of both complement C3 and factor H in these cases (less than 5% to 10% of normal). Homozygous or compound heterozygous deficiency of factor H is estimated to occur in approximately 1 in 400,000 Caucasian residents in the United States (160).
Autoantibodies against factor H
Acquired factor H deficiency due to immunoglobulin G (IgG) autoantibodies against factor H has been described in 3 out of 48 children (6%) with sporadic D-HUS (161). The plasma factor H activity was decreased, whereas the plasma level of factor H antigen was normal and no factor H gene mutation was identified. The IgG antibodies were proposed to inhibit the binding of factor H to the C3 convertase complex, compromising the decay-accelerating activity of factor H in vivo (161). No such anti-factor H IgG was detected in a large series of plasma samples from healthy controls and patients with various autoimmune disorders, suggesting that anti-factor H antibodies are specific to a particular form of D-HUS. The finding of the acquired factor H deficiency caused by IgG autoantibodies against factor H may provide a rationale for designing new diagnostic tests and for considering plasma exchange or immunosuppressive therapy in some cases.
Complement membrane cofactor protein
MCP (CD46), an integral membrane protein, is expressed on all human cells with the exception of erythrocytes. Four isoforms of MCP are expressed owing to alternative splicing. The extracellular region of MCP is composed of four SCR domains and a repeat region rich in serine, threonine, and proline residues (Figure 5). Like factor H, MCP inhibits complement activation by regulating C3b deposition on target cells. More than 25 mutations have been identified in patients with D-HUS, accounting for 10% and 13% of cases in two large series (Figure 5) (148, 162). Small in-frame deletions, frameshifts with premature terminations, and missense mutations have been observed, and the penetrance of MCP mutations appears to be approximately 54% (148). Most mutations (93%) cluster in the four extracellular SCR domains required for C3b binding and cofactor activity (148). Many mutants of MCP (75%) have reduced cell surface expression with MCP levels between 0% and 50% of normal as determined by fluorescence-activated cell sorting analysis of peripheral blood mononuclear cells or transfected Chinese hamster ovarian cells (162–164), suggesting a quantitative deficiency of MCP in a majority of cases. Two mutations (S206P and F208C) do not reduce protein expression, but cause reduced C3b binding and cofactor activity (148, 164). One mutation (E145Q) increases surface expression of MCP protein by threefold, but the mutant MCP has reduced C4b cofactor activity. Thus, quantitative or qualitative MCP defects may compromise the regulation of complement activation on cell surfaces, allowing endothelial injury and renal microvascular thrombosis in patients with D-HUS.
Figure 5.
The domain structure and mutations in membrane cofactor protein associated with diarrhea-negative hemolytic uremic syndrome (D-HUS). Membrane cofactor protein is an integral membrane protein composed of four short consensus repeat domains numbered 1 through 4, and O-glycosylated segments B and C rich in serine, threonine, and proline. Mutations associated with D-HUS or with familial HUS are indicated. An asterisk (*) indicates mutations that cause reduced levels of secretion.
Complement factor I
Factor I, an 88-kDa plasma GP, is predominantly synthesized in the liver, and its plasma concentration is 35 μg ml−1. The gene encoding factor I is located on chromosome 4q25 and spans 63 kb. Factor I is heterodimeric and consists of a noncatalytic heavy chain, linked by a disulfide bond to a catalytic light chain. The heavy chain contains a factor I module (found only in complement factor I, C6, and C7), a CD5 domain, and two low-density lipoprotein receptor repeats. The light chain contains a serine protease domain in which residues H362, D411, and S507 form the catalytic triad (Figure 5). Factor I cleaves the α-chains of C3b and C4b in the presence of one of its cofactors: factor H, C4 binding protein (C4BP), and MCP, or complement receptor 1 (CR1). These cofactors have slightly different functions: factor H promotes cleavage of C3b; C4BP promotes cleavage of C4b; MCP and CR1 promote cleavage of both C3b and C4b. By inactivating the C3b and the C4b, factor I prevents the formation of the C3 and C5 convertases and inhibits both the alternative and the classical complement pathways.
In 1970, Alper et al. (165) described the first case of factor I deficiency. Since then, at least 31 cases have been reported. These patients have all had virtually complete deficiency of factor I, owing presumably to mutations in both factor I alleles. The clinical manifestations are present from early childhood and include increased susceptibility to recurrent infection with encapsulated microorganisms such as Neisseria meningitides and Streptococcus pneumoniae. Heterozygous nonsense, missense, and frameshift mutations in factor I gene were recently reported in between 3% and 12% of patients with D-HUS (166–169). Incomplete penetrance is common. Most of the mutations (more than 70%) are found in the protease domain, which may result in reduced serum levels of factor I protein (Figure 6) (148, 150, 151). Most of the heavy chain mutations observed in D-HUS reduce factor I activity for the degradation of C3b and C4b, but at least one (G261D) does not (148–151).
Figure 6.
Domain structure and mutations of factor I associated with diarrhea-negative hemolytic uremic syndrome. The heavy and light chains of factor I are linked by a disulfide bond (S-S). Most of the mutations in factor I are clustered in the serine protease domain. The mutations associated with reduced serum levels of factor I are marked with an asterisk (*).
Complement factor B
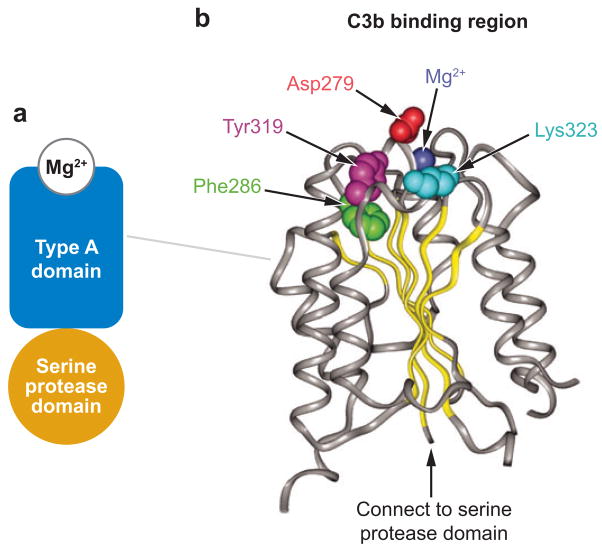
Recently, heterozygous gain-of-function missense mutations in factor B have been described in three patients with D-HUS (152). Two patients have the mutation F286L and one has the mutation K323E; both mutations are within the von Willebrand type A domain of factor B (Figure 7).
Figure 7.
The domain structure of complement factor B and mutations associated with diarrhea-negative hemolytic uremic syndrome. The schematic diagram of the factor B domain composition and the residues that participate in the Mg2+ binding are shown in panel a. The residues altered in patients with diarrhea-negative hemolytic uremic syndrome are shown in panel b.
Complement factor B is a zymogen that carries the catalytic sites of the complement alternative pathway convertase C3bBb. Upon interaction with C3b, factor B is cleaved by factor D into two fragments, Ba and Bb. Ba is released, whereas Bb remains bound to C3b, forming the C3bBb alternative pathway C3 convertase, an active serine protease that cleaves additional C3 into C3b. The Bb fragment of factor B comprises two protein domains (Figure 7): a VWF-A domain and a serine protease domain (170). The factor B VWF-A domain interacts with C3bBb, and the D-HUS mutations F286L and K323E alter residues at the C3bBb interface, in close proximity to residue Asp279, which is part of a Mg2+ binding site. Functional analysis showed that each of the D-HUS-associated mutations causes increased alternative complement pathway activation. Factor B (F286L) binds C3b to form an active, rapidly cycling C3 convertase and to enhance the generation of additional C3b. Factor B (K323E) binds C3b to form a C3bBb enzyme resistant to inactivation by decay-accelerating factor and factor H. In either case, increased complement activation presumably injures renal endothelial cells, promotes the attachment of neutrophils and macrophages, and causes microvascular thrombosis.
SUMMARY POINTS
Profound thrombocytopenia, hemolytic anemia, and fragmentation of red blood cells, without an obvious cause (e.g., autoimmune hemolysis, cancer, sepsis, malignant hypertension, organ transplantation, cyclosporine, antecedent bloody diarrhea), are sufficient to diagnose TMA and initiate empiric plasma exchange therapy.
TTP and HUS usually are pathologically distinct diseases: Thrombi rich in VWF and platelets occur in TTP, and fibrin-rich hyaline thrombi occur in D+HUS, suggesting (at least) two distinct pathways that cause microvascular thrombosis. Congenital TTP and acquired idiopathic TTP are usually caused by deficiency of plasma ADAMTS13 activity. HUS is usually caused either by infection with Shiga-toxin-producing E. coli (D+HUS) or by complement dysregulation owing to mutations in the genes encoding factor H, MCP, factor I, and factor B (D-HUS).
For patients diagnosed with TTP, the identification of autoantibodies against ADAMTS13 can help predict relapse and prognosis; for patients with D-HUS, the identification of mutations in factor H, MCP, and factor I is critical for predicting the response to liver or kidney transplantation.
FUTURE ISSUES
On the basis of current clinical or laboratory criteria, the differentiation of TTP and HUS is not always possible, so that empiric treatment with plasma exchange should be strongly considered if the distinction is uncertain.
Better criteria for diagnosis are needed.
The underlying pathogenesis of nonidiopathic TTP remains elusive, in part because it is heterogeneous and independent of ADAMTS13 deficiency.
For most children or adults with D-HUS, the underlying etiology is yet to be identified because mutations in genes encoding complements factor H, MCP, factor I, and factor B account for only 30% to 50% of cases.
Acknowledgments
The authors acknowledge support in part by the National Institutes of Health grants HL079027 to X.L.Z. and P50HL081012 to Joel Bennett (Principal Investigator)/X.L.Z. (Co-Investigator); and by grants HL72917 and HL89746 to J.E.S. J.E.S. is also an investigator of the Howard Hughes Medical Institute.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any biases that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Moschcowitz E. Hyaline thrombosis of the terminal arterioles and capillaries: a hitherto undescribed disease. Proc NY Pathol Soc. 1924;24:21–24. [Google Scholar]
- 2.Amorosi EL, Ultmann JE. Thrombocytopic purpura: report of 16 cases and review of the literature. Medicine. 1966;45:139–59. [Google Scholar]
- 3.George JN, Gilcher RO, Smith JW, Chandler L, Duvall D, et al. Thrombotic thrombocytopenic purpura-hemolytic uremic syndrome: diagnosis and management. J Clin Apher. 1998;13:120–25. doi: 10.1002/(sici)1098-1101(1998)13:3<120::aid-jca5>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 4.George JN. How I treat patients with thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. Blood. 2000;96:1223–29. [PubMed] [Google Scholar]
- 5.Bell WR, Braine HG, Ness PM, Kickler TS. Improved survival in thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. Clinical experience in 108 patients. N Engl J Med. 1991;325:398–403. doi: 10.1056/NEJM199108083250605. [DOI] [PubMed] [Google Scholar]
- 6.Gasser C, Gautier E, Steck A, Siebenmann RE, Oechslin R. Hemolyticuremic syndrome: bilateral necrosis of the renal cortex in acute acquired hemolytic anemia. Schweiz Med Wochenschr. 1955;85:905–9. [PubMed] [Google Scholar]
- 7.Vesely SK, George JN, Lammle B, Studt JD, Alberio L, et al. ADAMTS13 activity in thrombotic thrombocytopenic purpura-hemolytic uremic syndrome: relation to presenting features and clinical outcomes in a prospective cohort of 142 patients. Blood. 2003;102:60–68. doi: 10.1182/blood-2003-01-0193. [DOI] [PubMed] [Google Scholar]
- 8.Zheng X, Sadler JE. Thrombotic thrombocytopenic purpura and hemolytic uremic syndrome. In: Young NS, Gerson SL, High KA, editors. Clinical Hematology. Philadelphia: Mosby Elsevier; 2006. pp. 802–13. [Google Scholar]
- 9.Thompson CE, Damon LE, Ries CA, Linker CA. Thrombotic microangiopathies in the 1980s: clinical features, response to treatment, and the impact of the human immunodeficiency virus epidemic. Blood. 1992;80:1890–95. [PubMed] [Google Scholar]
- 10.Bueno D, Jr, Sevigny J, Kaplan AA. Extracorporeal treatment of thrombotic microangiopathy: a ten year experience. Ther Apher. 1999;3:294–97. doi: 10.1046/j.1526-0968.1999.00170.x. [DOI] [PubMed] [Google Scholar]
- 11.Cleary TG. Cytotoxin-producing Escherichia coli and the hemolytic uremic syndrome. Pediatr Clin N Am. 1988;35:485–501. doi: 10.1016/s0031-3955(16)36467-7. [DOI] [PubMed] [Google Scholar]
- 12.Tarr PI, Neill MA, Clausen CR, Watkins SL, Christie DL, et al. Escherichia coli O157:H7 and the hemolytic uremic syndrome: importance of early cultures in establishing the etiology. J Infect Dis. 1990;162:553–56. doi: 10.1093/infdis/162.2.553. [DOI] [PubMed] [Google Scholar]
- 13.Barre P, Kaplan BS, de Chadarevian JP, Drummond KN. Hemolytic uremic syndrome with hypocomplementemia, serum C3NeF, and glomerular deposits of C3. Arch Pathol Lab Med. 1977;101:357–61. [PubMed] [Google Scholar]
- 14.Roodhooft AM, McLean RH, Elst E, Van Acker KJ. Recurrent hemolytic uraemic syndrome and acquired hypomorphic variant of the third component of complement. Pediatr Nephrol. 1990;4:597–99. doi: 10.1007/BF00858631. [DOI] [PubMed] [Google Scholar]
- 15.Pichette V, Querin S, Schurch W, Brun G, Lehner-Netsch G, et al. Familial hemolytic-uremic syndrome and homozygous factor H deficiency. Am J Kidney Dis. 1994;24:936–41. doi: 10.1016/s0272-6386(12)81065-1. [DOI] [PubMed] [Google Scholar]
- 16.Furlan M, Robles R, Solenthaler M, Wassmer M, Sandoz P, Lammle B. Deficient activity of von Willebrand factor-cleaving protease in chronic relapsing thrombotic thrombocytopenic purpura. Blood. 1997;89:3097–103. [PubMed] [Google Scholar]
- 17.Furlan M, Lammle B. Deficiency of von Willebrand factor-cleaving protease in familial and acquired thrombotic thrombocytopenic purpura. Baillieres Clin Haematol. 1998;11:509–14. doi: 10.1016/s0950-3536(98)80064-4. [DOI] [PubMed] [Google Scholar]
- 18.Furlan M, Robles R, Galbusera M, Remuzzi G, Kyrle PA, et al. von Willebrand factor-cleaving protease in thrombotic thrombocytopenic purpura and the hemolytic-uremic syndrome. N Engl J Med. 1998;339:1578–84. doi: 10.1056/NEJM199811263392202. [DOI] [PubMed] [Google Scholar]
- 19.Furlan M, Robles R, Morselli B, Sandoz P, Lammle B. Recovery and half-life of von Willebrand factor-cleaving protease after plasma therapy in patients with thrombotic thrombocytopenic purpura. Thromb Haemost. 1999;81:8–13. [PubMed] [Google Scholar]
- 20.Furlan M, Robles R, Solenthaler M, Lammle B. Acquired deficiency of von Willebrand factor-cleaving protease in a patient with thrombotic thrombocytopenic purpura. Blood. 1998;91:2839–46. [PubMed] [Google Scholar]
- 21.Tsai HM, Lian EC. Antibodies to von Willebrand factor-cleaving protease in acute thrombotic thrombocytopenic purpura. N Engl J Med. 1998;339:1585–94. doi: 10.1056/NEJM199811263392203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elliott MA, Nichols WL, Jr, Plumhoff EA, Ansell SM, Dispenzieri A, et al. Post-transplantation thrombotic thrombocytopenic purpura: a single-center experience and a contemporary review. Mayo Clin Proc. 2003;78:421–30. doi: 10.4065/78.4.421. [DOI] [PubMed] [Google Scholar]
- 23.George JN, Selby GB. Thrombotic microangiopathy after allogeneic bone marrow transplantation: a pathologic abnormality associated with diverse clinical syndromes. Bone Marrow Transplant. 2004;33:1073–74. doi: 10.1038/sj.bmt.1704513. [DOI] [PubMed] [Google Scholar]
- 24.Gharpure VS, Devine SM, Holland HK, Geller RB, O’Toole K, et al. Thrombotic thrombocytopenic purpura associated with FK506 following bone marrow transplantation. Bone Marrow Transplant. 1995;16:715–16. [PubMed] [Google Scholar]
- 25.Moake JL, Byrnes JJ. Thrombotic microangiopathies associated with drugs and bone marrow transplantation. Hematol Oncol Clin N Am. 1996;10:485–97. doi: 10.1016/s0889-8588(05)70348-8. [DOI] [PubMed] [Google Scholar]
- 26.Medina PJ, Sipols JM, George JN. Drug-associated thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. Curr Opin Hematol. 2001;8:286–93. doi: 10.1097/00062752-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Pisoni R, Ruggenenti P, Remuzzi G. Drug-induced thrombotic microangiopathy: incidence, prevention and management. Drug Saf. 2001;24:491–501. doi: 10.2165/00002018-200124070-00002. [DOI] [PubMed] [Google Scholar]
- 28.Fontana S, Gerritsen HE, Kremer Hovinga J, Furlan M, Lammle B. Microangiopathic hemolytic anaemia in metastasizing malignant tumours is not associated with a severe deficiency of the von Willebrand factor-cleaving protease. Br J Haematol. 2001;113:100–2. doi: 10.1046/j.1365-2141.2001.02704.x. [DOI] [PubMed] [Google Scholar]
- 29.Mannucci PM, Karimi M, Mosalaei A, Canciani MT, Peyvandi F. Patients with localized and disseminated tumors have reduced but measurable levels of ADAMTS-13 (von Willebrand factor cleaving protease) Haematologica. 2003;88:454–58. [PubMed] [Google Scholar]
- 30.May HV, Jr, Harbert GM, Jr, Thornton WN., Jr Thrombotic thrombocytopenic purpura associated with pregnancy. Am J Obstet Gynecol. 1976;126:452–58. doi: 10.1016/0002-9378(76)90638-4. [DOI] [PubMed] [Google Scholar]
- 31.Atlas M, Barkai G, Menczer J, Houlu N, Lieberman P. Thrombotic thrombocytopenic purpura in pregnancy. Br J Obstet Gynaecol. 1982;89:476–79. doi: 10.1111/j.1471-0528.1982.tb03640.x. [DOI] [PubMed] [Google Scholar]
- 32.McCrae KR, Cines DB. Thrombotic microangiopathy during pregnancy. Semin Hematol. 1997;34:148–58. [PubMed] [Google Scholar]
- 33.Terrell DR, Williams LA, Vesely SK, Lammle B, Hovinga JA, George JN. The incidence of thrombotic thrombocytopenic purpura-hemolytic uremic syndrome: all patients, idiopathic patients, and patients with severe ADAMTS-13 deficiency. J Thromb Haemost. 2005;3:1432–36. doi: 10.1111/j.1538-7836.2005.01436.x. [DOI] [PubMed] [Google Scholar]
- 34.Torok TJ, Holman RC, Chorba TL. Increasing mortality from thrombotic thrombocytopenic purpura in the United States: analysis of national mortality data, 1968–1991. Am J Hematol. 1995;50:84–90. doi: 10.1002/ajh.2830500203. [DOI] [PubMed] [Google Scholar]
- 35.Lohrmann HP, Adam W, Heymer B, Kubanek B. Microangiopathic hemolytic anemia in metastatic carcinoma. Report of eight cases. Ann Intern Med. 1973;79:368–75. doi: 10.7326/0003-4819-79-3-368. [DOI] [PubMed] [Google Scholar]
- 36.George JN, Li X, McMinn JR, Terrell DR, Vesely SK, Selby GB. Thrombotic thrombocytopenic purpura-hemolytic uremic syndrome following allogeneic HPC transplantation: a diagnostic dilemma. Transfusion. 2004;44:294–304. doi: 10.1111/j.1537-2995.2004.00700.x. [DOI] [PubMed] [Google Scholar]
- 37.Leaf AN, Laubenstein LJ, Raphael B, Hochster H, Baez L, et al. Thrombotic thrombocytopenic purpura associated with human immunodeficiency virus type 1 (HIV-1) infection. Ann Intern Med. 1988;109:194–97. doi: 10.7326/0003-4819-109-3-194. [DOI] [PubMed] [Google Scholar]
- 38.Becker S, Fusco G, Fusco J, Balu R, Gangjee S, et al. HIV-associated thrombotic microangiopathy in the era of highly active antiretroviral therapy: an observational study. Clin Infect Dis. 2004;39(Suppl 5):S267–75. doi: 10.1086/422363. [DOI] [PubMed] [Google Scholar]
- 39.Esplin MS, Branch DW. Diagnosis and management of thrombotic microangiopathies during pregnancy. Clin Obstet Gynecol. 1999;42:360–67. doi: 10.1097/00003081-199906000-00020. [DOI] [PubMed] [Google Scholar]
- 40.George JN. The association of pregnancy with thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. Curr Opin Hematol. 2003;10:339–44. doi: 10.1097/00062752-200309000-00003. [DOI] [PubMed] [Google Scholar]
- 41.Dashe JS, Ramin SM, Cunningham FG. The long-term consequences of thrombotic microangiopathy (thrombotic thrombocytopenic purpura and hemolytic uremic syndrome) in pregnancy. Obstet Gynecol. 1998;91:662–68. doi: 10.1016/s0029-7844(98)00031-3. [DOI] [PubMed] [Google Scholar]
- 42.Mannucci PM, Canciani MT, Forza I, Lussana F, Lattuada A, Rossi E. Changes in health and disease of the metalloprotease that cleaves von Willebrand factor. Blood. 2001;98:2730–35. doi: 10.1182/blood.v98.9.2730. [DOI] [PubMed] [Google Scholar]
- 43.Tsai HM, Rice L, Sarode R, Chow TW, Moake JL. Antibody inhibitors to von Willebrand factor metalloproteinase and increased binding of von Willebrand factor to platelets in ticlopidine-associated thrombotic thrombocytopenic purpura. Ann Intern Med. 2000;132:794–99. doi: 10.7326/0003-4819-132-10-200005160-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bennett CL, Connors JM, Carwile JM, Moake JL, Bell WR, et al. Thrombotic thrombocytopenic purpura associated with clopidogrel. N Engl J Med. 2000;342:1773–77. doi: 10.1056/NEJM200006153422402. [DOI] [PubMed] [Google Scholar]
- 45.Jonas S, Grieco G. Editorial comment–an approach to the estimation of the risk of TTP during clopidogrel therapy. Stroke. 2004;35:537–38. [PubMed] [Google Scholar]
- 46.Zakarija A, Bandarenko N, Pandey DK, Auerbach A, Raisch DW, et al. Clopidogrel-associated TTP: an update of pharmacovigilance efforts conducted by independent researchers, pharmaceutical suppliers, and the Food and Drug Administration. Stroke. 2004;35:533–37. doi: 10.1161/01.STR.0000109253.66918.5E. [DOI] [PubMed] [Google Scholar]
- 47.Hosler GA, Cusumano AM, Hutchins GM. Thrombotic thrombocytopenic purpura and hemolytic uremic syndrome are distinct pathologic entities. A review of 56 autopsy cases. Arch Pathol Lab Med. 2003;127:834–39. doi: 10.5858/2003-127-834-TTPAHU. [DOI] [PubMed] [Google Scholar]
- 48.Asada Y, Sumiyoshi A, Hayashi T, Suzumiya J, Kaketani K. Immunohistochemistry of vascular lesion in thrombotic thrombocytopenic purpura, with special reference to factor VIII related antigen. Thromb Res. 1985;38:469–79. doi: 10.1016/0049-3848(85)90180-x. [DOI] [PubMed] [Google Scholar]
- 49.Katoh M, Shigematsu H. Renal involvement of thrombotic thrombocytopenic purpura: special reference to the glomeruloid structures. Pathol Int. 1999;49:638–42. doi: 10.1046/j.1440-1827.1999.00903.x. [DOI] [PubMed] [Google Scholar]
- 50.Padilla A, Moake JL, Bernardo A, Ball C, Wang Y, et al. P-selectin anchors newly released ultralarge von Willebrand factor multimers to the endothelial cell surface. Blood. 2004;103:2150–56. doi: 10.1182/blood-2003-08-2956. [DOI] [PubMed] [Google Scholar]
- 51.Tsai HM. Physiologic cleavage of von Willebrand factor by a plasma protease is dependent on its conformation and requires calcium ion. Blood. 1996;87:4235–44. [PubMed] [Google Scholar]
- 52.Sadler JE. von Willebrand factor. J Biol Chem. 1991;266:22777–80. [PubMed] [Google Scholar]
- 53.Tsai HM, Sussman I, Nagel RL. Shear stress enhances the proteolysis of von Willebrand factor in normal plasma. Blood. 1994;83:2171–79. [PubMed] [Google Scholar]
- 54.Furlan M, Robles R, Lammle B. Partial purification and characterization of a protease from human plasma cleaving von Willebrand factor to fragments produced by in vivo proteolysis. Blood. 1996;87:4223–34. [PubMed] [Google Scholar]
- 55.Tsai HM, Sussman II, Ginsburg D, Lankhof H, Sixma JJ, Nagel RL. Proteolytic cleavage of recombinant type 2A von Willebrand factor mutants R834W and R834Q: inhibition by doxycycline and by monoclonal antibody VP-1. Blood. 1997;89:1954–62. [PubMed] [Google Scholar]
- 56.Moake JL, Rudy CK, Troll JH, Weinstein MJ, Colannino NM, et al. Unusually large plasma factor VIII: von Willebrand factor multimers in chronic relapsing thrombotic thrombocytopenic purpura. N Engl J Med. 1982;307:1432–35. doi: 10.1056/NEJM198212023072306. [DOI] [PubMed] [Google Scholar]
- 57.Hulstein JJ, de Groot PG, Silence K, Veyradier A, Fijnheer R, Lenting PJ. A novel nanobody that detects the gain-of-function phenotype of von Willebrand factor in ADAMTS13 deficiency and von Willebrand disease type 2B. Blood. 2005;106:3035–42. doi: 10.1182/blood-2005-03-1153. [DOI] [PubMed] [Google Scholar]
- 58.Fujikawa K, Suzuki H, McMullen B, Chung D. Purification of human von Willebrand factor-cleaving protease and its identification as a new member of the metalloproteinase family. Blood. 2001;98:1662–66. doi: 10.1182/blood.v98.6.1662. [DOI] [PubMed] [Google Scholar]
- 59.Gerritsen HE, Robles R, Lammle B, Furlan M. Partial amino acid sequence of purified von Willebrand factor-cleaving protease. Blood. 2001;98:1654–61. doi: 10.1182/blood.v98.6.1654. [DOI] [PubMed] [Google Scholar]
- 60.Plaimauer B, Zimmermann K, Volkel D, Antoine G, Kerschbaumer R, et al. Cloning, expression, and functional characterization of the von Willebrand factor-cleaving protease (ADAMTS13) Blood. 2002;100:3626–32. doi: 10.1182/blood-2002-05-1397. [DOI] [PubMed] [Google Scholar]
- 61.Soejima K, Mimura N, Hirashima M, Maeda H, Hamamoto T, et al. A novel human metalloprotease synthesized in the liver and secreted into the blood: possibly, the von Willebrand factor-cleaving protease? J Biochem. 2001;130:475–80. doi: 10.1093/oxfordjournals.jbchem.a003009. [DOI] [PubMed] [Google Scholar]
- 62.Zheng X, Chung D, Takayama TK, Majerus EM, Sadler JE, Fujikawa K. Structure of von Willebrand factor-cleaving protease (ADAMTS13), a metalloprotease involved in thrombotic thrombocytopenic purpura. J Biol Chem. 2001;276:41059–63. doi: 10.1074/jbc.C100515200. [DOI] [PubMed] [Google Scholar]
- 63.Levy GG, Nichols WC, Lian EC, Foroud T, McClintick JN, et al. Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature. 2001;413:488–94. doi: 10.1038/35097008. [DOI] [PubMed] [Google Scholar]
- 64.Bork P, Beckmann G. The CUB domain. A widespread module in developmentally regulated proteins. J Mol Biol. 1993;231:539–45. doi: 10.1006/jmbi.1993.1305. [DOI] [PubMed] [Google Scholar]
- 65.Shang D, Zheng XW, Niiya M, Zheng XL. Apical sorting of ADAMTS13 in vascular endothelial cells and Madin-Darby canine kidney cells depends on the CUB domains and their association with lipid rafts. Blood. 2006;108:2207–15. doi: 10.1182/blood-2006-02-002139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Turner N, Nolasco L, Tao Z, Dong JF, Moake J. Human endothelial cells synthesize and release ADAMTS-13. J Thromb Haemost. 2006;4:1396–404. doi: 10.1111/j.1538-7836.2006.01959.x. [DOI] [PubMed] [Google Scholar]
- 67.Liu L, Choi H, Bernardo A, Bergeron AL, Nolasco L, et al. Platelet-derived VWF-cleaving metalloprotease ADAMTS-13. J Thromb Haemost. 2005;3:2536–44. doi: 10.1111/j.1538-7836.2005.01561.x. [DOI] [PubMed] [Google Scholar]
- 68.Suzuki M, Murata M, Matsubara Y, Uchida T, Ishihara H, et al. Detection of von Willebrand factor-cleaving protease (ADAMTS-13) in human platelets. Biochem Biophys Res Commun. 2004;313:212–16. doi: 10.1016/j.bbrc.2003.11.111. [DOI] [PubMed] [Google Scholar]
- 69.Zheng XW, Niiya M, Zheng X, Pollak E. Isolation and characterization of human ADAMTS13 gene promoter region-control of ADAMTS13 gene expression by hepatic transcription factors and inflammatory cytokines. Blood. 2006;108:459a. [Google Scholar]
- 70.Soejima K, Nakamura H, Hirashima M, Morikawa W, Nozaki C, et al. Analysis on the molecular species and concentration of circulating ADAMTS13 in blood. J Biochem. 2006;139:147–54. doi: 10.1093/jb/mvj013. [DOI] [PubMed] [Google Scholar]
- 71.Crawley JT, Lam JK, Rance JB, Mollica LR, O’Donnell JS, Lane DA. Proteolytic inactivation of ADAMTS13 by thrombin and plasmin. Blood. 2005;105:1085–93. doi: 10.1182/blood-2004-03-1101. [DOI] [PubMed] [Google Scholar]
- 72.Ono T, Mimuro J, Madoiwa S, Soejima K, Kashiwakura Y, et al. Severe secondary deficiency of von Willebrand factor-cleaving protease (ADAMTS13) in patients with sepsis-induced disseminated intravascular coagulation: its correlation with development of renal failure. Blood. 2006;107:528–34. doi: 10.1182/blood-2005-03-1087. [DOI] [PubMed] [Google Scholar]
- 73.Zhou W, Inada M, Lee TP, Benten D, Lyubsky S, et al. ADAMTS13 is expressed in hepatic stellate cells. Lab Invest. 2005;85:780–88. doi: 10.1038/labinvest.3700275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Uemura M, Tatsumi K, Matsumoto M, Fujimoto M, Matsuyama T, et al. Localization of ADAMTS13 to the stellate cells of human liver. Blood. 2005;106:922–24. doi: 10.1182/blood-2005-01-0152. [DOI] [PubMed] [Google Scholar]
- 75.Niiya M, Uemura M, Zheng XW, Pollak ES, Dockal M, et al. Increased ADAMTS13 proteolytic activity in rat hepatic stellate cells upon activation in vitro and in vivo. J Thromb Haemost. 2006;4:1063–70. doi: 10.1111/j.1538-7836.2006.01893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Soejima K, Matsumoto M, Kokame K, Yagi H, Ishizashi H, et al. ADAMTS-13 cysteine-rich/spacer domains are functionally essential for von Willebrand factor cleavage. Blood. 2003;102:3232–37. doi: 10.1182/blood-2003-03-0908. [DOI] [PubMed] [Google Scholar]
- 77.Zheng X, Nishio K, Majerus EM, Sadler JE. Cleavage of von Willebrand factor requires the spacer domain of the metalloprotease ADAMTS13. J Biol Chem. 2003;278:30136–41. doi: 10.1074/jbc.M305331200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Anderson PJ, Kokame K, Sadler JE. Zinc and calcium ions cooperatively modulate ADAMTS13 activity. J Biol Chem. 2006;281:850–57. doi: 10.1074/jbc.M504540200. [DOI] [PubMed] [Google Scholar]
- 79.Ai J, Smith P, Wang S, Zhang P, Zheng XL. The proximal carboxyl-terminal domains of ADAMTS13 determine substrate specificity and are all required for cleavage of von Willebrand factor. J Biol Chem. 2005;280:29428–34. doi: 10.1074/jbc.M505513200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gao W, Anderson PJ, Majerus EM, Tuley EA, Sadler JE. Exosite interactions contribute to tension-induced cleavage of von Willebrand factor by the antithrombotic ADAMTS13 metalloprotease. Proc Natl Acad Sci USA. 2006;103:19099–104. doi: 10.1073/pnas.0607264104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kokame K, Matsumoto M, Fujimura Y, Miyata T. VWF73, a region from D1596 to R1668 of von Willebrand factor, provides a minimal substrate for ADAMTS-13. Blood. 2003;103:607–12. doi: 10.1182/blood-2003-08-2861. [DOI] [PubMed] [Google Scholar]
- 82.Zhou W, Tsai HM. An enzyme immunoassay of ADAMTS13 distinguishes patients with thrombotic thrombocytopenic purpura from normal individuals and carriers of ADAMTS13 mutations. Thromb Haemost. 2004;91:806–11. doi: 10.1160/TH03-11-0675. [DOI] [PubMed] [Google Scholar]
- 83.Wu JJ, Fujikawa K, McMullen BA, Chung DW. Characterization of a core binding site for ADAMTS-13 in the A2 domain of von Willebrand factor. Proc Natl Acad Sci USA. 2006;103:18470–74. doi: 10.1073/pnas.0609190103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tao Z, Wang Y, Choi H, Bernardo A, Nishio K, et al. Cleavage of ultralarge multimers of von Willebrand factor by C-terminal-truncated mutants of ADAMTS-13 under flow. Blood. 2005;106:141–43. doi: 10.1182/blood-2004-11-4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pimanda JE, Maekawa A, Wind T, Paxton J, Chesterman CN, et al. Congenital thrombotic thrombocytopenic purpura in association with a mutation in the second CUB domain of ADAMTS13. Blood. 2004;103:627–29. doi: 10.1182/blood-2003-04-1346. [DOI] [PubMed] [Google Scholar]
- 86.Shelat SG, Ai J, Zheng XL. Molecular biology of ADAMTS13 and diagnostic utility of ADAMTS13 proteolytic activity and inhibitor assays. Semin Thromb Hemost. 2005;31:659–72. doi: 10.1055/s-2005-925472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Klaus C, Plaimauer B, Studt JD, Dorner F, Lammle B, et al. Epitope mapping of ADAMTS13 autoantibodies in acquired thrombotic thrombocytopenic purpura. Blood. 2004;103:4514–19. doi: 10.1182/blood-2003-12-4165. [DOI] [PubMed] [Google Scholar]
- 88.Bernardo A, Nolasco L, Wang Y, Ball C, Moake J, et al. Peptides from the C-terminal regions of ADAMTS-13 specifically block cleavage of ultralarge von Wille-brand factor multimers on the endothelial surface under flow. J Thromb Haemost. 2003;1:405a. [Google Scholar]
- 89.Tao Z, Peng Y, Nolasco L, Cal S, Lopez-Otin C, et al. Role of the CUB-1 domain in docking ADAMTS-13 to unusually large von Willebrand factor in flowing blood. Blood. 2005;106:4139–45. doi: 10.1182/blood-2005-05-2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang P, Pan W, Rux AH, Sachais BS, Zheng X. The cooperative activity between the carboxyl-terminal TSP1 repeats and the CUB domains of ADAMTS13 is crucial for recognition of von Willebrand factor under flow. Blood. 2007;110:1887–94. doi: 10.1182/blood-2007-04-083329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Majerus EM, Anderson PJ, Sadler JE. Binding of ADAMTS13 to von Willebrand factor. J Biol Chem. 2005;280:71773–78. doi: 10.1074/jbc.M502529200. [DOI] [PubMed] [Google Scholar]
- 92.Dong JF, Moake JL, Bernardo A, Fujikawa K, Ball C, et al. ADAMTS-13 metalloprotease interacts with the endothelial cell-derived ultralarge von Willebrand factor. J Biol Chem. 2003;278:29633–39. doi: 10.1074/jbc.M301385200. [DOI] [PubMed] [Google Scholar]
- 93.Jenkins PV, Pasi KJ, Perkins SJ. Molecular modeling of ligand and mutation sites of the type A domains of human von Willebrand factor and their relevance to von Willebrand’s disease. Blood. 1998;91:2032–44. [PubMed] [Google Scholar]
- 94.Tsai HM. Shear stress and von Willebrand factor in health and disease. Semin Thromb Hemost. 2003;29:479–88. doi: 10.1055/s-2003-44556. [DOI] [PubMed] [Google Scholar]
- 95.Nishio K, Anderson PJ, Zheng XL, Sadler JE. Binding of platelet glycoprotein Ibα to von Willebrand factor domain A1 stimulates the cleavage of the adjacent domain A2 by ADAMTS13. Proc Natl Acad Sci USA. 2004;101:10578–83. doi: 10.1073/pnas.0402041101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.De Cristofaro R, Peyvandi F, Palla R, Lavoretano S, Lombardi R, et al. Role of chloride ions in the modulation of the interaction between von Willebrand factor and ADAMTS-3. J Biol Chem. 2005;280:23295–302. doi: 10.1074/jbc.M501143200. [DOI] [PubMed] [Google Scholar]
- 97.Bernardo A, Ball C, Nolasco L, Moake JF, Dong JF. Effects of inflammatory cytokines on the release and cleavage of the endothelial cell-derived ultralarge von Willebrand factor multimers under flow. Blood. 2004;104:100–6. doi: 10.1182/blood-2004-01-0107. [DOI] [PubMed] [Google Scholar]
- 98.Studt JD, Hovinga JA, Antoine G, Hermann M, Rieger M, et al. Fatal congenital thrombotic thrombocytopenic purpura with apparent ADAMTS13 inhibitor: in vitro inhibition of ADAMTS13 activity by hemoglobin. Blood. 2005;105:542–44. doi: 10.1182/blood-2004-06-2096. [DOI] [PubMed] [Google Scholar]
- 99.Kokame K, Matsumoto M, Soejima K, Yagi H, Ishizashi H, et al. Mutations and common polymorphisms in ADAMTS13 gene responsible for von Willebrand factor-cleaving protease activity. Proc Natl Acad Sci USA. 2002;99:11902–7. doi: 10.1073/pnas.172277399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Antoine G, Zimmermann K, Plaimauer B, Grillowitzer M, Studt JD, et al. ADAMTS13 gene defects in two brothers with constitutional thrombotic thrombocytopenic purpura and normalization of von Willebrand factor-cleaving protease activity by recombinant human ADAMTS13. Br J Haematol. 2003;120:821–24. doi: 10.1046/j.1365-2141.2003.04183.x. [DOI] [PubMed] [Google Scholar]
- 101.Assink K, Schiphorst R, Allford S, Karpman D, Etzioni A, et al. Mutation analysis and clinical implications of von Willebrand factor-cleaving protease deficiency. Kidney Int. 2003;63:1995–99. doi: 10.1046/j.1523-1755.63.6s.1.x. [DOI] [PubMed] [Google Scholar]
- 102.Matsumoto M, Kokame K, Soejima K, Miura M, Hayashi S, et al. Molecular characterization of ADAMTS13 gene mutations in Japanese patients with Upshaw-Schulman syndrome. Blood. 2003;103:1305–10. doi: 10.1182/blood-2003-06-1796. [DOI] [PubMed] [Google Scholar]
- 103.Savasan S, Lee SK, Ginsburg D, Tsai HM. ADAMTS13 gene mutation in congenital thrombotic thrombocytopenic purpura with previously reported normal VWF cleaving protease activity. Blood. 2003;101:4449–51. doi: 10.1182/blood-2002-12-3796. [DOI] [PubMed] [Google Scholar]
- 104.Schneppenheim R, Budde U, Oyen F, Angerhaus D, Aumann V, et al. von Willebrand factor cleaving protease and ADAMTS13 mutations in childhood TTP. Blood. 2003;101:1845–50. doi: 10.1182/blood-2002-08-2399. [DOI] [PubMed] [Google Scholar]
- 105.Licht C, Stapenhorst L, Simon T, Budde U, Schneppenheim R, et al. Two novel ADAMTS13 gene mutations in thrombotic thrombocytopenic purpura/hemolyticuremic syndrome (TTP/HUS) Kidney Int. 2004;66:955–58. doi: 10.1111/j.1523-1755.2004.00841.x. [DOI] [PubMed] [Google Scholar]
- 106.Uchida T, Wada H, Mizutani M, Iwashita M, Ishihara H, et al. Identification of novel mutations in ADAMTS13 in an adult patient with congenital thrombotic thrombocytopenic purpura. Blood. 2004;104:2081–83. doi: 10.1182/blood-2004-02-0715. [DOI] [PubMed] [Google Scholar]
- 107.Noris M, Bucchioni S, Galbusera M, Donadelli R, Bresin E, et al. Complement factor H mutation in familial thrombotic thrombocytopenic purpura with ADAMTS13 deficiency and renal involvement. J Am Soc Nephrol. 2005;16:1177–83. doi: 10.1681/ASN.2005010086. [DOI] [PubMed] [Google Scholar]
- 108.Furlan M, Lammle B. Aetiology and pathogenesis of thrombotic thrombocytopenic purpura and hemolytic uraemic syndrome: the role of von Willebrand factor-cleaving protease. Best Pract Res Clin Haematol. 2001;14:437–54. doi: 10.1053/beha.2001.0142. [DOI] [PubMed] [Google Scholar]
- 109.Kokame K, Miyata T. Genetic defects leading to hereditary thrombotic thrombocytopenic purpura. Semin Hematol. 2004;41:34–40. doi: 10.1053/j.seminhematol.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 110.Bongers TN, De Maat MP, Dippel DW, Uitterlinden AG, Leebeek FW. Absence of Pro475Ser polymorphism in ADAMTS-13 in Caucasians. J Thromb Haemost. 2005;3:805. doi: 10.1111/j.1538-7836.2005.01239.x. [DOI] [PubMed] [Google Scholar]
- 111.Ruan C, Dai L, Su J, Wang Z. The frequency of P475S polymorphism in von Willebrand factor-cleaving protease in the Chinese population and its relevance to arterial thrombotic disorders. Thromb Haemost. 2004;91:1257–58. [PubMed] [Google Scholar]
- 112.Yoo G, Blomback M, Schenck-Gustafsson K, He S. Decreased levels of von Willebrand factor-cleaving protease in coronary heart disease and thrombotic thrombocytopenic purpura: study of a simplified method for assaying the enzyme activity based on ristocetin-induced platelet aggregation. Br J Haematol. 2003;121:123–29. doi: 10.1046/j.1365-2141.2003.04257.x. [DOI] [PubMed] [Google Scholar]
- 113.Plaimauer B, Fuhrmann J, Mohr G, Wernhart W, Bruno K, et al. Modulation of ADAMTS13 secretion and specific activity by a combination of common amino acid polymorphisms and a missense mutation. Blood. 2006;107:118–25. doi: 10.1182/blood-2005-06-2482. [DOI] [PubMed] [Google Scholar]
- 114.Kinoshita S, Yoshioka A, Park YD, Ishizashi H, Konno M, et al. Upshaw-Schulman syndrome revisited: a concept of congenital thrombotic thrombocytopenic purpura. Int J Hematol. 2001;74:101–8. doi: 10.1007/BF02982558. [DOI] [PubMed] [Google Scholar]
- 115.Savasan S, Taub JW, Buck S, Botterill M, Furlan M, Ravindranath Y. Congenital microangiopathic hemolytic anemia and thrombocytopenia with unusually large von Willebrand factor multimers and von Willebrand factor-cleaving protease. J Pediatr Hematol Oncol. 2001;23:364–67. doi: 10.1097/00043426-200108000-00008. [DOI] [PubMed] [Google Scholar]
- 116.Studt JD, Kremer Hovinga JA, Alberio L, Bianchi V, Lammle B. Von Willebrand factor-cleaving protease (ADAMTS-13) activity in thrombotic microangiopathies: diagnostic experience 2001/2002 of a single research laboratory. Swiss Med Wkly. 2003;133:325–32. doi: 10.4414/smw.2003.10242. [DOI] [PubMed] [Google Scholar]
- 117.Zheng XL, Richard KM, Goodnough LT, Sadler JE. Effect of plasma exchange on plasma ADAMTS13 metalloprotease activity, inhibitor level, and clinical outcome in patients with idiopathic and nonidiopathic thrombotic thrombocytopenic purpura. Blood. 2004;103:4043–49. doi: 10.1182/blood-2003-11-4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rieger M, Mannucci PM, Kremer Hovinga JA, Herzog A, Gerstenbauer G, et al. ADAMTS13 autoantibodies in patients with thrombotic microangiopathies and other immunomediated diseases. Blood. 2005;106:1262–67. doi: 10.1182/blood-2004-11-4490. [DOI] [PubMed] [Google Scholar]
- 119.Scheiflinger F, Knoebl P, Trattner B, Plaimauer B, Mohr G, et al. Non-neutralizing IgM and IgG antibodies to von Willebrand factor-cleaving protease (ADAMTS-13) in a patient with thrombotic thrombocytopenic purpura (TTP) Blood. 2003;102:3241–43. doi: 10.1182/blood-2003-05-1616. [DOI] [PubMed] [Google Scholar]
- 120.Sugio Y, Okamura T, Shimoda K, Matsumoto M, Yagi H, et al. Ticlopidine-associated thrombotic thrombocytopenic purpura with an IgG-type inhibitor to von Willebrand factor-cleaving protease activity. Int J Hematol. 2001;74:347–51. doi: 10.1007/BF02982073. [DOI] [PubMed] [Google Scholar]
- 121.Luken BM, Turenhout EA, Hulstein JJ, Van Mourik JA, Fijnheer R, Voorberg J. The spacer domain of ADAMTS13 contains a major binding site for antibodies in patients with thrombotic thrombocytopenic purpura. Thromb Haemost. 2005;93:267–74. doi: 10.1160/TH04-05-0301. [DOI] [PubMed] [Google Scholar]
- 122.Luken BM, Kaijen PH, Turenhout EA, Kremer Hovinga JA, Van Mourik JA, et al. Multiple B-cell clones producing antibodies directed to the spacer and disintegrin/thrombospondin type-1 repeat 1 (TSP1) of ADAMTS13 in a patient with acquired thrombotic thrombocytopenic purpura. J Thromb Haemost. 2006;4:2355–64. doi: 10.1111/j.1538-7836.2006.02164.x. [DOI] [PubMed] [Google Scholar]
- 123.Siegel D, Ostertag E. Inhibitory ADAMTS13 monoclonal autoantibodies cloned from TTP patients show restricted gene usage, inhibit murine ADAMTS13, and are blocked by rabbit anti-idiotypic antibodies. Blood. 2006;108:31a. [Google Scholar]
- 124.Raife TJ, Lentz SR, Atkinson BS, Vesely SK, Hessner MJ. Factor V Leiden: a genetic risk factor for thrombotic microangiopathy in patients with normal von Willebrand factor-cleaving protease activity. Blood. 2002;99:437–42. doi: 10.1182/blood.v99.2.437. [DOI] [PubMed] [Google Scholar]
- 125.Krieg S, Studt JD, Sulzer I, Lammle B, Hovinga JA. Is factor V Leiden a risk factor for thrombotic microangiopathies without severe ADAMTS 13 deficiency? Thromb Haemost. 2005;94:1186–89. [PubMed] [Google Scholar]
- 126.Joseph G, Smith KJ, Hadley TJ, Djulbegovic B, Troup GM, et al. HLA-DR53 protects against thrombotic thrombocytopenic purpura/adult hemolytic uremic syndrome. Am J Hematol. 1994;47:189–93. doi: 10.1002/ajh.2830470308. [DOI] [PubMed] [Google Scholar]
- 127.Praprotnik S, Blank M, Levy Y, Tavor S, Boffa MC, et al. Anti-endothelial cell antibodies from patients with thrombotic thrombocytopenic purpura specifically activate small vessel endothelial cells. Int Immunol. 2001;13:203–10. doi: 10.1093/intimm/13.2.203. [DOI] [PubMed] [Google Scholar]
- 128.Jimenez JJ, Jy W, Mauro LM, Horstman LL, Ahn YS. Elevated endothelial microparticles in thrombotic thrombocytopenic purpura: findings from brain and renal microvascular cell culture and patients with active disease. Br J Haematol. 2001;112:81–90. doi: 10.1046/j.1365-2141.2001.02516.x. [DOI] [PubMed] [Google Scholar]
- 129.Jimenez JJ, Jy W, Mauro LM, Horstman LL, Soderland C, et al. Endothelial microparticles released in thrombotic thrombocytopenic purpura express von Willebrand factor and markers of endothelial activation. Br J Haematol. 2003;123:896–902. doi: 10.1046/j.1365-2141.2003.04716.x. [DOI] [PubMed] [Google Scholar]
- 130.Schultz DR, Arnold PI, Jy W, Valant PA, Gruber J, et al. Anti-CD36 autoantibodies in thrombotic thrombocytopenic purpura and other thrombotic disorders: Identification of an 85 kD form of CD36 as a target antigen. Br J Haematol. 1998;103:849–57. doi: 10.1046/j.1365-2141.1998.01070.x. [DOI] [PubMed] [Google Scholar]
- 131.Wright JF, Wang H, Hornstein A, Hogarth M, Mody M, et al. Characterization of platelet glycoproteins and platelet/endothelial cell antibodies in patients with thrombotic thrombocytopenic purpura. Br J Haematol. 1999;107:546–55. doi: 10.1046/j.1365-2141.1999.01751.x. [DOI] [PubMed] [Google Scholar]
- 132.Combes V, Simon AC, Grau GE, Arnoux D, Camoin L, et al. In vitro generation of endothelial microparticles and possible prothrombotic activity in patients with lupus anticoagulant. J Clin Invest. 1999;104:93–102. doi: 10.1172/JCI4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Carter AO, Borczyk AA, Carlson JA, Harvey B, Hockin JC, et al. A severe outbreak of Escherichia coli O157:H7–associated hemorrhagic colitis in a nursing home. N Engl J Med. 1987;317:1496–500. doi: 10.1056/NEJM198712103172403. [DOI] [PubMed] [Google Scholar]
- 134.Griffin PM, Tauxe RV. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol Rev. 1991;13:60–98. doi: 10.1093/oxfordjournals.epirev.a036079. [DOI] [PubMed] [Google Scholar]
- 135.Alon U, Adler SP, Chan JC. Hemolytic-uremic syndrome associated with Streptococcus pneumoniae. Report of a case and review of the literature. Am J Dis Child. 1984;138:496–99. doi: 10.1001/archpedi.1984.02140430072019. [DOI] [PubMed] [Google Scholar]
- 136.Neuhaus TJ, Calonder S, Leumann EP. Heterogeneity of atypical hemolytic uraemic syndromes. Arch Dis Child. 1997;76:518–21. doi: 10.1136/adc.76.6.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tarr PI. Escherichia coli O157:H7: clinical, diagnostic, and epidemiological aspects of human infection. Clin Infect Dis. 1995;20:1–8. doi: 10.1093/clinids/20.1.1. [DOI] [PubMed] [Google Scholar]
- 138.Le Saux N, Spika JS, Friesen B, Johnson I, Melnychuck D, et al. Ground beef consumption in noncommercial settings is a risk factor for sporadic Escherichia coli O157:H7 infection in Canada. J Infect Dis. 1993;167:500–2. doi: 10.1093/infdis/167.2.500. [DOI] [PubMed] [Google Scholar]
- 139.Wong CS, Jelacic S, Habeeb RL, Watkins SL, Tarr PI. The risk of the hemolytic-uremic syndrome after antibiotic treatment of Escherichia coli O157:H7 infections. N Engl J Med. 2000;342:1930–36. doi: 10.1056/NEJM200006293422601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Morel-Maroger L, Kanfer A, Solez K, Sraer JD, Richet G. Prognostic importance of vascular lesions in acute renal failure with microangiopathic hemolytic anemia (hemolytic-uremic syndrome): clinicopathologic study in 20 adults. Kidney Int. 1979;15:548–58. doi: 10.1038/ki.1979.70. [DOI] [PubMed] [Google Scholar]
- 141.Tsai HM, Chandler WL, Sarode R, Hoffman R, Jelacic S, et al. von Willebrand factor and von Willebrand factor-cleaving metalloprotease activity in Escherichia coli O157:H7-associated hemolytic uremic syndrome. Pediatr Res. 2001;49:653–59. doi: 10.1203/00006450-200105000-00008. [DOI] [PubMed] [Google Scholar]
- 142.Heptinstall RH. Hemolytic uremic syndrome, thrombotic thrombocytopenic purpura, and systemic sclerosis (systemic scleroderma) In: Heptinstall RH, editor. Pathology of the Kidney. Vol. 2. Boston/Toronto/London: Little, Brown; 1992. pp. 1163–233. [Google Scholar]
- 143.Taylor CM. Hemolytic-uremic syndrome and complement factor H deficiency: clinical aspects. Semin Thromb Hemost. 2001;27:185–90. doi: 10.1055/s-2001-15247. [DOI] [PubMed] [Google Scholar]
- 144.Moake JL. Thrombotic microangiopathies. N Engl J Med. 2002;347:589–600. doi: 10.1056/NEJMra020528. [DOI] [PubMed] [Google Scholar]
- 145.Nolasco LH, Turner NA, Bernardo A, Tao Z, Cleary TG, et al. Hemolytic uremic syndrome-associated Shiga toxins promote endothelial-cell secretion and impair ADAMTS13 cleavage of unusually large von Willebrand factor multimers. Blood. 2005;106:4199–209. doi: 10.1182/blood-2005-05-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Caprioli J, Castelletti F, Bucchioni S, Bettinaglio P, Bresin E, et al. Complement factor H mutations and gene polymorphisms in hemolytic uraemic syndrome: the C-257T, the A2089G and the G2881T polymorphisms are strongly associated with the disease. Hum Mol Genet. 2003;12:3385–95. doi: 10.1093/hmg/ddg363. [DOI] [PubMed] [Google Scholar]
- 147.Richards A, Goodship JA, Goodship TH. The genetics and pathogenesis of hemolytic uraemic syndrome and thrombotic thrombocytopenic purpura. Curr Opin Nephrol Hypertens. 2002;11:431–35. doi: 10.1097/00041552-200207000-00010. [DOI] [PubMed] [Google Scholar]
- 148.Caprioli J, Noris M, Brioschi S, Pianetti G, Castelletti F, et al. Genetics of HUS: the impact of MCP, CFH, and IF mutations on clinical presentation, response to treatment, and outcome. Blood. 2006;108:1267–79. doi: 10.1182/blood-2005-10-007252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nilsson SC, Karpman D, Vaziri-Sani F, Kristoffersson AC, Salomon R, et al. A mutation in factor I that is associated with atypical hemolytic uremic syndrome does not affect the function of factor I in complement regulation. Mol Immunol. 2007;44:1835–44. doi: 10.1016/j.molimm.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 150.Fremeaux-Bacchi V, Dragon-Durey MA, Blouin J, Vigneau C, Kuypers D, et al. Complement factor I: a susceptibility gene for atypical hemolytic uraemic syndrome. J Med Genet. 2004;41:e84. doi: 10.1136/jmg.2004.019083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kavanagh D, Goodship TH. Membrane cofactor protein and factor I: mutations and transplantation. Semin Thromb Hemost. 2006;32:155–59. doi: 10.1055/s-2006-939771. [DOI] [PubMed] [Google Scholar]
- 152.Goicoechea de Jorge E, Harris CL, Esparza-Gordillo J, Carreras L, Arranz EA, et al. Gain-of-function mutations in complement factor B are associated with atypical hemolytic uremic syndrome. Proc Natl Acad Sci USA. 2007;104:240–45. doi: 10.1073/pnas.0603420103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kavanagh D, Kemp EJ, Richards A, Burgess RM, Mayland E, et al. Does complement factor B have a role in the pathogenesis of atypical HUS? Mol Immunol. 2006;43:856–59. doi: 10.1016/j.molimm.2005.06.041. [DOI] [PubMed] [Google Scholar]
- 154.Kim Y, Miller K, Michael AF. Breakdown products of C3 and factor B in hemolytic-uremic syndrome. J Lab Clin Med. 1977;89:845–50. [PubMed] [Google Scholar]
- 155.Neumann HP, Salzmann M, Bohnert-Iwan B, Mannuelian T, Skerka C, et al. Haemolytic uraemic syndrome and mutations of the factor H gene: a registry-based study of German speaking countries. J Med Genet. 2003;40:676–81. doi: 10.1136/jmg.40.9.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Saland JM, Emre SH, Shneider BL, Benchimol C, Ames S, et al. Favorable long-term outcome after liver-kidney transplant for recurrent hemolytic uremic syndrome associated with a factor H mutation. Am J Transplant. 2006;6:1948–52. doi: 10.1111/j.1600-6143.2006.01375.x. [DOI] [PubMed] [Google Scholar]
- 157.Thompson RA, Winterborn MH. Hypocomplementaemia due to a genetic deficiency of beta 1H globulin. Clin Exp Immunol. 1981;46:110–19. [PMC free article] [PubMed] [Google Scholar]
- 158.Buddles MR, Donne RL, Richards A, Goodship J, Goodship TH. Complement factor H gene mutation associated with autosomal recessive atypical hemolytic uremic syndrome. Am J Hum Genet. 2000;66:1721–22. doi: 10.1086/302877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Taylor CM. Complement factor H and the hemolytic uraemic syndrome. Lancet. 2001;358:1200–2. doi: 10.1016/s0140-6736(01)06339-5. [DOI] [PubMed] [Google Scholar]
- 160.Ault BH. Factor H and the pathogenesis of renal diseases. Pediatr Nephrol. 2000;14:1045–53. doi: 10.1007/s004670050069. [DOI] [PubMed] [Google Scholar]
- 161.Dragon-Durey MA, Loirat C, Cloarec S, Macher MA, Blouin J, et al. Anti-Factor H autoantibodies associated with atypical hemolytic uremic syndrome. J Am Soc Nephrol. 2005;16:555–63. doi: 10.1681/ASN.2004050380. [DOI] [PubMed] [Google Scholar]
- 162.Fremeaux-Bacchi V, Moulton EA, Kavanagh D, Dragon-Durey MA, Blouin J, et al. Genetic and functional analyses of membrane cofactor protein (CD46) mutations in atypical hemolytic uremic syndrome. J Am Soc Nephrol. 2006;17:2017–25. doi: 10.1681/ASN.2005101051. [DOI] [PubMed] [Google Scholar]
- 163.Noris M, Brioschi S, Caprioli J, Todeschini M, Bresin E, et al. Familial hemolytic uraemic syndrome and an MCP mutation. Lancet. 2003;362:1542–47. doi: 10.1016/S0140-6736(03)14742-3. [DOI] [PubMed] [Google Scholar]
- 164.Richards A, Kemp EJ, Liszewski MK, Goodship JA, Lampe AK, et al. Mutations in human complement regulator, membrane cofactor protein (CD46), predispose to development of familial hemolytic uremic syndrome. Proc Natl Acad Sci USA. 2003;100:12966–71. doi: 10.1073/pnas.2135497100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Alper CA, Abramson N, Johnston RB, Jr, Jandl JH, Rosen FS. Increased susceptibility to infection associated with abnormalities of complement-mediated functions and of the third component of complement (C3) N Engl J Med. 1970;282:350–54. doi: 10.1056/nejm197002122820701. [DOI] [PubMed] [Google Scholar]
- 166.Proesmans W. Typical and atypical hemolytic uremic syndrome. Kidney Blood Press Res. 1996;19:205–8. doi: 10.1159/000174075. [DOI] [PubMed] [Google Scholar]
- 167.Siegler RL, Pavia AT, Hansen FL, Christofferson RD, Cook JB. Atypical hemolytic-uremic syndrome: a comparison with postdiarrheal disease. J Pediatr. 1996;128:505–11. doi: 10.1016/s0022-3476(96)70361-x. [DOI] [PubMed] [Google Scholar]
- 168.Tonshoff B, Sammet A, Sanden I, Mehls O, Waldherr R, et al. Outcome and prognostic determinants in the hemolytic uremic syndrome of children. Nephron. 1994;68:63–70. doi: 10.1159/000188221. [DOI] [PubMed] [Google Scholar]
- 169.Zipfel PF, Neumann HP, Jozsi M. Genetic screening in hemolytic uraemic syndrome. Curr Opin Nephrol Hypertens. 2003;12:653–57. doi: 10.1097/00041552-200311000-00014. [DOI] [PubMed] [Google Scholar]
- 170.Smith CA, Vogel CW, Muller-Eberhard HJ. MHC Class III products: an electron microscopic study of the C3 convertases of human complement. J Exp Med. 1984;159:324–29. doi: 10.1084/jem.159.1.324. [DOI] [PMC free article] [PubMed] [Google Scholar]