Abstract
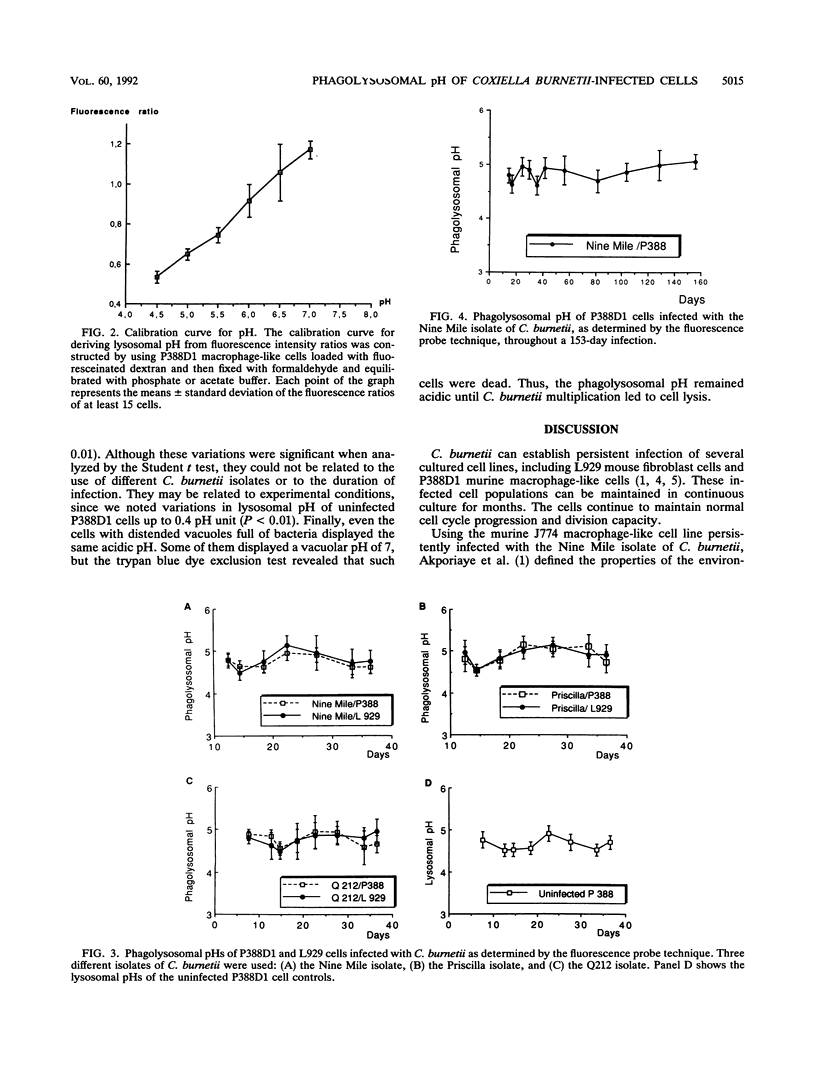
Coxiella burnetii, the agent of Q fever, is an obligate intracellular bacterium that multiples within vacuoles of phagolysosomal origin. Persistently infected cell lines were maintained in continuous culture for months. We studied the pH of the phagolysosomes by using two murine cell lines during early propagation of the bacteria and after establishment of persistent infection. Three strains of C. burnetii were studied because of the purported propensity of each strain to cause acute or chronic disease and to be resistant or susceptible to antibiotics. The pHs were calculated from fluorescence experiments with fluoresceinated dextran as a lysosomal probe. Phagolysosomal vacuoles maintained an acidic pH during a 36-day infection. Minimal variation of the pH occurred over the duration of the experiment with strains that caused either acute or chronic disease. Phagolysosomal pH remained stable for as long as 153 days with the Nine Mile phase II isolate. Thus, neither the course of C. burnetti infection nor the diversity of antibiotic susceptibility of the strains is related to variations in the phagolysosomal pH.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akporiaye E. T., Rowatt J. D., Aragon A. A., Baca O. G. Lysosomal response of a murine macrophage-like cell line persistently infected with Coxiella burnetii. Infect Immun. 1983 Jun;40(3):1155–1162. doi: 10.1128/iai.40.3.1155-1162.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- André P., Benoliel A. M., Capo C., Foa C., Buferne M., Boyer C., Schmitt-Verhulst A. M., Bongrand P. Use of conjugates made between a cytolytic T cell clone and target cells to study the redistribution of membrane molecules in cell contact areas. J Cell Sci. 1990 Oct;97(Pt 2):335–347. doi: 10.1242/jcs.97.2.335. [DOI] [PubMed] [Google Scholar]
- Antoine J. C., Prina E., Jouanne C., Bongrand P. Parasitophorous vacuoles of Leishmania amazonensis-infected macrophages maintain an acidic pH. Infect Immun. 1990 Mar;58(3):779–787. doi: 10.1128/iai.58.3.779-787.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baca O. G., Scott T. O., Akporiaye E. T., DeBlassie R., Crissman H. A. Cell cycle distribution patterns and generation times of L929 fibroblast cells persistently infected with Coxiella burnetii. Infect Immun. 1985 Feb;47(2):366–369. doi: 10.1128/iai.47.2.366-369.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton P. R., Stueckemann J., Welsh R. M., Paretsky D. Some ultrastructural effects of persistent infections by the rickettsia Coxiella burnetii in mouse L cells and green monkey kidney (Vero) cells. Infect Immun. 1978 Aug;21(2):556–566. doi: 10.1128/iai.21.2.556-566.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackstadt T., Williams J. C. Biochemical stratagem for obligate parasitism of eukaryotic cells by Coxiella burnetii. Proc Natl Acad Sci U S A. 1981 May;78(5):3240–3244. doi: 10.1073/pnas.78.5.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackstadt T., Williams J. C. pH dependence of the Coxiella burnetii glutamate transport system. J Bacteriol. 1983 May;154(2):598–603. doi: 10.1128/jb.154.2.598-603.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix L., Mallavia L. P. Active transport of proline by Coxiella burnetii. J Gen Microbiol. 1984 Nov;130(11):2857–2863. doi: 10.1099/00221287-130-11-2857. [DOI] [PubMed] [Google Scholar]
- Levy P. Y., Drancourt M., Etienne J., Auvergnat J. C., Beytout J., Sainty J. M., Goldstein F., Raoult D. Comparison of different antibiotic regimens for therapy of 32 cases of Q fever endocarditis. Antimicrob Agents Chemother. 1991 Mar;35(3):533–537. doi: 10.1128/aac.35.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder J. W. Comparative biology of intracellular parasitism. Microbiol Rev. 1985 Sep;49(3):298–337. doi: 10.1128/mr.49.3.298-337.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukkada A. J., Meade J. C., Glaser T. A., Bonventre P. F. Enhanced metabolism of Leishmania donovani amastigotes at acid pH: an adaptation for intracellular growth. Science. 1985 Sep 13;229(4718):1099–1101. doi: 10.1126/science.4035350. [DOI] [PubMed] [Google Scholar]
- Ohkuma S., Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raoult D., Drancourt M., Vestris G. Bactericidal effect of doxycycline associated with lysosomotropic agents on Coxiella burnetii in P388D1 cells. Antimicrob Agents Chemother. 1990 Aug;34(8):1512–1514. doi: 10.1128/aac.34.8.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman M. J., Coriz P. D., Baca O. G. A proposed model to explain persistent infection of host cells with Coxiella burnetii. J Gen Microbiol. 1986 May;132(5):1415–1422. doi: 10.1099/00221287-132-5-1415. [DOI] [PubMed] [Google Scholar]
- Samuel J. E., Frazier M. E., Mallavia L. P. Correlation of plasmid type and disease caused by Coxiella burnetii. Infect Immun. 1985 Sep;49(3):775–779. doi: 10.1128/iai.49.3.775-779.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodkin M. H., Williams J. C., Stephenson E. H. Genetic heterogeneity among isolates of Coxiella burnetii. J Gen Microbiol. 1986 Feb;132(2):455–463. doi: 10.1099/00221287-132-2-455. [DOI] [PubMed] [Google Scholar]
- Yeaman M. R., Baca O. G. Mechanisms that may account for differential antibiotic susceptibilities among Coxiella burnetii isolates. Antimicrob Agents Chemother. 1991 May;35(5):948–954. doi: 10.1128/aac.35.5.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman M. R., Roman M. J., Baca O. G. Antibiotic susceptibilities of two Coxiella burnetii isolates implicated in distinct clinical syndromes. Antimicrob Agents Chemother. 1989 Jul;33(7):1052–1057. doi: 10.1128/aac.33.7.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuerner R. L., Thompson H. A. Protein synthesis by intact Coxiella burnetii cells. J Bacteriol. 1983 Oct;156(1):186–191. doi: 10.1128/jb.156.1.186-191.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]