Abstract
Human mitochondrial methionine transfer RNA (hmtRNAMetCAU) has a unique post-transcriptional modification, 5-formylcytidine, at the wobble position-34 (f5C34). The role of this modification in (hmtRNAMetCAU) for the decoding of AUA, as well as AUG, in both the peptidyl- and aminoacyl-sites of the ribosome in either chain initiation or chain elongation is still unknown. We report the first synthesis and analyses of the tRNA's anticodon stem and loop domain containing the 5-formylcytidine modification. The modification contributes to the tRNA's anticodon domain structure, thermodynamic properties and its ability to bind codons AUA and AUG in translational initiation and elongation.
INTRODUCTION
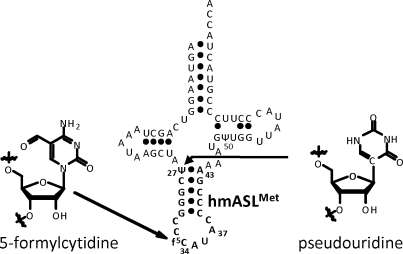
Mitochondria generate over 90% of the energy used by mammalian cells through oxidative phosphorylation. Thirteen proteins, components of the electron transfer chain and the ATP synthase, are the products of mitochondrial DNA. The synthesis of these proteins is carried out by a specific protein synthesizing machinery within this organelle. During the almost-three decades since the sequencing of the human mitochondrial genome (1), the mitochondrial genetic code has been found to differ significantly from the universal code. The human mitochondrial gene for the one methionine specific tRNA (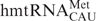 , where CAU is the anticodon) plays a unique role since it must provide the tRNA used for both the initiation of protein synthesis and the elongation of the protein chain by responding to the codon AUA, normally an isoleucine codon in the cytoplasm, as well as the universal methionine code, AUG. This is highly unusual since all cytoplasmic protein biosynthetic systems employ two different tRNAMet species, one for initiation and one for elongation, and both respond to the single methionine codon, AUG. Maternally inherited mutations in the gene of this tRNA, including an A37 to G37 mutation adjacent to the anticodon nucleosides that read the two codons (Figure 1), are responsible for some devastating diseases (2–5). Moreover, the
, where CAU is the anticodon) plays a unique role since it must provide the tRNA used for both the initiation of protein synthesis and the elongation of the protein chain by responding to the codon AUA, normally an isoleucine codon in the cytoplasm, as well as the universal methionine code, AUG. This is highly unusual since all cytoplasmic protein biosynthetic systems employ two different tRNAMet species, one for initiation and one for elongation, and both respond to the single methionine codon, AUG. Maternally inherited mutations in the gene of this tRNA, including an A37 to G37 mutation adjacent to the anticodon nucleosides that read the two codons (Figure 1), are responsible for some devastating diseases (2–5). Moreover, the 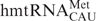 has a unique modification, 5-formylcytidine (Figure 1), at the wobble position-34 (f5C34) seen only in one other tRNA, a bovine liver, cytoplasmic tRNALeu with a f5C34 further modified with a 2′O-methyl (6). Nothing, however, is known about the decoding characteristics of
has a unique modification, 5-formylcytidine (Figure 1), at the wobble position-34 (f5C34) seen only in one other tRNA, a bovine liver, cytoplasmic tRNALeu with a f5C34 further modified with a 2′O-methyl (6). Nothing, however, is known about the decoding characteristics of 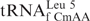 . Since its discovery in bovine and nematode mitochondrial tRNAMet in 1994 (7,8), f5C34 also has been found in the mitochondrial tRNAMet of squids, frogs, chickens, rats and fruit flies (9–11). The contribution of the f5C34 modification to the structure of the
. Since its discovery in bovine and nematode mitochondrial tRNAMet in 1994 (7,8), f5C34 also has been found in the mitochondrial tRNAMet of squids, frogs, chickens, rats and fruit flies (9–11). The contribution of the f5C34 modification to the structure of the 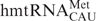 , its role in the decoding of AUG and AUA and its possible participation in either chain initiation or chain elongation by this unique tRNAMet is still unknown.
, its role in the decoding of AUG and AUA and its possible participation in either chain initiation or chain elongation by this unique tRNAMet is still unknown.
Figure 1.
Human mitochondrial 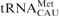 . The sequence and secondary structure of
. The sequence and secondary structure of 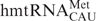 are shown with the heptadecamer, anticodon stem and loop domain in bold. Nucleosides 27 and 50 are modified to pseudouridine, Ψ. The wobble position-34 modification is 5-formylcytidine, f5C34.
are shown with the heptadecamer, anticodon stem and loop domain in bold. Nucleosides 27 and 50 are modified to pseudouridine, Ψ. The wobble position-34 modification is 5-formylcytidine, f5C34.
We speculate that mitochondria have a unique mechanism to partition this single 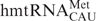 species between initiation and elongation. While a
species between initiation and elongation. While a 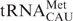 unmodified at the wobble position-34 can read AUG, we hypothesize that the 5-formyl modification allows one tRNAMet to expand codon reading to include recognition of the AUA codon in mitochondrial mRNAs. Toward proving the hypothesis that this wobble modification affords the single tRNAMet the ability to decode AUG and AUA, we are reporting the first synthesis of a 5-formylcytidine-modified RNA and the initial structural and biological investigations. Previously, bovine mitochondrial
unmodified at the wobble position-34 can read AUG, we hypothesize that the 5-formyl modification allows one tRNAMet to expand codon reading to include recognition of the AUA codon in mitochondrial mRNAs. Toward proving the hypothesis that this wobble modification affords the single tRNAMet the ability to decode AUG and AUA, we are reporting the first synthesis of a 5-formylcytidine-modified RNA and the initial structural and biological investigations. Previously, bovine mitochondrial 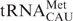 had been shown to translate AUG and AUA in an Escherichia coli translational system in vitro, where AUG coded for methionine and AUA for isoleucine (12). However, site-specific binding was not investigated. Here we compare the codon binding affinities of the f5C34-modified anticodon stem and loop of human mitochondrial tRNAMet (hmtASLMet-f5C34) with that of the unmodified ASL. Our results for both AUA and AUG codons at both the A and P-site of E. coli ribosomes increase our understanding of the modification's contributions to decoding and are consistent with previous results from the translation of poly(AUA) by bovine m
had been shown to translate AUG and AUA in an Escherichia coli translational system in vitro, where AUG coded for methionine and AUA for isoleucine (12). However, site-specific binding was not investigated. Here we compare the codon binding affinities of the f5C34-modified anticodon stem and loop of human mitochondrial tRNAMet (hmtASLMet-f5C34) with that of the unmodified ASL. Our results for both AUA and AUG codons at both the A and P-site of E. coli ribosomes increase our understanding of the modification's contributions to decoding and are consistent with previous results from the translation of poly(AUA) by bovine m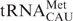 on E. coli ribosomes (12).
on E. coli ribosomes (12).
MATERIALS AND METHODS
Experimental procedures and analytical data for the synthesis of the f 5C phosphoramidite (9)
All reagents used in the following experiments are of the highest purity and dryness possible. Before use, glassware was thoroughly cleaned and dried (oven at 110°C for 30 min). NMR analysis of intermediates was conducted in the appropriate deuterated solvent (referenced accordingly for CDCl3: 1H 7.24 p.p.m., 13C 77.23 p.p.m.; and CD3CN: 1H 1.94 p.p.m.) using a Bruker Avance Ultrashield 300 MHZ spectrometer. Phosphorus, 31P, NMR experiments were referenced according to an external H3PO4 standard (0.00 p.p.m.). Mass-spec analysis of the samples was performed on a Micromass LCT ESI-TOF or an Agilent LC-TOF. Analytes were dissolved in acetonitrile and flown against a Leucin Enkephalin lock mass standard. Chemical and physical properties of the intermediates were those of the expected compounds (Supplementary Data).
2′,3′-O-isopropylidenecytidine (2)
To a suspension of cytidine (1, Figure 21 g, 4.1 mmol) in 50 ml of acetone was added 2,2-dimethoxypropane (6 ml, 5.1 g, 49 mmol). HClO4 was then added dropwise until the solution turned clear. The reaction mixture was stirred at room temperature for 12 h, after which it was neutralized by addition of Ca(OH)2, filtered and evaporated. Purification by silica gel chromatography using CHCl3 : MeOH (80 : 20) with 2% TEA, afforded 966 mg of 2 as a white foam (83% yield). The analytical data obtained matched known literature data for 2 (13).
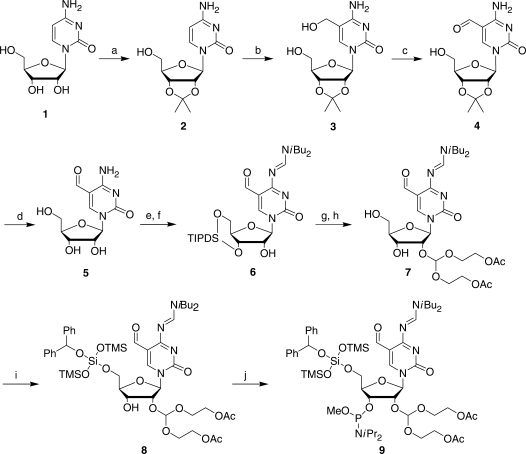
Figure 2.
Synthesis of the 5-formylcytidine phosphoramidite. The starting compound cytidine is numbered compound 1, intermediates are numbered 2–8 and the protected f5C phosphoramidite is compound 9. The synthetic transformations are: (a) acetone, dimethoxypropane, cat HClO4 (83%); (b) paraformaldehyde, 0.5 M KOH, 55°C (38%, 60% brsm); (c) RuO2 xH2O, dioxane, reflux (82%); (d) 1 M HCl (95%); (e) TIPDSCl2, Pyr, DMF; (f) DBF-CH(OMe)2, DMF (71% over 2 steps); (g) ACE-orthoester, PPTS, TBDMS-pentanedione, DCM; (h) HF-TEMED, CH3CN (53% over 2 steps); (i) BZH-Cl, DIA, DCM (84%); (j) P(OMe)(DIA)2, DIA, S-Et Tetrazole, DCM (93%).
5-(Hydroxymethyl)- 2′, 3′-O-isopropylidenecytidine (3)
To a solution of 2 (Figure 2, 1 g, 3.5 mmol) in 15 ml of 0.5 M KOH was added paraformaldehyde (1.05 g, 35 mmol). The reaction was stirred at 55°C for 36 h, after which it was cooled to room temperature and neutralized with 6 M HCl. The solution was filtered and evaporated. The oily residue was dissolved in MeOH : DCM (40 : 60), filtered and evaporated again. Purification by silica gel chromatography using MeOH : DCM (gradient 3 : 97, 8 : 92, 12 : 88) with 2% TEA, afforded 416 mg of 5-(hydroxymethyl)-2′,3′-O-isopropylidenecytidine (3) as a white foam (38% yield, 60% yield based on 357 mg of recovered starting material 2′,3′-O-isopropylidenecytidine (2).
5-Formyl-2′,3-O-isopropylidenecytidine (4)
To 3 (Figure 2, 100 mg, 0.32 mmol) in 3 ml dioxane was added 500 mg ruthenium dioxide hydrate (five weight equivalents). The reaction mixture was refluxed for 12 h and filtered. Purification by silica gel chromatography MeOH : DCM (5 : 95) with 2% TEA, afforded 81 mg of the 5-formyl-2′,3′-O-isopropylidenecytidine (4) as a white solid (82% yield).
5-Formylcytidine (5)
The acetonide, 5-formyl-2′,3′-O-isopropylidenecytidine (4, Figure 2, 500 mg, 1.61 mmol) was suspended in 1 M HCl (15 ml) at room temperature. The reaction progress was monitored by TLC. Upon disappearance of the starting material, the solution was neutralized with TEA. Water was subsequently evaporated. Recrystalization from MeOH afforded 414 mg of f5C (5) as a white solid (95% yield).
N4-[(Diisobutylamino)methylidene]-3′,5′-O-(1,1,3,3-tetraisopropyl-1,3-disiloxanediyl)-5-formylcytidine (6)
5-Formylcytidine (5, Figure 2, 0.47 g, 1.72 mmol) was dissolved in a mixture of 20 ml of pyridine and 2 ml of DMF. The solution was cooled to 0°C and TIPDSCl2 (0.59 g, 1.89 mmol) in 2 ml of pyridine was added dropwise over a period of 1 h. The reaction was allowed to gradually warm to room temperature overnight. The following morning the reaction was quenched with 5 ml of MeOH and evaporated to dryness. The resulting paste was coevaporated twice with 20 ml of toluene and the crude material was purified by flash chromatography on 30 ml of silica gel using a gradient of MeOH in DCM (3–4%). Product fractions were pooled and evaporated to afford the TIPDS protected intermediate (0.88 g, 100%) as light yellow oil that is contaminated with residual pyridinium salts. The above compound was used as is without further purification to remove the residual pyridinium salts. TIPDS protected f5C (5) (0.88 g, 1.72 mmol) was dissolved in 20 ml DMF and N,N-diisobutylformamidine dimethyl acetal (14) (0.70 g, 3.44 mmol) was added. The reaction was stirred for 16 h and evaporated under high vacuum. The resulting loose oil was coevaporated twice with 20 ml of toluene and the crude material was purified by flash chromatography on 30 ml of silica gel using a gradient of MeOH in DCM (1–2%). Product fractions were pooled and evaporated to afford 0.80 g of 6 as light yellow oil in 71% overall yield from f5C (5).
2′-O-[Bis(2-acetoxyethoxy)methyl]-N4-[(diisobutylamino) methylidene]-5-formylcytidine (7)
A mixture of 6 (Figure 2, 0.80 g, 1.23 mmol), pyridinium para-toluenesulfonate (0.31 g, 1.23 mmol), and Tris(2-acetoxyethoxy)methyl orthoformate (1.98 g, 6.15 mmol) was dissolved in 5 ml of DCM and stirred at room temperature. After 2 days, TBDMS-pentanedione (0.53 g, 2.46 mmol) was added and the reaction was stirred at ambient temperature. After stirring for an additional day, the reaction was quenched with 1 ml of TEMED. The crude material was separated from excess reagents by flash chromatography on 50 ml silica gel using a gradient of 25% ethyl acetate in hexanes with 0.1% TEMED to 50% ethyl acetate in hexanes with 0.1% TEMED. This material was concentrated to near dryness and taken directly onto the desilylation reaction.
A freshly made solution of TEMED (0.71 g, 6.15 mmol) in 10 ml of acetonitrile at 0°C was added 48% HF (0.15 ml, 4.30 mmol). This solution was allowed to stir for 5 min and added to the foregoing material from above at room temperature. The reaction was stirred for 2 h and concentrated to dryness. The crude material was purified by flash chromatography on 50 ml silica gel using a gradient of 20% heaxanes in ethyl acetate with 0.1% TEMED to 1% methanol in ethyl acetate with 0.1% TEMED. Product fractions were pooled and evaporated to leave the 2′-O-protected compound [7] as a light yellow oil (0.41 g) in 53% yield from the nucleobase protected compound 6.
5′-O-[Benzyhydryloxy-bis(trimethylsilyloxy)silyl]-2′-O-[bis(2-acetoxyethoxy)-methyl]-N4-[(diisobutyl-amino)methylidene]-5-formylcytidine (8)
Diisopropylamine (0.07 g, 0.65 mmol) was added to a solution of the 2′-O- and N4-protected nucleoside, 7 (Figure 2, 0.41 g, 0.65 mmol) in 7 ml of DCM and the solution was cooled to 0°C. In a separate flask BZHCl (0.34 g, 0.81 mmol) was diluted in 5 ml of DCM. Diisopropylamine (0.10 g, 0.98 mmol) was added to the silylating solution and the solution was allowed to stir for 2 min before being added dropwise to the nucleoside solution. The addition was completed within 30 min and the reaction was allowed to stir for 3 h and the reaction was quenched with 1 ml of MeOH and evaporated to dryness. The crude material was purified by flash chromatography on 30 ml silica gel using a gradient of 10% acetone in hexanes containing 0.1% (v/v) TEA to 20% acetone in hexanes containing 0.1% (v/v) TEA. Product fractions were pooled and evaporated to afford the 5′-O- protected compound 8 as a colorless oil. The yield was 0.56 g (84%).
5′-O-[Benzyhydryloxy-bis(trimethylsilyloxy)silyl]-2′-O-[bis(2-acetoxyethoxy)-methyl]-N4-[(diisobutyl-amino)methylidene]-5-formylcytidine-3′-(methyl-N,N-diisopropyl) phosphoramidite (9)
Bis(diisopropyl-amino) methoxy phosphine (0.21 g, 0.82 mmol) was dissolved in 3 ml of DCM and a 0.5-M solution of 5-ethylthio-1-H-tetrazole in anhydrous acetonitrile (0.08 ml, 0.55 mmol) was added. Diisopropylamine (0.06 g, 0.55 mmol) was then added and the phosphine solution was allowed to stir for 5 min at ambient temperature. In a separate flask, the 2′-O-, 5′-O- and N4-protected f5C, compound 8 (Figure 2, 0.56 g, 0.55mmol) and diisopropylamine (0.06 g, 0.55 mmol) were dissolved in 5 ml of DCM. The activated phosphine solution was added into the nucleoside solution and the reaction was stirred at room temperature. After 16 h the reaction was quenched with 2 ml of absolute ethanol and concentrated to dryness. The resulting white paste was purified by flash chromatography on 30 ml of silica gel using a mixture of DCM in hexanes [5 : 95 (v/v)] containing 2% (v/v) TEA followed by acetone in hexanes [1 : 9 (v/v)] to 2 : 8 (v/v) containing 0.5% (v/v) TEA. Product fractions were pooled and evaporated to afford the protected f5C phosphoramidite 9 (Figure 2), as a colorless oil.
Polyribonucleotide synthesis of 5′-ΨCGGGCC-f5C-AUACCCCCGA-3′
The above sequence was synthesized on a 1-μmol scale using a ABI 394 DNA synthesizer using previously published procedures (15,16). The f5C phosphoramidite (9, 0.067 M in anhydrous acetonitrile) was coupled to the growing polyribonucleotide chain for 3.5 min using 5-ethylthio-1H-tetrazole (0.5 M in anhydrous acetonitrile) as the activator. Once the synthesis of the polyribonucleotide chain was completed, the phosphate protecting groups were removed from the immobilized polyribonucleotide by treatment with disodium 2-carbamoyl-2-cyanoethylene-1,1-dithiolate trihydrate in DMF for 10 minutes. The support was washed excessively with water for 5 min and then flushed with Argon gas for 5 min to dry the support. The support was then transferred to a 2-ml Eppendorf tube and the polyribonucleotide was cleaved from the support and the exocyclic amine protecting groups were removed with 1 : 3 (v/v) tert-butylamine:water for 6 h at 60°C. The sample was cooled to room temperature, filtered and lyophilized to obtain the crude polyribonucleotide. The  -Ψ27;f5C34 was deprotected with acetate/TEMED according to standard Dharmacon protocols, purified by ion exchange HPLC (17), and dialyzed extensively against H2O. The
-Ψ27;f5C34 was deprotected with acetate/TEMED according to standard Dharmacon protocols, purified by ion exchange HPLC (17), and dialyzed extensively against H2O. The  -Ψ27 was synthesized and deprotected under standard conditions (18).
-Ψ27 was synthesized and deprotected under standard conditions (18).
Confirmation of nucleoside composition by nucleoside HPLC and NMR of the  constructs
constructs
Incorporation of f5C modification within the hmASLMet was confirmed by NMR, including the two-dimensional NOESY (Figures 3 and 4). The nucleoside composition of the hmASLMet products was confirmed by enzymatic hydrolysis of the RNA to its constituent nucleosides (17) and then subjected to HPLC monitored by diode array UV spectrometry, the peaks identified, integrated and quantified (19) (Figure 4).
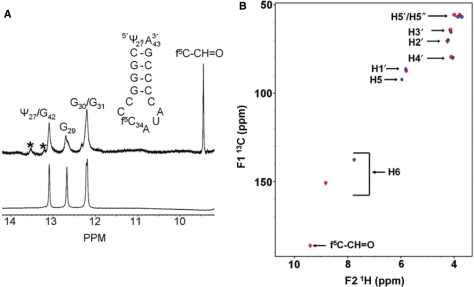
Figure 3.
NMR spectra of the 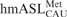 . (A) One-dimensional 1H-NMR spectrum (in H2O) of
. (A) One-dimensional 1H-NMR spectrum (in H2O) of  -Ψ27;f5C34 (top) is compared to that of the unmodified
-Ψ27;f5C34 (top) is compared to that of the unmodified  -Ψ27 (bottom). The formyl proton's chemical shift in the RNA is almost identical to that of the mononucleoside f5C. *Denotes impurities. (B) Superimposed 1H-13C HMQC spectra of cytidine (blue) and 5-formylcytidine (red).
-Ψ27 (bottom). The formyl proton's chemical shift in the RNA is almost identical to that of the mononucleoside f5C. *Denotes impurities. (B) Superimposed 1H-13C HMQC spectra of cytidine (blue) and 5-formylcytidine (red).
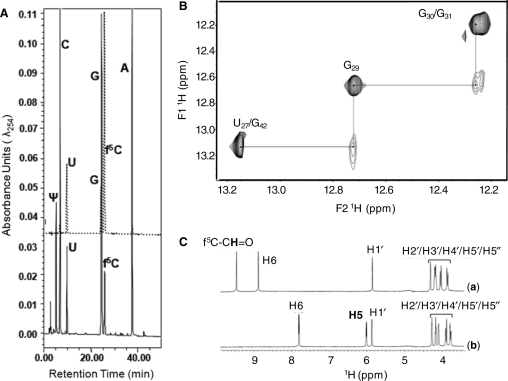
Figure 4.
HPLC nucleoside composition and NMR analyses of  and f5C. (A) The upper section of the figure depicts the HPLC separation of standard ribonucleosides where C, G and A (solid line) had been injected together, and U, G and f5C (dotted line) had been injected in a separate control experiment. The lower section depicts the chromatography of nucleoside composing the
and f5C. (A) The upper section of the figure depicts the HPLC separation of standard ribonucleosides where C, G and A (solid line) had been injected together, and U, G and f5C (dotted line) had been injected in a separate control experiment. The lower section depicts the chromatography of nucleoside composing the  to include Ψ, as well as f5C. (B) The NOESY connectivities between imino protons at 500 MHz in a 2D 1H NOESY NMR spectrum (mix = 250 ms) of
to include Ψ, as well as f5C. (B) The NOESY connectivities between imino protons at 500 MHz in a 2D 1H NOESY NMR spectrum (mix = 250 ms) of  (90% 1H2O + 10% 2H2O; 20 mM PO43–, 50 mM NaCl, pH 6.2; 2°C) with water suppression using the WATERGATE sequence. (C) 1D 1H NMR spectra of (a) cytidine (1) and (b) 5-formylcytidine (5) (500 MHz; 100% 2H2O; 20 mM PO43–, 50 mM Na+, 50 mM K+; pH = 6.2; 25°C) using the presaturation NMR sequence to suppress the water peak. The spectral regions and peaks corresponding to the various proton types in the molecules are labeled.
(90% 1H2O + 10% 2H2O; 20 mM PO43–, 50 mM NaCl, pH 6.2; 2°C) with water suppression using the WATERGATE sequence. (C) 1D 1H NMR spectra of (a) cytidine (1) and (b) 5-formylcytidine (5) (500 MHz; 100% 2H2O; 20 mM PO43–, 50 mM Na+, 50 mM K+; pH = 6.2; 25°C) using the presaturation NMR sequence to suppress the water peak. The spectral regions and peaks corresponding to the various proton types in the molecules are labeled.
Analysis of thermodynamic stability, circular dichroism and molecular dynamics simulations
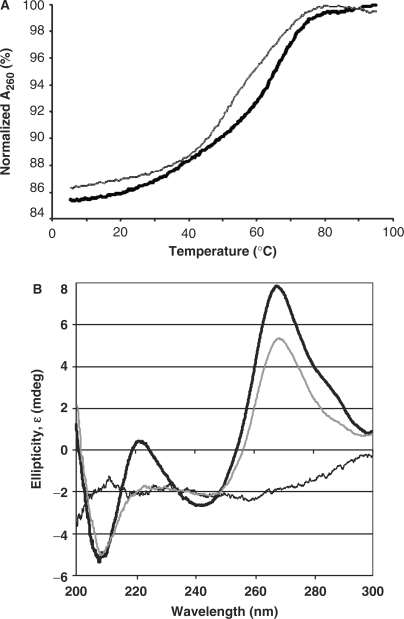
The ASL samples were dissolved to obtain a concentration of ∼4 µM in 20 mM Na–K phosphate buffer (pH 6.8). UV-monitored, thermal denaturations and renaturations were replicated five times and monitored by measuring UV absorbance (260 nm) using a Cary 3 spectrophotometer as published (20,21). The data points were averaged over 20 s and collected four times a minute with a temperature change of 0.5°C/min from 4 to 90°C. The data were analyzed (22), and the thermodynamic parameters were determined (Origin software, Microcal, Inc.) (Figure 5A). CD spectral ellipticity data were collected using a Jasco 600 spectropolarimeter and an interfaced computer (Jasco, Inc.).  -Ψ27 or the
-Ψ27 or the  -Ψ 27;f5C34 (0.2 A260/ml, 20 mM Na–K phosphate buffer, pH 6.8) was placed in a temperature-regulated, 1-cm path-length quartz cell. Each sample was scanned 10 times at 25°C. The final data are an average of the 10 scans (Figure 5B). The molecular dynamics simulation (MDS) were performed by following standard published protocol (23) with the exception of using a truncated octahedral TIP3P water box (24).
-Ψ 27;f5C34 (0.2 A260/ml, 20 mM Na–K phosphate buffer, pH 6.8) was placed in a temperature-regulated, 1-cm path-length quartz cell. Each sample was scanned 10 times at 25°C. The final data are an average of the 10 scans (Figure 5B). The molecular dynamics simulation (MDS) were performed by following standard published protocol (23) with the exception of using a truncated octahedral TIP3P water box (24).
Figure 5.
Thermal denaturations and circular dichroism spectra of the  -Ψ27 and the
-Ψ27 and the  -Ψ27;f5C34. (A) Thermodynamic stability of the ASLs. UV-monitored, thermal data were averaged from three denaturations and two renaturations for the
-Ψ27;f5C34. (A) Thermodynamic stability of the ASLs. UV-monitored, thermal data were averaged from three denaturations and two renaturations for the  -Ψ27;f5C34 (thin gray line) and for the
-Ψ27;f5C34 (thin gray line) and for the  -Ψ27 (thick black line). (B) Circular dichroism spectra. Spectra of the
-Ψ27 (thick black line). (B) Circular dichroism spectra. Spectra of the  -Ψ27;f5C34 (thin gray line) and that of the
-Ψ27;f5C34 (thin gray line) and that of the  -Ψ27 (thick black line) at the approximately equal concentrations of 2 µM were collected over the wavelength range of 200 to 300 nm.
-Ψ27 (thick black line) at the approximately equal concentrations of 2 µM were collected over the wavelength range of 200 to 300 nm.
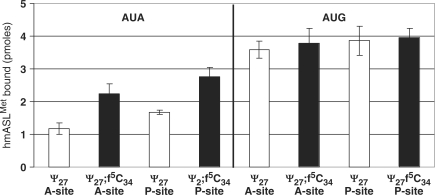
Ribosomal binding assay
The 27-mer mRNA oligos used in codon binding assays were designed from that of T4 gp32 mRNA (25) and purchased (Dharmacon RNA Technologies). They were chemically deprotected and HPLC-purified in our lab. Each mRNA sequence was entered into the program RNA Structure 4.2 (26) and was found to have a low probability of folding into any stable conformation. The mRNA sequences are as follows (mitochondrial methionine codons AUA and AUG are in bold):
5′-GGCAAGGAGGUAAAAAUAGUAGCACGU-3′;
5′-GGCAAGGAGGUAAAAAUGGUAGCACGU-3′;
5′-GGCAAGGAGGUAAAAGUAAUAGCACGU-3′;
5′-GGCAAGGAGGUAAAAGUAAUGGCACGU-3′.
The 70S ribosomes were prepared from E. coli MRE600 (27). The ASLs were 5′-end 32P-labeled using [ϒ-32P] ATP (MP Biomedicals). Unlabeled ASLs (5 μM) were mixed with 10 000 CPM of 5′-end 32P-labeled ASL. The assay was performed in ribosomal binding buffer (50 mM HEPES, pH 7.0; 30 mM KCl; 70 mM NH4Cl; 1 mM DTT; 100 μM EDTA; 20 mM MgCl2). The ribosomes, activated at 42°C for 10 min and then slowly cooled to 37°C, were then programmed with 2.5 μM mRNA for 15 min at 37°C. The ribosomal site not in observation was saturated with 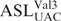 (unmodified) for 15 min at 37°C. P-site binding was performed prior to A-site binding.
(unmodified) for 15 min at 37°C. P-site binding was performed prior to A-site binding. 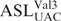 binds to the Val codon GUA; see underlined codons of the mRNA sequences above. Binding of
binds to the Val codon GUA; see underlined codons of the mRNA sequences above. Binding of 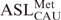 in either A- or P-site was allowed to proceed for 30 min at 37°C. The reaction mixtures (20 μl each) were then placed on ice for 20 min and filtered through nitrocellulose in a modified Whatman Schleicher and Schuell (Brentford, UK) 96-well filtration apparatus (28). Prior to filtration of experimental samples, the nitrocellulose filter was equilibrated in binding buffer at 4°C for at least 20 min and each well of the filtration apparatus was washed with 100 μl of cold binding buffer. Cold binding buffer (100 μl) was added to each sample, and the entire 120-μl volume was quickly filtered. Each well was then washed twice with 100 μl of cold binding buffer. The nitrocellulose was dried out on ‘kim’ wipes, and the radioactivity was measured using a phosphorimager (Molecular Dynamics, GE Healthcare). Data were measured for radioactive intensity using ImageQuant (Amersham). Nonspecific binding was determined by the binding of ASLs to ribosomes without mRNA and subtracted from the experimental data. The final data are a result of at least three separate experiments, each done with samples in triplicate, i.e. minimally nine determinations for each binding (Figure 6).
in either A- or P-site was allowed to proceed for 30 min at 37°C. The reaction mixtures (20 μl each) were then placed on ice for 20 min and filtered through nitrocellulose in a modified Whatman Schleicher and Schuell (Brentford, UK) 96-well filtration apparatus (28). Prior to filtration of experimental samples, the nitrocellulose filter was equilibrated in binding buffer at 4°C for at least 20 min and each well of the filtration apparatus was washed with 100 μl of cold binding buffer. Cold binding buffer (100 μl) was added to each sample, and the entire 120-μl volume was quickly filtered. Each well was then washed twice with 100 μl of cold binding buffer. The nitrocellulose was dried out on ‘kim’ wipes, and the radioactivity was measured using a phosphorimager (Molecular Dynamics, GE Healthcare). Data were measured for radioactive intensity using ImageQuant (Amersham). Nonspecific binding was determined by the binding of ASLs to ribosomes without mRNA and subtracted from the experimental data. The final data are a result of at least three separate experiments, each done with samples in triplicate, i.e. minimally nine determinations for each binding (Figure 6).
Figure 6.
Codon binding by  -Ψ27 and the
-Ψ27 and the  -Ψ27;f5C34. The equilibrium binding of the two ASLs to the cognate and noncognate codons, AUG and AUA respectively, was assessed using programmed E. coli ribosomes. (The ASL unmodified and the wobble position is designated ‘Ψ27’; ASL modified at the wobble position is designated as ‘Ψ27;f5C34’.) The ASLs were bound to the A-site with E. coli
-Ψ27;f5C34. The equilibrium binding of the two ASLs to the cognate and noncognate codons, AUG and AUA respectively, was assessed using programmed E. coli ribosomes. (The ASL unmodified and the wobble position is designated ‘Ψ27’; ASL modified at the wobble position is designated as ‘Ψ27;f5C34’.) The ASLs were bound to the A-site with E. coli
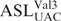 bound to its cognate codon in the P-site. The ASLs were bound to the P-site with the
bound to its cognate codon in the P-site. The ASLs were bound to the P-site with the 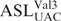 bound to its cognate codon in the A-site.
bound to its cognate codon in the A-site.
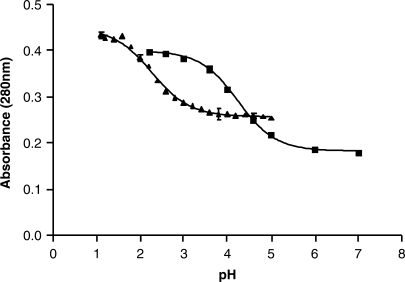
Analysis of the f5C pKa
UV spectra were compiled (220–320 nm) using a Varian Cary3 Spectrophotometer at different pH values for cytidine and 5-formylcytidine. The spectra were normalized to 0.2 OD at 260 nm. Entire spectra were collected to ensure that they all intersected at 260 nm at an OD of ∼0.2. However, the absorbance maximum at 280 nm was plotted against the pH, a previously published method of assessing the pKa of nucleosides (29). A pH range of 2.2–7.0 (citrate-phosphate buffer) was used for cytidine, and a pH range of 1.1–5.0 was used for f5C (KCl–HCl buffer for pH values between 1.1 and 2.0 and citrate–phosphate buffer for pH values between 2.2 and 5.0). The line fitting and data analysis was conducted with Prism v3.00 (Graphpad Software, Inc.) (Figure 7).
Figure 7.
Analysis of the pKa of (filled squares) cytidine in comparison to that of (filled triangles) 5-formylcytidine. UV spectra were collected for the two nucleosides over a range of pH values, pH 2.2–7.0 for cytidine, and a pH 1.1–5.0 for f5C. The absorbance at 280 nm was normalized and plotted against the pH.
RESULTS AND DISCUSSION
A 5-formylcytidine (f5C) has previously been synthesized from 5-(hydroxymethyl)cytosine (13) and from 5-methyluridine (30), but not incorporated into an RNA sequence. First, we developed a short (four steps) and facile synthesis of f5C (compound 5, Figure 2) from commercially available cytidine (1, Figure 2), starting by protecting cytidine as the acetonide 2 under standard acid catalysis with an 83% yield. Installation of the hydroxymethylene unit occurred through an assisted Baylis-Hillman-type reaction with formaldehyde (3, 38% yield, 60% yield based on recovered starting material; Supplementary Data). Selective oxidation of the allylic alcohol with RuO2 to the aldehyde 4 proceeded with an 82% yield. The acetonide protecting group was subsequently removed to deliver f5C (5) in 95% yield. The comparison between the NMR signals of C and those of f5C clearly demonstrated that the C-5 position of f5C was substituted (Figures 3B and 4C). This substitution was further confirmed to be the formyl group by the presence of a low field shifted signal at (F1 = 185 p.p.m.; F2 = 9.40 p.p.m.) corresponding to the CH group of the f5C modification. A complete and unambiguous assignment of the non-exchangeable protons was achieved by using the two dimensional (2D) 1H-13C Heteronuclear Multiple Quantum Correlation (HMQC) method (31,32). As expected, the NMR peaks observed between (F1 = 50.00–85.00 p.p.m.; F2 = 4.50–6.00 p.p.m.) corresponded to the protons (H1′-H5′/H5′′) of the ribose moiety (Figure 3B) (33). Conversion of f5C into the 2′,5′-protected f5C phosphoramidite (9, Figure 2) commenced with the protection of the 3′and 5′ hydroxyl groups as a disiloxane followed by protection of the 4-NH2 group as the formamidine 6 (71% yield). Installation of a 2′-ACE orthoester [2′-O-bis(acetoxyethoxy)methyl-] followed by fluoride treatment delivered the diol 7 in 53% yield over two steps (15). The synthesis of the f5C phosphoramidite 9 was completed through 5′-BZH (5′-O-benzhydroxy-bis(trimethylsiloxy)silyl-) protection (8, 84% yield) and phosphor-amidite formation (93% yield). A major concern for the incorporation of f5C phosphoramidite into synthetic RNA oligomers was the formation of imine adducts with the formyl group under resin cleavage and deprotection conditions. However, we decided not to protect the formyl group since the final deprotection of the 2′-ACE groups under mildly acidic conditions would potentially hydrolyze any imine formation that results during base-deprotection back to the formyl group.
In order to investigate the contribution of f5C34 to the structure of the anticodon loop and to the decoding of both the AUG and AUA codons at both the A- and P-sites, f5C was incorporated into the anticodon stem and loop domain of 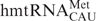 (
( ) at the wobble position 34, along with pseudouridine, Ψ27. The oligonucleotide was synthesized with Ψ27 and with and without f5C34 (
) at the wobble position 34, along with pseudouridine, Ψ27. The oligonucleotide was synthesized with Ψ27 and with and without f5C34 ( -Ψ27;f5C34, and
-Ψ27;f5C34, and  -Ψ27) using care not to oxidize the formyl group. Incorporation of f5C into RNA was accomplished by activating with S-ethyl tetrazole and coupling of the activated species for 3.5 min to the growing polyribonucleotide on the solid-support. Cleavage from the support and deprotection of the exocyclic amines was tested using NH4OH at room temperature for 24 h, methylamine at room temperature for 6 h, and t-butyl amine in water (1 : 3, v/v) at 60°C for 6 h. Only the t-butyl amine conditions resulted in the correct mass upon MALDI-TOF analysis of the crude products. There was no indication of any t-butyl-amine adducts present from the MALDI-TOF results. Successful incorporation of f5C was confirmed by NMR measurements (Figures 3 and 4) and HPLC of constituent nucleosides (Figure 4). The proton resonance of the formyl group is observed in the low field region of the 1D spectrum of
-Ψ27) using care not to oxidize the formyl group. Incorporation of f5C into RNA was accomplished by activating with S-ethyl tetrazole and coupling of the activated species for 3.5 min to the growing polyribonucleotide on the solid-support. Cleavage from the support and deprotection of the exocyclic amines was tested using NH4OH at room temperature for 24 h, methylamine at room temperature for 6 h, and t-butyl amine in water (1 : 3, v/v) at 60°C for 6 h. Only the t-butyl amine conditions resulted in the correct mass upon MALDI-TOF analysis of the crude products. There was no indication of any t-butyl-amine adducts present from the MALDI-TOF results. Successful incorporation of f5C was confirmed by NMR measurements (Figures 3 and 4) and HPLC of constituent nucleosides (Figure 4). The proton resonance of the formyl group is observed in the low field region of the 1D spectrum of  -Ψ27;f5C34 and absent from that of the
-Ψ27;f5C34 and absent from that of the  -Ψ27 (Figure 3). The formyl proton in
-Ψ27 (Figure 3). The formyl proton in  -Ψ27;f5C34 resonates at the same chemical shift as that of the mononucleoside f5C34, as observed in the superimposed 1H-13C HMQC spectra of cytidine and 5-formylcytidine (Figure 3B). The HPLC nucleoside composition analysis confirms the presence of the f5C (Figure 4A).
-Ψ27;f5C34 resonates at the same chemical shift as that of the mononucleoside f5C34, as observed in the superimposed 1H-13C HMQC spectra of cytidine and 5-formylcytidine (Figure 3B). The HPLC nucleoside composition analysis confirms the presence of the f5C (Figure 4A).
The structure of the resulting  -Ψ27;f5C34 was characterized by 1D 1H and 2D 1H NOESY NMR experiments conducted in H2O at 2°C (34), and by determining the thermodynamic contributions of f5C to the RNA. The formyl proton resonance was found at 9.45 p.p.m. corresponding almost exactly to that of the nucleoside alone (Figure 3A). The imino protons of the stem of
-Ψ27;f5C34 was characterized by 1D 1H and 2D 1H NOESY NMR experiments conducted in H2O at 2°C (34), and by determining the thermodynamic contributions of f5C to the RNA. The formyl proton resonance was found at 9.45 p.p.m. corresponding almost exactly to that of the nucleoside alone (Figure 3A). The imino protons of the stem of  -Ψ27;f5C34 were found to resonate between 12 and 13.5 p.p.m. on the 1H 1D NMR spectrum (Figure 3A). The NMR spin systems that involve the exchangeable imino protons of
-Ψ27;f5C34 were found to resonate between 12 and 13.5 p.p.m. on the 1H 1D NMR spectrum (Figure 3A). The NMR spin systems that involve the exchangeable imino protons of  -Ψ27;f5C34 were identified by conducting NMR experiments in H2O at 2°C (Figures 3A and Figure 4B). The identification and assignment of the exchangeable protons were indicative of the overall stability the
-Ψ27;f5C34 were identified by conducting NMR experiments in H2O at 2°C (Figures 3A and Figure 4B). The identification and assignment of the exchangeable protons were indicative of the overall stability the  -Ψ27;f5C34 in solution, and the comparison with
-Ψ27;f5C34 in solution, and the comparison with  -Ψ27 (Figure 3A) demonstrated the successful incorporation of f5C34 into the sequence of
-Ψ27 (Figure 3A) demonstrated the successful incorporation of f5C34 into the sequence of  .
.
The modified RNA synthesis has allowed us to begin examining the role of f5C34 in thermal stability and decoding activity of hmtRNAMet. Thermodynamic parameters were extracted from the repeated denaturations and renaturations of both  -Ψ27;f5C34 and
-Ψ27;f5C34 and  -Ψ27 (Table 1 and Figure 5A). Introduction of f5C34 lowered the melting temperature and standard free energy (ΔG°37) considerably, but did not alter the ASL's hyperchromicity. The circular dichroism spectrum of the
-Ψ27 (Table 1 and Figure 5A). Introduction of f5C34 lowered the melting temperature and standard free energy (ΔG°37) considerably, but did not alter the ASL's hyperchromicity. The circular dichroism spectrum of the  -Ψ27 exhibited a greater ellipticity at 270 nm than that of the
-Ψ27 exhibited a greater ellipticity at 270 nm than that of the  -Ψ27;f5C34. The lower degree of ellipticity of
-Ψ27;f5C34. The lower degree of ellipticity of  -Ψ27;f5C34 is indicative of a decrease in base stacking. The decreased base stacking must be attributed to the anticodon loop nucleosides because of the location of the modification. These differences in thermodynamics and circular dichroism ellipticity between
-Ψ27;f5C34 is indicative of a decrease in base stacking. The decreased base stacking must be attributed to the anticodon loop nucleosides because of the location of the modification. These differences in thermodynamics and circular dichroism ellipticity between  -Ψ27;f5C34 and
-Ψ27;f5C34 and  -Ψ27 indicated that f5C34 may enhance the motional dynamics of the loop. This difference in motional dynamics was observed by a molecular dynamics simulation (MDS) performed on the
-Ψ27 indicated that f5C34 may enhance the motional dynamics of the loop. This difference in motional dynamics was observed by a molecular dynamics simulation (MDS) performed on the  -Ψ27 and the
-Ψ27 and the  -Ψ27;f5C34 using AMBER 9 (35). The
-Ψ27;f5C34 using AMBER 9 (35). The  -Ψ27 displayed an average root mean square deviation from the starting structure of 2.18 ± 0.23 as opposed to
-Ψ27 displayed an average root mean square deviation from the starting structure of 2.18 ± 0.23 as opposed to  -Ψ27;f5C34 for which higher fluctuations of 2.60 ± 0.60 were detected (Supplementary Data). The enhanced motional dynamics may be important for the decoding of AUA, as well as AUG.
-Ψ27;f5C34 for which higher fluctuations of 2.60 ± 0.60 were detected (Supplementary Data). The enhanced motional dynamics may be important for the decoding of AUA, as well as AUG.
Table 1.
Thermodynamic contributions of f5C34
 |
Tm (°C) |
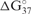 (kcal/mol) (kcal/mol) |
ΔH (kcal/mol) | ΔS (cal/K·mol) | Hyperchromicity (%) |
|---|---|---|---|---|---|
| Ψ27 | 67.7 ± 1.8 | –2.8 ± 0.1 | –31.1 ± 2.0 | –91.3 ± 6.3 | 16 ± 1 |
| Ψ27;f5C34 | 60.1 ± 0.8 | –1.6 ± 0.1 | –22.8 ± 1.2 | –68.4 ± 3.4 | 16 ± 1 |
The tRNAMet anticodon CAU is a cognate pair for the Met codon AUG. According to Crick's Wobble Hypothesis (36), the binding of anticodon CAU to codon AUA would be unlikely due to the C-A mismatch at the wobble position (wobble pair nucleosides in bold). However, the mitochondrial ribosome decodes both AUG and AUA using one tRNA with the anticodon CAU. This one tRNA consists of the modification f5C34. In contrast, two tRNAs decode the one Met codon AUG in the cytoplasm (37). One of the tRNAs is an initiator tRNA that decodes AUG in the ribosome's peptidyl- or P-site at the initiation of translation, where AUG is the first codon to be translated on the mRNA. This initiator 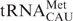 consists of an unmodified CAU anticodon. The second cytoplasmic
consists of an unmodified CAU anticodon. The second cytoplasmic 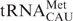 is responsible for elongation and recognizes AUG located within the mRNA, and thus responds only to the aminoacyl- or A-site codon. In E. coli, this elongator tRNAMet is modified with N4-acetylcytidine at the wobble position (ac4C34) (38). Thus, at the anticodon, one of the main distinguishing factors between the cytoplasmic initiator and elongator
is responsible for elongation and recognizes AUG located within the mRNA, and thus responds only to the aminoacyl- or A-site codon. In E. coli, this elongator tRNAMet is modified with N4-acetylcytidine at the wobble position (ac4C34) (38). Thus, at the anticodon, one of the main distinguishing factors between the cytoplasmic initiator and elongator 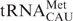 is the modification at the wobble position. We used a codon-binding assay to observe the affinity of the
is the modification at the wobble position. We used a codon-binding assay to observe the affinity of the  -Ψ27; f5C34 and
-Ψ27; f5C34 and  -Ψ27 for the codons AUA and AUG at either A-site or P-site of E. coli 70S ribosomes. To ensure binding of the two
-Ψ27 for the codons AUA and AUG at either A-site or P-site of E. coli 70S ribosomes. To ensure binding of the two 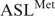 s to the A- or the P-site, the ribosomal site not in observation (P- or A-site, respectively) was saturated with the unmodified E. coli
s to the A- or the P-site, the ribosomal site not in observation (P- or A-site, respectively) was saturated with the unmodified E. coli
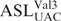 in response to its cognate codon GUA. The unmodified
in response to its cognate codon GUA. The unmodified 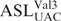 binds its cognate codon with high affinity and specificity (39).
binds its cognate codon with high affinity and specificity (39).
The  -Ψ27 bound AUG in the A-site and the P-site with an affinity comparable to what have observed previously for certain ASLs with unmodified wobble positions responding to cognate codons (Figure 6) (21,39). In contrast, the
-Ψ27 bound AUG in the A-site and the P-site with an affinity comparable to what have observed previously for certain ASLs with unmodified wobble positions responding to cognate codons (Figure 6) (21,39). In contrast, the  -Ψ27 bound poorly to AUA in both the A- and P-sites. Surprisingly, introduction of f5C34 enhanced binding to AUA by 2-fold (Figure 6). Our results indicated that of the two codons at either of the two ribosomal sites, the f5C34 modification appears to be most important for reading AUA.
-Ψ27 bound poorly to AUA in both the A- and P-sites. Surprisingly, introduction of f5C34 enhanced binding to AUA by 2-fold (Figure 6). Our results indicated that of the two codons at either of the two ribosomal sites, the f5C34 modification appears to be most important for reading AUA.
Both the  -Ψ27 and the fully modified
-Ψ27 and the fully modified  -Ψ27;f5C34 exhibited considerable affinity for AUG, and at both the A-site and the P-site. However, only the
-Ψ27;f5C34 exhibited considerable affinity for AUG, and at both the A-site and the P-site. However, only the  -Ψ27;f5C34 exhibited significant affinity for AUA. There was a doubling in the affinity of ASLMet for the AUA codon when f5C34 was present. This increase in affinity of the f5C34-modified ASL in comparison to that of the
-Ψ27;f5C34 exhibited significant affinity for AUA. There was a doubling in the affinity of ASLMet for the AUA codon when f5C34 was present. This increase in affinity of the f5C34-modified ASL in comparison to that of the  -Ψ27, unmodified at the wobble position, was not observed on AUG and may therefore be the sole contributor to the efficient translation of AUA codons. A 2-fold increase in affinity of tRNA toward a codon has been shown to be significant in translation (40). Although some ASL modifications cause small increases in codon-binding affinity, others can dramatically increase affinity to codons (39).
-Ψ27, unmodified at the wobble position, was not observed on AUG and may therefore be the sole contributor to the efficient translation of AUA codons. A 2-fold increase in affinity of tRNA toward a codon has been shown to be significant in translation (40). Although some ASL modifications cause small increases in codon-binding affinity, others can dramatically increase affinity to codons (39).
Of particular interest is the chemical and conformational mechanisms by which a stable, but noncanonical base pair occurs between f5C34 and the third base of the AUA codon, an adenosine, on the ribosome. C-A base pairs are extremely unusual. Although the C-A pairing has been found in the folded structure of some RNAs such as ribosomal RNAs (rRNAs), it is rarely found in anticodon:codon pairs. An anticodon:codon C-A mismatch has been detected when C34 of tRNAIleCAU, modified with lysidine (k2C34) at the wobble position, is paired to the cytoplasmic isoleucine codon AUA. The lysine moiety of C34 on the anticodon provides an amino group which hydrogen bonds to A of the codon, thus allowing the wobble position C-A mismatch to occur. One could imagine that the 5-formyl modification raises the pKa of cytidine's N3 to the physiological range where an additional hydrogen bond could be formed to AUA. However, the pKa of f5C determined by UV spectral analysis was lower than that of C (2.3 and 4.2, respectively; Figure 7), corresponding well with previous determinations (41) including those for df5C (42) and for f5U (43). Thus, f5C must contribute to the decoding of the mitochondrial genome through a different mechanism. Another C-A anticodon:codon mismatch may occur at the wobble position when 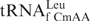 pairs with the leucine codon UUA. Similar to hmtRNAMet, this tRNALeu isoacceptor has a 5-formylated, 2′-O-methylated C at the wobble position, f5Cm34. The wobble modifications are thought to be a general characteristic of mammalian cytoplasmic tRNALeu that may aid in the decoding of leucine codons UUG and UUA and prevent the miscoding of the similar codons of phenylalanine, UUU and UUC. However, there is a lack of information on the decoding properties of
pairs with the leucine codon UUA. Similar to hmtRNAMet, this tRNALeu isoacceptor has a 5-formylated, 2′-O-methylated C at the wobble position, f5Cm34. The wobble modifications are thought to be a general characteristic of mammalian cytoplasmic tRNALeu that may aid in the decoding of leucine codons UUG and UUA and prevent the miscoding of the similar codons of phenylalanine, UUU and UUC. However, there is a lack of information on the decoding properties of 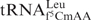 and therefore, there is the possibility that an isoacceptor other than
and therefore, there is the possibility that an isoacceptor other than 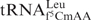 is responsible for specifically reading UUA (6,38).
is responsible for specifically reading UUA (6,38).
Eighty percent of the methionine codons internal to mitochondrial mRNA are the AUA codon. Thus, the enhanced affinity of  -Ψ27;f5C34 for AUA in the A-site of the E. coli ribosome has important implications for the affinity and kinetics of decoding AUA during elongation. The enhanced A-site binding of AUA by the f5C-modified,
-Ψ27;f5C34 for AUA in the A-site of the E. coli ribosome has important implications for the affinity and kinetics of decoding AUA during elongation. The enhanced A-site binding of AUA by the f5C-modified,  may be even more evident on the mitochondrial ribosome, a concept not studied here. Also, the disease-related A37-G37 (A4435G) mutation, associated with an increased penetrance and expression of the primary Leber hereditary optic neuropathy mutation (G11778A), LHON (44), may critically alter the anticodon architecture such that either or both decoding events do not occur. This has yet to be examined. The synthesis of the wild-type modified and unmodified anticodon stem and loops of the
may be even more evident on the mitochondrial ribosome, a concept not studied here. Also, the disease-related A37-G37 (A4435G) mutation, associated with an increased penetrance and expression of the primary Leber hereditary optic neuropathy mutation (G11778A), LHON (44), may critically alter the anticodon architecture such that either or both decoding events do not occur. This has yet to be examined. The synthesis of the wild-type modified and unmodified anticodon stem and loops of the  and their physical, chemical characterizations will be important in understanding the contributions of the modification to biological function and in characterization of the human disease-relevant mutant tRNA.
and their physical, chemical characterizations will be important in understanding the contributions of the modification to biological function and in characterization of the human disease-relevant mutant tRNA.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
The North Carolina State University RNA Biology Group (to A.D.); a grant from United Mitochondrial Disease Foundation, jointly with Dr. Linda Spremulli of the University of North Carolina–Chapel Hill (grant number 05-20 to P.F.A.); NSF (grant number MCB0548602 to P.F.A.); an NSF Graduate Fellowship (to E.M.G); and Dharmacon RNA Technologies, Inc. (ThermoFisher, Inc.). Funding for open access charge: National Science Foundation.
Conflict of interest statement. The perception of conflict may arise due to Drs. Michael O. Delaney and Rob Kaiser being employees of Dharmacon RNA Technologies (ThermoFisher). The company provided the polymer synthesis in which the f5C nucleotide was incorporated into RNA for the first time.
Supplementary Material
ACKNOWLEDGEMENTS
We are very grateful to Dr. Glenn Björk (University of Umeå, Sweden) for the HPLC analysis of the synthesized RNA. AD is a Beckman Young Investigator and a Cottrell Scholar. We thank Mr. Antonio M. Munoz for his contribution to the molecular dynamics simulations.
REFERENCES
- 1.Anderson S, Bankier AT, Barrell BG, de Bruijn MH, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, et al. Sequence and organization of the human mitochondrial genome. Nature. 1981;290:457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- 2.Enns GM. The contribution of mitochondria to common disorders. Mol. Genet. Metab. 2003;80:11–26. doi: 10.1016/j.ymgme.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Wittenhagen LM, Kelley SO. Impact of disease-related mitochondrial mutations on tRNA structure and function. Trends Biochem. Sci. 2003;28:605–611. doi: 10.1016/j.tibs.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 4.King MP, Koga Y, Davidson M, Schon EA. Defects in mitochondrial protein synthesis and respiratory chain activity segregate with the tRNA(Leu(UUR)) mutation associated with mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes. Mol. Cell Biol. 1992;12:480–490. doi: 10.1128/mcb.12.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lombes A, Bories D, Girodon E, Frachon P, Ngo MM, Breton-Gorius J, Tulliez M, Goossens M. The first pathogenic mitochondrial methionine tRNA point mutation is discovered in splenic lymphoma. Hum. Mutat. 1998;(Suppl 1):S175–S183. doi: 10.1002/humu.1380110158. [DOI] [PubMed] [Google Scholar]
- 6.Pais de Barros JP, Keith G, El Adlouni C, Glasser AL, Mack G, Dirheimer G, Desgres J. 2′-O-methyl-5-formylcytidine (f5Cm), a new modified nucleotide at the ‘wobble' of two cytoplasmic tRNAs Leu (NAA) from bovine liver. Nucleic Acids Res. 1996;24:1489–1496. doi: 10.1093/nar/24.8.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moriya J, Yokogawa T, Wakita K, Ueda T, Nishikawa K, Crain PF, Hashizume T, Pomerantz SC, McCloskey JA, Kawai G, et al. A novel modified nucleoside found at the first position of the anticodon of methionine tRNA from bovine liver mitochondria. Biochemistry. 1994;33:2234–2239. doi: 10.1021/bi00174a033. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe Y, Tsurui H, Ueda T, Furushima R, Takamiya S, Kita K, Nishikawa K, Watanabe K. Primary and higher order structures of nematode (Ascaris suum) mitochondrial tRNAs lacking either the T or D stem. J. Biol. Chem. 1994;269:22902–22906. [PubMed] [Google Scholar]
- 9.Tomita K, Ueda T, Watanabe K. 5-formylcytidine (f5C) found at the wobble position of the anticodon of squid mitochondrial tRNA(Met)CAU. Nucleic Acids Symp. Ser. 1997:197–198. [PubMed] [Google Scholar]
- 10.Takemoto C, Ueda T, Miura K, Watanabe K. Nucleotide sequences of animal mitochondrial tRNAs(Met) possibly recognizing both AUG and AUA codons. Nucleic Acids Symp. Ser. 1999:77–78. doi: 10.1093/nass/42.1.77. [DOI] [PubMed] [Google Scholar]
- 11.Tomita K, Ueda T, Ishiwa S, Crain PF, McCloskey JA, Watanabe K. Codon reading patterns in Drosophila melanogaster mitochondria based on their tRNA sequences: a unique wobble rule in animal mitochondria. Nucleic Acids Res. 1999;27:4291–4297. doi: 10.1093/nar/27.21.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takemoto C, Koike T, Yokogawa T, Benkowski L, Spremulli LL, Ueda TA, Nishikawa K, Watanabe K. The ability of bovine mitochondrial transfer RNAMet to decode AUG and AUA codons. Biochimie. 1995;77:104–108. doi: 10.1016/0300-9084(96)88112-0. [DOI] [PubMed] [Google Scholar]
- 13.Youssif S, Mohamed EK, Ahmed AFS, Ghoneim AA. Synthesis of some new cytidine derivatives. Afinidad. 2005;62:242–248. [Google Scholar]
- 14.Jurczyk SC, Kodra JT, Rozzell JD, Benner SA, Battersby TR. Synthesis of oligonucleotides containing 2′-deoxyisoguanosine and 2′-deoxy-5-methylisocytidine using phosphoramidite chemistry. Helv. Chim. Acta. 1998;81:793–811. [Google Scholar]
- 15.Scaringe SA, Kitchen D, Kaiser RJ, Marshall WS. Preparation of 5′-silyl-2′-orthoester ribonucleosides for use in oligoribonucleotide synthesis. Curr. Protoc. Nucleic Acid Chem. 2007 doi: 10.1002/0471142700.nc0210s16. , 2004 May; Chapter 2: Unit 2.10. [DOI] [PubMed] [Google Scholar]
- 16.Hartsel SA, Kitchen DE, Scaringe SA, Marshall WS. RNA oligonucleotide synthesis via 5′-silyl-2′-orthoester chemistry. Methods Mol. Biol. 2005;288:33–50. doi: 10.1385/1-59259-823-4:033. [DOI] [PubMed] [Google Scholar]
- 17.Gehrke CW, Kuo KC, McCune RA, Gerhardt KO, Agris PF. Quantitative enzymatic hydrolysis of tRNAs: reversed-phase high-performance liquid chromatography of tRNA nucleosides. J. Chromatogr. 1982;230:297–308. [PubMed] [Google Scholar]
- 18.Guenther RH, Gopal DH, Agris PF. Purification of transfer RNA species by single-step ion-exchange high-performance liquid chromatography. J. Chromatogr. 1988;444:79–87. doi: 10.1016/s0021-9673(01)94010-5. [DOI] [PubMed] [Google Scholar]
- 19.Zumwalt RW, Kuo KCT, Agris PF, Ehrlich M, Gehrke CW. High performance liquid chromatography of nucleosides in RNA and DNA. J. Liquid Chromatogr. 1982;5:2041–2060. [Google Scholar]
- 20.Ashraf SS, Guenther RH, Ansari G, Malkiewicz A, Sochacka E, Agris PF. Role of modified nucleosides of yeast tRNA(Phe) in ribosomal binding. Cell Biochem. Biophys. 2000;33:241–252. doi: 10.1385/cbb:33:3:241. [DOI] [PubMed] [Google Scholar]
- 21.Yarian CS, Basti MM, Cain RJ, Ansari G, Guenther RH, Sochacka E, Czerwinska G, Malkiewicz A, Agris PF. Structural and functional roles of the N1- and N3-protons of psi at tRNA's position 39. Nucleic Acids Res. 1999;27:3543–3549. doi: 10.1093/nar/27.17.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serra MJ, Turner DH. Predicting thermodynamic properties of RNA. Methods Enzymol. 1995;259:242–261. doi: 10.1016/0076-6879(95)59047-1. [DOI] [PubMed] [Google Scholar]
- 23.McCrate NE, Varner ME, Kim KI, Nagan MC. Molecular dynamics simulations of human tRNA Lys,3 UUU: the role of modified bases in mRNA recognition. Nucleic Acids Res. 2006;34:5361–5368. doi: 10.1093/nar/gkl580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jorgensen WL, Chandrasekhar J, Madura J, Klein ML. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983:926–935. [Google Scholar]
- 25.Fahlman RP, Dale T, Uhlenbeck OC. Uniform binding of aminoacylated transfer RNAs to the ribosomal A and P sites. Mol. Cell. 2004;16:799–805. doi: 10.1016/j.molcel.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 26.Mathews DH, Turner DH, Zuker M. RNA secondary structure prediction. Curr. Protoc. Nucleic Acid Chem. 2007 doi: 10.1002/0471142700.nc1102s28. , 2007 Mar; Chapter 11: Unit 11.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phelps SS, Jerinic O, Joseph S. Universally conserved interactions between the ribosome and the anticodon stem-loop of A site tRNA important for translocation. Mol. Cell. 2002;10:799–807. doi: 10.1016/s1097-2765(02)00686-x. [DOI] [PubMed] [Google Scholar]
- 28.Wong I, Lohman TM. A double-filter method for nitrocellulose-filter binding: application to protein-nucleic acid interactions. Proc. Natl Acad. Sci. USA. 1993;90:5428–5432. doi: 10.1073/pnas.90.12.5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Notari RE, Witiak DT, DeYoung JL, Lin AJ. Comparative kinetics of cytosine nucleosides. Influence of a 6-methyl substituent on degradation rates and pathways in aqueous buffers. J. Med. Chem. 1972;15:1207–1214. doi: 10.1021/jm00282a002. [DOI] [PubMed] [Google Scholar]
- 30.Abdel Rahman AAH, Wada T, Saigo K. Facile methods for the synthesis of 5-formylcytidine. Tetrahedron Lett. 2001;42:1061–1063. [Google Scholar]
- 31.Bax A, Griffey RH, Hawkins BL. Correlation of proton and N-15 chemical shifts by multiple quantum NMR. J. Magn. Reson. 1983;55:301–315. [Google Scholar]
- 32.Bax A, Subramanian S. Sensitivity-enhanced two-dimensional heteronuclear shift correlation NMR-spectroscopy. J. Magn. Reson. 1986;67:565–569. [Google Scholar]
- 33.Wuthrich K. NMR of Proteins and Nucleic Acids. New York, NY: Wiley-Interscience; 1986. [Google Scholar]
- 34.Jeener J, Meier BH, Bachmann P, Ernst RR. Investigation of exchange processes by two-dimensional NMR spectroscopy. J. Chem. Phys. 1979;69:4546–4553. [Google Scholar]
- 35.Case DA, Cheatham TE, III, Darden T, Gohlke H, Luo R, Merz K.M., Jr, Onufriev A, Simmerling C, Wang B, Woods RJ. The Amber biomolecular simulation programs. J. Comput. Chem. 2005;26:1668–1688. doi: 10.1002/jcc.20290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crick FHC. Codon–anticodon pairing: the wobble hypothesis. J. Mol. Biol. 1966;19:548–555. doi: 10.1016/s0022-2836(66)80022-0. [DOI] [PubMed] [Google Scholar]
- 37.Mayer C, Stortchevoi A, Kohrer C, Varshney U, RajBhandary UL. Initiator tRNA and its role in initiation of protein synthesis. Cold Spring Harb. Symp. Quant. Biol. 2001;66:195–206. doi: 10.1101/sqb.2001.66.195. [DOI] [PubMed] [Google Scholar]
- 38.Sprinzl M, Vassilenko KS. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 2005;33:D139–D140. doi: 10.1093/nar/gki012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vendeix FA, Dziergowska A, Gustilo EM, Graham WD, Sproat B, Malkiewicz A, Agris PF. Anticodon domain modifications contribute order to tRNA for ribosome-mediated codon binding. Biochemistry. 2008;47:6117–6129. doi: 10.1021/bi702356j. [DOI] [PubMed] [Google Scholar]
- 40.Kurata S, Weixlbaumer A, Ohtsuki T, Shimazaki T, Wada T, Kirino Y, Takai K, Watanabe K, Ramakrishnan V, Suzuki T. Modified uridines with C5-methylene substituents at the first position of the tRNA anticodon stabilize U.G wobble pairing during decoding. J. Biol. Chem. 2008;283:18801–18811. doi: 10.1074/jbc.M800233200. [DOI] [PubMed] [Google Scholar]
- 41.Kawai G, Yokogawa T, Nishikawa K, Hashizume T, McCloskey JA, Yokoyama S, Watanabe K. Conformatzonal properties of a novel modified nucleoside, 5-formylcytidine, found at the first position of the anticodon of bovine mitochondrial tRNAMet. Nucleosides Nucleotides. 1994;13:1189–1199. [Google Scholar]
- 42.Yoshida M, Makino K, Morita H, Terato H, Ohyama Y, Ide H. Substrate and mispairing properties of 5-formyl-2′-deoxyuridine 5′-triphosphate assessed by in vitro DNA polymerase reactions. Nucleic Acids Res. 1997;25:1570–1577. doi: 10.1093/nar/25.8.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karino N, Ueno Y, Matsuda A. Synthesis and properties of oligonucleotides containing 5-formyl-2′-deoxycytidine: in vitro DNA polymerase reactions on DNA templates containing 5-formyl-2′-deoxycytidine. Nucleic Acids Res. 2001;29:2456–2463. doi: 10.1093/nar/29.12.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qu J, Li R, Zhou X, Tong Y, Lu F, Qian Y, Hu Y, Mo JQ, West CE, Guan MX. The novel A4435G mutation in the mitochondrial tRNAMet may modulate the phenotypic expression of the LHON-associated ND4 G11778A mutation. Invest Ophthalmol. Vis. Sci. 2006;47:475–483. doi: 10.1167/iovs.05-0665. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.