Abstract
Endometrial carcinoma affects over 40,000 American women annually, making it the most common gynecologic malignancy. Over 80% of disease is diagnosed in the early stages, resulting in a generally favorable prognosis for most patients. However, discrepancies still exist with regard to primary surgical management and postoperative adjuvant therapies directed at reducing recurrence rates and improving survival. In this review, we outline the surgical management of newly diagnosed disease and review the risk factors that guide clinicians in the recommendation for postoperative adjuvant therapy.
Key words: Endometrial cancer, Hysterectomy, Lymphadenectomy, Radiation, Chemotherapy
Endometrial cancer is the most common gynecologic malignancy affecting American women and the fourth most common cancer overall. According to the American Cancer Society, 40,100 women will be diagnosed with endometrial carcinoma in 2008.1 Unlike many malignancies, this cancer has a bellwether: postmenopausal bleeding. Endometrial sampling via office Pipelle procedure or operative curettage can lead to early diagnosis and a more favorable prognosis. Survival data suggest that roughly 85% to 90% of all women will be alive at 5 years (Figure 1).2
Figure 1.
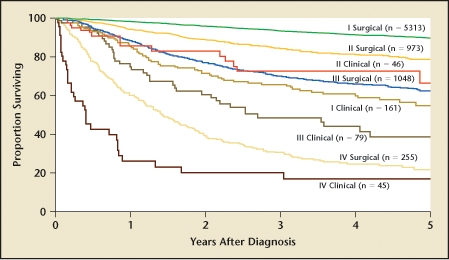
Carcinoma of the corpus uteri: patients treated in 1999–2001. Survival by mode of staging, N = 7920. Reprinted with permission from Creasman WT et al.2
After the diagnosis of endometrial cancer has been confirmed, hormonal manipulation may be considered in the reproductive aged patient who wishes fertility preservation. However, these cases are the exception and must be followed under close surveillance. Standard treatment almost universally begins with a total hysterectomy via any of a number of approaches—abdominal, vaginal, or minimally invasive. Comprehensive staging, including pelvic and para-aortic lymph node assessment, is crucial in guiding postoperative adjuvant treatment recommendations.
Adjuvant postoperative treatment recommendations in advanced stage disease are widely disparate and an area of active research. However, assuming an adequate performance status, virtually all women with advanced stage disease (stage III and IV) will be recommended for chemotherapy, external beam pelvic radiotherapy with or without an extended para-aortic field, or some combination of both modalities. These treatments are geared at improving disease-free and overall survival in a population in whom overall survival remains disappointing—as low as 20% in stage IV disease.2
Women with early stage disease present a clinical conundrum. Given the generally favorable prognosis overall, adjuvant treatment recommendations remain challenging. Minimal improvements in survival are difficult to demonstrate in large, randomized, controlled trials. Many variables weigh into a clinician’s decision on how to proceed surgically and whether to recommend adjuvant treatment in early stage disease, as well as the type of treatment recommended. In this review, we focus on primary surgical management and the outcome variables that may lead to the recommendation of adjuvant postoperative treatment.
Preoperative Risk Assessment
Numerous studies, both retrospectively and prospectively, have identified risk factors associated with metastatic spread of disease. One of the most reliable risk factors is tumor histology. Endometrioid histology is present in up to 80% of endometrial cancers and represents the natural progression of atypical complex hyperplasia due to estrogen imbalance. These type 1 tumors are thought to have slower growth and, anecdotally, their distribution is more commonly along lymphatic channels. Endometrioid tumors are graded on a scale of 1 to 3 based on the amount of solid growth and nuclear atypia, with lower grade tumors recapitulating native endometrium. High-grade tumors are associated with higher rates of deep myometrial invasion and lymphatic spread.3
Type 2 papillary serous and clear cell histologies represent a more aggressive malignancy. They arise in atrophic endometrium, and are frequently associated with mutations in the p53 tumor suppressor gene.4 Uterine papillary serous endometrial cancers behave in the same way as classic ovarian serous tumors, with predominantly peritoneal spread. As a result, type 2 tumors are thought to be high-risk histologies and postoperative management reflects the need for more aggressive therapy. The presence of these histologies on preoperative endometrial sampling warrants classifying these patients as high risk.5
Myometrial invasion is another important prognostic indicator and was incorporated into the surgical staging criteria adopted by the International Federation of Gynecology and Obstetrics (FIGO) in 1988 (Figure 2). Although some reports have stratified depth in thirds, most organizations (FIGO included) measure inner and outer halves. Regardless of which criteria utilized, it remains clear that the deeper the invasion, the greater the risk of lymphatic or metastatic spread.6,7
Figure 2.
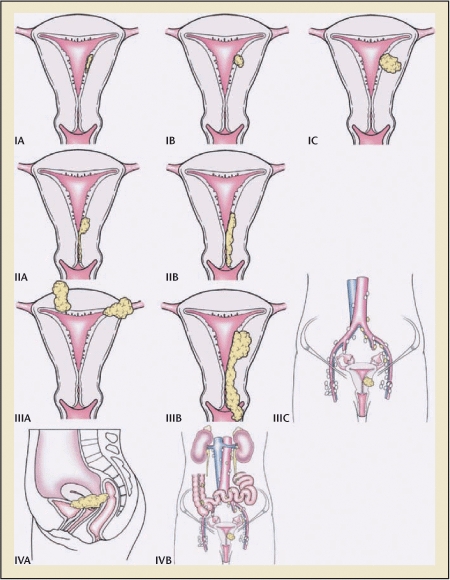
Carcinoma of the corpus uteri. Staging uterine cancer. Primary tumor and metastases (International Federation of Gynecology and Obstetrics). Reprinted with permission from Creasman WT et al.2
Some reports have attempted to incorporate the size of the primary tumor in its maximal dimension into risk stratification. The theory is that the larger the tumor, the greater the likelihood of lymphatic spread. The conventional threshold is a measure of 2 cm.8 Extending this concept further, some have attempted to quantify 3-dimensional tumor volumes and correlate this to risk of metastatic spread and survival.9
These risk factors can all be ascertained with the combined use of preoperative endometrial sampling and intraoperative frozen section analysis of the removed uterus. Intraoperative pathologic assessment of tumor grade and depth of invasion is accurate in 70% to 90% of cases.10,11 Some believe that these risk factors are enough to guide the intraoperative decision to perform or withhold pelvic and para-aortic lymphadenectomy, whereas others debate this contention and would recommend lymphadenectomy in all women with endometrial cancer.12–16
Surgical Management
Primary Disease
Conventional therapy for most endometrial cancer begins with a hysterectomy. In 1988, FIGO adopted a surgical staging technique for the classification of endometrial cancer. Surgical staging includes removal of the uterus, fallopian tubes, and ovaries; peritoneal cytology; and intraoperative assessment of the pelvic and para-aortic lymph nodes. After 20 years, this remains the standard of care for the vast majority of women with endometrial cancer. Surgical staging allows for full assessment of the abdominopelvic cavity, including visualization and palpation of tissues, collection of peritoneal cytology, and removal of the pelvic and para-aortic lymph nodes. Moreover, the surgeon can readily collect biopsies of suspicious-appearing areas, such as peritoneal implants, or the omentum.
In the past 10 years, surgical advances in minimally invasive surgery have provided new options in the treatment of endometrial cancer. Beginning with traditional laparoscopy and continuing with robotically assisted laparoscopy, the surgeon may be able to achieve the goal of a comprehensive surgical staging, including lymphadenectomy, with reduced surgical morbidity. Studies have demonstrated comparable perioperative complication rates with the laparoscopic surgical approach, as well as comparable lymph node counts. Long-term survival data are incomplete from a multi-institutional study comparing laparoscopy to open surgical staging. However, preliminary data supports the premise that laparoscopy is equivalent as described above.17 Robotically assisted laparoscopy may bring improvements to standard laparoscopy and further improve the quality of surgical care.
Various modifications have been proposed to this standard approach. For example, in select patients, a vaginal hysterectomy may be preferred. This surgical technique fails to account for the possibility of lymph node or peritoneal metastases. But when combined with modern imaging modalities, the clinician may believe that the risk of occult metastases is low enough to warrant consideration of a vaginal approach. This might affect the very elderly patient, patients with multiple medical morbidities, particularly cardiopulmonary disease, or the morbidly obese, whereby a vaginal approach would obviate the need for postoperative abdominal surgical wound healing. Several retrospective studies have demonstrated that in carefully selected patients, the overall cancer mortality is comparable with a standard abdominal approach.18–20
Finally, there may be a role for radical hysterectomy, with associated staging, in select patients with early stage endometrial cancer. Originally the radical hysterectomy was the preferred treatment for endometrial cancer cases. Over time, clinicians recognized that the increase in associated morbidity failed to achieve any improvement in survival. Today, the radical hysterectomy remains a reasonable option for patients with known cervical involvement of a primary endometrial lesion. This approach allows for complete excision of the tumor with a goal of negative surgical margins, and, ideally, precludes the need for postoperative radiation to the parametrial tissues in cases with cervical involvement.21–22 A final consideration in the patient with known cervical disease is preoperative pelvic radiation with intracavitary brachytherapy. This is particularly advantageous in the patient with bulky cervical disease that may not be amenable to gross resection. In this manner, the parametrium and pelvic lymph nodes are ideally sterilized, allowing an extrafascial hysterectomy to complete therapy.
Assessment of Metastatic Disease
The role of surgical lymphadenectomy has been continuously debated since the FIGO staging criteria were adopted. Assessment of the lymph node-bearing tissue has been interpreted by some to include lymph node inspection and/or palpation with selective biopsies of suspicious areas. Data suggest that this technique is of limited value as microscopic metastases can be frequently missed by inspection/palpation.23 These micrometastases also tend to be below the detectable limits of conventional imaging modalities such as computed tomography and magnetic resonance imaging. The role of positron emission tomography imaging in staging endometrial cancer remains unclear. Specifically identifying which patients benefit from lymphadenectomy represents a unique challenge.
Advances and breakthroughs in medicine have enabled surgeons to perform lymphadenectomies with minimal morbidity and increasing ease. Advances in modern anesthesia and surgical technology have reduced the risk of perioperative morbidity and mortality associated with lymphadenectomy.16,24,25 These developments, combined with the obvious mortality associated with untreated metastatic disease, have led most surgeons to advocate a comprehensive staging pelvic and para-aortic lymphadenectomy in the majority of patients with endometrial cancer. Furthermore, because 15% to 20% of women harbor metastatic disease in the para-aortic region alone, the need for complete lymphadenectomy cannot be underemphasized.3,26,27 Disparity exists on what constitutes a complete lymphadenectomy. Measuring nodal counts has an inherent variability from institution to institution, and may even vary from surgeon to surgeon and from 1 pathologist to the next within a single institution. It is generally agreed that the higher the nodal count, the more reliable the data, particularly as they relate to a negative lymphadenectomy, with 1 series suggesting an adequate nodal yield exceeds 21 nodes.28 Some studie have suggested a therapeutic benefit to lymphadenectomy beyond the diagnostic information it may provide.29
Acknowledging the lack of an established survival advantage derived from lymphadenectomy in low-risk patients, some gynecologic oncologists favor intraoperative triage based on preoperative and intraoperative risk factors.14 In this surgical approach, the tumor histology, grade, and depth of invasion are ascertained by frozen section pathology and used to guide a surgeon’s decision to proceed with lymphadenectomy (Table 1). Thus, in the lowest risk patients, the surgeon may forgo lymphadenectomy based on the low statistical risk of finding metastatic disease. At the time of publication (September 2008), there is no clearly adopted consensus on which low-risk patients benefit from lymphadenectomy.
Table 1.
Histologic Grade and Depth of Invasion
| Grade | ||||
| Depth | Gl(%) | G2(%) | G3(%) | Total(%) |
| Endometrium only | 44 (24) | 31 (11) | 11 (7) | 86 (14) |
| Superficial | 96 (53) | 131 (45) | 54 (35) | 281 (45) |
| Middle | 22 (12) | 69 (24) | 24 (16) | 115 (19) |
| Deep | 18 (10) | 57 (20) | 64 (42) | 139 (22) |
| Total | 180 (100) | 288 (100) | 153 (100) | 621 (100) |
Reprinted with permission from Creasman WT et al.3
The final surgical variable that must be considered is the role of surgical cytoreduction or debulking. This treatment is widely accepted in the management of serous ovarian cancer and has some traction with regard to management of endometrial cancer. The concept suggests that reducing large bulky disease to small volume might improve the efficacy of adjuvant therapy, either chemotherapy or radiation. In uterine papillary serous carcinoma, the paradigm seems reasonable in that the histology and distribution of disease are similar to serous ovarian cancer and both diseases tend to be treated comparably with chemotherapy.30,31 For endometrioid histology, there is less support based on biology. However, known limitations of radiation on bulky disease suggests that a survival advantage with debulking surgery for all histologies may exist, with some data to support this premise.30,32 As always, with radical surgical debulking, patient morbidity must be carefully weighed against any survival advantage.
Postoperative Risk Assessment
Risk stratification represents a guideline for a clinician’s postoperative decision regarding adjuvant therapies. Given the morbidity associated with adjuvant therapy and the associated health care expenditures, we are continuously looking to identify precisely which patients might benefit from these therapies. In light of this dilemma, additional risk factors may be identified postoperatively after thorough comprehensive pathologic specimen inspection.
The crux of any clinical algorithm hinges on the lymphadenectomy. In patients who have undergone a comprehensive staging procedure with confirmed extrauterine disease, adjuvant therapy will undoubtedly be recommended. In those with a thorough and negative evaluation of lymph node tissues, adjuvant treatment recommendations can be tailored towards the goal of preventing local recurrence. However, in those patients who have not under gone a full staging procedure with lymphadenectomy, postoperative adjuvant treatment recommendations present a conundrum.
In completely staged, node-negative patients, the risk of distant disease is minimal. However, in the unstaged patient, where the lymph node status is unknown and there is no gross evidence of metastatic disease, risk factors must be weighed more carefully against the morbidity of potential adjuvant therapies. The clinician must extrapolate the risk factors and postulate the likelihood of possible lymphatic spread. In the past, the lack of lymph node data would frequently lower the clinician’s threshold to recommend pelvic external beam therapy to sterilize the lymph node beds. These guidelines were based on large cooperative studies that have demonstrated over 10% of clinical stage I disease is associated with positive occult lymphatic spread.3 This radiation treatment schema theoretically would address the issue of occult metastatic disease in patients with known risk factors, such as high-grade histology and deep myoinvasion.
More recently, greater consideration is being given to a second surgical procedure for completion of lymphadenectomy and staging, typically by a gynecologic oncologist, especially in patients possessing 1 or more pathologic risk factors. Laparoscopic advances have increased the feasibility of this option and decreased the associated morbidity of the second procedure. Performing this additional procedure provides extra diagnostic information and may increase the confidence in postoperative treatment recommendations. Careful consideration of all known risk factors must guide clinicians in proceeding with the second surgical procedure, as surgical lymphadenectomy is not without risk. In the lowest risk unstaged patients, the likelihood of occult disease may be lower than the complication rates associated with either the procedure or potential adjuvant therapies. It is becoming increasingly clear that pelvic radiation is not an acceptable surrogate for comprehensive staging lymphadenectomy.
In attempts to narrow the range of patients receiving adjuvant therapy, clinicians continue to identify additional risk factors and use them to tailor treatment recommendations based on risk. These risk factors are most useful in 2 selected subgroups of patients: those with a complete negative pathologic evaluation of the lymph nodes or those who did not undergo lymphadenectomy. Conceptually, it is believed that lymphovascular space invasion (LVSI) identified on the uterine specimen represents a surrogate for distant lymphatic metastases. Specimen processing artifacts might mitigate this interpretation slightly, but the basic premise generally holds true. In a patient with positive lymph nodes, the significance of LVSI is moot. However, in the completely staged patient with negative nodes, LVSI may represent an increased risk of occult distant disease that was not identified during lymphadenectomy.
Lower uterine segment disease and occult cervical disease have been separately postulated to increase the risk of distant spread. The underlying biologic concept behind these variables is that tumor in the lower uterine segment and/or cervix is more prone to lymphatic spread via parametrial lymphatics when compared with exophytic fundal tumors.33,34 This theory has been contested, particularly with attempts at sentinel lymph node mapping and lymphoscintilliographic studies that have demonstrated tumor migration through collaterals along the fallopian tube and out the infundibulopelvic ligament, directly into the para-aortic chains.35 However, most clinicians would accept that disease in the cervix and/or lower uterine segment is associated with increased risk of lymphatic spread.
One final postoperative risk factor is pelvic cytology. Staging protocols dictate intraoperative collection of cytology from the pelvic cavity. Interpretation of this information is a challenge in its own right. In patients with known metastatic disease documented by peritoneal biopsies or positive lymph nodes, cytology adds little to clinical decision making. However, in unstaged patients, or patients with negative staging, positive cytology represents an additional risk factor for metastatic disease. Independently, its prognostic significance is unclear.36–38 Furthermore, in some cases positive cytology may potentially represent an artifact. Although cytology is typically collected at the beginning of a case, before intraoperative uterine manipulation occurs, positive washings may be the contaminated product of preoperative diagnostic procedures such as dilation and curettage, particularly those accompanied by hysteroscopy, that may extravasate malignant cells into the abdominopelvic cavity.
Postoperative Adjuvant Treatment
After surgery, interpreting the associated pathologic data and the associated risk of metastatic disease presents the most critical decision in management. Clinicians have 3 potential tools in their armamentarium for adjuvant treatment: systemic chemotherapy, external beam pelvic radiotherapy, or vaginal brachytherapy. Each treatment modality carries associated morbidities and, above all else, patients’ performance status must be considered and their autonomy must be respected. Chemotherapy is typically platinum based and although generally well tolerated, it can be complicated by myelosuppression, nausea, and neuropathy among several other potential acute or chronic toxicities. Current multi-institutional studies are investigating which chemotherapeutic regimens achieve the best survival with the least morbidity. Several retrospective reports have demonstrated the utility of cytotoxic chemotherapy for uterine papillary serous carcinomas of any stage.5,39,40 Patients with advanced endometrioid endometrial cancer, especially when intraperitoneal disease is present, may also benefit from systemic chemotherapy. In the presence of lymphatically disseminated advanced disease, pelvic radiation, with or without an extended field to the para-aortic region, is often considered. However, even in this patient population, chemotherapy is gaining traction as an upfront therapy.41–44 In the absence of high-risk histology or distant disease, the clinician must then assess the information available regarding the individual patient and consider the utility of the aforementioned therapies or vaginal brachytherapy. Vaginal brachytherapy has not been shown to improve survival in early stage, low-risk disease; however, it does reduce local recurrence rates and it is well tolerated with very little morbidity.45–47
Patterns of care studies have demonstrated a wide range of variability in postoperative treatment recommendations for patients with early stage disease.48,49 The early stage, low-risk patient population is heterogeneous and there are no clear studies to guide recommendations. One approach is to consider the total number of risk factors that are positive. For example, in a patient with only 1 risk factor such as positive LVSI or minimal myoinvasion or grade 2 histology, treatment might be withheld. However, if a patient has deep myometrial invasion and a high-grade histology and a tumor larger than 2 cm, adjuvant vaginal brachytherapy would almost always be recommended. Recent data suggest advanced age may also be an independent risk factor and should be considered in this clinical decision making.50
Conclusions
Despite the many medical advances of the past 20 years, the incidence of endometrial cancer remains on the rise. The preponderance of new cases will continue to be low-risk patients—patients without metastatic disease. It is extremely unlikely that prospective level I evidence will emerge to guide clinicians in their treatment recommendations either intraoperatively or postoperatively. Survival is already quite high in this population and clinical trials would require the enrollment of thousands of patients and prohibitive costs to demonstrate small differences in outcome. As a result, clinicians will continue to rely on the combination of preoperative and intraoperative risk factors to guide the decision to perform surgical lymphadenectomy. These data can then be combined with postoperative pathologic data to guide adjuvant treatment recommendations. Universal guidelines continue to remain elusive and the proverbial patient-tailored approach will often require clinicians to make recommendations on a case-by-case basis (Figure 3).
Figure 3.
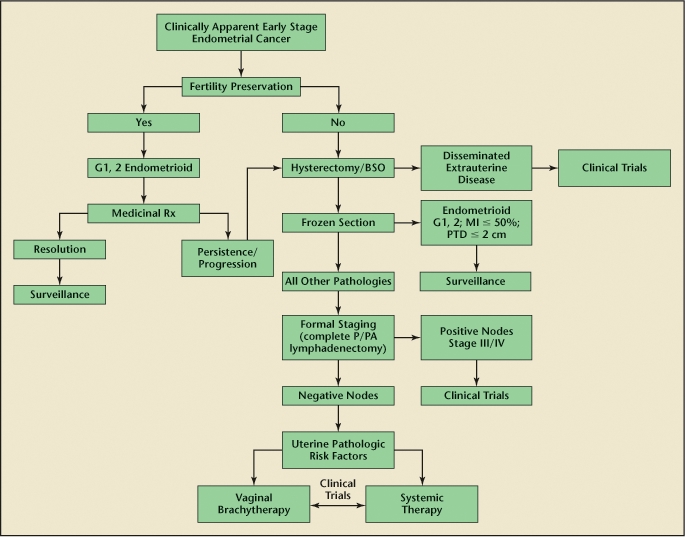
Treatment algorithm for early stage endometrical cancer. BSO, bilateral salpingo-oophorectomy; G, grade; MI, myometrial invasion; P/PA, pelvic/para-aortic; PTD, primary tumor diameter; Rx, prescription. Reprinted with permission from Greven KM, Podratz KC. Management of early-stage endometrial cancer. In: Gershenson D, McGuire W, Gore M, eds. Gynecologic Cancer: Controversies in Management. Philadelphia: Elsevier; 2004:259–273.
It remains unclear what future treatments hold promise. A large multi-institutional protocol is underway to assess competing chemotherapeutic regimens in advanced stage and recurrent disease. The optimal use of radiation remains unclear, and developments such as intensity modulated radiation therapy may help increase the ability to deliver dose with reduced morbidity. Hormones remain a well-tolerated option in selected cases and ongoing studies might better identify which patients benefit from them most. Lastly, new biologic agents are constantly under development. Anti-vascular endothelial growth factor antibodies, such as bevacizumab, are being utilized in several tumor types and will likely surface in endometrial protocols in the near future. As we better understand the molecular and genetic pathways responsible for endometrial cancer involving DNA mismatch repair genes, it seems plausible that individually tailored therapy might become more than a fantasy and our collective efforts might shift toward prevention.
Main Points.
Endometrial cancer is the most common gynecologic malignancy affecting American women and the fourth most common cancer overall.
Virtually all women with advanced stage disease will be recommended for chemotherapy, external beam pelvic radiotherapy with or without an extended para-aortic field, or some combination of both modalities. However, women with early stage disease present a clinical conundrum.
Numerous studies have identified risk factors associated with metastatic spread of disease: tumor histology, myometrial invasion, and the size of the primary tumor.
Conventional therapy for most endometrial cancer begins with a hysterectomy. Surgical staging allows for full assessment of the abdominopelvic cavity, including visualization and palpation of tissues, collection of peritoneal cytology, and removal of the pelvic and para-aortic lymph nodes.
In patients who have undergone a comprehensive staging procedure with confirmed extrauterine disease, adjuvant therapy is recommended.
In the unstaged patient, where the lymph nodes status is unknown and there is no gross evidence of metastatic disease, risk factors must be weighed more carefully against the morbidity of potential adjuvant therapies.
Interpreting the pathologic data and risk of metastatic disease presents the most critical decision in management.
Promising future treatments include intensity modulated radiation therapy, chemotherapy, hormones, and new biologic agents.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Creasman WT, Odicino F, Maisonneuve P, et al. Carcinoma of the corpus uteri. FIGO 6th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006;95(suppl 1):S105–S143. doi: 10.1016/S0020-7292(06)60031-3. [DOI] [PubMed] [Google Scholar]
- 3.Creasman WT, Morrow CP, Bundy BN, et al. Surgical pathologic spread patterns of endometrial cancer. A Gynecologic Oncology Group Study. Cancer. 1987;60(8 suppl):2035–2041. doi: 10.1002/1097-0142(19901015)60:8+<2035::aid-cncr2820601515>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 4.Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15:10–17. doi: 10.1016/0090-8258(83)90111-7. [DOI] [PubMed] [Google Scholar]
- 5.Havrilesky LJ, Secord AA, Bae-Jump V, et al. Outcomes in surgical stage I uterine papillary serous carcinoma. Gynecol Oncol. 2007;105:677–682. doi: 10.1016/j.ygyno.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 6.Frumovitz M, Singh DK, Meyer L, et al. Predictors of final histology in patients with endometrial cancer. Gynecol Oncol. 2004;95:463–468. doi: 10.1016/j.ygyno.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 7.Larson DM, Connor GP, Broste SK, et al. Prognostic significance of gross myometrial invasion with endometrial cancer. Obstet Gynecol. 1996;88:394–398. doi: 10.1016/0029-7844(96)00161-5. [DOI] [PubMed] [Google Scholar]
- 8.Mariani A, Webb MJ, Keeney GL, et al. Surgical stage I endometrial cancer: predictors of distant failure and death. Gynecol Oncol. 2002;87:274–280. doi: 10.1006/gyno.2002.6836. [DOI] [PubMed] [Google Scholar]
- 9.Shah C, Johnson EB, Everett E, et al. Does size matter? Tumor size and morphology as predictors of nodal status and recurrence in endometrial cancer. Gynecol Oncol. 2005;99:564–570. doi: 10.1016/j.ygyno.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 10.Case AS, Rocconi RP, Straughn JM, Jr, et al. A prospective blinded evaluation of the accuracy of frozen section for the surgical management of endometrial cancer. Obstet Gynecol. 2006;108:1375–1379. doi: 10.1097/01.AOG.0000245444.14015.00. [DOI] [PubMed] [Google Scholar]
- 11.Frumovitz M, Slomovitz BM, Singh DK, et al. Frozen section analyses as predictors of lymphatic spread in patients with early-stage uterine cancer. J Am Coll Surg. 2004;199:388–393. doi: 10.1016/j.jamcollsurg.2004.05.258. [DOI] [PubMed] [Google Scholar]
- 12.Aalders JG, Thomas G. Endometrial cancer-revisiting the importance of pelvic and para aortic lymph nodes. Gynecol Oncol. 2007;104:222–231. doi: 10.1016/j.ygyno.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 13.Chalas E. Endometrial cancer: what is a clinician to do? Obstet Gynecol. 2007;110:1222–1223. doi: 10.1097/01.AOG.0000296093.27006.e5. [DOI] [PubMed] [Google Scholar]
- 14.Mariani A, Dowdy SC, Cliby WA, et al. Prospective assessment of lymphatic dissemination in endometrial cancer: a paradigm shift in surgical staging. Gynecol Oncol. 2008;109:11–18. doi: 10.1016/j.ygyno.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orr JW. Surgical staging of endometrial cancer: does the patient benefit? Gynecol Oncol. 1998;71:335–339. doi: 10.1006/gyno.1998.5296. [DOI] [PubMed] [Google Scholar]
- 16.Orr JW , Jr, Taylor PT., Jr Surgical management of endometrial cancer: how much is enough? Gynecol Oncol. 2008;109:1–3. doi: 10.1016/j.ygyno.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Walker JL, Piedmonte M, Spiros N, et al. Phase III trial of laparoscopy versus laparotomy for surgical resection and comprehensive surgical staging of uterine cancer: a Gynecologic Oncology Group study funded by the National Cancer Institute. Gynecol Oncol. 2006;101(suppl 1):S11–S12. [Google Scholar]
- 18.Bloss JD, Berman ML, Bloss LP, Buller RE. Use of vaginal hysterectomy for the management of stage I endometrial cancer in the medically compromised patient. Gynecol Oncol. 1991;40:74–77. doi: 10.1016/0090-8258(91)90089-n. [DOI] [PubMed] [Google Scholar]
- 19.Peters WA , III, Andersen WA, Thornton WN , Jr, Morley GW. The selective use of vaginal hysterectomy in the management of adenocarcinoma of the endometrium. Am J Obstet Gynecol. 1983;146:285–289. doi: 10.1016/0002-9378(83)90750-0. [DOI] [PubMed] [Google Scholar]
- 20.Susini T, Massi G, Amunni G, et al. Vaginal hysterectomy and abdominal hysterectomy for treatment of endometrial cancer in the elderly. Gynecol Oncol. 2005;96:362–367. doi: 10.1016/j.ygyno.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Cohn DE, Woeste EM, Cacchio S, et al. Clinical and pathologic correlates in surgical stage II endometrial carcinoma. Obstet Gynecol. 2007;109:1062–1067. doi: 10.1097/01.AOG.0000260871.87607.25. [DOI] [PubMed] [Google Scholar]
- 22.Mariani A, Webb MJ, Keeney GL, et al. Role of wide/radical hysterectomy and pelvic lymph node dissection in endometrial cancer with cervical involvement. Gynecol Oncol. 2001;83:72–80. doi: 10.1006/gyno.2001.6346. [DOI] [PubMed] [Google Scholar]
- 23.Arango HA, Hoffman MS, Roberts WS, et al. Accuracy of lymph node palpation to determine need for lymphadenectomy in gynecologic malignancies. Obstet Gynecol. 2000;95:553–556. doi: 10.1016/s0029-7844(99)00607-9. [DOI] [PubMed] [Google Scholar]
- 24.Larson DM, Johnson K, Olson KA. Pelvic and para-aortic lymphadenectomy for surgical staging of endometrial cancer: morbidity and mortality. Obstet Gynecol. 1992;79:998–1001. [PubMed] [Google Scholar]
- 25.Fanning J. Long-term survival of intermediate risk endometrial cancer (stage IG3, IC, II) treated with full lymphadenectomy and brachytherapy without teletherapy. Gynecol Oncol. 2001;82:371–374. doi: 10.1006/gyno.2001.6276. [DOI] [PubMed] [Google Scholar]
- 26.McMeekin DS, Lashbrook D, Gold M, et al. Analysis of FIGO stage IIIc endometrial cancer patients. Gynecol Oncol. 2001;81:273–278. doi: 10.1006/gyno.2001.6157. [DOI] [PubMed] [Google Scholar]
- 27.Morrow CP, Bundy BN, Kurman RJ, et al. Relationship between surgical-pathological risk factors and outcome in clinical stage I and II carcinoma of the endometrium: a Gynecologic Oncology Group study. Gynecol Oncol. 1991;40:55–65. doi: 10.1016/0090-8258(91)90086-k. [DOI] [PubMed] [Google Scholar]
- 28.Chan JK, Urban R, Cheung MK, et al. Lymphadenectomy in endometrioid uterine cancer staging: how many lymph nodes are enough? A study of 11,443 patients. Cancer. 2007;109:2454–2460. doi: 10.1002/cncr.22727. [DOI] [PubMed] [Google Scholar]
- 29.Lutman CV, Havrilesky LJ, Cragun JM, et al. Pelvic lymph node count is an important prognostic variable for FIGO stage I and II endometrial carcinoma with high-risk histology. Gynecol Oncol. 2006;102:92–97. doi: 10.1016/j.ygyno.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 30.Bristow RE, Zahurak ML, Alexander CJ, et al. FIGO stage IIIC endometrial carcinoma: resection of macroscopic nodal disease and other determinants of survival. Int J Gynecol Cancer. 2003;13:664–672. doi: 10.1046/j.1525-1438.2003.13385.x. [DOI] [PubMed] [Google Scholar]
- 31.Memarzadeh S, Holschneider CH, Bristow RE, et al. FIGO stage III and IV uterine papillary serous carcinoma: impact of residual disease on survival. Int J Gynecol Cancer. 2002;12:454–458. doi: 10.1046/j.1525-1438.2002.01149.x. [DOI] [PubMed] [Google Scholar]
- 32.Lambrou NC, Gomez-Marin O, Mirhashemi R, et al. Optimal surgical cytoreduction in patients with stage III and stage IV endometrial carcinoma: a study of morbidity and survival. Gynecol Oncol. 2004;93:653–658. doi: 10.1016/j.ygyno.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 33.Dilek S, Dede M, Gezginc K, et al. Does the localisation of tumour at stage I endometrial endometrioid adenocarcinoma have an impact on invasion of the tumour and individualisation of the surgical procedure? Eur J Gynaecol Oncol. 2008;29:138–140. [PubMed] [Google Scholar]
- 34.Madom LM, Brown AK, Lui F, et al. Lower uterine segment involvement as a predictor for lymph node spread in endometrial carcinoma. Gynecol Oncol. 2007;107:75–78. doi: 10.1016/j.ygyno.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 35.Frumovitz M, Bodurka DC, Broaddus RR, et al. Lymphatic mapping and sentinel node biopsy in women with high-risk endometrial cancer. Gynecol Oncol. 2007;104:100–103. doi: 10.1016/j.ygyno.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 36.Lee CM, Slomovitz BM, Greer M, et al. Practice patterns of SGO members for stage IIIA endometrial cancer. Gynecol Oncol. 2005;98:77–83. doi: 10.1016/j.ygyno.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 37.Slomovitz BM, Ramondetta LM, Lee CM, et al. Heterogeneity of stage IIIA endometrial carcinomas: implications for adjuvant therapy. Int J Gynecol Cancer. 2005;15:510–516. doi: 10.1111/j.1525-1438.2005.15317.x. [DOI] [PubMed] [Google Scholar]
- 38.Havrilesky LJ, Cragun JM, Calingaert B, et al. The prognostic significance of positive peritoneal cytology and adnexal/serosal metastasis in stage IIIA endometrial cancer. Gynecol Oncol. 2007;104:401–405. doi: 10.1016/j.ygyno.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 39.Huh WK, Powell M, Leath CA, III, et al. Uterine papillary serous carcinoma: comparisons of outcomes in surgical stage I patients with and without adjuvant therapy. Gynecol Oncol. 2003;91:470–475. doi: 10.1016/j.ygyno.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 40.Kelly MG, O’Malley DM, Hui P, et al. Improved survival in surgical stage I patients with uterine papillary serous carcinoma (UPSC) treated with adjuvant platinum-based chemotherapy. Gynecol Oncol. 2005;98:353–359. doi: 10.1016/j.ygyno.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 41.Alvarez SA, Havrilesky LJ, Bae-Jump V, et al. The role of multi-modality adjuvant chemotherapy and radiation in women with advanced stage endometrial cancer. Gynecol Oncol. 2007;107:285–291. doi: 10.1016/j.ygyno.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 42.Arimoto T, Nakagawa S, Yasugi T, et al. Treatment with paclitaxel plus carboplatin, alone or with irradiation, of advanced or recurrent endometrial carcinoma. Gynecol Oncol. 2007;104:32–35. doi: 10.1016/j.ygyno.2006.07.038. [DOI] [PubMed] [Google Scholar]
- 43.Michener CM, Peterson G, Kulp B, et al. Carboplatin plus paclitaxel in the treatment of advanced or recurrent endometrial carcinoma. J Cancer Res Clin Oncol. 2005;131:581–584. doi: 10.1007/s00432-005-0676-x. [DOI] [PubMed] [Google Scholar]
- 44.Fleming GF, Filiaci VL, Bentley RC, et al. Phase III randomized trial of doxorubicin + cisplatin versus doxorubicin + 24-h paclitaxel + filgrastim in endometrial carcinoma: a Gynecologic Oncology Group study. Ann Oncol. 2004;15:1173–1178. doi: 10.1093/annonc/mdh316. [DOI] [PubMed] [Google Scholar]
- 45.Pearcey RG, Petereit DG. Post-operative high dose rate brachytherapy in patients with low to intermediate risk endometrial cancer. Radiother Oncol. 2000;56:17–22. doi: 10.1016/s0167-8140(00)00171-7. [DOI] [PubMed] [Google Scholar]
- 46.Petereit DG, Tannehill SP, Grosen EA, et al. Outpatient vaginal cuff brachytherapy for endometrial cancer. Int J Gynecol Cancer. 1999;9:456–462. doi: 10.1046/j.1525-1438.1999.99061.x. [DOI] [PubMed] [Google Scholar]
- 47.Randall ME, Greven KM. Brachytherapy: criteria for case selection. Cancer Invest. 1995;13:405–410. doi: 10.3109/07357909509031920. [DOI] [PubMed] [Google Scholar]
- 48.Naumann RW, Higgins RV, Hall JB. The use of adjuvant radiation therapy by members of the Society of Gynecologic Oncologists. Gynecol Oncol. 1999;75:4–9. doi: 10.1006/gyno.1999.5548. [DOI] [PubMed] [Google Scholar]
- 49.Naumann RW, Coleman RL. The use of adjuvant radiation therapy in early endometrial cancer by members of the Society of Gynecologic Oncologists in 2005. Gynecol Oncol. 2007;105:7–12. doi: 10.1016/j.ygyno.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 50.Keys HM, Roberts JA, Brunetto VL, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004;92:744–751. doi: 10.1016/j.ygyno.2003.11.048. [DOI] [PubMed] [Google Scholar]


