Abstract
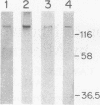
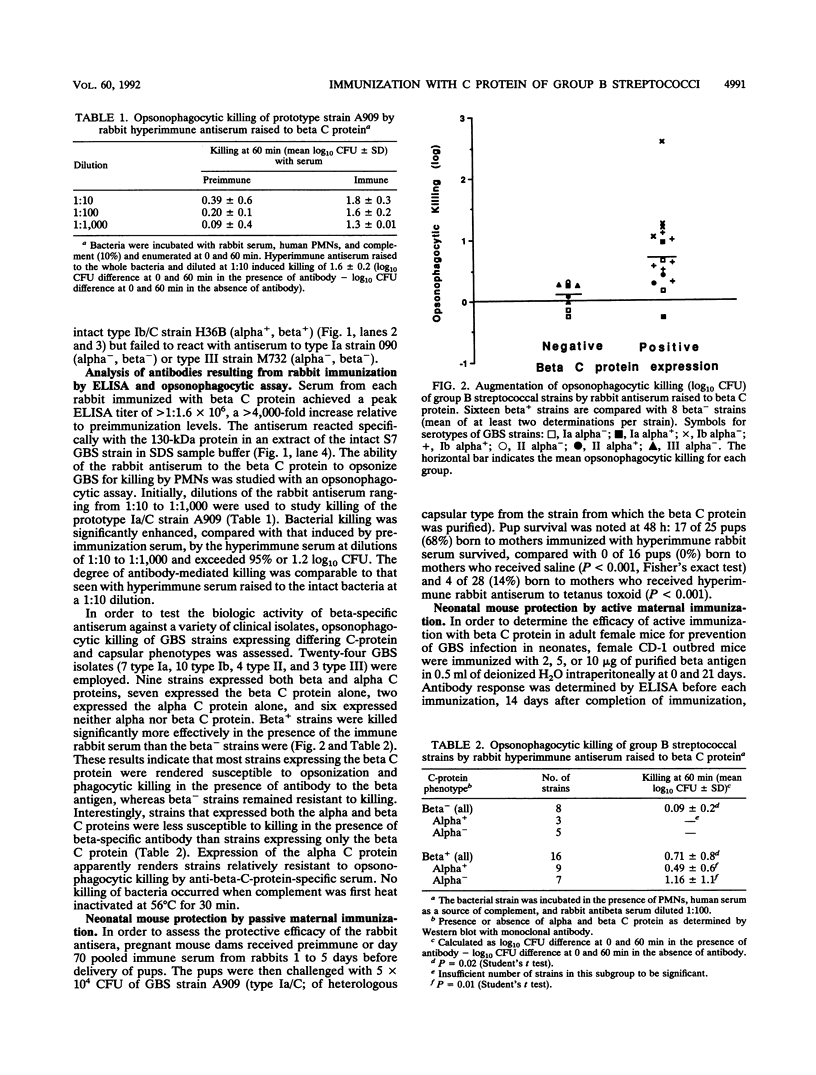
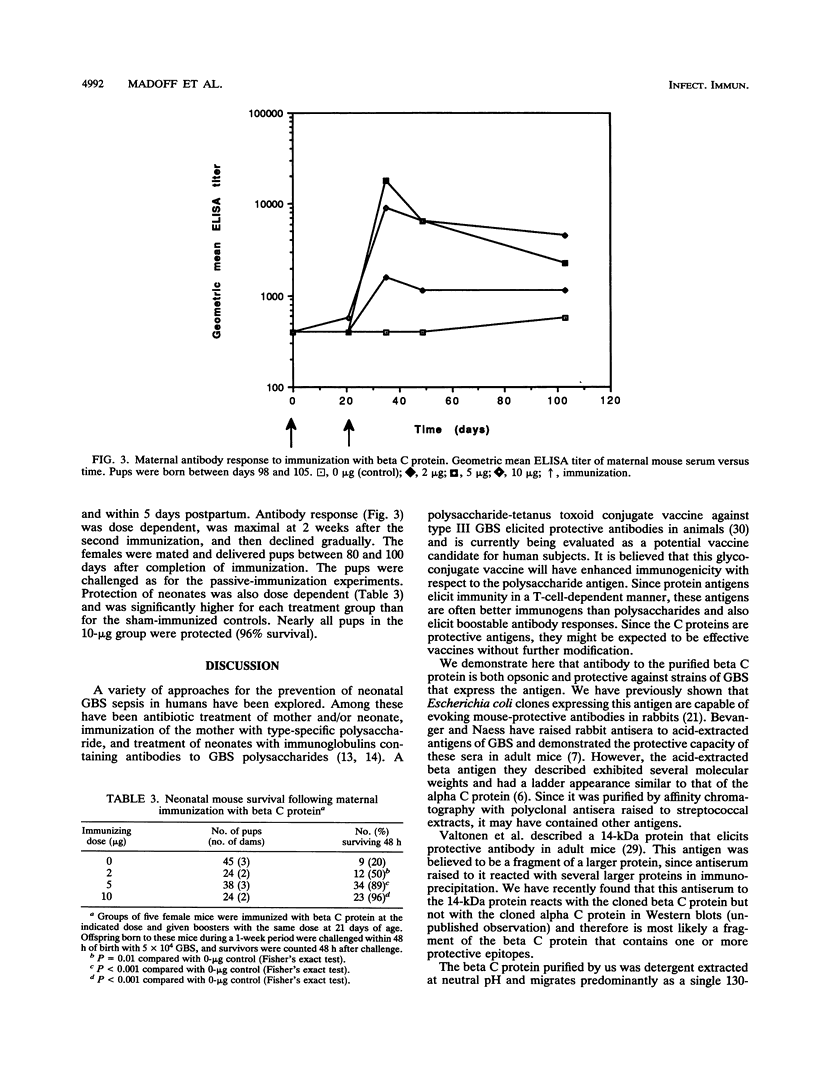
Group B streptococci (GBS) cause the majority of cases of neonatal sepsis and meningitis in the United States. Immunization of women of childbearing age is one strategy under consideration for the prevention of neonatal disease. The beta C protein, a 130-kDa antigen present in many clinical isolates of GBS, was purified from GBS by extraction into sodium dodecyl sulfate (SDS)-containing buffer, preparative SDS-polyacrylamide gel electrophoresis, and electroelution. Purified beta C protein antigen (25 micrograms) with Freund's adjuvant was used to immunize rabbits. Rabbits developed enzyme-linked immunosorbent assay titers of > 1:1.6 x 10(6), and sera from immunized rabbits were administered to pregnant mice. Their neonatal pups were then challenged with a strain of GBS expressing beta C protein; 68% of these pups were protected by immune antiserum, whereas no controls were protected (P < 0.001). The immune serum (diluted 1:100) facilitated opsonophagocytic killing of GBS strains expressing the beta C protein but not those that do not express the antigen (mean log kill +/- standard deviation = 0.71 +/- 0.8 log10 CFU for beta+ strains and 0.09 +/- 0.2 for beta- strains; P = 0.02). In subsequent experiments, adult female mice were actively immunized with two doses of 2, 5, or 10 micrograms of beta C protein 2 months prior to mating. One- to two-day-old offspring of these dams were challenged with GBS and were protected in a dose-dependent manner, with 96% survival in the high-dose (10-micrograms) group and 20% survival in a sham-immunized control group (P < 0.001). Thus, active immunization of mice with the GBS beta C protein confers protection against lethal infection with beta+ GBS to their offspring.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker C. J., Edwards M. S., Kasper D. L. Role of antibody to native type III polysaccharide of group B Streptococcus in infant infection. Pediatrics. 1981 Oct;68(4):544–549. [PubMed] [Google Scholar]
- Baker C. J., Kasper D. L. Vaccination as a measure for prevention of neonatal GBS infection. Antibiot Chemother (1971) 1985;35:281–290. doi: 10.1159/000410381. [DOI] [PubMed] [Google Scholar]
- Baker C. J., Rench M. A., Edwards M. S., Carpenter R. J., Hays B. M., Kasper D. L. Immunization of pregnant women with a polysaccharide vaccine of group B streptococcus. N Engl J Med. 1988 Nov 3;319(18):1180–1185. doi: 10.1056/NEJM198811033191802. [DOI] [PubMed] [Google Scholar]
- Baltimore R. S., Kasper D. L., Baker C. J., Goroff D. K. Antigenic specificity of opsonophagocytic antibodies in rabbit anti-sera to group B streptococci. J Immunol. 1977 Feb;118(2):673–678. [PubMed] [Google Scholar]
- Bevanger L. Ibc proteins as serotype markers of group B streptococci. Acta Pathol Microbiol Immunol Scand B. 1983 Aug;91(4):231–234. doi: 10.1111/j.1699-0463.1983.tb00038.x. [DOI] [PubMed] [Google Scholar]
- Bevanger L., Naess A. I. Mouse-protective antibodies against the Ibc proteins of group B streptococci. Acta Pathol Microbiol Immunol Scand B. 1985 Apr;93(2):121–124. doi: 10.1111/j.1699-0463.1985.tb02862.x. [DOI] [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Brady L. J., Boyle M. D. Identification of non-immunoglobulin A-Fc-binding forms and low-molecular-weight secreted forms of the group B streptococcal beta antigen. Infect Immun. 1989 May;57(5):1573–1581. doi: 10.1128/iai.57.5.1573-1581.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady L. J., Daphtary U. D., Ayoub E. M., Boyle M. D. Two novel antigens associated with group B streptococci identified by a rapid two-stage radioimmunoassay. J Infect Dis. 1988 Nov;158(5):965–972. doi: 10.1093/infdis/158.5.965. [DOI] [PubMed] [Google Scholar]
- Chun C. S., Brady L. J., Boyle M. D., Dillon H. C., Ayoub E. M. Group B streptococcal C protein-associated antigens: association with neonatal sepsis. J Infect Dis. 1991 Apr;163(4):786–791. doi: 10.1093/infdis/163.4.786. [DOI] [PubMed] [Google Scholar]
- Givner L. B., Baker C. J. The prevention and treatment of neonatal group B streptococcal infections. Adv Pediatr Infect Dis. 1988;3:65–90. [PubMed] [Google Scholar]
- Givner L. B., Edwards M. S., Baker C. J. A polyclonal human IgG preparation hyperimmune for type III, group B Streptococcus: in vitro opsonophagocytic activity and efficacy in experimental models. J Infect Dis. 1988 Oct;158(4):724–730. doi: 10.1093/infdis/158.4.724. [DOI] [PubMed] [Google Scholar]
- Jerlström P. G., Chhatwal G. S., Timmis K. N. The IgA-binding beta antigen of the c protein complex of Group B streptococci: sequence determination of its gene and detection of two binding regions. Mol Microbiol. 1991 Apr;5(4):843–849. doi: 10.1111/j.1365-2958.1991.tb00757.x. [DOI] [PubMed] [Google Scholar]
- Johnson D. R., Ferrieri P. Group B streptococcal Ibc protein antigen: distribution of two determinants in wild-type strains of common serotypes. J Clin Microbiol. 1984 Apr;19(4):506–510. doi: 10.1128/jcm.19.4.506-510.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancefield R. C., McCarty M., Everly W. N. Multiple mouse-protective antibodies directed against group B streptococci. Special reference to antibodies effective against protein antigens. J Exp Med. 1975 Jul 1;142(1):165–179. doi: 10.1084/jem.142.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madoff L. C., Hori S., Michel J. L., Baker C. J., Kasper D. L. Phenotypic diversity in the alpha C protein of group B streptococci. Infect Immun. 1991 Aug;59(8):2638–2644. doi: 10.1128/iai.59.8.2638-2644.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madoff L. C., Michel J. L., Kasper D. L. A monoclonal antibody identifies a protective C-protein alpha-antigen epitope in group B streptococci. Infect Immun. 1991 Jan;59(1):204–210. doi: 10.1128/iai.59.1.204-210.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel J. L., Madoff L. C., Kling D. E., Kasper D. L., Ausubel F. M. Cloned alpha and beta C-protein antigens of group B streptococci elicit protective immunity. Infect Immun. 1991 Jun;59(6):2023–2028. doi: 10.1128/iai.59.6.2023-2028.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti L. C., Kasper D. L., Michon F., DiFabio J., Holme K., Jennings H. J., Wessels M. R. An oligosaccharide-tetanus toxoid conjugate vaccine against type III group B Streptococcus. J Biol Chem. 1990 Oct 25;265(30):18278–18283. [PubMed] [Google Scholar]
- Payne N. R., Ferrieri P. The relation of the Ibc protein antigen to the opsonization differences between strains of type II group B streptococci. J Infect Dis. 1985 Apr;151(4):672–681. doi: 10.1093/infdis/151.4.672. [DOI] [PubMed] [Google Scholar]
- Payne N. R., Kim Y. K., Ferrieri P. Effect of differences in antibody and complement requirements on phagocytic uptake and intracellular killing of "c" protein-positive and -negative strains of type II group B streptococci. Infect Immun. 1987 May;55(5):1243–1251. doi: 10.1128/iai.55.5.1243-1251.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodewald A. K., Onderdonk A. B., Warren H. B., Kasper D. L. Neonatal mouse model of group B streptococcal infection. J Infect Dis. 1992 Sep;166(3):635–639. doi: 10.1093/infdis/166.3.635. [DOI] [PubMed] [Google Scholar]
- Russell-Jones G. J., Gotschlich E. C., Blake M. S. A surface receptor specific for human IgA on group B streptococci possessing the Ibc protein antigen. J Exp Med. 1984 Nov 1;160(5):1467–1475. doi: 10.1084/jem.160.5.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell-Jones G. J., Gotschlich E. C. Identification of protein antigens of group B streptococci, with special reference to the Ibc antigens. J Exp Med. 1984 Nov 1;160(5):1476–1484. doi: 10.1084/jem.160.5.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valtonen M. V., Kasper D. L., Levy N. J. Isolation of a C (Ibc) protein from group B Streptococcus which elicits mouse protective antibody. Microb Pathog. 1986 Apr;1(2):191–204. doi: 10.1016/0882-4010(86)90021-5. [DOI] [PubMed] [Google Scholar]
- Wessels M. R., Paoletti L. C., Kasper D. L., DiFabio J. L., Michon F., Holme K., Jennings H. J. Immunogenicity in animals of a polysaccharide-protein conjugate vaccine against type III group B Streptococcus. J Clin Invest. 1990 Nov;86(5):1428–1433. doi: 10.1172/JCI114858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson H. W., Eagon R. G. Type-specific antigens of group B type Ic streptococci. Infect Immun. 1971 Nov;4(5):596–604. doi: 10.1128/iai.4.5.596-604.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]