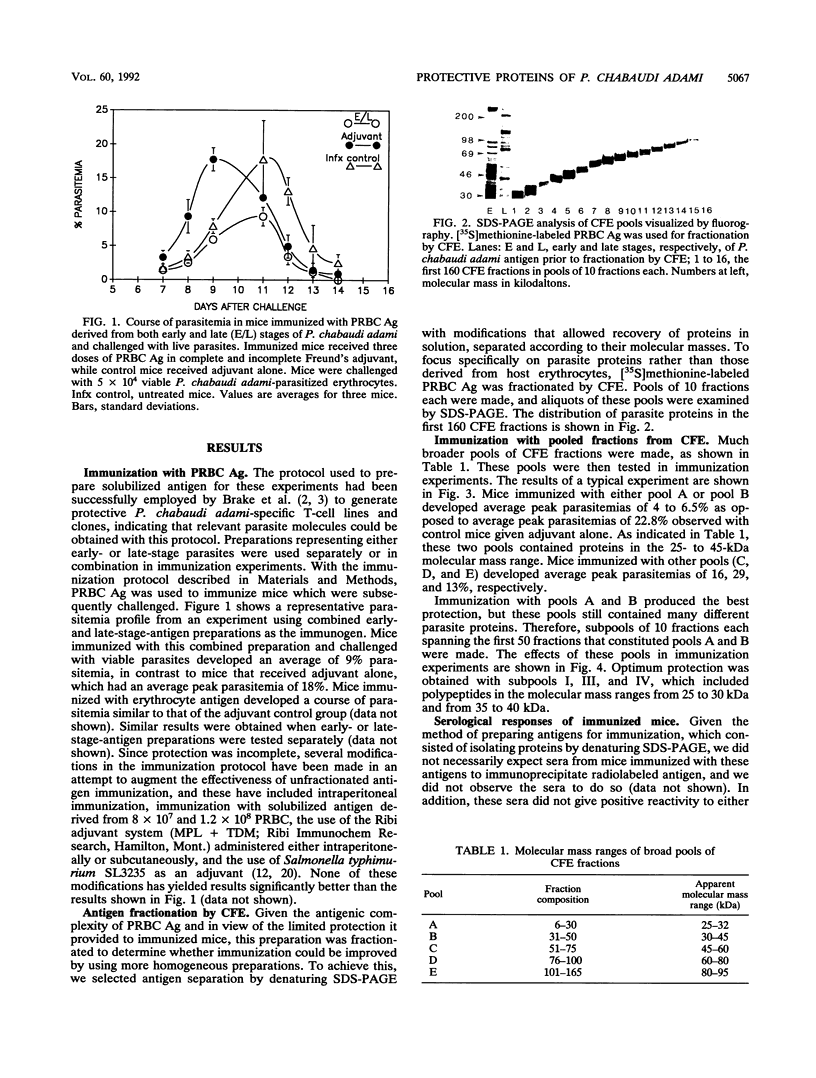
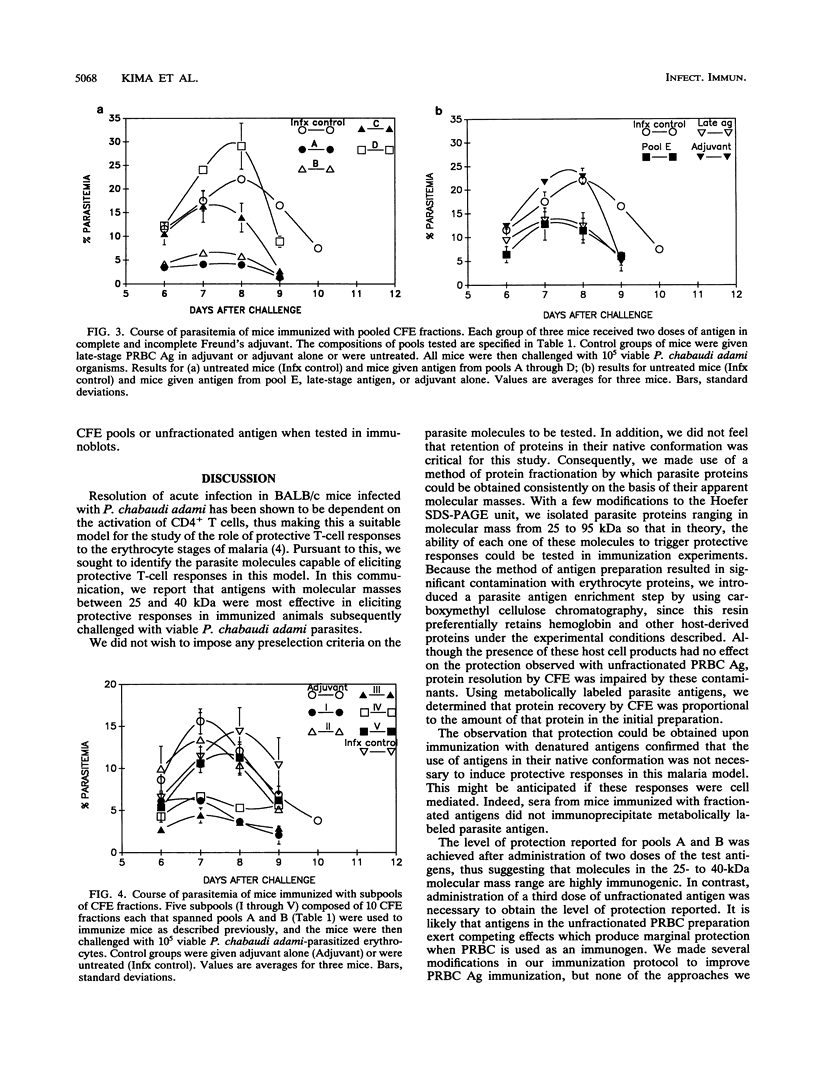
Abstract
The presence of the CD4+ T cell has been shown to be crucial for resolution of acute infection in the Plasmodium chabaudi adami murine malaria model. This model is, therefore, suitable for the isolation of malaria antigens that are capable of activating protective T cells. In light of this, we set out to identify P. chabaudi adami molecules that activate protective responses in this model. Denatured P. chabaudi adami proteins were isolated by continuous-flow electrophoresis on the basis of their apparent molecular masses and then sequentially assessed for the ability to protect mice in immunization experiments. We report here that low-molecular-mass P. chabaudi adami polypeptides in the range from 25 to 40 kDa are most effective at immunizing mice against a challenge infection with viable P. chabaudi adami. The method used to obtain these proteins could also be applied to identify molecules that activate protective cell-mediated responses in other infectious disease models.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benjamin D. C., Berzofsky J. A., East I. J., Gurd F. R., Hannum C., Leach S. J., Margoliash E., Michael J. G., Miller A., Prager E. M. The antigenic structure of proteins: a reappraisal. Annu Rev Immunol. 1984;2:67–101. doi: 10.1146/annurev.iy.02.040184.000435. [DOI] [PubMed] [Google Scholar]
- Brake D. A., Long C. A., Weidanz W. P. Adoptive protection against Plasmodium chabaudi adami malaria in athymic nude mice by a cloned T cell line. J Immunol. 1988 Mar 15;140(6):1989–1993. [PubMed] [Google Scholar]
- Brake D. A., Weidanz W. P., Long C. A. Antigen-specific, interleukin 2-propagated T lymphocytes confer resistance to a murine malarial parasite, Plasmodium chabaudi adami. J Immunol. 1986 Jul 1;137(1):347–352. [PubMed] [Google Scholar]
- Brown G. V., Anders R. F., Stace J. D., Alpers M. P., Mitchell G. F. Immunoprecipitation of biosynthetically-labelled proteins from different Papua New Guinea Plasmodium falciparum isolates by sera from individuals in the endemic area. Parasite Immunol. 1981 Winter;3(4):283–298. doi: 10.1111/j.1365-3024.1981.tb00407.x. [DOI] [PubMed] [Google Scholar]
- Brown K. N., Jarra W., Newbold C. I., Schryer M. Variability in parasite protein antigen structure and protective immunity to malaria. Ann Inst Pasteur Immunol. 1985 Jan-Feb;136C(1):11–23. [PubMed] [Google Scholar]
- Deans J. A., Cohen S. Immunology of malaria. Annu Rev Microbiol. 1983;37:25–49. doi: 10.1146/annurev.mi.37.100183.000325. [DOI] [PubMed] [Google Scholar]
- Fenton B., Clark J. T., Khan C. M., Robinson J. V., Walliker D., Ridley R., Scaife J. G., McBride J. S. Structural and antigenic polymorphism of the 35- to 48-kilodalton merozoite surface antigen (MSA-2) of the malaria parasite Plasmodium falciparum. Mol Cell Biol. 1991 Feb;11(2):963–971. doi: 10.1128/mcb.11.2.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grun J. L., Weidanz W. P. Immunity to Plasmodium chabaudi adami in the B-cell-deficient mouse. Nature. 1981 Mar 12;290(5802):143–145. doi: 10.1038/290143a0. [DOI] [PubMed] [Google Scholar]
- Hager D. A., Burgess R. R. Elution of proteins from sodium dodecyl sulfate-polyacrylamide gels, removal of sodium dodecyl sulfate, and renaturation of enzymatic activity: results with sigma subunit of Escherichia coli RNA polymerase, wheat germ DNA topoisomerase, and other enzymes. Anal Biochem. 1980 Nov 15;109(1):76–86. doi: 10.1016/0003-2697(80)90013-5. [DOI] [PubMed] [Google Scholar]
- Hoffmann E. J., Weidanz W. P., Long C. A. Susceptibility of CXB recombinant inbred mice to murine plasmodia. Infect Immun. 1984 Mar;43(3):981–985. doi: 10.1128/iai.43.3.981-985.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder A. A. The precursor to major merozoite surface antigens: structure and role in immunity. Prog Allergy. 1988;41:72–97. [PubMed] [Google Scholar]
- Kumar S., Gorden J., Flynn J. L., Berzofsky J. A., Miller L. H. Immunization of mice against Plasmodium vinckei with a combination of attenuated Salmonella typhimurium and malarial antigen. Infect Immun. 1990 Oct;58(10):3425–3429. doi: 10.1128/iai.58.10.3425-3429.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langhorne J., Evans C. B., Asofsky R., Taylor D. W. Immunoglobulin isotype distribution of malaria-specific antibodies produced during infection with Plasmodium chabaudi adami and Plasmodium yoelii. Cell Immunol. 1984 Sep;87(2):452–461. doi: 10.1016/0008-8749(84)90014-5. [DOI] [PubMed] [Google Scholar]
- Lew A. M., Langford C. J., Anders R. F., Kemp D. J., Saul A., Fardoulys C., Geysen M., Sheppard M. A protective monoclonal antibody recognizes a linear epitope in the precursor to the major merozoite antigens of Plasmodium chabaudi adami. Proc Natl Acad Sci U S A. 1989 May;86(10):3768–3772. doi: 10.1073/pnas.86.10.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majarian W. R., Daly T. M., Weidanz W. P., Long C. A. Passive immunization against murine malaria with an IgG3 monoclonal antibody. J Immunol. 1984 Jun;132(6):3131–3137. [PubMed] [Google Scholar]
- Meding S. J., Langhorne J. CD4+ T cells and B cells are necessary for the transfer of protective immunity to Plasmodium chabaudi chabaudi. Eur J Immunol. 1991 Jun;21(6):1433–1438. doi: 10.1002/eji.1830210616. [DOI] [PubMed] [Google Scholar]
- Saul A., Lord R., Jones G. L., Spencer L. Protective immunization with invariant peptides of the Plasmodium falciparum antigen MSA2. J Immunol. 1992 Jan 1;148(1):208–211. [PubMed] [Google Scholar]
- Smith B. P., Reina-Guerra M., Hoiseth S. K., Stocker B. A., Habasha F., Johnson E., Merritt F. Aromatic-dependent Salmonella typhimurium as modified live vaccines for calves. Am J Vet Res. 1984 Jan;45(1):59–66. [PubMed] [Google Scholar]
- Unanue E. R. Antigen-presenting function of the macrophage. Annu Rev Immunol. 1984;2:395–428. doi: 10.1146/annurev.iy.02.040184.002143. [DOI] [PubMed] [Google Scholar]
- Weidanz W. P., Long C. A. The role of T cells in immunity to malaria. Prog Allergy. 1988;41:215–252. [PubMed] [Google Scholar]
- Wilson C. M. Studies and critique of Amido Black 10B, Coomassie Blue R, and Fast Green FCF as stains for proteins after polyacrylamide gel electrophoresis. Anal Biochem. 1979 Jul 15;96(2):263–278. doi: 10.1016/0003-2697(79)90581-5. [DOI] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]



