Abstract
Vaccination against human papillomavirus (HPV) promises to dramatically decrease the incidence of HPV-related diseases, including cervical cancer. Although this vaccine is recommended by the Advisory Committee on Immunization Practices and The American College of Obstetricians and Gynecologists for all age-eligible women, challenges related to the vaccine’s high cost and the difficulty in reaching some patients for vaccination may make implementation of this recommendation difficult. As an alternative strategy, some may consider targeting HPV vaccines to specific patients based on their risk for HPV infection or HPV-related disease. This article reviews what is known about risk factors for HPV, and discusses why using risk factors as the basis for targeting HPV vaccination is unlikely to be a viable vaccination strategy.
Key words: Human papillomavirus, Risk factors, Vaccine, Vaccination strategies
In June 2006, the US Food and Drug Administration licensed the first vaccine against human papillomavirus (HPV) for use in the United States.1 This vaccine promises to dramatically reduce the incidence of HPV-related diseases such as cervical dysplasia, cervical and other anogenital cancers, and genital warts. The Advisory Committee on Immunization Practices (ACIP) recommends that all 11- to 12-year-old girls be vaccinated against HPV routinely, with comprehensive catch-up vaccination suggested for girls and women ages 13 to 26 who have not yet received the vaccine and vaccination allowable for girls as young as age 9 who are perceived to be at high risk.2 In keeping with this comprehensive approach, The American College of Obstetricians and Gynecologists (ACOG) also recommends vaccination of girls and women within the eligible age range.3
Although a comprehensive vaccination strategy would maximize the vaccine’s impact on HPV-related disease, implementation of this type of strategy may prove difficult as providers, patients, and parents of patients struggle to overcome challenges related to the vaccine’s high cost, and because of the logistical challenges of getting certain populations of patients vaccinated. Because of these obstacles, an alternative vaccination strategy that some might consider is to vaccinate only certain subpopulations of women based on their risk of HPV infection or HPV-related disease. A targeted approach such as this has been suggested by the American Cancer Society which, for women ages 19 to 26, recommends that HPV vaccination occur in the context of a discussion with the medical provider to weigh the likelihood of previous HPV exposure with the vaccine’s financial cost and the potential benefits of vaccination.4 This article reviews what is known about risk factors for HPV infection and disease, and discusses some of the pitfalls of using risk factors as a basis for targeted HPV vaccination approaches.
Risk Factors and the Cervical Oncogenesis Pathway
Cervical carcinogenesis following HPV infection is a multistep process that typically occurs over several years or even decades (Figure 1), although recent research suggests that in some women this process may be significantly accelerated.5,6 According to this multistep model, persistent infection with certain high-risk strains of HPV precedes the development of precancerous lesions, which, if left untreated, can develop into invasive cervical cancer. Progression through this pathway is limited, however. Despite more than 6.2 million new HPV infections each year in the United States,7 only a small proportion of women infected with high-risk HPV go on to develop precancerous lesions and even fewer develop invasive cervical cancer (11,070 cases of cervical cancer in the United States estimated for 2008).8 This pattern of vastly more HPV infections than HPV-related neoplasia appears to be independent of cervical cancer screening measures because it has been observed even in populations where routine cervical cancer screening is not widely available.9 This discrepancy has generated much interest in understanding the risk factors that affect the transitions between these various pathway elements. Thus, when discussing risk factors for HPV, it is useful to consider them in terms of the location(s) in the pathway they affect. However, it is important to note that because HPV vaccines must be provided before vaccine-type HPV infection occurs, any risk factor-based approaches to HPV vaccination would need to focus specifically on those factors associated with the acquisition of HPV infection, rather than downstream events.
Figure 1.
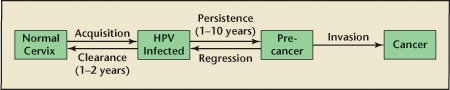
Stages in the development of cervical cancer. Acquisition of human papillomavirus (HPV) occurs via sexual contact. Most infections clear spontaneously, generally within 1 to 2 years. Persistent infections, which can last 10 or more years, lead to high-grade cervical lesions and eventually invasive cervical cancer if left untreated. Low-grade cervical lesions often regress spontaneously, but can also progress to higher grade lesions (pre-cancer) and cancer. Adapted from Burchell AN et al.20
Biologically Based Risk Factors
One useful way to categorize risk factors is based on whether they have primarily a biological or behavioral basis (Table 1). Biologically based risk factors primarily affect downstream transitions in the oncogenesis pathway, rather than risk for acquiring HPV, and include characteristics of the HPV virus itself and intrinsic host factors affecting immune response. Known or proposed host factors include immunosupression and human immunodeficiency virus infection, co-occurrence of other sexually transmitted infections such as Chlamydia trachomatis and herpes simplex virus, micronutrient deficiencies (eg, lycopene, beta-carotene, vitamins C and E, lutein), genetic polymorphisms in the human leukocyte antigen (HLA) system, and patient age.10–19 For most of these factors, it has been difficult to determine whether they affect the transition from incident to persistent infection, from persistent infection to high-grade cervical dysplasia, or both. A full description of the relationship between these various host factors and HPV is beyond the scope of this article, but more detailed information can be found in several recent reviews covering this topic.19–23
Table 1.
Risk Factors Known or Postulated to Be Associated With HPV Infection or HPV-Related Disease
| Biologically Based | Behaviorally Based |
| Host Factors | Sexual History-Related Factors |
| Immunosupression | Lifetime number of sex partners |
| HIV infection | Recent new partner |
| Coinfection with other STDs | Older sex partner |
| Micronutrient deficiencies | Oral contraceptive use |
| Genetic polymorphisms | Pattern of condom use |
| Age at exposure to HPV | Parity |
| Age at first menarche | Partner’s number of partners Marital status |
| Viral Factors | Substance Use-Related Factors |
| HPV type | Heavy alcohol use |
| Coinfection with multiple HPV types | Sex while impaired by alcohol |
| Viral load | Current or previous cigarette use |
| Current or previous illicit drug use |
HIV, human immunodeficiency virus; HPV, human papillomavirus; STD, sexually transmitted disease.
Age is an intrinsic host factor that merits extra attention because it is one of the few biologically based factors that is associated with risk for acquiring HPV infection in addition to its association with downstream events in the oncogenesis pathway. Because of this, age might be considered by providers who are attempting to target HPV vaccination based on a woman’s risk profile. Numerous studies have demonstrated an increased risk of HPV infection at younger ages—the highest prevalence of HPV occurs among adolescents and young adults between the ages of 15 and 25,20,24–26 and it is believed that more than 75% of new HPV infections occur in individuals of this age range.7 This increased risk for infection among younger women has been postulated to be related to the lack of adaptive immune responses and/or the relatively large area of cervical epithelium undergoing squamous metaplasia in this age group, which may enhance the opportunity for HPV DNA to infect the basal cell layer where it can then proliferate.27,28 It is notable that a second (though lower) peak in HPV prevalence also occurs in women over age 55 in some populations, possibly due to waning immunity, reactivation of latent infection, or birth-cohort effects.29,30
In addition to risk of HPV infection, age is also associated with risk of viral persistence. As age increases, so too does the possibility that a given high-risk HPV infection will persist. This relationship is postulated to be due to waning immune effects or characteristics of the virus itself,21,30,31 although the exact relationship between age, infection, and viral persistence is still being investigated.
Important viral factors include the specific type of HPV causing infection, whether coinfection with multiple HPVs occurs, and viral load. HPV types are generally classified as high risk or low risk depending on their association with cervical cancer. This classification represents the terminal end of the oncogenesis pathway and is mediated by upstream, HPV type-specific effects on risk for viral persistence and progression to high-grade dysplasia. HPV 16 in particular has been shown to take longer to clear and to be more frequently associated with high-grade cervical lesions and cancer in several populations than other HPV types.32–36 The effect of multiple infections on risk of infection acquisition and persistence has been less straightforward, with studies showing both an increased risk or no change in risk in these outcomes when coinfection with multiple HPV types was detected.22,35,37–39 Similarly, the role of viral load is also not clear. Increasing viral load has been shown in cross-sectional studies to correlate with an increased risk of cervical cancer, but longitudinal studies assessing patterns of viral load over time and their association with progression through the HPV oncogenesis pathway is less convincing (reviewed in Wang and Hildesheim19). Variations in the HPV detection techniques used in these studies make a direct comparison of their findings difficult, adding to the uncertainty regarding the relationship between HPV virus characteristics and cervical oncogenesis.
Behaviorally Based Risk Factors
Behaviorally based risk factors primarily affect the acquisition of HPV infection and are logical factors to consider in risk-based HPV vaccination strategies. Behaviorally based risk factors include characteristics of a woman’s sexual history (number of partners, characteristics of the partners, contraceptive use, parity) and substance use history (alcohol, cigarettes, illicit drugs). Of the sexual history characteristics, increasing number of lifetime sexual partners and having had a recent, new sexual partner are 2 factors that have been consistently shown to be associated with an increased risk of HPV infection.40–43 Having an older sexual partner (1.5–2 years older) also appears to be associated with increased infection with HPV, although fewer studies have investigated this relationship.26,41,44 The association between oral contraceptive (OCP) use and HPV has been less straightforward to assess, with some studies showing an increased association between current or long-term OCP use and HPV infection, persistence, or development of cervical lesions, whereas others do not.17,40,42,45–49 Similarly, the effect of regular condom use on preventing HPV infection or development of HPV-associated cervical lesions has also shown mixed results. A recent meta-analysis suggested that although condom use does not decrease risk of becoming infected with HPV, it may lower the chances of developing subsequent cervical neoplasia.50
The impact of substance use on risk for HPV infection and related disease has been similarly difficult to assess. Current or past cigarette smoking has been associated with acquisition of HPV infection, progression to precancerous lesions, and cervical cancer in a few studies, but most have failed to support this connection.40,42,45,51,52 Far fewer studies have assessed the role of illicit drug use and HPV infection and/or related diseases, and results of these studies also show mixed associations.26,44,53 One of the reasons these associations may be so varied is because substance use may in fact be a marker for other, unmeasured, sexual behaviors that influence risk of HPV infection.
Risk Factors and Targeted HPV Vaccination
Although much is now known about risk factors associated with HPV infection and progression through the cervical oncogenesis pathway, few studies have assessed the impact of using risk factors to target HPV vaccination to specific populations of women. Historically, risk factor-based approaches to vaccination in the United States have had mixed results. For example, risk-based approaches to hepatitis A vaccination of children were so successful that hepatitis A rates in high prevalence states where vaccination had been targeted fell below that of states with initially lower prevalence where the vaccine had not been systematically used. This eventually led the ACIP to recommend the hepatitis A vaccine for routine use in all children in all states, so that low levels of disease could be achieved nationally.54 In contrast, risk factor-based approaches to hepatitis B vaccination were largely unsuccessful. Rates of infection continued to rise several years after risk-based vaccination strategies were implemented.55 It was not until a universal strategy of routinely vaccinating all infants against hepatitis B was adopted that rates of infection began to decline dramatically.56
A recently published study attempted to evaluate the impact of using risk factors for HPV infection as a mechanism to select specific individuals for HPV vaccination.44 As in other studies, this study also found an association between current HPV infection and a variety of behaviorally based risk factors. However, when the authors estimated the population level impact of selectively vaccinating only those women with a particular behavioral risk factor (or as an alternative strategy, vaccinating only those women who did not have that risk factor), they found that a large proportion of HPV-naive women (ie, those that were not positive for HPV at the time of the study) were left unvaccinated. Taking an example from that study, the authors describe how selectively vaccinating women with a history of having an older sex partner would cause approximately 3 million women with this risk factor to be vaccinated, and approximately 1.2 million women without this risk factor to remain unvaccinated. Of the 1.2 million unvaccinated women, more than 95% (1.1 million) had no evidence of current HPV infection in the study. The impact of this latter finding is substantial because women without previous exposure to vaccine-type HPV are those with the greatest potential to benefit from HPV vaccination because the vaccine must be given prior to vaccine-type infection to be effective. Similar results were found for the other risk factors assessed, leading the authors to conclude that a woman’s risk profile lacked the sensitivity or specificity needed to accurately target women for HPV vaccination. These findings support the recommendations set forth by the ACIP and ACOG for universal HPV vaccination of all age-eligible women.2,3
In a separate, parallel study, the same authors also attempted to evaluate the relationship between behaviorally based risk factors in adolescence and subsequent risk for HPV infection as a young adult.57 The premise of this study was that current HPV vaccine recommendations focus primarily on adolescents, yet several studies had shown reluctance on the part of parents and physicians, including gynecologists, to vaccinate adolescents they believed to be at low risk for HPV.58–61 This study, which evaluated behaviorally based risk factors for sexually active and virginal adolescents separately, found essentially no association between these risk factors and future HPV infection as a sexually active young adult. Even sexual activity status during adolescence was not associated with this outcome. These results further underscore the conclusion that risk factors are not a viable mechanism to select women for HPV vaccination as risk factor assessment at an individual level does not appear to adequately target those who could most benefit from receiving the vaccine.
Conclusions
Given the ubiquity of HPV infection among young sexually active individuals, it is not all that surprising that risk factor-based strategies for HPV vaccination would be of little practical value. Accumulating evidence demonstrates that nearly all individuals who become sexually active are at substantial risk of acquiring HPV infection. Thus, defining the at-risk group under this condition is essentially impossible. Comprehensive vaccination of all age-eligible women remains the only viable alternative to ensure that the majority of women who will be exposed to HPV in their lifetime are afforded the protection provided by HPV vaccines.
Infection with HPV is, however, only the first step in the pathway to cervical cancer. Much research has been undertaken to understand the cofactors that cause an individual to progress through the various stages of the oncogenesis pathway. Despite the plethora of research, until the relationships between these factors and progression through the pathway are better understood, all women should continue to undergo routine cervical cancer screening, regardless of their HPV vaccination status.
Main Points.
Persistent infection with certain high-risk strains of HPV precedes the development of precancerous lesions, which, if left untreated, can develop into invasive cervical cancer.
A targeted approach to human papillomavirus (HPV) vaccination has been suggested by the American Cancer Society which, for women ages 19 to 26, recommends that HPV vaccination occur in the context of a discussion with the medical provider to weigh the likelihood of previous HPV exposure with the vaccine’s financial cost and the potential benefits of vaccination.
Biologically based risk factors primarily affect downstream transitions in the oncogenesis pathway, rather than risk for acquiring HPV, and include characteristics of the HPV virus itself and intrinsic host factors affecting immune response.
Known or proposed biologically based risk factors include immunosupression and human immunodeficiency virus infection, co-occurrence of other sexually transmitted infections such as Chlamydia trachomatis and herpes simplex virus, micronutrient deficiencies (eg, lycopene, beta-carotene, vitamins C and E, lutein), genetic polymorphisms in the human leukocyte antigen system, and patient age.
Behaviorally based risk factors primarily affect the acquisition of HPV infection and are logical factors to consider in risk-based HPV vaccination strategies.
Behaviorally based risk factors include characteristics of a woman’s sexual history (number of partners, characteristics of the partners, contraceptive use, parity) and substance use history (alcohol, cigarettes, illicit drugs).
Risk factor-based approaches to vaccination in the United States have had mixed results, and risk factors are not a viable mechanism to select women for HPV vaccination as risk factor assessment at an individual level does not appear to adequately target those who could most benefit from receiving the vaccine.
References
- 1. [Accessed August 1, 2008];Product approval information—licensing action, Gardasil® questions and answers. US Food and Drug Administration Web site. http://www.fda.gov/cber/products/hpvmer060806qa.htm. Updated June 8, 2006.
- 2.Markowitz LE, Dunne EF, Saraiya M, et al. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2007;56:1–24. [PubMed] [Google Scholar]
- 3.ACOG Committee Opinion No. 344: human papillomavirus vaccination. Obstet Gynecol. 2006;108:699–705. doi: 10.1097/00006250-200609000-00047. [DOI] [PubMed] [Google Scholar]
- 4.Saslow D, Castle PE, Cox JT, et al. American Cancer Society Guideline for human papillomavirus (HPV) vaccine use to prevent cervical cancer and its precursors. CA Cancer J Clin. 2007;57:7–28. doi: 10.3322/canjclin.57.1.7. [DOI] [PubMed] [Google Scholar]
- 5.Winer RL, Kiviat NB, Hughes JP, et al. Development and duration of human papillomavirus lesions, after initial infection. J Infect Dis. 2005;191:731–738. doi: 10.1086/427557. [DOI] [PubMed] [Google Scholar]
- 6.Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst. 2005;97:1072–1079. doi: 10.1093/jnci/dji187. [DOI] [PubMed] [Google Scholar]
- 7. [Accessed August 1, 2008];Genital HPV infection—CDC fact sheet. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/std/HPV/STDFact-HPV.htm. Updated April 10, 2008.
- 8. [Accessed August 1, 2008];Cervical cancer. National Cancer Institute Web site. http://www.cancer.gov/cancertopics/types/cervical.
- 9.Bosch FX, de Sanjose S. Chapter 1: human papillomavirus and cervical cancer—burden and assessment of causality. J Natl Cancer Inst Monogr. 2003:3–13. doi: 10.1093/oxfordjournals.jncimonographs.a003479. [DOI] [PubMed] [Google Scholar]
- 10.Silins I, Ryd W, Strand A, et al. Chlamydia trachomatis infection and persistence of human papillomavirus. Int J Cancer. 2005;116:110–115. doi: 10.1002/ijc.20970. [DOI] [PubMed] [Google Scholar]
- 11.Castle PE, Giuliano AR. Chapter 4: genital tract infections, cervical inflammation, and antioxidant nutrients—assessing their roles as human papillomavirus cofactors. J Natl Cancer Inst Monogr. 2003:29–34. doi: 10.1093/oxfordjournals.jncimonographs.a003478. [DOI] [PubMed] [Google Scholar]
- 12.Anttila T, Saikku P, Koskela P, et al. Serotypes of Chlamydia trachomatis and risk for development of cervical squamous cell carcinoma. JAMA. 2001;285:47–51. doi: 10.1001/jama.285.1.47. [DOI] [PubMed] [Google Scholar]
- 13.Strickler HD, Burk RD, Fazzari M, et al. Natural history and possible reactivation of human papillomavirus in human immunodeficiency virus-positive women. J Natl Cancer Inst. 2005;97:577–586. doi: 10.1093/jnci/dji073. [DOI] [PubMed] [Google Scholar]
- 14.Harris TG, Burk RD, Palefsky JM, et al. Incidence of cervical squamous intraepithelial lesions associated with HIV serostatus, CD4 cell counts, and human papillomavirus test results. JAMA. 2005;293:1471–1476. doi: 10.1001/jama.293.12.1471. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Closas R, Castellsague X, Bosch X, Gonzalez CA. The role of diet and nutrition in cervical carcinogenesis: a review of recent evidence. Int J Cancer. 2005;117:629–637. doi: 10.1002/ijc.21193. [DOI] [PubMed] [Google Scholar]
- 16.Giuliano AR, Siegel EM, Roe DJ, et al. Dietary intake and risk of persistent human papillomavirus (HPV) infection: the Ludwig-McGill HPV Natural History Study. J Infect Dis. 2003;188:1508–1516. doi: 10.1086/379197. [DOI] [PubMed] [Google Scholar]
- 17.Richardson H, Abrahamowicz M, Tellier PP, et al. Modifiable risk factors associated with clearance of type-specific cervical human papillomavirus infections in a cohort of university students. Cancer Epidemiol Biomarkers Prev. 2005;14:1149–1156. doi: 10.1158/1055-9965.EPI-04-0230. [DOI] [PubMed] [Google Scholar]
- 18.Maciag PC, Schlecht NF, Souza PS, et al. Polymorphisms of the human leukocyte antigen DRB1 and DQB1 genes and the natural history of human papillomavirus infection. J Infect Dis. 2002;186:164–172. doi: 10.1086/341080. [DOI] [PubMed] [Google Scholar]
- 19.Wang SS, Hildesheim A. Chapter 5: viral and host factors in human papillomavirus persistence and progression. J Natl Cancer Inst Monogr. 2003:35–40. doi: 10.1093/oxfordjournals.jncimonographs.a003480. [DOI] [PubMed] [Google Scholar]
- 20.Burchell AN, Winer RL, de Sanjose S, Franco EL. Chapter 6: epidemiology and transmission dynamics of genital HPV infection. Vaccine. 2006;24(suppl 3):S52–S61. doi: 10.1016/j.vaccine.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 21.Moscicki AB, Schiffman M, Kjaer S, Villa LL. Chapter 5: updating the natural history of HPV and anogenital cancer. Vaccine. 2006;24(suppl 3):S42–S51. doi: 10.1016/j.vaccine.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 22.Trottier H, Franco EL. The epidemiology of genital human papillomavirus infection. Vaccine. 2006;24(suppl 1):S1–S15. doi: 10.1016/j.vaccine.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 23.Baseman JG, Koutsky LA. The epidemiology of human papillomavirus infections. J Clin Virol. 2005;32(suppl 1):S16–S24. doi: 10.1016/j.jcv.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 24.Dunne EF, Unger ER, Sternberg M, et al. Prevalence of HPV infection among females in the United States. JAMA. 2007;297:813–819. doi: 10.1001/jama.297.8.813. [DOI] [PubMed] [Google Scholar]
- 25.Kahn JA, Lan D, Kahn RS. Sociodemographic factors associated with high-risk human papillomavirus infection. Obstet Gynecol. 2007;110:87–95. doi: 10.1097/01.AOG.0000266984.23445.9c. [DOI] [PubMed] [Google Scholar]
- 26.Manhart LE, Holmes KK, Koutsky LA, et al. Human papillomavirus infection among sexually active young women in the United States: implications for developing a vaccination strategy. Sex Transm Dis. 2006;33:502–508. doi: 10.1097/01.olq.0000204545.89516.0a. [DOI] [PubMed] [Google Scholar]
- 27.Trottier H, Franco EL. Human papillomavirus and cervical cancer: burden of illness and basis for prevention. Am J Manag Care. 2006;12(17 suppl):S462–S472. [PubMed] [Google Scholar]
- 28.Moscicki AB, Burt VG, Kanowitz S, et al. The significance of squamous metaplasia in the development of low grade squamous intraepithelial lesions in young women. Cancer. 1999;85:1139–1144. doi: 10.1002/(sici)1097-0142(19990301)85:5<1139::aid-cncr18>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 29.Herrero R, Hildesheim A, Bratti C, et al. Population-based study of human papillomavirus infection and cervical neoplasia in rural Costa Rica. J Natl Cancer Inst. 2000;92:464–474. doi: 10.1093/jnci/92.6.464. [DOI] [PubMed] [Google Scholar]
- 30.Castle PE, Schiffman M, Herrero R, et al. A prospective study of age trends in cervical human papillomavirus acquisition and persistence in Guanacaste, Costa Rica. J Infect Dis. 2005;191:1808–1816. doi: 10.1086/428779. [DOI] [PubMed] [Google Scholar]
- 31.Hildesheim A, Schiffman MH, Gravitt PE, et al. Persistence of type-specific human papillomavirus infection among cytologically normal women. J Infect Dis. 1994;169:235–240. doi: 10.1093/infdis/169.2.235. [DOI] [PubMed] [Google Scholar]
- 32.Molano M, Van den Brule A, Plummer M, et al. Determinants of clearance of human papillomavirus infections in Colombian women with normal cytology: a population-based, 5-year follow-up study. Am J Epidemiol. 2003;158:486–494. doi: 10.1093/aje/kwg171. [DOI] [PubMed] [Google Scholar]
- 33.Brisson J, Bairati I, Morin C, et al. Determinants of persistent detection of human papillomavirus DNA in the uterine cervix. J Infect Dis. 1996;173:794–799. doi: 10.1093/infdis/173.4.794. [DOI] [PubMed] [Google Scholar]
- 34.Richardson H, Kelsall G, Tellier P, et al. The natural history of type-specific human papillomavirus infections in female university students. Cancer Epidemiol Biomarkers Prev. 2003;12:485–490. [PubMed] [Google Scholar]
- 35.Liaw KL, Hildesheim A, Burk RD, et al. A prospective study of human papillomavirus (HPV) type 16 DNA detection by polymerase chain reaction and its association with acquisition and persistence of other HPV types. J Infect Dis. 2001;183:8–15. doi: 10.1086/317638. [DOI] [PubMed] [Google Scholar]
- 36.Munoz N, Bosch FX, de Sanjose S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 37.Ho GY, Bierman R, Beardsley L, et al. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. 1998;338:423–428. doi: 10.1056/NEJM199802123380703. [DOI] [PubMed] [Google Scholar]
- 38.Woodman CB, Collins S, Winter H, et al. Natural history of cervical human papillomavirus infection in young women: a longitudinal cohort study. Lancet. 2001;357:1831–1836. doi: 10.1016/S0140-6736(00)04956-4. [DOI] [PubMed] [Google Scholar]
- 39.Rousseau MC, Franco EL, Villa LL, et al. A cumulative case-control study of risk factor profiles for oncogenic and nononcogenic cervical human papillomavirus infections. Cancer Epidemiol Biomarkers Prev. 2000;9:469–476. [PubMed] [Google Scholar]
- 40.Burk RD, Ho GY, Beardsley L, et al. Sexual behavior and partner characteristics are the predominant risk factors for genital human papillomavirus infection in young women. J Infect Dis. 1996;174:679–689. doi: 10.1093/infdis/174.4.679. [DOI] [PubMed] [Google Scholar]
- 41.Tarkowski TA, Koumans EH, Sawyer M, et al. Epidemiology of human papillomavirus infection and abnormal cytologic test results in an urban adolescent population. J Infect Dis. 2004;189:46–50. doi: 10.1086/380466. [DOI] [PubMed] [Google Scholar]
- 42.Wheeler CM, Parmenter CA, Hunt WC, et al. Determinants of genital human papillomavirus infection among cytologically normal women attending the University of New Mexico student health center. Sex Transm Dis. 1993;20:286–289. doi: 10.1097/00007435-199309000-00009. [DOI] [PubMed] [Google Scholar]
- 43.Winer RL, Koutsay LA. The epidemiology of human papillomavirus infections. In: Rohan T, Shah K, editors. Cervical Cancer: From Etiology to Prevention. New York: Kluwer Academic Publishers; 2004. pp. 143–187. [Google Scholar]
- 44.Dempsey AF, Gebremariam A, Koutsky LA, Manhart L. Using risk factors to predict human papillomavirus infection: implications for targeted vaccination strategies in young adult women. Vaccine. 2008;26:1111–1117. doi: 10.1016/j.vaccine.2007.11.088. [DOI] [PubMed] [Google Scholar]
- 45.Wang SS, Schiffman M, Shields TS, et al. Seroprevalence of human papillomavirus-16, -18, -31, and -45 in a population-based cohort of 10000 women in Costa Rica. Br J Cancer. 2003;89:1248–1254. doi: 10.1038/sj.bjc.6601272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stone KM, Karem KL, Sternberg MR, et al. Seroprevalence of human papillomavirus type 16 infection in the United States. J Infect Dis. 2002;186:1396–1402. doi: 10.1086/344354. [DOI] [PubMed] [Google Scholar]
- 47.Green J, Berrington de Gonzalez A, Smith JS, et al. Human papillomavirus infection and use of oral contraceptives. Br J Cancer. 2003;88:1713–1720. doi: 10.1038/sj.bjc.6600971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Castellsague X, Munoz N. Chapter 3: cofactors in human papillomavirus carcinogenesis-role of parity, oral contraceptives, and tobacco smoking. J Natl Cancer Inst Monogr. 2003:20–28. [PubMed] [Google Scholar]
- 49.Vaccarella S, Lazcano-Ponce E, Castro-Garduno JA, et al. Prevalence and determinants of human papillomavirus infection in men attending vasectomy clinics in Mexico. Int J Cancer. 2006;119:1934–1939. doi: 10.1002/ijc.21992. [DOI] [PubMed] [Google Scholar]
- 50.Manhart LE, Koutsky LA. Do condoms prevent genital HPV infection, external genital warts, or cervical neoplasia? A meta-analysis. Sex Transm Dis. 2002;29:725–735. doi: 10.1097/00007435-200211000-00018. [DOI] [PubMed] [Google Scholar]
- 51.Winer RL, Lee SK, Hughes JP, et al. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol. 2003;157:218–226. doi: 10.1093/aje/kwf180. [DOI] [PubMed] [Google Scholar]
- 52.Syrjanen K, Shabalova I, Petrovichev N, et al. Smoking is an independent risk factor for oncogenic human papillomavirus (HPV) infections but not for high-grade CIN. Eur J Epidemiol. 2007;22:723–735. doi: 10.1007/s10654-007-9180-8. [DOI] [PubMed] [Google Scholar]
- 53.Syrjanen K, Naud P, Derchain S, et al. Drug addiction is not an independent risk factor for oncogenic human papillomavirus infections or high-grade cervical intraepithelial neoplasia: case-control study nested within the Latin American Screening study cohort. Int J STD AIDS. 2008;19:251–258. doi: 10.1258/ijsa.2007.007179. [DOI] [PubMed] [Google Scholar]
- 54.Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2006;55:1–23. [PubMed] [Google Scholar]
- 55.Alter MJ, Hadler SC, Margolis HS, et al. The changing epidemiology of hepatitis B in the United States. Need for alternative vaccination strategies. JAMA. 1990;263:1218–1222. [PubMed] [Google Scholar]
- 56.Wasley A, Grytdal S, Gallagher K. Surveillance for acute viral hepatitis-United States, 2006. MMWR Surveill Summ. 2008;57:1–24. [PubMed] [Google Scholar]
- 57.Dempsey AF, Gebremariam A, Koutsky L, Manhart L. Behavior in early adolescence and risk of human papillomavirus infection as a young adult: results from a population-based study. Pediatrics. 2008;122:1–7. doi: 10.1542/peds.2007-2515. [DOI] [PubMed] [Google Scholar]
- 58.Kahn JA, Rosenthal SL, Tissot AM, et al. Factors influencing pediatricians’ intention to recommend human papillomavirus vaccines. Ambul Pediatr. 2007;7:367–373. doi: 10.1016/j.ambp.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 59.Raley JC, Followwill KA, Zimet GD, Ault KA. Gynecologists’ attitudes regarding human papilloma virus vaccination: a survey of Fellows of the American College of Obstetricians and Gynecologists. Infect Dis Obstet Gynecol. 2004;12:127–133. doi: 10.1080/10647440400020661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mays RM, Sturm LA, Zimet GD. Parental perspectives on vaccinating children against sexually transmitted infections. Soc Sci Med. 2004;58:1405–1413. doi: 10.1016/S0277-9536(03)00335-6. [DOI] [PubMed] [Google Scholar]
- 61.Dempsey AF, Zimet GD, Davis RL, Koutsky L. Factors that are associated with parental acceptance of human papillomavirus vaccines: a randomized intervention study of written information about HPV. Pediatrics. 2006;117:1486–1493. doi: 10.1542/peds.2005-1381. [DOI] [PubMed] [Google Scholar]


