Abstract
For many practicing obstetrician-gynecologists, tubal ligation was the gold standard by which female sterilization techniques were measured. Yet gynecologic surgeons have simultaneously sought to occlude the fallopian tubes transcervically to avoid discomfort and complications associated with transabdominal approaches. In this review, the history of transcervical sterilization is discussed. Past, current, and upcoming techniques are reviewed. This article focuses on interval sterilization techniques, thus removing post-vaginal and post-cesarean delivery tubal ligations from the discussion.
Key words: Laparoscopic tubal ligation, Transcervical sterilization, Quinacrine
For many practicing obstetrician-gynecologists (ob-gyns), tubal ligation was the gold standard by which female sterilization techniques were measured. Since the early 1970s, tubal ligations—both laparoscopic and postpartum—have been performed safely and efficiently, and nearly all ob-gyns are well versed in the procedure.
Yet gynecologic surgeons have simultaneously sought to occlude the fallopian tubes transcervically to avoid the discomfort and complications associated with transabdominal approaches. Different techniques and devices have been tried, and varying degrees of success have been reported. From a practical perspective, to be considered an acceptable technique for permanent female contraception, transcervical sterilization needs to be judged against tubal ligation on 4 criteria: (1) effectiveness, (2) safety, (3) discomfort and pain, and (4) cost. In this review, the history of transcervical sterilization is discussed. Past, current, and upcoming techniques are reviewed to determine how they measure up to tubal ligation. For simplicity’s sake, this review focuses on interval sterilization techniques, thus removing post-vaginal and post-cesarean delivery tubal ligations from much of the discussion.
Defining the “Gold Standard”
Laparoscopic Tubal Ligation
As previously noted, laparoscopic tubal ligation (LTL) was considered the gold standard against which other methods for permanent female sterilization were judged. With this technique, intra-abdominal access is most frequently obtained through the use of a laparoscope. Given the need for pneumoperitoneum and its associated discomfort, LTL is usually performed using general anesthesia in the outpatient setting. Almost all patients are candidates for this procedure, except for those women with profound medical problems that preclude them from receiving general anesthesia, even for a short duration. Typically, an umbilical incision is used for primary abdominal access with 1 or 2 small ancillary incisions in the midline or lower quadrants.
Once abdominal access is achieved, both fallopian tubes are identified and occluded under direct visualization. Methods of tubal occlusion include electrosurgical methods using unipolar or bipolar electrocoagulation or mechanical methods such as the Hulka-Clemens spring clip, the Filshie hinged clip, or the Falope or Yoon silastic ring/band. Patients usually have a 48- to 72-hour recovery period with mild-to-moderate abdominal pain at the incision sites and from residual pneumoperitoneum. Contraception is considered immediate following occlusion of the fallopian tubes.
Effectiveness
The effectiveness of tubal ligation has been most extensively studied in the US Collaborative Review of Sterilization (CREST) study.1 This study followed 10,685 sterilized women for up to 14 years following their tubal ligation. The findings demonstrated that tubal ligation is highly effective, although effectiveness varies by the ligation method employed and by patient age, race, and ethnicity. In the CREST study,1 the cumulative 10-year probability of pregnancy following tubal ligation was 18.5 per 1000 procedures (95% confidence interval, 15.1–21.8). Postprocedural pregnancy rates were highest following laparoscopic Hulka-Clemens clip sterilization (36.5 pregnancies per 1000 procedures) and lowest following unipolar coagulation and postpartum partial salpingectomy (each 7.5 pregnancies per 1000 procedures) (Figure 1). Sterilization failure occurred more commonly in women who underwent the procedure at a younger age due to increased fertility in younger women.1 The risk of pregnancy during the first year is about 0.5% (Table 1).2
Figure 1.
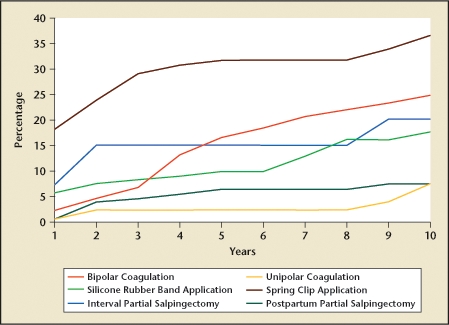
Efficacy of tubal ligation in the 10 years following the procedure. Data from Peterson HB et al.1
Table 1.
Percentage of US Women Experiencing an Unintended Pregnancy During the First Year of Typical Use and the First Year of Perfect Use of Contraception and the Percentage Continuing Use at the End of the First Year
| Women Experiencing an Unintended Pregnancy Within the First year of Use (%) | |||
| Method | Typical Use | Perfect Use | Women Continuing Use at 1 Year (%) |
| No method | 85 | 85 | |
| Spermicides | 29 | 18 | 42 |
| Withdrawal | 27 | 4 | 43 |
| Periodic abstinence | 25 | 51 | |
| Calendar | 9 | ||
| Ovulation method | 3 | ||
| Symptothermal | 2 | ||
| Postovulation | 1 | ||
| Cap | |||
| Parous women | 32 | 26 | 46 |
| Nulliparous women | 16 | 9 | 57 |
| Sponge | |||
| Parous women | 32 | 20 | 46 |
| Nulliparous women | 16 | 9 | 57 |
| Diaphragm | 16 | 6 | 57 |
| Condom | |||
| Female (Reality) | 21 | 5 | 49 |
| Male | 15 | 2 | 53 |
| Combined pill and minipill | 8 | 0.3 | 68 |
| Ortho Evra® patch | 8 | 0.3 | 68 |
| NuvaRing® | 8 | 0.3 | 68 |
| Depo-Provera® | 3 | 0.3 | 56 |
| Lunelle™ | 3 | 0.05 | 56 |
| IUD | |||
| ParaGard (copper T) | 0.8 | 0.6 | 78 |
| Mirena (levonorgesterel containing intrauterine system) | 0.1 | 0.1 | 81 |
| Norplant® and Norplant-2® | 0.05 | 0.05 | 84 |
| Female sterilization | 0.5 | 0.5 | 100 |
| Male sterilization | 0.15 | 0.10 | 100 |
Reality, Female Health Company, UK; Ortho Evra, Ortho-McNeil-Janssen Pharmaceuticals, Inc., Raritan, NJ; NuvaRing, Organon USA Inc., Roseland, NJ; Depo-Provera, Pfizer Inc, New York, NY; Lunelle, Pharmacia Corporation, Peapack, NJ; ParaGard, Duramed Pharmaceuticals, Inc., Pomona, NY; Mirena, Bayer Healthcare Pharmaceuticals, Montville, NJ; Norplant and Norplant-2, Wyeth Pharmaceuticals Inc., Philadelphia, PA.
Reprinted from Contraception, Vol. 70, Trussel J, Contraceptive failure in the United States, pp. 89–96, Copyright 2004, with permission from Elsevier.2
Safety
The safety of LTL is well established. Injuries to major blood vessels, bowel, and/or bladder are uncommon. The most significant risks are likely related to the general anesthetic rather than to the surgical techniques involved. Between 1977 and 1981, there were 29 deaths reported to the Centers for Disease Control and Prevention after tubal sterilization, of which 3 were major vessel injuries secondary to laparoscopic procedures.3 A prospective study of 9475 LTLs found no deaths and no definite major vascular injuries, although the power to detect these events was limited.4 In another trial, no life-threatening events or deaths were observed in a cohort of 3500 women undergoing interval laparoscopic sterilizations, and only 1.7% of women undergoing laparoscopy experienced any intra- or postoperative complications.5 Finally, in a trial sponsored by the World Health Organization, 819 women undergoing LTL with electrocautery in 8 centers around the world were evaluated. Major surgical complications occurred in 0.9% of the women and another 0.9% experienced significant anesthetic-related complications.6
Discomfort and pain
Although discomfort and pain are clearly more difficult to objectively evaluate than effectiveness or complications, several authors have approached this topic and concluded that although LTL is generally well tolerated, patients do often experience significant discomfort, especially when rings or clips are used.7 Pain from LTL is generally attributed to 3 components: (1) incisional pain, (2) pain from CO2 gas irritation to the abdominal peritoneum and phrenic nerve, and (3) pain from fallopian tube tissue necrosis. “Gas pain” in the upper abdomen and shoulders alone has been shown to persist for up to 3 days.8 In a 1989 study of 54 Canadian women undergoing LTL with electrocautery, Fraser and colleagues9 noted that 85% of their sample reported that pain and/or fatigue impacted their recovery and contributed to an average delay of return to normal activity level of 4.4 days, not including the day of surgery.
Cost
In many aspects, evaluating cost is even more challenging than evaluating pain because the true cost of medical care is rarely known, as it is often too complex to calculate. As an example, studies looking at surgical costs frequently employ hourly hospital charges for operating rooms because determining the true cost of an operating room would involve amortizing the costs of the equipment, the building, and personnel, as well as a host of other variables. Rather, most authors look at hospital and physician charges as interchangeable with cost even though this is clearly not the case and unquestionably yields inaccurate conclusions. For the purposes of this review, I use what available data I have, which are usually charges based.
In the United States, in most circumstances the cost of LTL with electrocautery is a compilation of 3 charges: (1) the surgeon’s fee, (2) the anesthesiologist’s fee, and (3) the hospital fee for use of the operating room, which includes all nonphysician hospital charges. Using these criteria, Hopkins and colleagues10 looked at LTL costs at the Mayo Clinic in Rochester, MN, in 2003 and 2004 and determined the median cost to be $2880, whereas Levie and Chudnoff11 at Montifiore Medical Center in New York City, in a meticulously designed cost analysis, determined that the cost was $3449.
Past Methods
Electrocautery
Blind attempts at electrocautery of the uterotubal junction were reported as early as 187812 and in further detail by Dickenson13 in 1916. According to Cooper,14 in 1927, Schroeder suggested the use of the hysteroscope for direct visualization of the tubal ostia for the purpose of sterilization and recorded the first 2 attempts at hysteroscopic female sterilization using electrocautery in 1934. With this technique, an electrode is passed into the intramural portion of the tube under hysteroscopic guidance, and a coagulating current is applied for several seconds. Despite a mixed effectiveness and safety profile, this method continued to have advocates around the world until the late 1970s, when reports of complications and poor effectiveness became better known.
Effectiveness
In the largest report of electrocautery for hysteroscopic sterilization, Quinones and colleagues15 detailed the treatment of 1284 patients. In this series, both tubes were occluded in only 80% of the patients. Other authors reported patency rates as high 26%. Although these failure rates may have been acceptable in a pre-antibiotic, pre-general anesthesia era in which laparotomy and laparoscopy entailed considerable morbidity, they are insufficient when compared with modern LTL.
Safety
In addition to poor effectiveness rates, transcervical hysteroscopic sterilization using electrocautery was also notable for patient safety concerns, with authors reporting uterine perforation and thermal injury to the bowel.16 Echoing these concerns, March and Israel17 concluded in 1975 that the technique was both unsafe and insufficiently effective.
Neodysmium:Yttrium-Aluminum-Garnet Laser
For those familiar with its design and use, the neodysmium:yttrium-aluminum-garnet (Nd:YAG) laser would seem the ideal means by which to hysteroscopically occlude the fallopian tubes. The Nd:YAG laser is delivered through a long, flexible, quartz fiber and applies consistent thermal energy to tissues to a depth of 5 mm. To this end, Brumsted and colleagues18 attempted using the Nd:YAG laser for tubal occlusion in a carefully designed clinical trial reported in 1991. Despite high hopes, the trial was terminated after enrolling only 17 subjects due to patency rates of 74%! Although there were no safety concerns with this technique, the authors concluded that “[t]o date, none of the methods tested have been effective enough to replace laparoscopic tubal ligation.”
Current Methods Approved for Use in the United States
Transcervical Sterilization
Currently in the United States, Essure® (Conceptus, Inc., Mountain View, CA) is the only available approved method of transcervical sterilization. It was approved by the European Union in 2001 and received US Food and Drug Administration (FDA) approval in November 2002. In this nonincisional method of sterilization, a metal microinsert is placed under hysteroscopic guidance into the interstitial portion of each fallopian tube. The insert comes loaded in a single-use delivery system and consists of an inner coil of stainless steel and polyethylene terephthalate (PET) fibers and an outer coil of nickel-titanium (nitinol). PET fibers were chosen because of their known success in causing tissue in-growth into medical devices in other procedures such as arterial grafts. The device is placed in the proximal fallopian tube in the wound down state and then deployed to an expanded state that anchors the insert in the tube (Figure 2).19 Subsequent to placement, the PET fibers stimulate a benign tissue response that elicits the invasion of macrophages, fibroblasts, foreign body giant cells, and plasma cells. Within several weeks, the fibrotic in-growth around the device results in complete tubal occlusion.20 Tubal occlusion and proper positioning are confirmed 12 weeks following microinsert placement by hysterosalpingogram (HSG) (Figure 3). Backup birth control must be used until bilateral tubal occlusion and proper position is confirmed by HSG.
Figure 2.
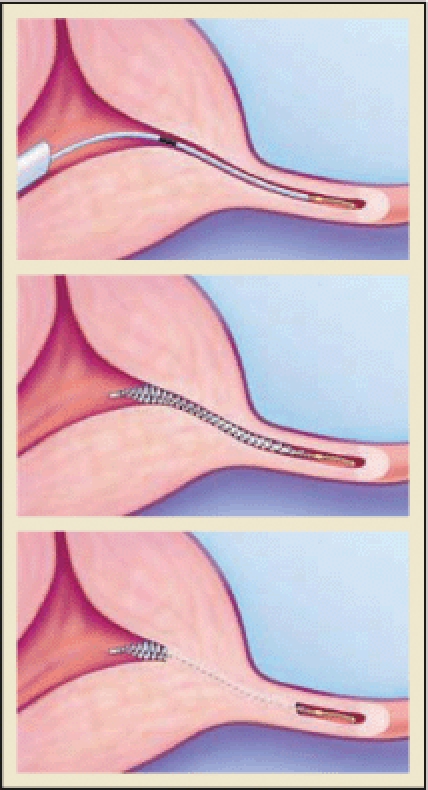
The Essure (Conceptus, Inc., Mountain View, CA) procedure for permanent birth control. Copyright 2006 Conceptus Incorporated. All rights reserved.
Figure 3.
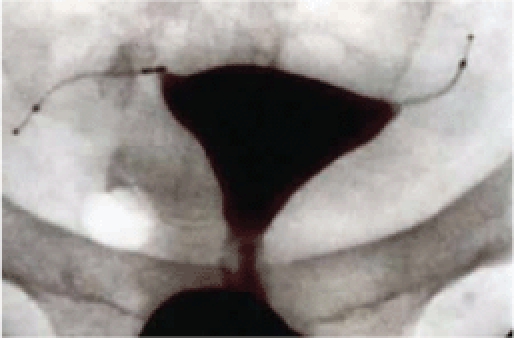
Tubal occlusion is confirmed 12 weeks following Essure (Conceptus, Inc., Mountain View, CA) microinsert placement by hysterosalpingogram. Copyright Conceptus Incorporated. All rights reserved.
Effectiveness
According to the phase II multicenter trial of effectiveness, no pregnancies were reported in 6015 woman-months of exposure to intercourse following documented bilateral tubal occlusion.21 In a more recent review, Levy and colleagues22 analyzed 64 pregnancies that were reported to the device manufacturer as of December 2005. A breakdown of these pregnancies is detailed in Table 2. Applying these data to the CREST study, transcervical tubal occlusion is second only to unipolar tubal ligation in terms of effectiveness (Table 3). To date, when all reported pregnancies with a confirmatory HSG are analyzed,23 hysteroscopic tubal occlusion represents the most effective of all female or male sterilization techniques at the observed follow-up times.
Table 2.
Causes of Reported Pregnancies
| Reason Pregnancy Occurred | N | % of Total |
| Patient or physician noncompliance | 30 | 47 |
| Misread radiograph or HSG | 18 | 28 |
| Pregnant at time of placement | 8 | 12.5 |
| Prior device design | 1 | 1.5 |
| Other | 7 | 11 |
| Total | 64 |
HSG, hysterosalpingogram.
Reprinted from Journal of Minimally Invasive Gynecology, Vol. 14, Levy B et al, A summary of reported pregnancies after hysteroscopic sterilization, pp. 271–274, Copyright 2007, with permission from Elsevier.22
Table 3.
Comparison of Cumulative Risk of Pregnancy in CREST Study Versus Essure Sterilization
| Method | 5 Years of Follow-Up |
| Bipolar | 16.5 (10.6–22.4) |
| Unipolar | 2.3 (0.0–4.8) |
| Silicone band | 10.0 (6.4–13.5) |
| Spring clip | 31.7 (22.6–40.7) |
| Interval salpingectomy | 15.1 (3.1–27.1) |
| Postpartum salpingectomy | 6.3 (2.2–10.3) |
| All CREST average | 13.1 (10.8–15.4) |
| Essure, posterior mean | 2.6 (0.0–7.9)* |
Cumulative number of pregnancies/1000 procedures and 95% confidence intervals.
Represents 75 phase II clinical trial patients who have completed 5-year follow-up. No patients in the pivotal study have reached the 5-year follow-up visit.
CREST, US Collaborative Review of Sterilization.
Reprinted from Journal of Minimally Invasive Gynecology, Vol. 14, Levy B et al, A summary of reported pregnancies after hysteroscopic sterilization, pp. 271–274, Copyright 2007, with permission from Elsevier.22
Safety
There were no major adverse events reported in the phase II and Privotal trial data obtained from 745 women undergoing placement of Essure between 1998 and 2001, although uterine perforation was noted in 2.8% of the patients.24 Similarly, Chern and Siow25 did not encounter any significant safety concerns in their review of 80 patients who underwent the Essure procedure in Singapore. A review of the FDA’s Manufacturer and User Facility Device Experience database from the introduction of Essure in 2002 to July 2008 also did not reveal any major adverse events (death, bowel injury, or major vascular injury), but there were 2 reports of devices embedding into abdominal structures and requiring removal after procedures complicated by uterine perforations.26 Finally, for women with significant medical problems (such as severe cardiac disease) who require permanent contraception but might otherwise carry considerable surgical risks, Essure has been shown to be a safe alternative to tubal ligation.27
Discomfort and pain
As compared with LTL, significant advantages for patients can be achieved when the Essure procedure is performed in the office under local anesthesia rather than in the operating room. Aside from the convenience of the setting and the elimination of the recovery time from the anesthetic agents, several authors have reported very favorable pain profiles and satisfaction data with this technique. In a study from Spain on 1615 patients undergoing placement of Essure with only oral ibuprofen and oral benzodiazepine, Arjona and colleagues28 reported that “1398 (86.5%) of the 1615 women with Essure microinserts inserted considered it excellent or very good, 10.2% (166) felt pain similar to normal menstruation (good), and only 3.1% felt more pain than with menstruation (fair or poor).” These data are further bolstered by a French study of 1032 women undergoing the Essure procedure, in which 90% reported a return to their everyday life within 24 hours and 80% of the local anesthesia patients reported returning to work the next day.29 Finally, in a direct comparison against LTL in a prospective cohort trial of 89 women, Duffy and colleagues30 showed that 82% of the Essure patients considered their tolerance of the procedure to be “excellent to good,” as compared with only 41% of the LTL patients. And, at 90 days, 100% of the Essure patients were satisfied with recovery as compared with only 80% of the LTL subjects.
Cost
The outpatient, in-office nature of transcervical sterilization with Essure gives this method a very favorable cost profile as compared with other methods. In a 2005 study of female sterilization techniques, Levie and Chudnoff11 demonstrated significant cost savings with transcervical sterilization when compared with LTL as long as the transcervical procedure was performed in the office setting, despite the relatively high cost of the device. Similarly, Hopkins and colleagues31 demonstrated savings of $180 per patient (P = .038) when in-office hysteroscopic sterilization was compared with tubal ligation with electrocautery. Echoing these findings, Thiel and Carson32 also demonstrated significant cost savings with Essure as compared with LTL and added, “[c]arrying out the Essure procedure in an ambulatory setting frees space in the operating room for other types of cases, improving access to care for more patients.”
Future Possibilities
Adiana
The Adiana® (Hologic, Inc., Bedford, MA) sterilization method is a combination of controlled thermal damage to the lining of the fallopian tube followed by insertion of a nonabsorbable biocompatible silicone elastomer matrix within the tubal lumen. Under hysteroscopic guidance, a delivery catheter is introduced into the tubal os. Once placement inside the intramural section of the fallopian tube is confirmed, the distal tip of the catheter delivers radiofrequency (RF) energy, causing a lesion within the fallopian tube. Following thermal injury, the silicone matrix is deployed in the region of the tube where the lesion was formed and the catheter and hysteroscope are removed. Over the next few weeks, occlusion is achieved by fibroblast ingrowth into the matrix, which serves as permanent scaffolding and allows for “space-filling.”33 The mean procedure time (scope in, scope out) is about 12 minutes.34 Patients require local anesthesia with occasional intravenous sedation. Occlusion of tubes is assessed by HSG 3 months after device placement.
Effectiveness
Data from the pivotal study called the Evaluation of the Adiana System for Sterilization Using Electrothermal Energy (EASE) trial was presented to the Obstetrics and Gynecology Devices Panel of the Medical Devices Advisory Committee for the FDA in December 2007. In this study, the primary endpoint was to demonstrate the effectiveness of the Adiana system. In this trial of 570 women, the cumulative failure rates were 1.08% at 1 year and 1.82% at 2 years.35 These effectiveness data suggest rates more than double that of LTL and are further disappointing when evaluated vis-à-vis the data for Essure®.
Safety
In the limited data available from the EASE trial, Adiana seems to be a relatively safe procedure. Of the 653 procedures performed in the study, the only notable significant complication was a case of hyponatremia that was treated with “medication prior to patient discharge on the same day of the procedure.”36 Although the details of this complication are not explained, the presumed cause is excessive absorption of glycine, which is needed as a distention media given the use of the RF energy. As more data emerge, the safety profile of the Adiana device and its use with glycine should be further clarified.
Discomfort and pain
As with other transcervical sterilization techniques, Adiana offers the advantage of being a procedure that can be performed in the office under local anesthesia. Again looking at data from the EASE trial, of the 653 subjects who underwent the Adiana procedure, 98% reported they tolerated it “well” to “excellent.” Twenty-five percent reported some cramping with the procedure, and only 2% complained of postprocedural pain.37
Cost
The costs associated with Adiana are, as of yet, unknown. Although there will be costs associated with the device itself, the RF generator, and possibly a fluid management system, the presumed in-office location of the procedure should yield a favorable cost profile relative to LTL.
Quinacrine
In 1973, Zipper and colleagues38 demonstrated the sclerosing effects of intrauterine quinacrine on the tubal ostia of rats. From these early animal experiments and the accepted clinical application of quinacrine for inducing pleural sclerosis, Zipper and associates proceeded to investigate the potential use of transcervical quinacrine as a method of sterilization in humans. In their initial trials, the quinacrine was delivered as a slurry with dilutions of 125 mg/mL and 250 mg/mL. Despite promising early effectiveness results, this method was abandoned after 3 deaths were reported that were attributed to rapid absorption of the slurry through endometrial capillaries.39
Refining the technique, in 1977 Zipper and colleagues40 developed a new pellet-based method in which 7 pellets of 36 mg of quinacrine (252 mg total) are placed into the uterus using a tube similar to a copper T intrauterine device (IUD) inserter for 2 to 3 doses 1 month apart.
Effectiveness
Because of its profound implications for permanent contraception worldwide, quinacrine has been extensively studied, most frequently in poorer nations where access to expensive medical technology is limited. In a study of 2592 Chilean women followed over 25 years, quinacrine pellets offered a cumulative pregnancy rate of 4.6%,41 which was corroborated by findings from Indonesia, where a study of 200 women followed over 10 years revealed a pregnancy rate of 4.3%.42 However, recent 10-year data from Vietnam has questioned these data with reported pregnancy rates of 12.1%43 (Figure 4). Although pregnancy rates of 4.3% to 4.6% are not outstanding, they do compare reasonably with the 3.6% rates of laparoscopically applied Hulka clips. Nonetheless, pregnancy rates over 10% seem excessive, even by developing world standards. Given the complexity of assessing effectiveness data due to variations in techniques and follow-up methodology, the final words on the effectiveness of quinacrine sterilization cannot be fairly written until better data are obtained.
Figure 4.
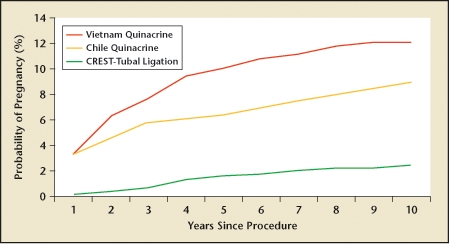
Vietnamese quinacrine cumulative probability of pregnancy and estimates from Chile (quinacrine) and US Collaborative Review of Sterilization (CREST) study (tubal ligation). Reprinted from Contraception, Vol. 78, Sokal DC et al, Contraceptive effectiveness of two insertions of quinacrine: results from 10-year follow-up in Vietnam, pp. 61–65, Copyright 2008, with permission from Elsevier.43
Safety
Quinacrine is a mutagen. It acts by chelation of DNA forming quinacrine-DNA complexes. Because of its role as a mutagen, it has been implicated as a potential carcinogen, although carcinogenicity in either humans or animals has never been established.44 Rather, since the 1930s, quinacrine has been used as an antimalarial agent in over 100 million people without any associated increases in cancers.45 With respect to its potential as a teratogen, in over 130,000 quinacrine sterilizations worldwide there have been no reports of birth defects in any of the involved pregnancies. Finally, with the important exception of the 3 deaths reported with the early use of the quinacrine slurry, no deaths have been reported in over 130,000 cases using the pellets, whereas 3 to 10 deaths could have been expected in the same population undergoing tubal ligation in an industrialized country and perhaps 25 deaths in a less developed nation.46
Discomfort and pain
In general, quinacrine sterilization is generally well tolerated although the insertion can be associated with cramping similar to that experienced with an IUD insertion. In the large Vietnamese trial noted earlier, major complications and side effects were not identified, and only a small number of women were seen for problems such as salpingitis (3%), menstrual disorders (2.7%), and dysmenorrhea (2%).47
Cost
The most attractive feature of quinacrine sterilization from a world health perspective is cost. When quinacrine was manufactured by SIPHARM (Sisseln, Switzerland), the cost in Asia for the inserter and quinacrine pellets was less than $1 per sterilization.48 Compared with the cost of tubal ligation, the difference is dramatic and needs no further elaboration.
Summary
After over 100 years of seeking a safe and effective method for female sterilization that avoids entry into the abdomen, transcervical sterilization is today a reality. By every criterion except immediacy, the Essure transcervical tubal occlusion technique appears equal or superior to LTL. In addition to safety and effectiveness, transcervical tubal occlusion offers the benefits of an in-office, local anesthetic procedure that includes lower total costs and better resource utilization. Looking to the future, both physicians and patients can expect further advances in both implantable devices and chemical technologies that should broaden the options for this area of women’s health.
Main Points.
Laparoscopic tubal ligation (LTL) was considered the gold standard against which other methods for permanent female sterilization were judged. Almost all patients are candidates for this procedure, except for women with profound medical problems that preclude them from receiving general anesthesia, even for a short duration.
Methods of tubal occlusion using electrocautery or Nd:YAG laser proved ineffective and were abandoned.
Transcervical sterilization using Essure® has proved effective and safe. According to phase II multicenter trial results, no pregnancies were reported in 6015 woman-months of exposure to intercourse following documented bilateral tubal occlusion. No major adverse events were reported.
The Adiana® sterilization method is a combination of controlled thermal damage to the lining of the fallopian tube followed by insertion of a nonabsorbable silicone elastomer matrix within the tubal lumen. Failure rates were less than 2% after 2 years, and the procedure is safe and well tolerated. Cost data are as yet unknown, but should be favorable relative to LTL.
Because of its profound implications for permanent contraception worldwide, quinacrine has been extensively studied, most frequently in poorer nations where access to expensive medical technology is limited. Because of its role as a mutagen, it has been implicated as a potential carcinogen, although carcinogenicity in either humans or animals has never been established. The most attractive feature of quinacrine sterilization from a world health perspective is cost, about $1 per sterilization.
References
- 1.Peterson HB, Xia Z, Hughes JM, et al. The risk of pregnancy after tubal sterilization: findings from the U.S. Collaborative Review of Sterilization. Am J Obstet Gynecol. 1996;174:1161–1170. doi: 10.1016/s0002-9378(96)70658-0. [DOI] [PubMed] [Google Scholar]
- 2.Trussell J. Contraceptive failure in the United States. Contraception. 2004;70:89–96. doi: 10.1016/j.contraception.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 3.Peterson HB, DeStefano F, Rubin GL, et al. Deaths attributable to tubal sterilization in the United States, 1977 to 1981. Am J Obstet Gynecol. 1983;145:131–136. doi: 10.1016/0002-9378(83)91040-2. [DOI] [PubMed] [Google Scholar]
- 4.Jamieson DJ, Hillis SD, Duerr A, et al. Complications of interval laparoscopic tubal sterilization findings from the United States Collaborative Review of Sterilization. Obstet Gynecol. 2000;96:997–1002. doi: 10.1016/s0029-7844(00)01082-6. [DOI] [PubMed] [Google Scholar]
- 5.Destefano F, Greenspan JR, Dicker RC, et al. Complications of interval laparoscopic tubal sterilization. Obstet Gynecol. 1983;61:153–158. [PubMed] [Google Scholar]
- 6.World Health Organization, |Task Force on Female Sterilization, |Special Programme of Research, |Development and Research Training in Human Reproduction, authors. Minilaparotomy or laparoscopy for sterilization. Am J Obstet Gynecol. 1982;143:645–652. [PubMed] [Google Scholar]
- 7.Van Ee R, Hemrika DJ, De Blok S, et al. Effects of ketoprofen and mesosalpinx infiltration on postoperative pain after laparoscopic sterilization. Obstet Gynecol. 1996;88:568–572. doi: 10.1016/0029-7844(96)00222-0. [DOI] [PubMed] [Google Scholar]
- 8.Alexander JI. Pain after laparoscopy. Br J Anaesth. 1997;79:369–378. doi: 10.1093/bja/79.3.369. [DOI] [PubMed] [Google Scholar]
- 9.Fraser RA, Hotz SB, Hurtig JB, et al. The prevalence and impact of pain after day-care tubal ligation surgery. Pain. 1989;39:189–201. doi: 10.1016/0304-3959(89)90006-7. [DOI] [PubMed] [Google Scholar]
- 10.Hopkins MR, Creedon DJ, Wagie AE, et al. Retrospective cost analysis comparing Essure hysteroscopic sterilization and laparoscopic bilateral tubal coagulation. J Minim Invasive Gynecol. 2007;14:97–102. doi: 10.1016/j.jmig.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Levie MD, Chudnoff SG. Office hysteroscopic sterilization compared with laparoscopic sterilization: a critical cost analysis. J Minim Invasive Gynecol. 2005;12:318–322. doi: 10.1016/j.jmig.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 12.Kocks J. Eine neue Methode der Sterilsation der Frauen. Zentbl Gynäkol. 1878;26:617. (Ger). [Google Scholar]
- 13.Dickenson RL. Simple sterilization of women by cautery stricture at the intra uterine tubal openings, compared with other methods. Surg Gynecol Obstet. 1916;23:203. [Google Scholar]
- 14.Cooper JM. Hysteroscopic sterilization. Clin Obstet Gynecol. 1992;35:282. [PubMed] [Google Scholar]
- 15.Quinones R, Alvarado D, Ley E. Hysteroscopic sterilization. Int J Gynaecol Obstet. 1976;14:27–34. doi: 10.1002/j.1879-3479.1976.tb00562.x. [DOI] [PubMed] [Google Scholar]
- 16.Neubüser D, Bailer P, Bosselmann K. [Experience with the trans-uterine tubal coagulation with high frequency current and the thermo method under hysteroscopic control.] Geburtshilfe Frauenheilkd. 1977;37:809–812. [PubMed] [Google Scholar]
- 17.March CM, Israel R. A critical appraisal of hysteroscopic tubal fulguration for sterilization. Contraception. 1975;11:261. doi: 10.1016/0010-7824(75)90034-7. [DOI] [PubMed] [Google Scholar]
- 18.Brumsted JR, Shirk G, Soderling MJ, Reed T. Attempted transcervical occlusion of the fallopian tube with the Nd:YAG laser. Obstet Gynecol. 1991;77:327–328. doi: 10.1097/00006250-199102000-00036. [DOI] [PubMed] [Google Scholar]
- 19.Cooper JM, Carignan CS, Cher D, et al. Microinsert nonincisional hysteroscopic sterilization. Obstet Gynecol. 2003;102:59–67. doi: 10.1016/s0029-7844(03)00373-9. [DOI] [PubMed] [Google Scholar]
- 20.Valle RF, Carigan CS, Wright TC, et al. Tissue response to the STOP microcoil transcervical permanent contraceptive device: results from a prehysterectomy study. Fertil Steril. 2001;76:974–980. doi: 10.1016/s0015-0282(01)02858-8. [DOI] [PubMed] [Google Scholar]
- 21.Kerin JF, Cooper JM, Price T, et al. Hysteroscopic sterilization using a micro-insert device: results of a multicentre phase II study. Hum Reprod. 2003;18:1223–1230. doi: 10.1093/humrep/deg256. [DOI] [PubMed] [Google Scholar]
- 22.Levy B, Levie MD, Childers ME. A summary of reported pregnancies after hysteroscopic sterilization. J Minim Invasive Gynecol. 2007;14:271–274. doi: 10.1016/j.jmig.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 23.Ory EM, Hines RS, Cleland WH, Rehberg JF. Pregnancy after microinsert sterilization with tubal occlusion confirmed by hysterosalpingogram. Obstet Gynecol. 2008;111:508–510. doi: 10.1097/01.AOG.0000296487.36158.41. [DOI] [PubMed] [Google Scholar]
- 24.Essure. Mountain View, CA: Conceptus, Inc.; 2007. [package insert] [Google Scholar]
- 25.Chern B, Siow A. Initial Asian experience in hysteroscopic sterilisation using the Essure permanent birth control device. BJOG. 2005;112:1322–1327. doi: 10.1111/j.1471-0528.2005.00436.x. [DOI] [PubMed] [Google Scholar]
- 26.MAUDE: US Food and Drug Administration Center for Devices and Radiological Health. Rockville, MD: Center for Devices and Radiological Health; [Accessed July 27, 2008]. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfMAUDE/search.CFM. [Google Scholar]
- 27.Famuyide AO, Hopkins MR, El-Nashar SA, et al. Hysteroscopic sterilization in women with severe cardiac disease: experience at a tertiary center. Mayo Clin Proc. 2008;83:431–438. doi: 10.4065/83.4.431. [DOI] [PubMed] [Google Scholar]
- 28.Arjona JE, Miño M, Cordón J, et al. Satisfaction and tolerance with office hysteroscopic tubal sterilization. Fertil Steril. 2008 Jan 17; doi: 10.1016/j.fertnstert.2007.08.007. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 29.Scarabin C, Dhainaut C. The ESTHYME study. Women’s satisfaction after hysteroscopic sterilization (Essure micro-insert). A retrospective multicenter survey. Gynecol Obstet Fertil. 2007;35:1123–1128. doi: 10.1016/j.gyobfe.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 30.Duffy S, Marsh F, Rogerson L, et al. Female sterilisation: a cohort controlled comparative study of ESSURE versus laparoscopic sterilisation. BJOG. 2005;112:1522–1528. doi: 10.1111/j.1471-0528.2005.00726.x. [DOI] [PubMed] [Google Scholar]
- 31.Hopkins MR, Creedon DJ, Wagie AE, et al. Retrospective cost analysis comparing Essure hysteroscopic sterilization and laparoscopic bilateral tubal coagulation. J Minim Invasive Gynecol. 2007;14:97–102. doi: 10.1016/j.jmig.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 32.Thiel JA, Carson GD. Cost-effectiveness analysis comparing the Essure tubal sterilization procedure and laparoscopic tubal sterilization. J Obstet Gynaecol Can. 2008;30:581–585. doi: 10.1016/S1701-2163(16)32891-2. [DOI] [PubMed] [Google Scholar]
- 33. [Accessed July 29, 2008];US Food and Drug Administration Obstetrics and Gynecology Devices Panel. Adiana Transcervical Sterilization System PMA P070022 Panel Package. - pg 13–15, OB-GYN, December 14, 2007. http://www.fda.gov/ohrms/dockets/ac/07/briefing/2007-4334b1-00-index.html.
- 34.Johns DA. Advances in hysteroscopic sterilization: report on 600 patients enrolled in the Adiana EASE trial. J Min Inv Gynecol. 2005;12:S39–S40. [Google Scholar]
- 35. [Accessed July 29, 2008];US Food and Drug Administration Obstetrics and Gynecology Devices Panel. Adiana Transcervical Sterilization System PMA P070022 Panel Package. - pg 48, OB-GYN, December 14, 2007. http://www.fda.gov/ohrms/dockets/ac/07/briefing/2007-4334b1-00-index.html.
- 36. [Accessed July 29, 2008];US Food and Drug Administration Obstetrics and Gynecology Devices Panel. Adiana Transcervical Sterilization System PMA P070022 Panel Package. - pg 49–50, OB-GYN, December 14, 2007. http://www.fda.gov/ohrms/dockets/ac/07/briefing/2007-4334b1-00-index.html.
- 37. [Accessed July 29, 2008];US Food and Drug Administration Obstetrics and Gynecology Devices Panel. Adiana Transcervical Sterilization System PMA P070022 Panel Package. - pg 49, OB-GYN, December 14, 2007. http://www.fda.gov/ohrms/dockets/ac/07/briefing/2007-4334b1-00-index.html.
- 38.Zipper J, Prager R, Medel M. Biological changes induced by unilateral instillations of quinacrine in the rat: reversion through the use of estrogen and progesterone. Fertil Steril. 1973;24:48–53. doi: 10.1016/s0015-0282(16)39434-1. [DOI] [PubMed] [Google Scholar]
- 39.Zipper J, Medel M, Goldsmith A, et al. The clinical efficacy of the repeated transcervical instillation of quinacrine for female sterilization. Int J Gynaecol Obstet. 1976;14:499–502. doi: 10.1002/j.1879-3479.1976.tb00093.x. [DOI] [PubMed] [Google Scholar]
- 40.Zipper J, Cole LP, Goldsmith A, et al. Quinacrine hydrochloride pellets: preliminary data on a nonsurgical method of female sterilization. Int J Gynaecol Obstet. 1980;18:275–290. doi: 10.1002/j.1879-3479.1980.tb00496.x. [DOI] [PubMed] [Google Scholar]
- 41.Zipper J, Trujillo V. 25 years of quinacrine sterilization experience in Chile: review of 2,592 cases. Int J Gynaecol Obstet. 2003;83(suppl 2):S23–S29. doi: 10.1016/S0020-7292(03)90086-5. [DOI] [PubMed] [Google Scholar]
- 42.Suhadi A, Anwar M, Soejoenoes A. 10-year follow-up of women who elected quinacrine sterilization (QS) in Wonosobo, Central Java, Indonesia. Int J Gynaecol Obstet. 2003;83(suppl 2):S137–S139. doi: 10.1016/S0020-7292(03)90106-8. [DOI] [PubMed] [Google Scholar]
- 43.Sokal DC, Hieu do T, Loan ND, et al. Contraceptive effectiveness of two insertions of quinacrine: results from 10-year follow-up in Vietnam. Contraception. 2008;78:61–65. doi: 10.1016/j.contraception.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 44.Kessel E. Quinacrine sterilization: an assessment of risks for ectopic pregnancy, birth defects and cancer. Adv Contracept. 1998;14:81–90. doi: 10.1023/a:1006556331674. [DOI] [PubMed] [Google Scholar]
- 45.Lippes J, Brar M, Gerbracht K, et al. An FDA phase I clinical trial of quinacrine sterilization (QS) Int J Gynaecol Obstet. 2003;83(suppl 2):S45–S49. doi: 10.1016/S0020-7292(03)90089-0. [DOI] [PubMed] [Google Scholar]
- 46.Informed Consent Working Group, authors. Quinacrine sterilization (QS): informed consent. Int J Gynaecol Obstet. 2003;83(suppl 2):S147–S159. doi: 10.1016/S0020-7292(03)90108-1. [DOI] [PubMed] [Google Scholar]
- 47.Sokal DC, Hieu do T, Loan ND, et al. Safety of quinacrine contraceptive pellets: results from 10-year follow-up in Vietnam. Contraception. 2008;78:66–72. doi: 10.1016/j.contraception.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 48.Lippes J. Quinacrine sterilization: the imperative need for American clinical trials. Fertil Steril. 2002;77:1106–1109. doi: 10.1016/s0015-0282(02)03089-3. [DOI] [PubMed] [Google Scholar]


