Abstract
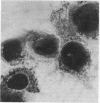
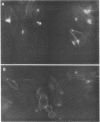
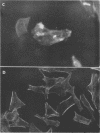
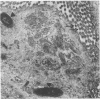
Providencia alcalifaciens is a member of the family Enterobacteriaceae. There are reports that P. alcalifaciens can cause diarrhea, but the mechanism(s) by which it causes diarrhea is known. We studied P. alcalifaciens isolated from a child and two adults with diarrhea for enteropathogenicity. The three isolates did not exhibit any characteristic adherence to cultured HEp-2 cell monolayers, and they did not produce enterotoxins, cytotoxins, or keratoconjunctivitis in the Sereny test. Two isolates invaded cultured HEp-2 cell monolayers, producing localized bacterial clusters and actin condensation. The pattern of actin condensation was different from that produced by enteropathogenic Escherichia coli but similar to that produced by Shigella flexneri. Invasion and actin condensation were poor for the third isolate. Histology of adult rabbit small intestinal loops inoculated with all three isolates revealed bacterial attachment to, penetration of, and microulcer formation on the surface epithelium and hyperemia, edema, and polymorphonuclear cell infiltration of lamina propria. All the isolates produced diarrhea in rabbits with removable intestinal ties, and some of these rabbits developed hindlimb paralysis. Intestinal histology of the rabbits with removable intestinal ties which developed diarrhea showed changes similar to that in adult rabbits on which ileal loop assays had been performed. Transmission electron microscopy of intestinal tissues also confirmed tissue penetration by the isolates. Nerve tissue histology of two rabbits that developed hindlimb paralysis showed focal mononuclear cell infiltration around peripheral nerve sheaths. It is concluded that some strains of P. alcalifaciens are enteropathogenic and that they cause diarrhea by invading the intestinal mucosal epithelium. However, the relevance to human disease of the hindlimb paralysis observed in this animal model is not clear.
Full text
PDF

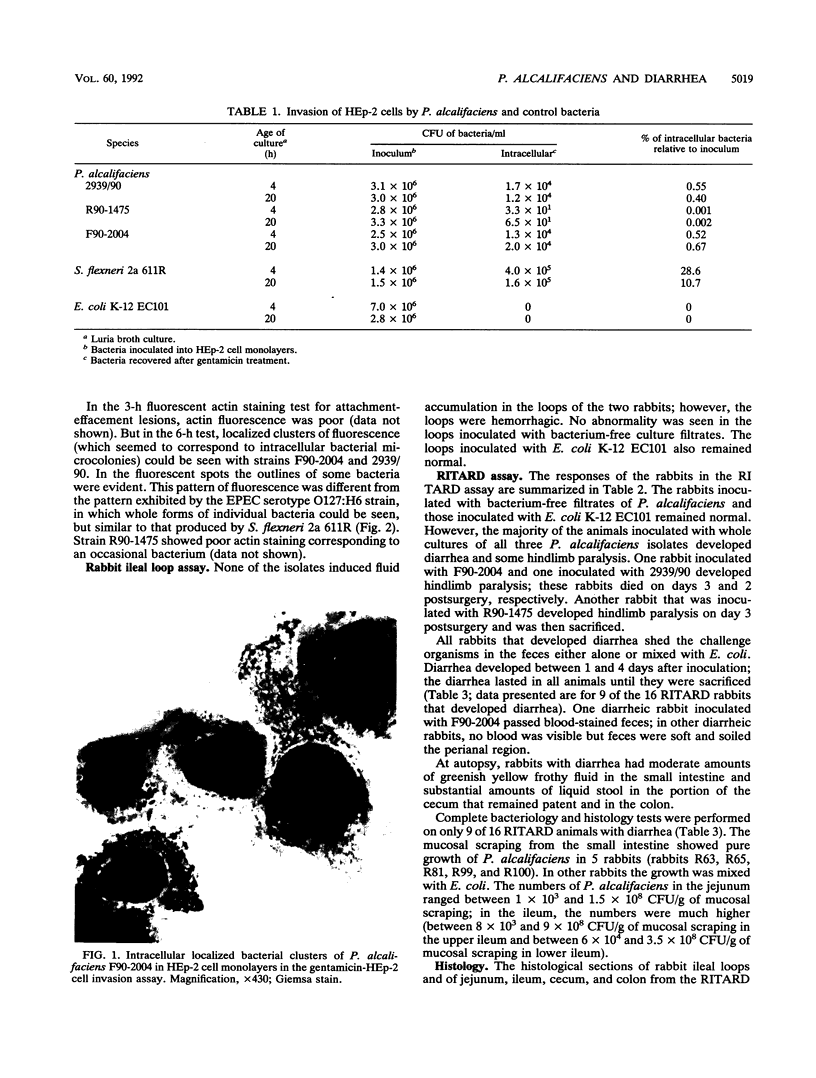
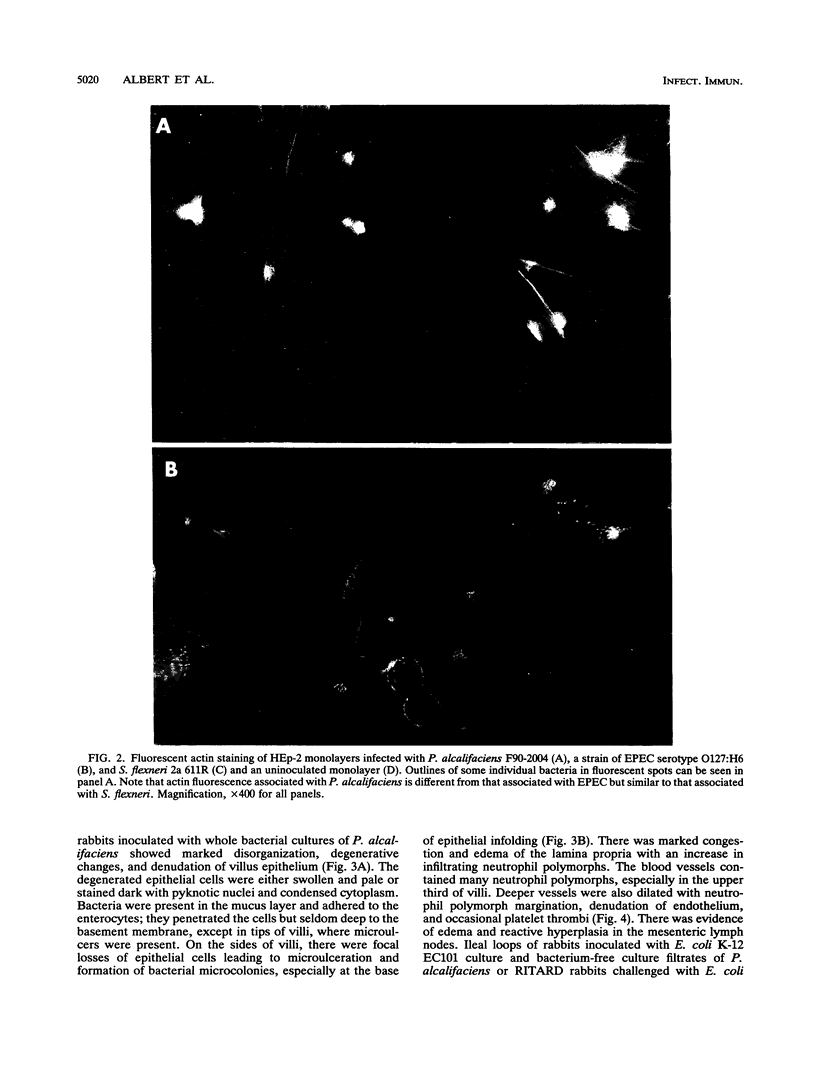
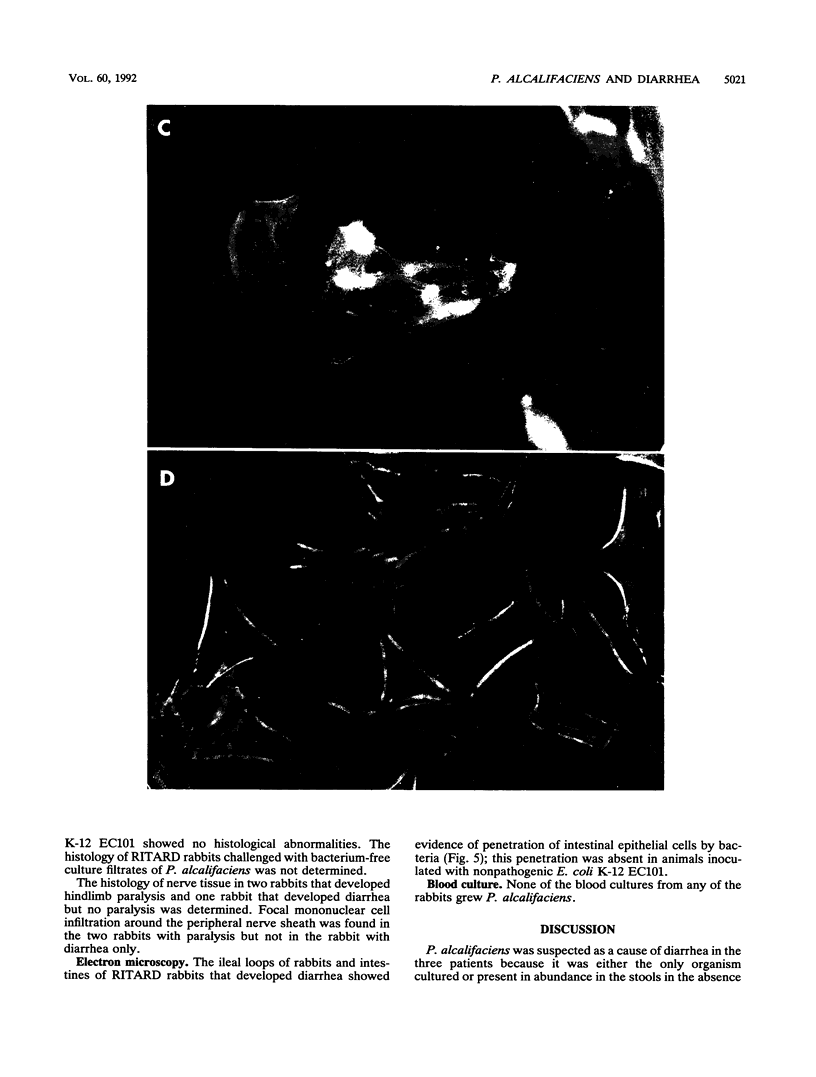
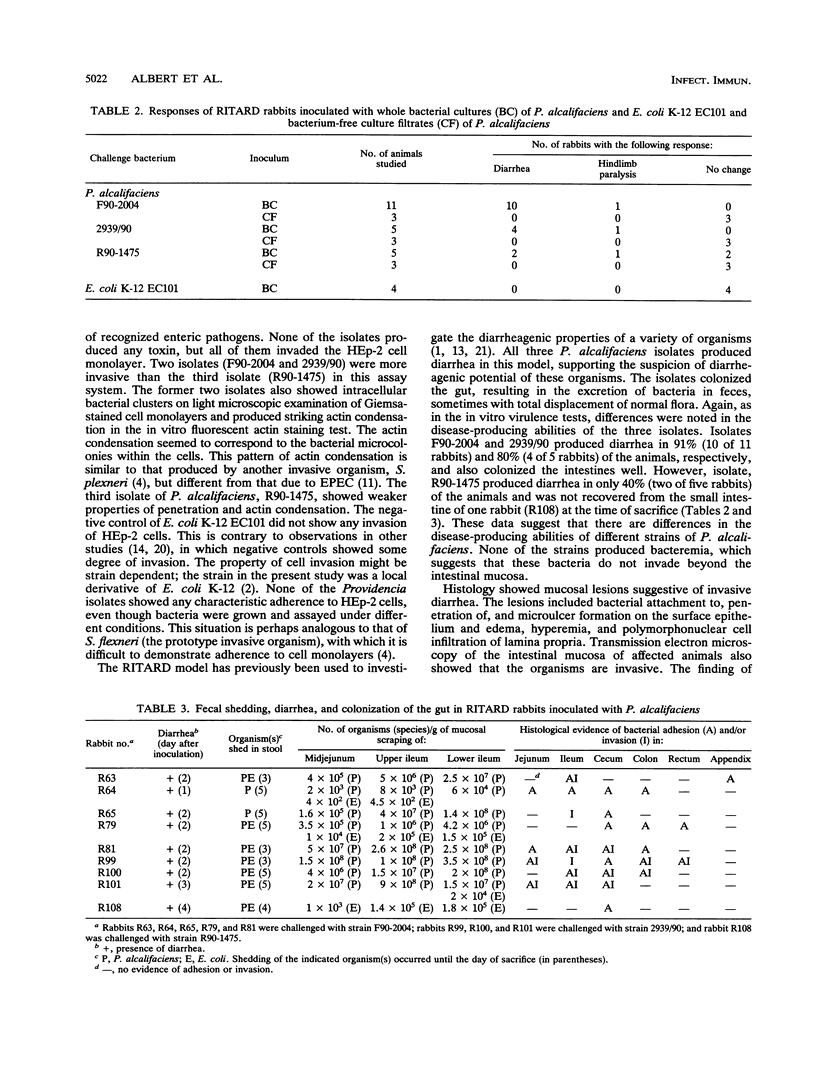


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert M. J., Alam K., Islam M., Montanaro J., Rahaman A. S., Haider K., Hossain M. A., Kibriya A. K., Tzipori S. Hafnia alvei, a probable cause of diarrhea in humans. Infect Immun. 1991 Apr;59(4):1507–1513. doi: 10.1128/iai.59.4.1507-1513.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf M. M., Ahmed Z. U., Sack D. A. Unusual association of a plasmid with nalidixic acid resistance in an epidemic strain of Shigella dysenteriae type 1 from Asia. Can J Microbiol. 1991 Jan;37(1):59–63. doi: 10.1139/m91-009. [DOI] [PubMed] [Google Scholar]
- CAVANAGH J. B., HOWARD J. G., WHITBY J. L. The neurotoxin of Shigella shigae; a comparative study of the effects produced in various laboratory animals. Br J Exp Pathol. 1956 Jun;37(3):272–278. [PMC free article] [PubMed] [Google Scholar]
- Clerc P., Sansonetti P. J. Entry of Shigella flexneri into HeLa cells: evidence for directed phagocytosis involving actin polymerization and myosin accumulation. Infect Immun. 1987 Nov;55(11):2681–2688. doi: 10.1128/iai.55.11.2681-2688.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean A. G., Ching Y. C., Williams R. G., Harden L. B. Test for Escherichia coli enterotoxin using infant mice: application in a study of diarrhea in children in Honolulu. J Infect Dis. 1972 Apr;125(4):407–411. doi: 10.1093/infdis/125.4.407. [DOI] [PubMed] [Google Scholar]
- Gentry M. K., Dalrymple J. M. Quantitative microtiter cytotoxicity assay for Shigella toxin. J Clin Microbiol. 1980 Sep;12(3):361–366. doi: 10.1128/jcm.12.3.361-366.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin D. E., Gemski P. Release of Shiga toxin from Shigella dysenteriae 1 by polymyxin B. Infect Immun. 1983 Apr;40(1):425–428. doi: 10.1128/iai.40.1.425-428.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes J., Hawkey P. M. Providencia alcalifaciens and travellers' diarrhoea. BMJ. 1989 Jul 8;299(6691):94–95. doi: 10.1136/bmj.299.6691.94-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutton S., Phillips A. D., Smith H. R., Gross R. J., Shaw R., Watson P., Price E. Screening for enteropathogenic Escherichia coli in infants with diarrhea by the fluorescent-actin staining test. Infect Immun. 1991 Jan;59(1):365–371. doi: 10.1128/iai.59.1.365-371.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon H. W., Whipp S. C., Argenzio R. A., Levine M. M., Giannella R. A. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect Immun. 1983 Sep;41(3):1340–1351. doi: 10.1128/iai.41.3.1340-1351.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazzaglia G., Sack R. B., Bourgeois A. L., Froehlich J., Eckstein J. Diarrhea and intestinal invasiveness of Aeromonas strains in the removable intestinal tie rabbit model. Infect Immun. 1990 Jun;58(6):1924–1931. doi: 10.1128/iai.58.6.1924-1931.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins-Browne R. M., Bennett-Wood V. Quantitative assessment of the ability of Escherichia coli to invade cultured animal cells. Microb Pathog. 1992 Feb;12(2):159–164. doi: 10.1016/0882-4010(92)90119-9. [DOI] [PubMed] [Google Scholar]
- SEN R. Isolation of strains of the Providence group from cases with diarrhoea in Ibadan, Nigeria, West Africa. Indian J Med Res. 1962 Jul;50:622–626. [PubMed] [Google Scholar]
- SERENY B. Experimental keratoconjunctivitis shigellosa. Acta Microbiol Acad Sci Hung. 1957;4(4):367–376. [PubMed] [Google Scholar]
- SINGER J., BAR-CHAY J. Biochemical investigation of Providence strains and their relationship to the Proteus group. J Hyg (Lond) 1954 Mar;52(1):1–8. doi: 10.1017/s0022172400027194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack D. A., Sack R. B. Test for enterotoxigenic Escherichia coli using Y-1 adrenal cells in miniculture. Infect Immun. 1975 Feb;11(2):334–336. doi: 10.1128/iai.11.2.334-336.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small P. L., Falkow S. Identification of regions on a 230-kilobase plasmid from enteroinvasive Escherichia coli that are required for entry into HEp-2 cells. Infect Immun. 1988 Jan;56(1):225–229. doi: 10.1128/iai.56.1.225-229.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small P. L., Isberg R. R., Falkow S. Comparison of the ability of enteroinvasive Escherichia coli, Salmonella typhimurium, Yersinia pseudotuberculosis, and Yersinia enterocolitica to enter and replicate within HEp-2 cells. Infect Immun. 1987 Jul;55(7):1674–1679. doi: 10.1128/iai.55.7.1674-1679.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira W. M., Sack R. B., Froehlich J. L. Simple adult rabbit model for Vibrio cholerae and enterotoxigenic Escherichia coli diarrhea. Infect Immun. 1981 May;32(2):739–747. doi: 10.1128/iai.32.2.739-747.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]