Abstract
Currently, virtually all clinical diagnostic ultrasound systems used in ophthalmology are based on fixed-focus, single-element transducers. High-frequency (≥20-MHz) transducers introduced to ophthalmology during the last decade have led to improved resolution and diagnostic capabilities for assessment of the anterior segment and the retina. However, single-element transducers are restricted to a small depth of field, limiting their capacity to image the eye as a whole. We fabricated a 20-MHz annular array probe prototype consisting of 5 concentric transducer elements and scanned an ex vivo human eye. Synthetically focused images of the bank eye showed improved depth of field and sensitivity, allowing simultaneous display of the anterior and posterior segments and the full lens contour. This capability may be useful in assessment of vitreoretinal pathologies and investigation of the accommodative mechanism.
Ophthalmic ultrasonography offers real-time examination of ocular tissues and pathologies even in the presence of optical opacities. Since their introduction by Baum and Greenwood in 1958,1 mechanically scanned 10-MHz transducers have largely been the norm, providing clinically useful images of the eye and orbit. In the 1990s, ultrasound biomicroscopy, employing 35- to 50-MHz transducers, was introduced by Pavlin et al.2 Ultrasound biomicroscopy systems provide significantly improved resolution compared with 10-MHz systems but are limited to the anterior segment owing to attenuation, which increases exponentially with frequency. Recently introduced 20-MHz systems offer improved resolution compared with 10-MHz systems and provide sufficient penetration for imaging of the retina and deeper tissues.3
While virtually all current ophthalmic ultrasound systems use mechanically scanned single element–focused transducers, array-based ultrasound probes are the dominant technology in other clinical specialties. Arrays typically consist of a linear arrangement of more than 100 independent transducer elements. By controlling pulse emission timing of the many elements and adding appropriate time delays to echo data received by each element, not only can mechanical scanning be done away with, but dynamic focusing can also be achieved, with a resultant improvement in lateral resolution. However, because element dimensions scale with wavelength, fabrication of probes becomes progressively more difficult and expensive with increasing frequency. Commercial array probes with center frequency higher than 15 MHz are not currently available.
A limitation of single element–focused transducers is their small axial depth of field, which restricts the best-obtainable resolution to a small depth range around the focal plane. Depth of field, defined as the axial range around the focus where echo amplitude is one-half maximum or more, is a function of the F ratio (focal length divided by aperture) and the frequency of the transducer. A 10-MHz transducer with an F ratio of 2:1 might have a depth of field of more than 9 mm. Because depth of field gets progressively smaller with increasing frequency, a 20-MHz probe with the same aperture and focal length would have a depth of field less than 5 mm. In practical clinical terms for ophthalmology, the restricted depth of field characteristic of singleelement high-frequency transducers precludes simultaneous imaging of the anterior and posterior segments of the eye without degradation of resolution and sensitivity. This is illustrated in Figure 1, which shows an in vivo immersion scan of a human eye, which was obtained using a clinical scanner (Cinescan A/B-S; Quantel Medical, Bozeman, Montana) equipped with a 20-MHz single-element transducer (20-mm focal length). In this case, the focal zone was placed in the central vitreous. With a lateral resolution near 0.5 mm and a depth of field near 5 mm, features of the anterior segment, which is in the near field well outside the focal plane, are visible only as specular reflections from surfaces at a right angle to the probe axis.
Figure 1.
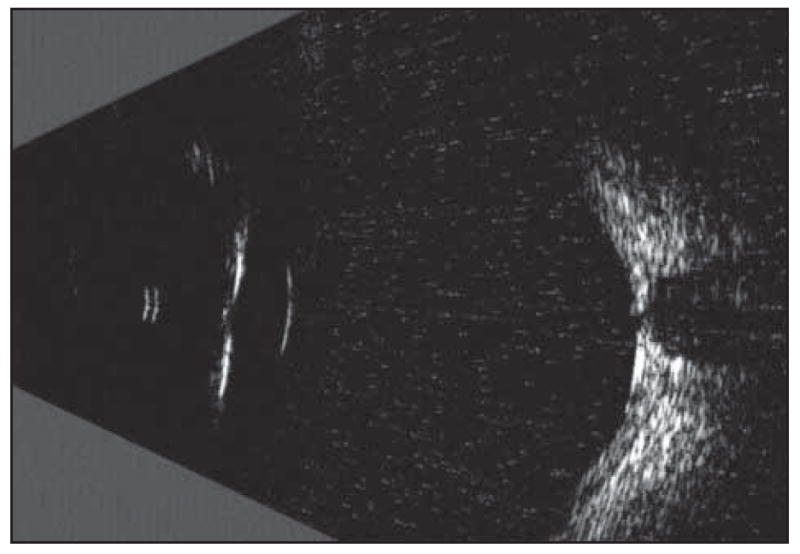
A 20-MHz immersion scan image of a healthy eye in vivo. Typical clinical sector-scan systems use single-element, fixed-focus transducers designed to place the focal zone near the retina in a contact scan. While this provides satisfactory images of the posterior segment, the eye as a whole is poorly depicted owing to the limited depth of field of this type of transducer.
Most 10- to 20-MHz commercial ophthalmic ultrasound systems are designed so that, in a contact examination (where the probe is placed against the eyelid), the focus falls in the vitreous just anterior to the retina. This design paradigm maximizes resolution at the vitreoretinal interface but provides reduced resolution and sensitivity to more anterior structures. While, in principle, it is possible to improve visualization of the midvitreous and anterior segment by performing an immersion examination with a fixed-focus probe, this is not a practical option in most clinical settings.
While high-frequency linear and phased arrays offer one solution to improving depth of field, their development has proven to be technically challenging4,5 and their implementation for ophthalmic imaging is problematic because of limited focusing in the elevation axis and refraction by anterior segment structures. Annular arrays, consisting of a series of concentric rings, can produce synthetically focused images without the complexity of linear or phased arrays and with far fewer elements.6-8 Annular arrays, however, must be mechanically scanned to create an image. We have recently described a 35-MHz annular array for imaging of the anterior segment.9,10 Herein, we present a 20-MHz annular array probe prototype that permits imaging of the full cross section of the eye with improved sensitivity and resolution in comparison with current single-element probes.
METHODS
The annular array prototype used in these studies had a 10-mm aperture divided into 5 concentric rings of equal surface area. A 25-μm-thick polymer membrane was used as the piezoelectric material. The array surface was focused to a 31-mm radius of curvature. The fabrication process has been previously described in detail.7
The transducer array was mounted onto a scanning system consisting of 3 orthogonal (x, y, and z) computer-controlled linear translation stages, each providing a 1-μm positional precision. Each image was formed from 5 consecutive scans across the target tissue, with each of the 5 elements acting in turn as the ultrasound emitter. During scan motion, acoustic pulses were emitted by the active element at evenly spaced intervals and echo data from all 5 elements were recorded (200-MHz sample rate, 8 bits per sample). Thus, radiofrequency echo data for all 25 possible transmit/receive element combinations were acquired.9,10 By simply adding the 25 waveforms, the array behaves as a single-element transducer with a fixed focal point defined by its radius of curvature. However, in a process called synthetic focusing, the focal point is shifted in range by introduction of appropriate time shifts to the echo data from each channel before they are added. We postprocessed the radio-frequency data with a synthetic-focusing algorithm that produced a series of focal zones along each line of sight (vector).9 A composite image was then formed by merging the bands of synthetically focused data. The time required to perform the series of the 5 scans needed for each image was approximately 8 seconds. The processing time to generate a synthetically focused image from the stored echo data was about 5 seconds.
We characterized the depth of field of the array in simulated single-element and synthetic focus modes by scanning thin (25-μm-diameter) wire targets at various ranges from the transducer. We determined reflection amplitude and beam width as a function of range from these data.
A 1-day-old bank eye from a 73-year-old man was immersed in normal saline and scanned as described previously. Institutional review board approval was obtained for the use of human tissues.
RESULTS
In simulated single-element mode, the measured depth of field of the array was 4 mm, with a best lateral resolution of 0.22 mm in the focal plane. At the limits of this depth of field, lateral resolution degraded to 0.5 mm. With synthetic focusing, lateral resolution ranged from 0.17 to 0.27 mm across an 18-mm depth of field. While addition of the 25 waveforms acquired along each vector (in both simulated single-element and synthetic focus modes) reduces incoherent background noise, only synthetic focusing reinforces echoes generated from coherently reflecting tissue structures. This is the primary factor leading to extension of the depth of field and improved sensitivity. The 4.5-fold increase in depth of field obtained with synthetic focusing confers improved sensitivity outside the small focal region of a comparable single-element transducer of similar frequency, aperture, and focal length.
Figure 2 shows bank eye images obtained with the annular array probe. Simulated, single-element, fixedfocus and synthetically focused images were created from the acquired radiofrequency data (281 vectors at 100-μm intervals). The simulated single-element image (Figure 2A), with the geometric focus positioned just beyond the cornea, resolved details of the cornea and anterior chamber, but little was visualized at greater depths. With synthetic focusing (Figure 2B), the anterior and posterior segments were simultaneously in focus, the entire lens circumference was visualized, a cataract was observed within the lens, and the iris was well resolved.
Figure 2.
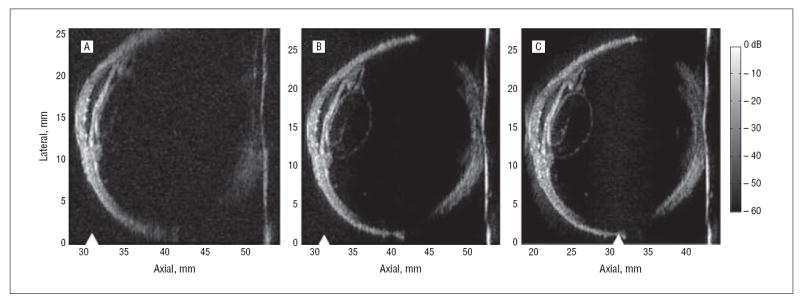
Bank eye images were acquired with the 20-MHz annular array for cases of fixed focusing (A) and synthetic focusing with the 31-mm geometric focus (triangles) positioned just beyond the cornea (B) and with the geometric focus placed 1 cm deeper into the eye (C).
The advantage of the annular array was further demonstrated by moving the geometric focus 10 mm deeper into the eye and then forming another synthetically focused image (Figure 2C). The resulting image was nearly identical to that in Figure 2B, except for a few differences that can be attributed to the improved lateral resolution that resulted from the shift of the eye toward the transducer. These differences include a better delineation of the lens boundary, an improved resolution of the posterior segment, and a near-field artifact visible at 20 mm axially.
The improved image quality obtained with the annular array probe facilitates 3-dimensional imaging. A 3-dimensional image stack composed of 113 parallel scan planes at 250-μm intervals was acquired for the bank eye. The images were then manually segmented to identify the retina, lens, ciliary body, sclera, and iris (Figure 3). The pupil, which is outside the scan plane in Figure 2, is readily seen in the 3-dimensional reconstruction, as are the lens and ciliary body. Figure 2B highlights the ability of the array to volumetrically create an image of the lens.
Figure 3.
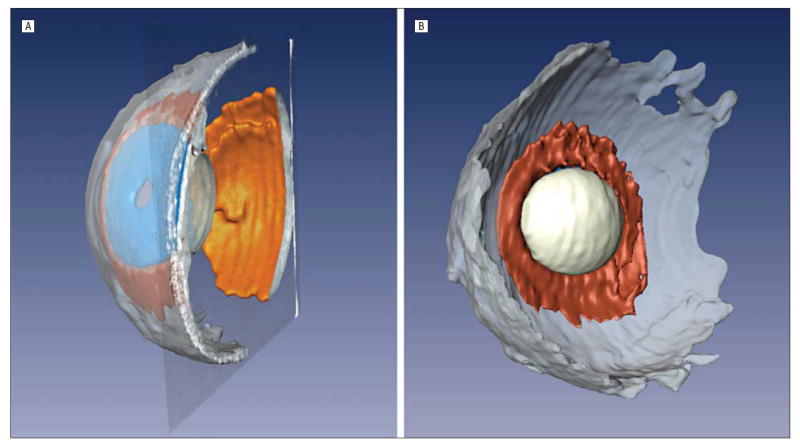
A, Manually segmented stack of bank eye images permitted a 3-dimensional visualization of the retina (orange), iris (blue), lens (white), ciliary body (brown), and sclera (gray). B, Anterior segment from a posterior perspective shows the lens and ciliary body.
CONCLUSIONS
The annular array images represent the first clinical-quality, high-frequency ultrasound images of the full globe acquired with an array probe. The annular array greatly improves current high-resolution ultrasound technology and provides a practical near-term solution to the problem of improving depth of field and lateral resolution. Although the prototype device described here has a limited scan rate, real-time imaging (10 frames/s) is feasible using currently available technologies.
The ability to visualize the complete globe with fine resolution and to demarcate the lens margins in their entirety will permit complex biophysical processes, such as accommodation, to be studied in real time. It may also be of value in the sizing of intraocular lens implants, especially phakic lenses, and in the management of presbyopia. While the vitreous is responsible for most retinal detachments and is the preferred surgical corrective route for retinal surgery, it is poorly understood and woefully inadequately imaged because of its optical transparency and low acoustic reflectivity. The enhanced sensitivity and depth of field offered by the annular array probe may provide improved visualization of retinal traction with hemorrhage, posterior vitreous detachment, and floaters and may thus offer improved management of vitreoretinal disease.
Acknowledgments
Funding/Support: This work was funded in part by grants EY014371 and EB000238 from the National Institutes of Health; Research to Prevent Blindness; and the Dyson Foundation.
Footnotes
Financial Disclosure: None reported.
References
- 1.Baum G, Greenwood I. The application of ultrasonic locating techniques to ophthalmology, part 2: ultrasonic visualization of soft tissues. Arch Ophthalmol. 1958;60:263–279. doi: 10.1001/archopht.1958.00940080279015. [DOI] [PubMed] [Google Scholar]
- 2.Pavlin CJ, Harasiewicz K, Sherar MD, Foster ES. Clinical use of ultrasound biomicroscopy. Ophthalmology. 1991;98(3):287–295. doi: 10.1016/s0161-6420(91)32298-x. [DOI] [PubMed] [Google Scholar]
- 3.Coleman DJ, Silverman RH, Chabi A, et al. High resolution ultrasonic imaging of the posterior segment. Ophthalmology. 2004;111(7):1344–1351. doi: 10.1016/j.ophtha.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 4.Cannata JM, Williams JA, Zhou Q, Ritter TA, Shung KK. Development of a 35-MHz piezo-composite ultrasound array for medical imaging. IEEE Trans Ultrason Ferroelectr Freq Control. 2006;53(1):224–236. doi: 10.1109/tuffc.2006.1588408. [DOI] [PubMed] [Google Scholar]
- 5.Ritter TA, Shrout TR, Tutwiler R, Shung KK. A 30-MHz piezo-composite ultrasound array for medical imaging applications. IEEE Trans Ultrason Ferroelectr Freq Control. 2002;49(2):217–230. doi: 10.1109/58.985706. [DOI] [PubMed] [Google Scholar]
- 6.Brown JA, Démoré CEM, Lockwood GR. Design and fabrication of annular arrays for high-frequency ultrasound. IEEE Trans Ultrason Ferroelectr Freq Control. 2004;51(8):1010–1017. doi: 10.1109/tuffc.2004.1324405. [DOI] [PubMed] [Google Scholar]
- 7.Ketterling JA, Aristizábal O, Turnbull DH, Lizzi FL. Design and fabrication of a 40-MHz annular array transducer. IEEE Trans Ultrason Ferroelectr Freq Control. 2005;52(4):672–681. doi: 10.1109/tuffc.2005.1428050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Snook KA, Hu CH, Shrout TR, Shung KK. High-frequency ultrasound annular array imaging, part I: array design and fabrication. IEEE Trans Ultrason Ferroelectr Freq Control. 2006;53(2):300–308. doi: 10.1109/tuffc.2006.1593368. [DOI] [PubMed] [Google Scholar]
- 9.Ketterling JA, Ramachandran S, Aristizábal O. Operational verification of a 40-MHz annular array transducer. IEEE Trans Ultrason Ferroelectr Freq Control. 2006;53(3):623–630. doi: 10.1109/tuffc.2006.1610571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silverman RH, Ketterling JA, Coleman DJ. High-frequency ultrasonic imaging of the anterior segment using an annular array transducer. Ophthalmology. 2007;114(4):816–822. doi: 10.1016/j.ophtha.2006.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]


