Abstract
IL-7 and IL-15 are key cytokines involved in the generation and maintenance of memory CD8+ T-cells. We evaluated these cytokines as molecular adjuvants for topical HIV-1 DermaVir vaccine. We found that mice receiving DermaVir formulated with HIV-1 Gag plasmid in the presence of IL-7- or IL-15-encoding plasmid significantly enhanced Gag-specific central memory T-cells, as measured by a peptide-based cultured IFN-γ ELISPOT. Additionally, IL-15 significantly improved DermaVir-induced Gag-specific effector memory CD8+ T-cell responses, measured by standard IFN-γ ELISPOT. In a DermaVir prime/vaccinia vector boost regimen, the inclusion of IL-15 together with DermaVir significantly improved Gag-specific effector memory T-cell responses. Our study demonstrates that IL-15 is more potent than IL-7 in enhancing HIV-1-specific central memory T-cells induced by topical DermaVir. IL-15 adjuvanted DermaVir might be an alternative prime in a prophylactic vaccine regimen.
Keywords: topical HIV-1 DermaVir, cytokine adjuvant, memory T-cells
1. Introduction
DNA vaccines represent a potent platform for immunization due to the advantages of allowing repeated administrations and ease up-scalable manufacturing. Naked DNA vaccines have been shown to safely induce antigen-specific immunity against a variety of pathogens [1, 2]; however, it has become clear that their potency should be improved to elicit antigen-specific memory T-cell responses. To induce such responses, a topical plasmid DNA-containing vaccine, DermaVir, has been developed to deliver DNA-encoded antigens into Langerhans cells (LC), the dendritic cell (DC) precursors in the skin. Unlike naked DNA vaccines, DermaVir consists of gene-expressing plasmid DNA formulated in a nanoparticle with polyethylenimine-mannose (PEIm) and glucose solution [3–6]. PEIm is a cationic polymer known to be an efficient carrier of DNA that protects the DNA from enzymatic degradation, promotes the uptake by cells and facilitates the trafficking of DNA into the nucleus, thus augmenting gene expression [7, 8]. We have shown that PEIm-DNA nanoparticles deliver HIV-1 genes-expressing plasmid DNA, driven by HIV-1-long terminal repeats (LTR), to cultured autologous monocyte-derived DC, which induce HIV-1-specific T-cell-restricted immunity in vitro and ex vivo in macaques [9]. Following topical vaccine administration, LC take up DermaVir nanoparticles and the subsequent DNA gene expression triggers antigen production and maturation of LC to DC, which in turn migrate to the draining lymph nodes and present the antigen to naïve T-cells [3, 6]. Topical DermaVir immunization with a LTR-driven plasmid DNA expressing the majority of simian human immunodeficiency virus (SHIV) proteins has been shown to induce both CD8+ and CD4+ SIV-specific T-cell responses in naïve rhesus macaques [4], enhance SIV-specific T-cell responses and control viral rebound during treatment interruptions in chronically SIV-infected rhesus macaques [5].
Cytokines are a group of soluble small proteins that play a crucial role in the development of an immune response against pathogens. The incorporation of cytokines as vaccine adjuvants has received great attention in order to enhance the potency of vaccine-induced immune responses and to modulate the host immune responses for specific situations. Particularly, cytokines have been added as cytokine-encoding plasmid DNA and used as molecular adjuvants for naked DNA vaccines [10]. We have shown that topical DermaVir induces significantly higher HIV-1-specific memory T-cell responses than a naked DNA vaccine given intramuscularly in a murine model [11]. Therefore, we hypothesized that selected cytokines might be useful for enhancing DermaVir-induced memory T-cell responses. Recently, IL-7 and IL-15 have emerged as key cytokines involved in regulating the homeostatic turnover of memory CD8+ T-cells [12–15]. Both naïve and memory T-cell populations are highly dependent on the presence of IL-7 for their persistence and survival [14, 16]. Additionally, IL-7 is critical for the transition from CD8+ effector to memory T-cells [17]. IL-15 is involved in the generation and maintenance of CD8+ memory T-cells [18, 19].
Current studies evaluating HIV-1 vaccine candidates use the standard IFN-γ ELISPOT assay, which measures IFN-γ secreted by effector and effector memory T-cells. We included in our study the cultured IFN-γ ELISPOT assay in order to quantify central memory T-cell responses [20–24]. Studies in the malaria field have shown that the cultured ELISPOT, but not the standard assay, identified long-lasting protective anti-malarial T-cell responses [22–25]. In the HIV/AIDS macaque model, recent studies indicate that antigen-specific central memory T-cells induced by a vaccine regimen are essential for a better outcome and survival after pathogenic SIV challenge [26, 27], consistent with the concept that effective and long-term antigen-specific immune responses should rely on cells that can rapidly expand after antigen challenge, that is central memory T-cells [28–30].
The aim of the present study was to evaluate the memory T-cell adjuvant activity of plasmid DNA encoding IL-7 or IL-15 in the DermaVir nanoparticle in the murine model. Additionally, we investigated the effect of IL-7 or IL-15 formulated in the DermaVir nanoparticle in a new DermaVir prime/vaccinia vector boost regimen.
2. Materials and methods
2.1. DNA constructs used for DermaVir formulation
The plasmid DNA used in this study contained either HIV-1 Gag (pGag) [31] or the murine IL-7 (pIL-7) [32] or IL-15 (pIL-15) [33], under the control of the human CMV immediate early promoter. The pVax DNA was used as a control vector. DermaVir was formulated with a total of 25 µg DNA/200 µL with PEIm and dextrose as previously described [4].
2.2. Topical DermaVir immunization
Female 6–8 week-old Balb/C mice (The Jackson Laboratory, Bar Harbor, ME) were anesthetized using Avertin (Sigma-Aldrich, St. Louis, MO). The hair of the dorsum was removed by shaving and the skin was exfoliated with a sponge as previously described [4]. DermaVir (200 µL) was applied on the entire treated area; after air-drying, mice were returned to the corresponding cages. Animals were cared for in accordance with the guidelines of the National Institutes of Health (NIH) and the University of Pennsylvania Institutional Care and Use Committee.
2.3. Study design
Mice (four per group) were topically immunized with control pVax or pGag DermaVir, either alone or co-formulated with pIL-7 or pIL-15. Immunizations were performed at weeks 0 and 2, without or with an intraperitoneal (i.p.) boost at week 6 with 2.2 × 106 PFU VacV-Gag [34] (provided by Dr. S. Isaacs, University of Pennsylvania, PA).
2.4. Splenocytes and peripheral cells isolation
Spleens were removed and a single-cell suspension was prepared for each group of mice. The pooled spleens from each group were crushed and passed trough a 70-□m cell strainer. Cells were then incubated for 5 min at room temperature with ACK lysing buffer (Biosource, Rockville, MD) and then washed. Splenocytes were resuspended in culture medium (RPMI 1640 medium containing 2 mM L-glutamine and supplemented with 10% heat inactivated FBS, 100 IU/mL penicillin, 100 µg/mL streptomycin). Retro-orbital bleeding was performed at two points and a single-cell suspension was prepared for each group of mice. The pooled blood from each group was incubated for 7 min at room temperature with ACK lysing buffer, washed and the procedure was repeated twice. Cells were then resuspended in culture medium.
2.5. Synthetic peptides
A total of 123 peptides (15 amino acids in length with an 11-amino acid overlap) corresponding to the complete HIV-1 Consensus Subtype B Gag sequence were obtained from the NIH AIDS Research and Reference Reagent Program, suspended in DMSO and divided into four pools, with 31 (pools 1–3) or 30 (pool 4) peptides/pool, and stored at −20°C until use.
2.6. Standard IFN-γ ELISPOT assay
MultiScreen-IP 96-well plates (Millipore, Bedford, MA) were coated with a monoclonal anti-mouse IFN-γ capture antibody (Mabtech, Cincinnati, OH), overnight at 4°C. Plates were then washed and blocked for 2 h at room temperature with culture medium. Cells (2 × 105/well) were added in triplicate (splenocytes) or in duplicate (peripheral cells) and stimulated in the presence of culture medium only (negative control), Con A (positive control, 5 µg/mL; Sigma-Aldrich) or HIV-1 Gag peptide pools (10 µg/mL). After a 24-h incubation at 37°C, cells were washed followed by an overnight incubation at 4°C with a monoclonal anti-mouse IFN-γ biotinylated detection antibody (Mabtech). Plates were washed and strepatavidin-alkaline phosphatase (Mabtech) was added and incubated at room temperature for 1 h. Plates were washed and the substrate solution (BCIP/NBT, Sigma) was added. The colorimetric reaction was terminated by washing with tap water; after air-drying, spots were counted using an automated ELISPOT reader system (CTL Analyzers, Cleveland, OH) with the ImmunoSpot software. The mean number of spots from triplicate or duplicate wells was adjusted to 1 × 106 splenocytes. Spots formed in control medium wells ranged from 1 to 5/well (splenocytes) and from 0 to 5/well (peripheral cells). Assays were also performed after depletion of CD8+ T-cells from splenocytes by using CD8a (Ly-2) MicroBeads specific for mouse cells (Miltenyi Biotec, Auburn, CA) following manufacturer’s instructions. Magnetic separations were done using autoMACS (Miltenyi Biotec). The depleted fractions contained < 3% CD8+ cells, as determined by flow cytometry.
2.7. Cultured IFN-γ ELISPOT assay
Splenocytes (1 × 106/mL) were plated in each of four wells in a 48-well plate. HIV-1 Gag peptide pools were added, one pool/well, and cells were cultured at 37°C for 6 days. On day 6, cells from each well were washed twice, counted and suspended at 1 × 106/mL. Cells (1 × 105/well) were then tested in the same way as the standard ELISPOT assay in response to the corresponding HIV-1 Gag peptide pool or control medium only. Background levels ranged from 0 to 3 spots/well.
2.8. IL-4 ELISPOT assay
ELISPOT assays for the detection of IL-4 production were carried out as described in the standard IFN-γ ELISPOT but using anti-mouse IL-4 ELISPOT kit (Mabtech). Spots formed in control medium wells ranged from 3 to 9/well.
2.9. Data analysis
Data are expressed as the mean ± SD calculated from triplicate or duplicate wells of pooled cells from each group. The net number of spots was calculated after subtraction of spots formed in control medium wells from the number of spots formed in response to each of the four HIV-1 Gag peptide pools used for stimulation. Background immune responses from each control group was subtracted from the corresponding experimental group (i.e., pGag minus pVax; pGag + pIL-7 minus pVax + pIL-7; pGag + pIL-15 minus pVax + pIL-15) and the resulting Gag-specific immune responses between mice immunized with pGag DermaVir alone and mice co-immunized with pGag + the corresponding IL-encoding plasmid DNA DermaVir (without or with heterologous boost) were compared. Statistical analysis was determined by nonparametric Mann-Whitney U test using Statistica for Windows Software (StatSoft, Inc., Tulsa, OK). A p value < 0.05 was considered statistically significant.
3. Results
3.1. IL-15 significantly improves DermaVir-induced Gag-specific effector memory T-cell responses
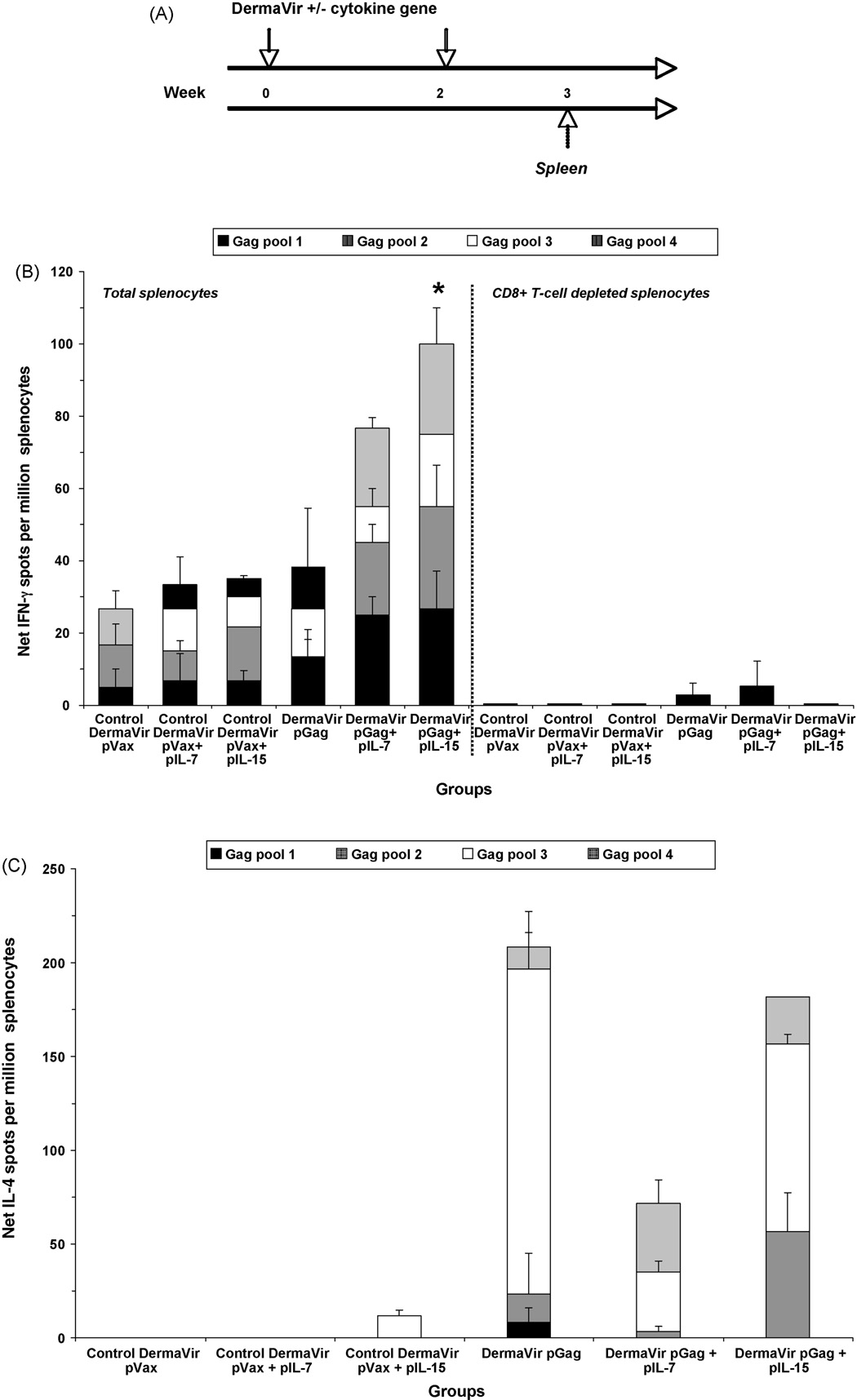
We examined the capacity of pIL-7 and pIL-15 to enhance DermaVir-induced cellular immune responses. Mice were topically immunized with control pVax or pGag DermaVir, either alone or formulated together with pIL-7 or pIL-15, at weeks 0 and 2 (Fig. 1A). At week 3, a pool of splenocytes obtained from each group was assayed for antigen-specific effector memory T-cells by standard IFN-γ ELISPOT in response to HIV-1 Gag peptide pools (Fig. 1B). A low number of IFN-γ spots were detected in the control mice immunized with pVax, pVax + pIL-7, or pVax + pIL-15 DermaVir. While the antigen-specific IFN-γ response detected in mice immunized with pGag DermaVir alone was 38.4 spots/million splenocytes, a higher response was detected in mice co-immunized with pGag + pIL-7 (76.7 spots/million splenocytes). However, co-immunization with pGag + pIL-15 resulted in the highest antigen-specific IFN-γ production (100 spots/million splenocytes). After subtracting the background control group immune response from the corresponding experimental group, Gag-specific IFN-γ response detected in mice co-immunized with pGag + pIL-15 was significantly higher compared to mice immunized with pGag (p = 0.009) while the higher response observed in mice receiving pGag + pIL-7 compared to pGag DermaVir-immunized mice was not significant (p = 0.104). CD8+ T-cells mainly mediated the IFN-γ response (Fig. 1B, right panel).
Fig. 1.
Antigen-specific effector memory T-cell responses induced by topical DermaVir adjuvanted with IL-7 or IL-15. (A) Mice (four per group) were topically immunized at weeks 0 and 2 with control pVax or pGag DermaVir, either alone or co-formulated with pIL-7 or pIL-15 (25 µg total DNA/immunization). Analyses were performed at week 3 in response to four HIV-1 Gag peptide pools by standard ELISPOT. (B) IFN-γ production in total (left panel) and CD8+ T-cell depleted (right panel) splenocytes. (C) IL-4 production in total splenocytes. Net number of spots was determined by subtracting the number of spots formed in control medium wells. Asterisk indicates a Gag-specific response (calculated by subtracting the background responses detected in each control group from the corresponding experimental group) that is significantly different (p < 0.05) between pGag DermaVir-immunized mice and mice co-immunized with pGag + the corresponding IL-encoding plasmid DNA DermaVir.
The frequencies of Gag-specific T-cells producing IL-4 were also assessed by standard ELISPOT (Fig. 1C). No or low antigen-specific IL-4 response was detected in the control groups. Mice immunized with pGag DermaVir alone showed an antigen-specific IL-4 response of 208.3 spots/million splenocytes. Co-immunization with pGag + pIL-7 or pIL-15 also resulted in a higher IL-4 response (71.7 or 181.7 spots/million splenocytes, respectively) than the corresponding control group (pVax + pIL-7 or pIL-15, respectively). However, IL-4 production was not enhanced in mice co-immunized with pGag + pIL-7 or pIL-15 compared to pGag alone; conversely, the inclusion of pIL-7 or pIL-15 slightly decreased Gag-specific IL-4 responses albeit not significantly (p = 0.22 or p = 0.42, respectively).
3.2. IL-7 and IL-15 significantly enhance DermaVir-induced Gag-specific central memory T-cell responses
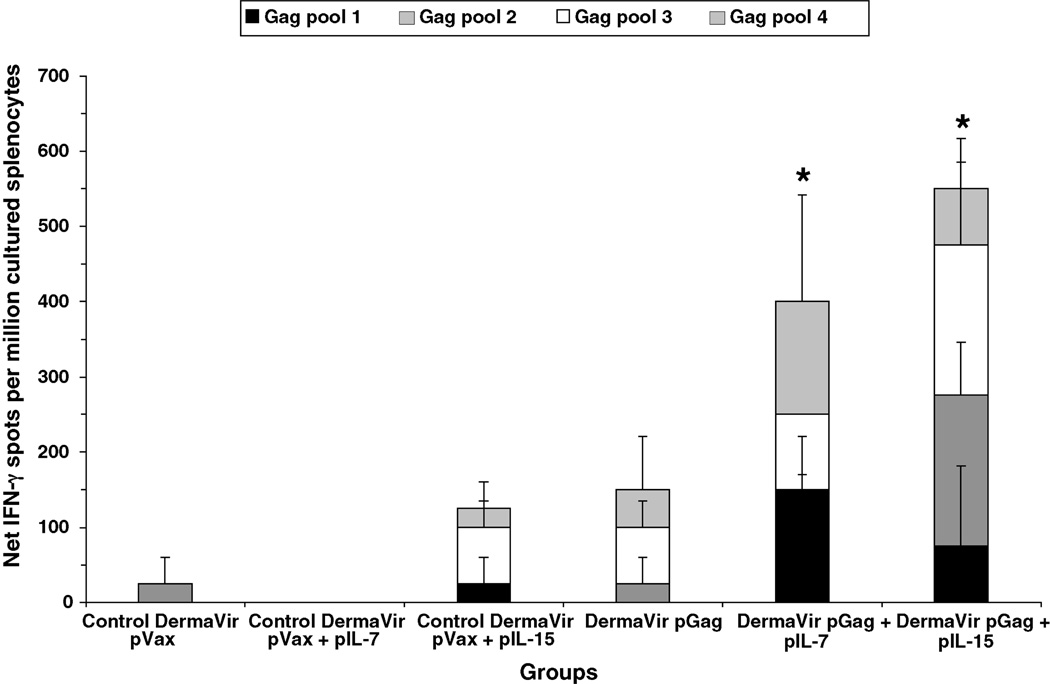
Unlike the standard ELIPSOT assay (that mainly detects antigen-specific effector cells) the cultured ELISPOT assay detects antigen-specific IFN-γ secreting central memory T-cells. Pools of splenocytes obtained from each group, at week 3, were first stimulated for 6 days with each HIV-1 Gag peptide pool and then tested against the corresponding Gag peptide pool by ELISPOT (Fig. 2). Low or no IFN-γ spots were detected in mice immunized with pVax or pVax + pIL-7, respectively, while a background level of 125 spots/million cultured splenocytes was observed in mice co-immunized with pVax + pIL-15. In mice immunized with pGag DermaVir alone, a value of 150 spots/million cultured splenocytes was documented which was 3.9-fold greater than that detected in the standard ELISPOT (see Fig. 1A). In mice co-immunized with pGag + pIL-7 or pIL-15, antigen-specific IFN-γ responses increased up to 400 and 550 spots/million cultured splenocytes, respectively; these responses were 5.2-fold or 5.5-fold higher than those detected in the standard ELISPOT (compare with Fig. 1A). The Gag-specific IFN-γ response induced by pIL-7 and pIL-15 was significantly higher when compared to pGag alone (p = 0.024 and p = 0.033, respectively). Similarly, experiments performed to evaluate Gag-specific T-cell proliferative responses by CFSE staining showed that IL-15 had the highest impact on T-cell expansion, followed by IL-7 (data not shown). This is consistent with the notion that the higher number of spots detected by the cultured ELISPOT assay is consequent to proliferation of antigen-specific T-cells during the culture.
Fig. 2.
Antigen-specific central memory T-cell responses induced by topical DermaVir adjuvanted with IL-7 or IL-15. Mice (four per group) were topically immunized at weeks 0 and 2 with control pVax or pGag DermaVir, either alone or co-formulated with pIL-7 or pIL-15 (25 µg total DNA/immunization). IFN-γ production in response to four HIV-1 Gag peptide pools was evaluated by cultured ELISPOT in total splenocytes at week 3. Net number of spots was determined by subtracting the number of spots formed in control medium wells. Asterisk indicates a Gag-specific response (calculated by subtracting the background responses detected in each control group from the corresponding experimental group) that is significantly different (p < 0.05) between pGag DermaVir-immunized mice and mice co-immunized with pGag + the corresponding IL-encoding plasmid DNA DermaVir.
3.3. IL-15 significantly improves Gag-specific effector memory T-cell responses in DermaVir prime/VacV boost regimen
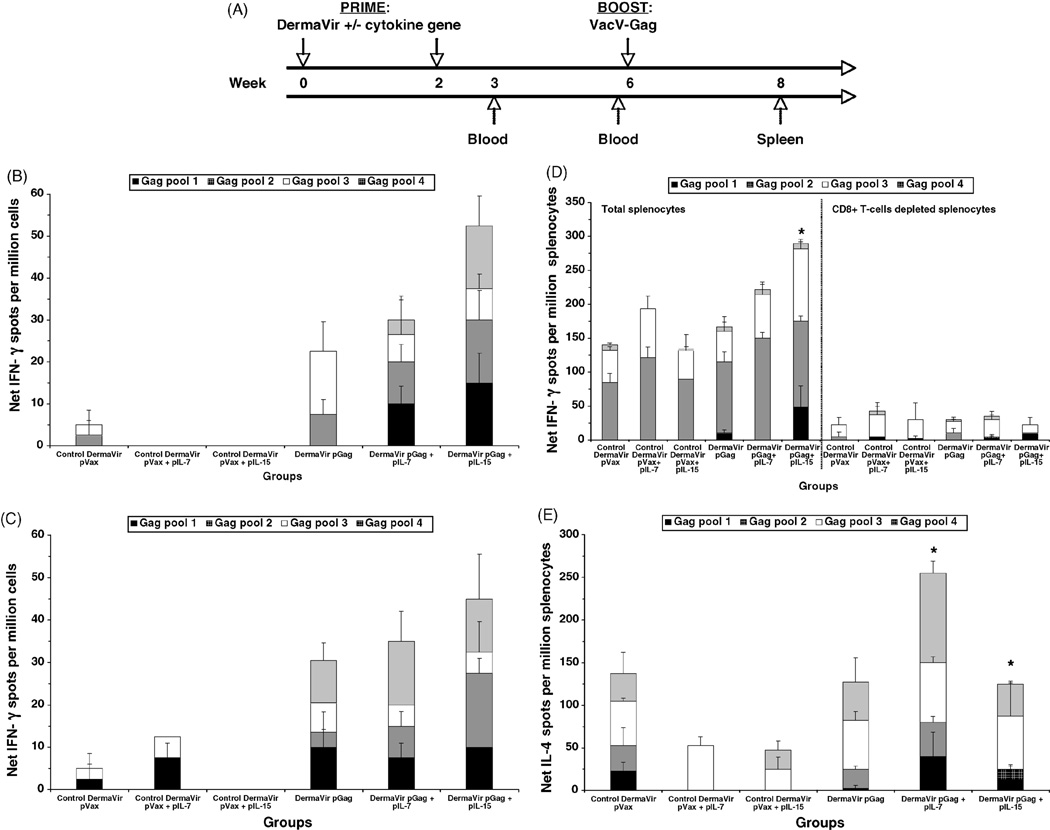
Since both IL-7 and IL-15 enhanced DermaVir-induced antigen-specific memory T-cell responses, we then investigated the effect of these cytokines when co-formulated with DermaVir and administered during the prime phase followed by a VacV boost. Mice were topically DermaVir immunized at weeks 0 and 2 and boosted i.p. at week 6 with an HIV-1 Gag-expressing VacV (VacV-Gag) (Fig. 3A). A pool of peripheral cells (obtained by retro-orbital bleeding) from each group was assayed for IFN-γ production in response to HIV-1 Gag peptide pools by standard ELISPOT at weeks 3 and 6 (before VacV-Gag boost). As shown in Fig. 3B, the pattern of antigen-specific IFN-γ responses observed at week 3 in the peripheral blood resembles that detected in splenocytes at the same time point (compare with Fig. 1A), although at a lower magnitude. Specifically, co-immunization with pGag + pIL-15 DermaVir enhanced (2.3-fold) antigen-specific IFN-γ production compared to pGag alone, while co-immunization with pIL-7 resulted in a slight enhancement (1.3-fold). At week 6, and before VacV-Gag boost, mice co-immunized with pGag + pIL-15 still showed the highest antigen-specific IFN-γ production (Fig. 3C). All groups were then VacV-Gag boosted at week 6 and the splenocytes harvested at week 8. As shown in Fig. 3D (left panel), boosting with VacV-Gag enhanced antigen-specific IFN-γ responses in all groups; the number of spots detected in mice primed with pGag or pGag + pIL-7 were quite similar to that detected in the corresponding control group (pVax or pVax + pIL-7, respectively) while mice primed with pGag + pIL-15 mounted a 2.1-fold higher number of spots than pVax + pIL-15 primed mice. Only mice primed with pGag + pIL-15 mounted a Gag-specific IFN-γ response significantly higher than the pGag group (p = 0.03), while in mice primed with pGag + pIL-7 the increase was not significant (p = 0.44). CD8+ T-cells mostly mediated the Gag-specific IFN-γ T-cell responses in all groups (Fig. 3D, right panel).
Fig. 3.
Antigen-specific effector memory T-cell responses induced by topical DermaVir prime/VacV boost regimen. (A) Mice (four per group) were topically immunized at weeks 0 and 2 with control pVax or pGag DermaVir, either alone or co-formulated with pIL-7 or pIL-15 (25 µg total DNA/immunization) and boosted at week 6 with 2.2 × 106 PFU VacV-Gag (i.p.). IFN-γ production in response to four HIV-1 Gag peptide pools was evaluated by standard ELISPOT at weeks (B) 3 and (C) 6 (before boost) in peripheral cells obtained by retro-orbital bleeding. At week 8, (D) IFN-γ production in total (left panel) and CD8+ T-cell depleted splenocytes (right panel) and (E) IL-4 production in total splenocytes were evaluated by standard ELISPOT. Net number of spots was determined by subtracting the number of spots formed in control medium wells. Asterisk indicates a Gag-specific response (calculated by subtracting the background responses detected in each control group from the corresponding experimental group) that is significantly different (p < 0.05) between pGag DermaVir-immunized mice and mice co-immunized with pGag + the corresponding IL-encoding plasmid DNA DermaVir.
VacV-Gag also enhanced antigen-specific IL-4 responses at week 8 (Fig. 3E); the number of IL-4 spots/million splenocytes was 137.5, 52.5 and 47.5 in mice primed with pVax, pVax + pIL-7 and pVax + pIL-15, respectively. VacV-Gag boost did not increase IL-4 responses in mice primed with pGag DermaVir. We noted an enhanced Gag-specific IL-4 response in mice primed with pGag + pIL-7 compared to mice immunized with pGag alone (p = 0.026), while a slight augmentation was observed in mice primed with pGag + pIL-15 compared to pGag alone (p = 0.043).
3.4. IL-15 improves antigen-specific memory T-cell responses in DermaVir prime/VacV boost regimen
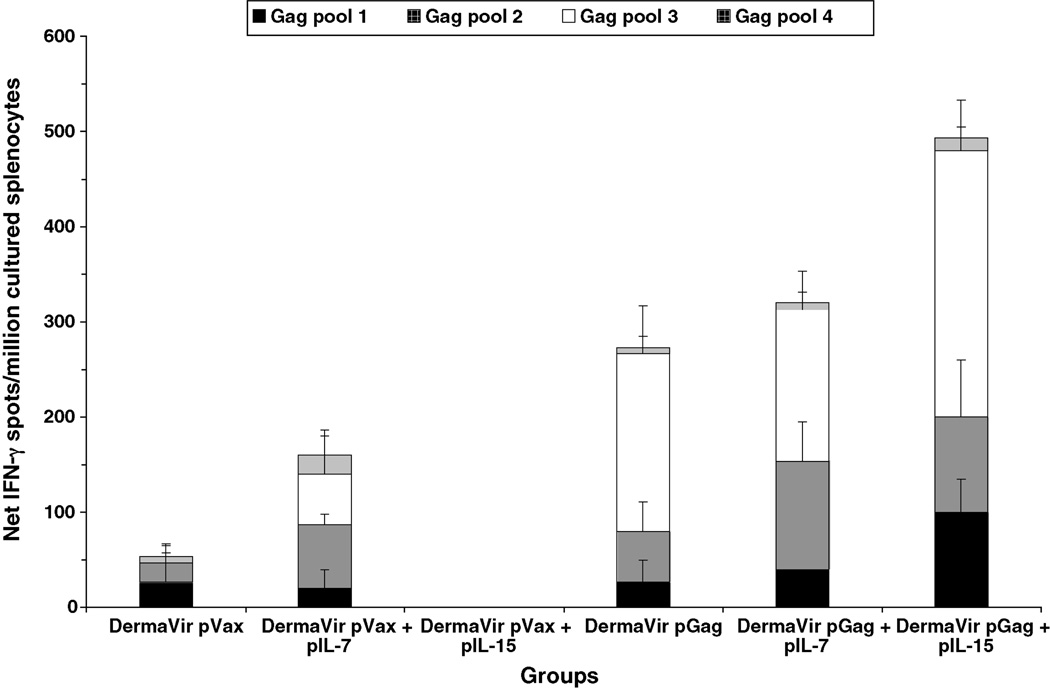
Cultured ELISPOT assays were also performed at week 8 (Fig. 4). Mice primed with pGag DermaVir mounted a 5.1-fold higher antigen-specific IFN-γ response compared to pVax control group. Compared to mice primed with pGag DermaVir, mice receiving pGag + pIL-7 DermaVir during the prime mounted a Gag-specific IFN-γ response not significantly different (p = 0.41), while a 1.8-fold increase of Gag-specific IFN-γ response was detected in mice primed with pGag + pIL-15 DermaVir, although not significant when compared to mice primed with pGag DermaVir alone (p = 0.19). These findings are consistent with the concept that the boost effect recruits memory T-cells to become effectors.
Fig. 4.
Antigen-specific central memory T-cell responses in splenocytes induced by topical DermaVir prime/VacV boost regimen. Mice (four per group) were topically immunized at weeks 0 and 2 with control pVax or pGag DermaVir, either alone or co-formulated with pIL-7 or pIL-15 (25 µg total DNA/immunization) and boosted at week 6 with 2.2 × 106 PFU VacV-Gag (i.p.). IFN-γ production in response to four HIV-1 Gag peptide pools was evaluated at week 8 in total splenocytes by cultured ELISPOT. Net number of spots was determined by subtracting the number of spots formed in control medium wells.
Recent studies have shown the importance of investigating, together with IFN-γ, other CD8+ T-cell functions, such as IL-2 secretion [35, 36]. We also analyzed dual IFN-γ/IL-2-secreting CD8+ T-cells in all groups after VacV-Gag boost in response to HIV-1 Gag peptides. After background responses from each control group was subtracted from the corresponding experimental group, the highest percentage of Gag-specific IFN-γ/IL-2-secreting CD8+ T-cells was detected in mice primed with pGag + pIL-15 DermaVir (0.12%) while in mice primed with pGag and pGag + pIL-7 was 0.05% and 0%, respectively (data not shown).
Discussion
Considerable evidence suggests that cytokine adjuvants can be used to enhance naked DNA vaccines immunogenicity. Although DermaVir is a DNA-containing vaccine, it differs from naked DNA vaccines: (i) the plasmid DNA is not naked but complexed with a chemical vector, PEIm, to form a nanoparticle that mimics a pathogen [3–6]; (ii) through this chemical vector, topically administered DermaVir vaccine specifically targets LC that are distributed in a dense network throughout the epidermis [3, 6]; (iii) the expression of the DNA-encoded antigen occurs in the draining lymph nodes [3, 6]; (iv) antigen-specific memory T-cell responses are induced [11, 37]. We examined the in vivo activity of cytokine-encoding plasmid adjuvants on antigen-specific T-cell responses in mice topically immunized with DermaVir. By co-formulating pIL-7 or pIL-15 in the HIV-1 DermaVir nanoparticle, we show that both IL-7 and IL-15 prime memory responses, but only IL-15 boosts them.
IL-15 appears to be an important T-cell immunity adjuvant [33, 38, 39]. It has effects on memory T-cell responses when administered as a plasmid DNA [33], or encoded by a VacV [40], or as a recombinant protein [41]. Few studies have evaluated the activity of IL-7 as a plasmid DNA [32] or recombinant protein [41] as vaccine adjuvants. We found that adjuvantation of DermaVir vaccination with pIL-7 or pIL-15 results in enhanced antigen-specific effector CD8+ T-cells, but does not increase IL-4 responses, consistent with the concept that these cytokines exert a Th1 biased adjuvant effect. Importantly, the quality of these responses was affected by the vaccination regimen. In the DermaVir prime/VacV boost regimen, antigen-specific IL-4 responses were enhanced after the boost in mice primed with pIL-7 adjuvanted DermaVir. Sin et al. [32] reported that pIL-7 co-injected with antigen in a naked DNA form could drive antigen-specific Th2-type and/or CTL responses. Since we did not observe a Th2-type response after pIL-7 adjuvanted DermaVir vaccination in the absence of heterologous boost, it is possible that VacV contributed to the IL-7 capability to activate T-cells and generate the Th2-type cytokine production. This is consistent with the observation that the incorporation of pIL-7 in the DermaVir prime/VacV boost did not enhance Gag-specific IFN-γ response, as IL-4 suppresses Th1-type cytokine expression by CD8+ T-cells [42–44].
In our experimental conditions IL-15 is superior to IL-7 as a Th1 adjuvant. IL-15 promotes monocyte differentiation into DC with LC-like characteristics and enhances their antigen presenting functions [45]. DermaVir has been designed to target LC [3–6]. It is possible that pIL-15 facilitates the activation and maturation of DC after DermaVir vaccination, resulting in an enhanced immune response.
We have recently shown that topical DermaVir prime/intramuscular protein boost prophylactic vaccine regimen in the macaque model elicits antigen-specific central memory T-cell responses that are effective in controlling pathogenic SHIV infection [37]. Here, we show that DermaVir elicits antigen-specific central memory T-cell response, which can be enhanced by IL-15 or IL-7, suggesting that DermaVir prime might be efficient in a prophylactic vaccine regimen.
In summary, our studies demonstrate that IL-15 is a promising DermaVir adjuvant to enhance antigen-specific central memory-type T-cells in a prime-boost setting. Additional studies in primates are required to establish the role of this cytokine as adjuvant in prophylactic DermaVir vaccination.
Acknowledgements
This work was supported by NIH grant R01 AI05698201 to F.L.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Laddy DJ, Weiner DB. From plasmids to protection: a review of DNA vaccines against infectious diseases. Int Rev Immunol. 2006;25:99–123. doi: 10.1080/08830180600785827. [DOI] [PubMed] [Google Scholar]
- 2.Ulmer JB, Wahren B, Liu MA. Gene-based vaccines: recent technical and clinical advances. Trends Mol Med. 2006;12:216–222. doi: 10.1016/j.molmed.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Lori F, Trocio J, Bakare N, Kelly LM, Lisziewicz J. DermaVir, a novel HIV immunization technology. Vaccine. 2005;23:2030–2034. doi: 10.1016/j.vaccine.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Lisziewicz J, Trocio J, Whitman L, Varga G, Xu J, Bakare N, et al. DermaVir: a novel topical vaccine for HIV/AIDS. J Invest Dermatol. 2005;124:160–169. doi: 10.1111/j.0022-202X.2004.23535.x. [DOI] [PubMed] [Google Scholar]
- 5.Lisziewicz J, Trocio J, Xu J, Whitman L, Ryder A, Bakare N, et al. Control of viral rebound through therapeutic immunization with DermaVir. AIDS. 2005;19:35–43. doi: 10.1097/00002030-200501030-00004. [DOI] [PubMed] [Google Scholar]
- 6.Lisziewicz J, Kelly L, Lori F. Topical DermaVir vaccine targeting dendritic cells. Curr Drug Deliv. 2006;3:83–88. doi: 10.2174/156720106775197574. [DOI] [PubMed] [Google Scholar]
- 7.Boussif O, Lezoualc'h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci USA. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pollard H, Remy JS, Loussouarn G, Demolombe S, Behr JP, Escande D. Polyethylenimine but not cationic lipids promotes transgene delivery to the nucleus in mammalian cells. J Biol Chem. 1998;273:7507–7511. doi: 10.1074/jbc.273.13.7507. [DOI] [PubMed] [Google Scholar]
- 9.Lisziewicz J, Gabrilovich DI, Varga G, Xu J, Greenberg PD, Arya SK, et al. Induction of potent human immunodeficiency virus type 1-specific T-cell-restricted immunity by genetically modified dendritic cells. J Virol. 2001;75:7621–7628. doi: 10.1128/JVI.75.16.7621-7628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calarota SA, Weiner DB. Enhancement of human immunodeficiency virus type 1-DNA vaccine potency through incorporation of T-helper 1 molecular adjuvants. Immunol Rev. 2004;199:84–99. doi: 10.1111/j.0105-2896.2004.00150.x. [DOI] [PubMed] [Google Scholar]
- 11.Calarota SA, Weiner DB, Lori F, Lisziewicz J. Induction of HIV-specific memory T-cell responses by topical DermaVir vaccine. Vaccine. 2007;25:3070–3074. doi: 10.1016/j.vaccine.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 12.Becker TC, Wherry EJ, Boone D, Murali-Krishna K, Antia R, Ma A, et al. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T-cells. J Exp Med. 2002;195:154–158. doi: 10.1084/jem.20020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldrath AW, Sivakumar PV, Glaccum M, Kennedy MK, Bevan MJ, Benoist C, et al. Cytokine requirements for acute and basal homeostatic proliferation of naïve and memory CD8+ T cells. J Exp Med. 2002;195:1515–1522. doi: 10.1084/jem.20020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naïve and memory T-cells in vivo. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 15.Schluns KS, Williams K, Ma A, Zheng XX, Lefrancois L. Cutting edge: requirement for IL-15 in the generation of primary and memory antigenspecific CD8 T cells. J Immunol. 2002;168:4827–4831. doi: 10.4049/jimmunol.168.10.4827. [DOI] [PubMed] [Google Scholar]
- 16.Tan JT, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg KI, et al. IL-7 is critical for homeostatic proliferation and survival of naïve T cells. Proc Natl Acad Sci USA. 2001;98:8732–8737. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 18.Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T-cells in vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 19.Ku CC, Murakami M, Sakamoto A, Kappler J, Marrack P. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science. 2000;288:675–678. doi: 10.1126/science.288.5466.675. [DOI] [PubMed] [Google Scholar]
- 20.Godkin AJ, Thomas HC, Openshaw PJ. Evolution of epitope-specific memory CD4(+) T cells after clearance of hepatitis C virus. J Immunol. 2002;169:2210–2214. doi: 10.4049/jimmunol.169.4.2210. [DOI] [PubMed] [Google Scholar]
- 21.Vuola JM, Keating S, Webster DP, Berthoud T, Dunachie S, Gilbert SC, et al. Differential immunogenicity of various heterologous prime-boost vaccine regimens using DNA and viral vectors in healthy volunteers. J Immunol. 2005;174:449–455. doi: 10.4049/jimmunol.174.1.449. [DOI] [PubMed] [Google Scholar]
- 22.Reece WH, Pinder M, Gothard PK, Milligan P, Bojang K, Doherty T, et al. A CD4(+) T-cell immune response to a conserved epitope in the circumsporozoite protein correlates with protection from natural Plasmodium falciparum infection and disease. Nat Med. 2004;10:406–410. doi: 10.1038/nm1009. [DOI] [PubMed] [Google Scholar]
- 23.Keating SM, Bejon P, Berthoud T, Vuola JM, Todryk S, Webster DP, et al. Durable human memory T cells quantifiable by cultured enzyme-linked immunospot assays are induced by heterologous prime boost immunization and correlate with protection against malaria. J Immunol. 2005;175:5675–5680. doi: 10.4049/jimmunol.175.9.5675. [DOI] [PubMed] [Google Scholar]
- 24.Webster DP, Dunachie S, Vuola JM, Berthoud T, Keating S, Laidlaw SM, et al. Enhanced T cell-mediated protection against malaria in human challenges by using the recombinant poxviruses FP9 and modified vaccinia virus Ankara. Proc Natl Acad Sci USA. 2005;102:4836–4841. doi: 10.1073/pnas.0406381102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun P, Schwenk R, White K, Stoute JA, Cohen J, Ballou WR, et al. Protective immunity induced with malaria vaccine, RTS,S, is linked to plasmodium falciparum circumsporozoite protein-specific CD4+ and CD8+ T cells producing IFN-g. J Immunol. 2003;171:6961–6967. doi: 10.4049/jimmunol.171.12.6961. [DOI] [PubMed] [Google Scholar]
- 26.Letvin NL, Mascola JR, Sun Y, Gorgone DA, Buzby AP, Xu L, et al. Preserved CD4+ central memory T cells and survival in vaccinated SIV-challenged monkeys. Science. 2006;312:1530–1533. doi: 10.1126/science.1124226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mattapallil JJ, Douek DC, Buckler-White A, Montefiori D, Letvin NL, Nabel GJ, et al. Vaccination preserves CD4 memory T cells during acute simian immunodeficiency virus challenge. J Exp Med. 2006;203:1533–1541. doi: 10.1084/jem.20060657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2:251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 29.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 30.Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 31.Kim JJ, Nottingham LK, Sin JI, Tsai A, Morrison L, Oh J, et al. CD8 positive T cells influence antigen-specific immune responses through the expression of chemokines. J Clin Invest. 1998;102:1112–1124. doi: 10.1172/JCI3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sin JI, Kim J, Pachuk CJ, Weiner DB. Interleukin 7 can enhance antigen-specific cytotoxic-T-lymphocyte and/or Th2-type immune responses in vivo. Clin Diagn Lab Immunol. 2000;7:751–758. doi: 10.1128/cdli.7.5.751-758.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kutzler MA, Robinson TM, Chattergoon MA, Choo DK, Choo AY, Choe PY, et al. Coimmunization with an optimized IL-15 plasmid results in enhanced function and longevity of CD8 T cells that are partially independent of CD4 T cell help. J Immunol. 2005;175:112–123. doi: 10.4049/jimmunol.175.1.112. [DOI] [PubMed] [Google Scholar]
- 34.Kwak H, Mustafa W, Speirs K, Abdool AJ, Paterson Y, Isaacs SN. Improved protection conferred by vaccination with a recombinant vaccinia virus that incorporates a foreign antigen into the extrecellular enveloped virion. Virology. 2004;322:337–348. doi: 10.1016/j.virol.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 35.Zimmerli SC, Harari A, Cellerai C, Vallelian F, Bart PA, Pantaleo G. HIV-1-specific IFN-g/IL-2-secreting CD8 T cells support CD4-independent proliferation of HIV-1-specific CD8 T cells. Proc Natl Acad Sci USA. 2005;102:7239–7244. doi: 10.1073/pnas.0502393102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cristillo AD, Lisziewicz J, He L, Lori F, Galmin L, Trocio JN, et al. HIV-1 prophylactic vaccine comprised of topical DermaVir prime and protein boost elicits cellular immune responses and controls pathogenic R5 SHIV162P3. Virology. 2007 doi: 10.1016/j.virol.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 38.Kim JJ, Trivedi NN, Nottingham LK, Morrison L, Tsai A, Hu Y, et al. Modulation of amplitude and direction of in vivo immune responses by co-administration of cytokine gene expression cassettes with DNA immunogens. Eur J Immunol. 1998;28:1089–1103. doi: 10.1002/(SICI)1521-4141(199803)28:03<1089::AID-IMMU1089>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 39.Xin KQ, Hamajima K, Sasaki S, Tsuji T, Watabe S, Okada E, et al. IL-15 expression plasmid enhances cell-mediated immunity induced by an HIV-1 DNA vaccine. Vaccine. 1999;17:858–866. doi: 10.1016/s0264-410x(98)00271-0. [DOI] [PubMed] [Google Scholar]
- 40.Oh S, Berzofsky A, Burke DS, Waldmann TA, Perera LP. Coadministration of HIV vaccine vectors with vaccinia viruses expressing IL-15 but not IL-2 induces long-lasting cellular immunity. Proc Natl Acad Sci USA. 2003;100:3392–3397. doi: 10.1073/pnas.0630592100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Melchionda F, Fry TJ, Milliron MJ, McKirdy MA, Tagaya Y, Mackall CL. Adjuvant IL-7 or IL-15 overcomes immunodominance and improves survival of the CD8+ memory cell pool. J Clin Invest. 2005;115:1177–1187. doi: 10.1172/JCI23134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Croft M, Carter L, Swain SL, Dutton RW. Generation of polarized antigen-specific CD8 effector populations: reciprocal action of interleukin (IL)-4 and IL-12 in promoting type 2 versus type 1 cytokine profiles. J Exp Med. 1994;180:1715–1728. doi: 10.1084/jem.180.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Noble A, Macary PA, Kemeny DM. IFN-gamma and IL-4 regulate the growth and differentiation of CD8+ T cells into subpopulations with distinct cytokine profiles. J Immunol. 1995;155:2928–2937. [PubMed] [Google Scholar]
- 44.Sharma DP, Ramsay AJ, Maguire DJ, Rolph MS, Ramshaw IA. Interleukin-4 mediates down regulation of antiviral cytokine expression and cytotoxic T-lymphocyte responses and exacerbates vaccinia virus infection in vivo. J Virol. 1996;70:7103–7107. doi: 10.1128/jvi.70.10.7103-7107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mohamadzadeh M, Berard F, Essert G, Chalouni C, Pulendran B, Davoust J, et al. Interleukin 15 skews monocyte differentiation into dendritic cells with features of Langerhans cells. J Exp Med. 2001;194:1013–1020. doi: 10.1084/jem.194.7.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]