Abstract
Background:
Even though time-to-treatment has been shown to be a determinant of mortality in primary angioplasty, the potential benefits from early pharmacological reperfusion by glycoprotein (Gp) IIb–IIIa inhibitors are still unclear. The aim of this meta-analysis was to combine individual data from all randomised trials conducted on facilitated primary angioplasty by the use of early Gp IIb–IIIa inhibitors.
Methods and results:
The literature was scanned by formal searches of electronic databases (MEDLINE, EMBASE) from January 1990 to October 2007. All randomised trials on facilitation by the early administration of Gp IIb–IIIa inhibitors in ST-segment elevation myocardial infarction (STEMI) were examined. No language restrictions were enforced. Individual patient data were obtained from 11 out of 13 trials, including 1662 patients (840 patients (50.5%) randomly assigned to early and 822 patients (49.5%) to late Gp IIb–IIIa inhibitor administration). Preprocedural Thrombolysis in Myocardial Infarction Study (TIMI) grade 3 flow was more frequent with early Gp IIb–IIIa inhibitors. Postprocedural TIMI 3 flow and myocardial blush grade 3 were higher with early Gp IIb–IIIa inhibitors but did not reach statistical significance except for abciximab, whereas the rate of complete ST-segment resolution was significantly higher with early Gp IIb–IIIa inhibitors. Mortality was not significantly different between groups, although early abciximab demonstrated improved survival compared with late administration, even after adjustment for clinical and angiographic confounding factors.
Conclusions:
This meta-analysis shows that pharmacological facilitation with the early administration of Gp IIb–IIIa inhibitors in patients undergoing primary angioplasty for STEMI is associated with significant benefits in terms of preprocedural epicardial recanalisation and ST-segment resolution, which translated into non-significant mortality benefits except for abciximab.
Several randomised trials1 have shown that primary angioplasty is superior to thrombolysis in terms of survival in the treatment of ST-segment elevation myocardial infarction (STEMI). The attempts to extend primary angioplasty to the vast majority of STEMI patients may, however, be associated with longer delays to treatment, with a negative impact on survival.2–5 Adjunctive abciximab has been shown to reduce mortality in patients undergoing primary angioplasty.6 7 The early administration of glycoprotein (Gp) IIb–IIIa inhibitors seems even more attractive for the potential benefits expected from early recanalisation, which might overcome any potential delay to mechanical reperfusion.8 9 The Early Glycoprotein IIb–IIIa Inhibitors in Primary Angioplasty (EGYPT) cooperation aimed at performing a comprehensive meta-analysis of randomised trials based on individual patient data to evaluate the benefits of pharmacological facilitation with Gp IIb–IIIa inhibitors in patients undergoing primary angioplasty for STEMI.
METHODS
Eligibility and search strategy
We identified all randomised trials comparing pharmacological facilitation by the early administration of Gp IIb–IIIa inhibitors versus its periprocedural administration in STEMI patients undergoing primary angioplasty. The literature was scanned by formal searches of electronic databases (MEDLINE, EMBASE) from January 1990 to October 2007, the scientific session abstracts in Circulation, Journal of College of Cardiology, European Heart Journal and American Journal of Cardiology from January 1990 to October 2007. The following key words were used: randomised trial, myocardial infarction, reperfusion, primary angioplasty, facilitated angioplasty, Gp IIb–IIIa inhibitors, abciximab, eptifibatide, tirofiban. No language restrictions were enforced. All principal investigators were contacted in order to provide individual patient data, which were transferred without patient identifiers (initials and birthday) to the Eastern Piedmont University, Novara, Italy. The dataset was checked for completeness and consistency and compared with the results of any publications. Queries were resolved by direct correspondence with the study investigator responsible. Data were managed according to the intention-to-treat principle.
Angiograms and ECG were not analysed by a central core laboratory, but data were provided by each principal investigator. Analysis of angiograms was based on standard definitions.10–12 In particular, distal embolisation was defined as an abrupt “cutoff” in the main vessel or one of the coronary branches of the infarct-related artery, distal to the angioplasty site.12 Even though ST-segment analysis was performed according to the pre-specified criteria of each trial, data were provided according to uniform thresholds (<30% no resolution; 30%–70% partial resolution; >70% complete resolution).
Outcome measures
Angiographic endpoints were preprocedural and postprocedural Thrombolysis in Myocardial Infarction Study (TIMI) grade 3 flow distal embolisation. Myocardial perfusion was evaluated by myocardial blush grade (MBG) 3 and post-procedural electrocardiograms were evaluated for complete (>70%) ST-segment resolution. Infarct size was estimated by using peak creatine kinase levels. The primary clinical endpoint was mortality. We also analysed the rate of major bleeding complications (defined as retroperitoneal, intracranial bleeding, or a drop in haemoglobin >5 g/dl) as the major safety endpoint.
Data analysis
Statistical analysis was performed using the Review Manager 4.27 freeware package and SPSS 15.0 statistical package. The pooled odds ratio (OR) for categorical variables was calculated by using the modified Mantel–Haenszel method with “observed minus expected” values for each trial, whereas a weighted mean difference was used for continuous variables.24 We performed survival analyses with the use of Cox regression analysis stratified according to trial.25 Survival was defined as the interval from randomisation until the event of interest. Survival curves are presented as non-stratified Kaplan–Meier across trials. Heterogeneity across trials was assessed by the I2 statistics. Prespecified subgroup analyses were performed according to the molecule (abciximab, tirofiban and eptifibatide). Additional subgroup analyses were performed for mortality according to diabetic status, age (>65 or <65 years) and time to treatment (>3 or <3 h), gender and infarct location (anterior versus non-anterior).
A multivariate adjustment of mortality benefits was finally performed for major clinical or angiographic characteristics, such as age, gender, diabetes, hypertension, smoking, previous revascularisation, previous myocardial infarction (MI), anterior MI, Killip class at presentation, time to treatment, time from symptom onset to Gp IIb–IIIa inhibitor administration, duration of preprocedural drug administration (from Gp IIb–IIIa inhibitor administration to balloon angioplasty), type of drug, multivessel disease, coronary stenting and interaction between molecules and early drug administration, by using a Cox regression analysis stratified according to trial (all covariates were entered in block in the model).26
RESULTS
Eligible studies
Individual patient data were obtained from 1113–17 19–23,27 out of 1425 28 29 trials. A total of 1662 patients were included, 840 patients (50.5%) were randomly assigned to early (administration started in the ambulance, in the community hospital before/during transportation to percutaneous coronary intervention (PCI) centres, or in the emergency room/intensive care unit of PCI hospitals) and 822 patients (49.5%) were randomly assigned to late (periprocedural) Gp IIb–IIIa inhibitor administration.
Study characteristics are reported in table 1. Baseline patient characteristics are reported in table 2. A total of six trials was conducted on abciximab (n = 612, 36.8%), three trials on tirofiban (n = 632, 38%) and two trials on eptifibatide (n = 418, 25.2%). Baseline patient characteristics according to study drug are reported in table 3.
Table 1. Characteristics of randomised trials comparing early versus late Gp IIb–IIIa inhibitor administration in primary angioplasty.
| Study | Period | Study design (no of patients) | Symptom duration, hours | Stent | Primary endpoints | Follow-up duration |
| ReoPro-BRIDGING13 | 2003–4 | Early (n = 28) versus late (n = 27) abciximab* | <6 | Yes | Preprocedural TIMI 3 flow, cTFC and MACE | 1 year |
| RELAx-AMI14 | 2003–4 | Early (n = 105) versus late (n = 105) abciximab* | <6 | Yes | Preprocedural TIMI 3 flow, ST resolution, myocardial salvage | 30 days |
| Rakowski et al15 | 2004 | Early (n = 25) versus late (n = 30) abciximab* | <12 | Yes | Preprocedural TIMI 3 flow, ST resolution, LVF | 1 year |
| ERAMI16 | 2001–2 | Early (n = 36) versus late (n = 38) abciximab)* | <12 | nr | Preprocedural TIMI flow | 1 year |
| Zorman et al17 | 1998–2001 | Early (n = 56) versus late (n = 56) abciximab* | <12 | Yes | Early (60 minutes) ST-segment resolution, preprocedural 3 TIMI flow | 6 months |
| REOMOBILE18 | 2001–2 | Early (n = 52) versus late (n = 48) abciximab* | <6 | Yes | Preprocedural TIMI flow | 1 year |
| Cutlip et al19 | 2001–2 | Early (n = 28) versus late or no (n = 30) tirofiban† | <12 | Yes | Preprocedural TIMI flow | 30 days |
| On-TIME20 | 2001–2 | Early (n = 251) versus late (n = 256) tirofiban† | <6 | Yes | Preprocedural TIMI flow | 1 year |
| Emre et al21 | 2002–3 | Early (n = 32) versus late (n = 34) tirofiban† | <6 | Yes | Myocardial perfusion and functional recovery at 30 days | 30 days |
| INTAMI22 | 2002–4 | Early (n = 53) versus late or no (n = 49) eptifibatide‡ | <12 | Yes | Preprocedural TIMI 3 flow | 1 year |
| TITAN-TIMI 3423 | 2004–5 | Early (n = 180) versus late or no (n = 163) eptifibatide‡ | <6 | Yes | Preprocedural TIMI frame count | 30 days |
*0.25 mg/kg intravenous bolus, followed by 0.125 μg/kg per minute infusion (12 h).
†10 μg/kg intravenous bolus followed by 0.15 μg/kg per minute infusion (24 h).
‡180 μg/kg intravenous double bolus followed by 2.0 μg/kg per minute infusion (12–24 h).
cTFC, corrected TIMI frame count; Gp, glycoprotein; LVF, left ventricular function; MACE, major adverse cardiac events; nr, not reported; TIMI 3, Thrombolysis in Myocardial Infarction Study grade 3 flow.
Table 2. Patient demographic and clinical characteristics.
| Variables | Early Gp IIb–IIIa inhibitors | Late Gp IIb–IIIa inhibitors |
| (n = 840) | (n = 822) | |
| Age, years | ||
| Median | 61 | 61 |
| Range | 52–70 | 52–70 |
| Sex, n (%) | 642/840 (76.4%) | 641/822 (78.0%) |
| Hypertension, n (%) | 353/838 (42.1%) | 347/822 (42.2%) |
| Diabetes, n (%) | 123/840 (14.6%) | 135/822 (16.4%) |
| Previous MI, n (%) | 67/838 (8.0%) | 80/822 (9.7%) |
| Previous revascularisation, n (%) | 61/792 (7.7%) | 59/770 (7.7%) |
| Smoking, n (%) | 440/840 (52.4%) | 419/822 (51.0%) |
| Hypercholesterolemia, n (%) | 298/840 (35.5%) | 309/820 (37.7%) |
| Killip class III/IV, n (%) | 33/722 (4.6%) | 33/705 (4.7%) |
| Anterior MI, n (%) | 361/831 (43.4%) | 369/819 (45.1%) |
| Symptom onset to Gp IIb–IIIa inhibitor time, minutes* | ||
| Median | 100 | 197 |
| 25–75th percentiles | 65–178 | 144–275 |
| Ischaemia time, minutes | ||
| Median | 193 | 203 |
| 25–75th percentiles | 146–270 | 150–285 |
| Infarct-related artery | ||
| LAD, n (%) | 351(41.7%) | 361 (43.9%) |
| CX, n (%) | 124 (14.7%) | 100 (12.1%) |
| RCA, n (%) | 339 (40.3%) | 336 (40.9%) |
| GRAFT, n (%) | 6 (0.7%) | 6 (0.7%) |
| LM, n (%) | 4 (0.5%) | 5 (0.6%) |
| Multivessel disease, n (%) | 437/757 (57.7%) | 433/737 (58.8%) |
| Follow-up | ||
| Median | 330 | 347 |
| 25–75th percentiles | 30–360 | 30–360 |
All p values are not significant except for the time from symptom onset to administration of Gp IIb–IIIa inhibitors* (p<0.001).
CX, circumflex artery; Gp, glycoprotein; LAD, left descending coronary artery; LM, left main artery; MI, myocardial infarction; RCA, right coronary artery.
Table 3. Patient demographic and clinical characteristics according to study drug.
| Variables | Early abciximab | Late abciximab | Early tirofiban | Late tirofiban | Early eptifibatide | Late eptifibatide |
| (n = 302) | (n = 310) | (n = 311) | (n = 321) | (n = 227) | (n = 191) | |
| Age, years | ||||||
| Median | 61 | 62 | 62 | 62 | 58 | 59 |
| 25–75th percentiles | 52–69 | 52–72 | 54–70 | 52–70 | 50–70 | 51–68 |
| Sex, n (%) | 228/302 (75.5%) | 239/310 (77.1%) | 249/311 (80.1%) | 259/321 (80.7%) | 165/227 (72.7%) | 143/191 (74.9%) |
| Hypertension, n (%) | 143/301 (47.5%) | 143/310 (46.1%) | 97/311 (31.2%) | 110/321 (34.3%) | 113/226 (50.0%) | 94/191 (49.2%) |
| Diabetes, n (%) | 61/302 (20.2%) | 69/310 (22.3%) | 37/311 (11.9%) | 42/321 (13.1%) | 35/227 (15.4%) | 37/191 (19.4%) |
| Previous MI, n (%) | 14/301 (4.7%) | 26/310 (8.4%) | 21/310 (6.8%) | 31/321 (9.7%) | 32/227 (14.1%) | 23/191 (12.0%) |
| Previous revascularisation, n (%) | 9/254 (3.5%) | 13/258 (5.0%) | 22/311 (7.1%) | 22/321 (6.9%) | 30/227 (13.2%) | 24/191 (12.6%) |
| Smoking, n (%) | 151/302 (50.0%) | 146/310 (47.1%) | 176/311 (56.6%) | 196/321 (61.1%) | 113/227 (49.8%) | 77/191 (40.3%) |
| Hypercholesterolemia, n (%) | 135/302 (44.7%) | 142/309 (46.0%) | 78/311 (25.1%) | 90/321 (28.0%) | 85/227 (37.4%) | 77/190 (40.5%) |
| Killip class III/IV, n (%) | 15/302 (4.9%) | 14/310 (4.4%) | 3/251 (1.2%) | 4/256 (1.6%) | 15/169 (9.2%) | 15/139 (10.8%) |
| Anterior MI, n (%) | 153/302 (50.7%) | 157/310 (50.6%) | 127/311 (40.8%) | 138/321 (43.0%) | 81/218 (37.2%) | 74/188 (39.4%) |
| Symptom onset to Gp IIb–IIIa inhibitor time, minutes* | ||||||
| Median | 130 | 203 | 64 | 191 | 150 | 199 |
| 25–75th percentiles | 800–203 | 145–300 | 50–85 | 145–264 | 95–257 | 138–267 |
| Ischaemia time, minutes | ||||||
| Median | 194 | 208 | 193 | 198 | 191 | 210 |
| 25–75th percentiles | 145–271 | 150–300 | 150–249 | 150–270 | 138–295 | 147–303 |
| Infarct-related artery | ||||||
| LAD, n (%) | 151 (50%) | 153 (49.4%) | 123 (39.5%) | 134 (41.7%) | 77 (33.9%) | 74 (38.7%) |
| CX, n (%) | 34 (11.3%) | 30(9.7%) | 51 (16.4%) | 41 (12.8%) | 39 (17.2%) | 29 (15.2%) |
| RCA, n (%) | 113 (37.4%) | 122 (39.4%) | 123 (39.5%) | 131 (40.8%) | 103 (45.3%) | 83 (43.5%) |
| GRAFT, n (%) | 0 | 6 (0.7%) | 2 (0.6%) | 4 (1.2%) | 4 (1.8%) | 2 (1.0%) |
| LM, n (%) | 0 | 3 (1.0%) | 4 (1.3%) | 1 (0.3%) | 0 | 1 (0.5%) |
| Multivessel disease, n (%) | 120/254 (47.2%) | 142/258 (55.0%) | 150/282 (53.2%) | 147/289 (50.9%) | 167/221 (75.6%) | 144/190 (75.8%) |
| Follow-up | ||||||
| Median | 180 | 180 | 357 | 356 | 30 | 30 |
| 25–75th percentiles | 30–360 | 30–360 | 346–360 | 342–360 | 30–30 | 30–30 |
All p values are not significant except for the time from symptom onset to administration of Gp IIb–IIIa inhibitors* (p<0.001).
CX, circumflex artery; Gp, glycoprotein; LAD, left descending coronary artery; LM, left main artery; MI, myocardial infarction; RCA, right coronary artery.
Angiographic endpoints
Preprocedural TIMI 3 flow
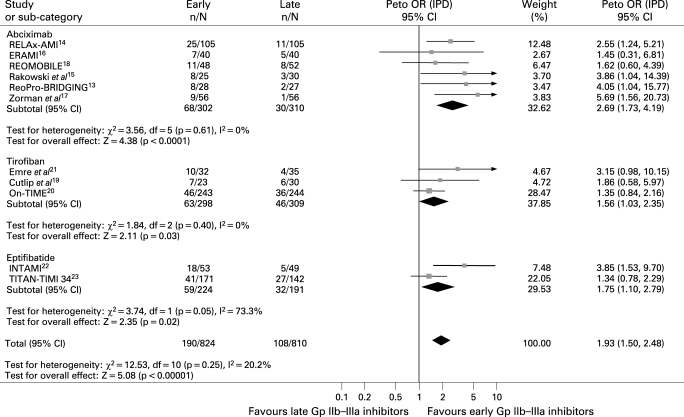
Data were available fot 1634 patients. As shown in fig 1, early Gp IIb–IIIa inhibitors were associated with a significantly improved preprocedural TIMI 3 flow (23.0% versus 13.3%, Peto OR 1.93; 95% CI 1.50 to 2.48; p<0.001, phet = 0.25) with similar benefits across the three molecules.
Figure 1. Facilitation with early glycoprotein IIb–IIIa inhibitors and preprocedural TIMI flow with Peto odds ratios and 95% CI. The size of the data markers (squares) is approximately proportional to the statistical weight of each trial. Gp, glycoprotein; IPD, individual patient data; OR, odds ratio; TIMI, Thrombolysis in Myocardial Infarction Study.
Postprocedural TIMI 3 flow
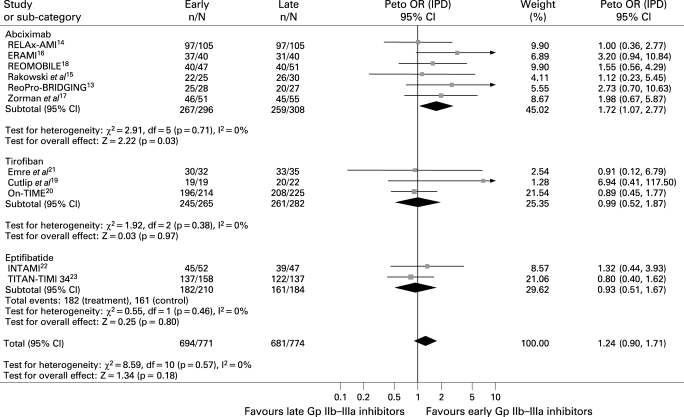
Data were available for 1551 patients. As shown in fig 2, no difference was observed in the rate of postprocedural TIMI 3 flow (90% versus 87.9%, Peto OR 1.24; 95% CI 0.90 to 1.71; p = 0.18, phet = 0.57). Early abciximab was, however, associated with a significant improvement in postprocedural TIMI 3 flow (90.2% versus 84.1%, Peto OR 1.72; 95% CI 1.07 to 2.77; p = 0.03, p interaction of abciximab versus small molecules 0.057).
Figure 2. Facilitation with early Gp IIb–IIIa inhibitors and postprocedural TIMI 3 flow with Peto odds ratios and 95% CI. The size of the data markers (squares) is approximately proportional to the statistical weight of each trial. Gp, glycoprotein; IPD, individual patient data; OR, odds ratio; TIMI, Thrombolysis in Myocardial Infarction Study.
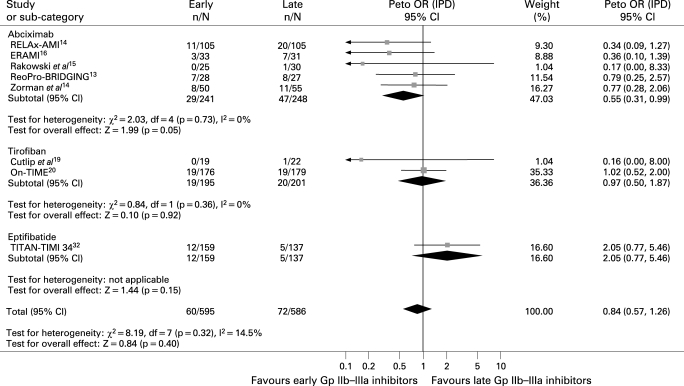
Distal embolisation
Data were available for 1181 patients. Early Gp IIb–IIIa inhibitors were not associated with significant benefits in terms of distal embolisation (10.1% versus 12.3%, Peto OR 0.84; 95% CI 0.57 to 1.26; p = 0.4, phet = 0.32; fig 3), except for abciximab (12% versus 19%, Peto OR 0.55; 95% CI 0.31 to 0.99; p = 0.05, p interaction of abciximab versus small molecules 0.057).
Figure 3. Facilitation with early Gp IIb–IIIa inhibitors and distal embolisation with Peto odds ratios and 95% CI. The size of the data markers (squares) is approximately proportional to the statistical weight of each trial. Gp, glycoprotein; IPD, individual patient data; OR, odds ratio.
Myocardial perfusion
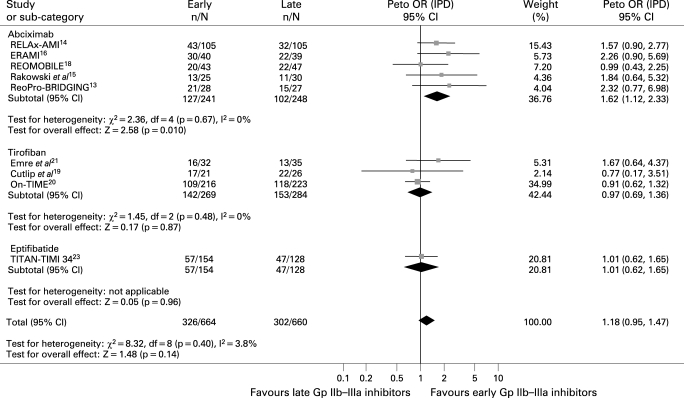
Myocardial blush
Data were available for 1324 patients. As shown in fig 4, early Gp IIb–IIIa inhibitors were associated with slight benefits in final MBG 3 (49.1% versus 45.8%, Peto OR 1.18; 95% CI 0.95 to 1.47; p = 0.14, phet = 0.40, number needed to treat 30.3). In an analysis limited to abciximab trials, the benefits achieved statistical significance (52.7% versus 41.1%, Peto OR 1.62; 95% CI 1.12 to 2.33; p = 0.01; p interaction of abciximab versus small molecules 0.02).
Figure 4. Facilitation with early Gp IIb–IIIa inhibitors and myocardial blush grade 3 with Peto odds ratios and 95% CI. The size of the data markers (squares) is approximately proportional to the statistical weight of each trial. *Myocardial perfusion evaluated by myocardial perfusion grade. Gp, glycoprotein; IPD, individual patient data; OR, odds ratio.
ST-segment resolution
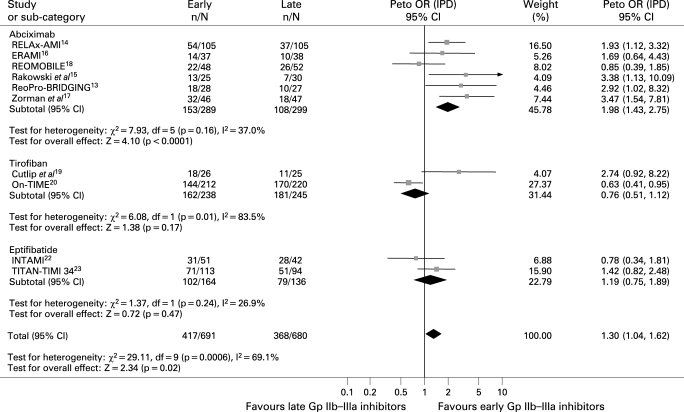
Data were available for 1371 patients. As shown in fig 5, early Gp IIb–IIIa inhibitors were associated with significant benefits in terms of complete ST-segment resolution (60.3% versus 54.1%, Peto OR 1.30; 95% CI 1.04 to 1.62; p = 0.02, phet<0.001). This difference was greater for early versus later abciximab (52.9% versus 36.1%, Peto OR 1.98; 95% CI 1.43 to 2.75; p<0.001; p interaction of abciximab versus small molecules <0.001).
Figure 5. Facilitation with early Gp IIb–IIIa inhibitors and complete ST-segment resolution with Peto odds ratios and 95% CI. The size of the data markers (squares) is approximately proportional to the statistical weight of each trial. Gp, glycoprotein; IPD, individual patient data; OR, odds ratio.
Enzymatic infarct size and abortion of MI
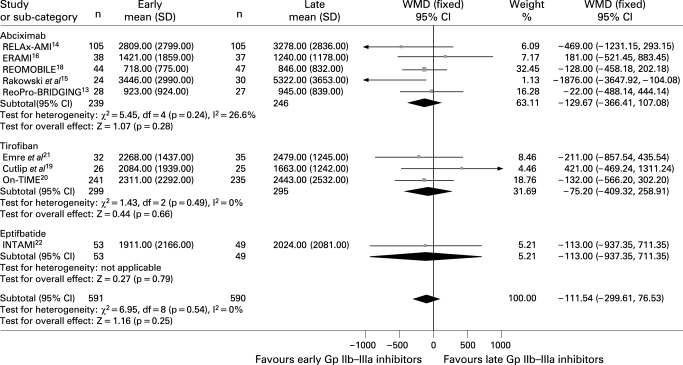
Data on creatine kinase levels were available for 1181 patients. As shown in fig 6, early Gp IIb–IIIa inhibitors were associated with a trend in benefits in terms of enzymatic infarct size (weighted mean difference −111.5; 95% CI −229.6 to 76.5; p = 0.25, phet = 0.54).
Figure 6. Facilitation with early Gp IIb–IIIa inhibitors and enzymatic infarct size (peak creatine kinase) with weighted mean difference and 95% CI. The size of the data markers (squares) is approximately proportional to the statistical weight of each trial. Gp, glycoprotein; OR, odds ratio; WMD, weighted mean difference.
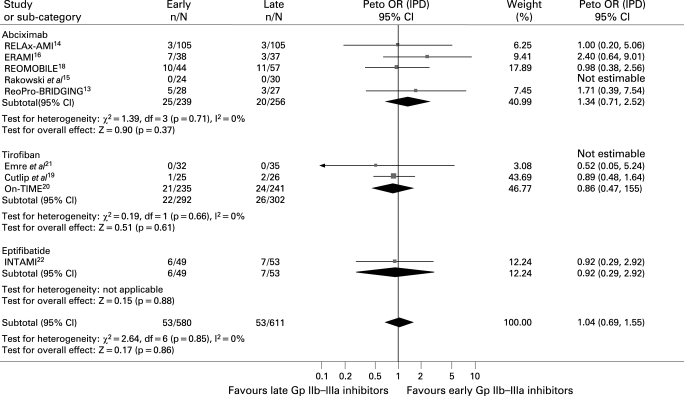
Early Gp IIb–IIIa inhibitors were associated with a non-significantly higher rate of abortion of MI (9.7% versus 8.1%, Peto OR 1.04; 95% CI 0.69 to 1.55; p = 0.86, phet = 0.85; fig 7).
Figure 7. Facilitation with early Gp IIb–IIIa inhibitors and myocardial abortion with Peto odds ratios and 95% CI. The size of the data markers (squares) is approximately proportional to the statistical weight of each trial. Gp, glycoprotein; IPD, individual patient data; OR, odds ratio.
Clinical endpoints
Mortality
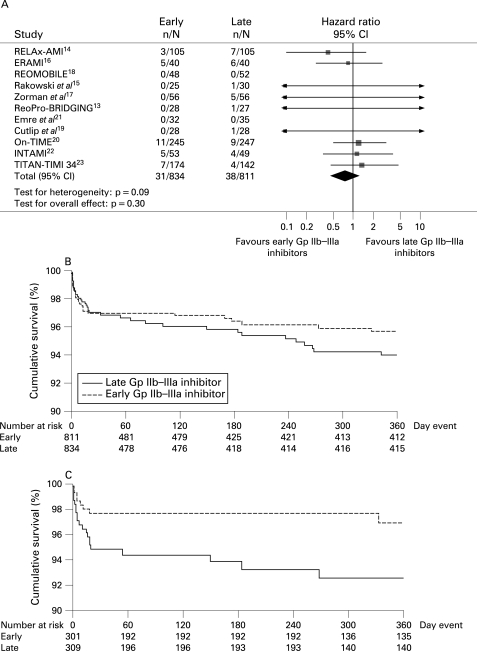
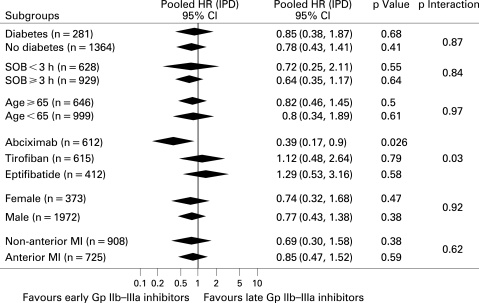
Follow-up data were available at 30 days in four trials,14 19 21 23 at 6 months in one trial17 and at one year in six trials13 15 16 18 20 22 (table 1). As shown in fig 8, early Gp IIb–IIIa inhibitors were associated with non-significantly larger benefits in mortality (3.7% versus 4.7%; hazard ratio (HR) 0.78; 95% CI 0.49 to 1.26; p = 0.3, phet = 0.09), which were more pronounced with abciximab (2.6% versus 6.5%; HR 0.39; 95% CI 0.17 to 0.9; p = 0.026, phet = 0.76; p interaction of abciximab versus small molecules 0.034; fig 9). The results did not change for either overall or abciximab after multivariate adjustment (adjusted HR 0.32; 95% CI 0.24 to 4.13; p = 0.38; adjusted HR 0,38; 95% CI 0.15 to 1.00; p = 0.05, respectively). Additional subgroup analyses did not show any difference in treatment response according to the high-risk subsets of patients.
Figure 8. (A) Facilitation with early Gp IIb–IIIa inhibitors and mortality with pooled hazard ratios and 95% CI. The size of the data markers (squares) is approximately proportional to the statistical weight of each trial. (B) Kaplan–Meier survival curves according to early versus late Gp IIb–IIIa inhibitors. (C) Kaplan–Meier survival curves according to early versus late Gp IIb–IIIa inhibitors in trials with abciximab. Gp, glycoprotein.
Figure 9. Facilitation with early Gp IIb–IIIa inhibitors and mortality with pooled hazard ratios and 95% CI in subgroups of patients. Gp, glycoprotein; HR, hazard ratio; IPD, individual patient data; SOB, symptom onset-to-balloon time.
Safety endpoint
No difference was observed in terms of major bleeding complications (3.2% versus 2.9%, Peto OR 1.13; 95% CI 0.62 to 2.06; p = 0.68, phet = 0.51).
DISCUSSION
The EGYPT cooperation aimed at performing a meta-analysis to evaluate the benefits from the early administration of Gp IIb–IIIa inhibitors in patients undergoing primary angioplasty, based on individual data of 1662 patients enrolled in 11 randomised trials.13–23 The main finding of this meta-analysis is that facilitation with Gp IIb–IIIa inhibitors improved preprocedural recanalisation. In the analysis limited to the abciximab trials, there was a significant mortality reduction with early versus late abciximab administration. It is of note that other subanalyses also demonstrated improvement in postprocedural TIMI 3 flow, MBG, distal embolisation and ST-segment resolution achieved with early abciximab, although the interaction was statistically significant only for MBG and improved ST-segment resolution. Comparisons between agents must be made with extreme caution given the uncontrolled features of the trials, the possible subtherapeutic dosages utilised in some of the studies and the differences in timing of early administration. Also, it must be noted that none of the studies compared agents, so inferences are based on differences between early versus late administration for each agent. Nevertheless, this is the first demonstration of mortality benefits from pharmacological facilitation in STEMI patients undergoing primary angioplasty and indicates a need for further studies to identify the best strategy.
Recent investigations have demonstrated that time to treatment is a relevant issue in primary angioplasty, with a significant impact on mortality.3–5 It has been hypothesised that the early administration of pharmacological therapy may induce earlier reperfusion, resulting in reduced infarct size and improved survival, particularly when long-distance transportation is required.8 9
The ASSENT-4 trial30 showed harmful effects from facilitation with full-dose tenecteplase in patients undergoing primary angioplasty, despite improved preprocedural recanalisation. These data have been explained by a potential intracoronary prothrombotic rebound at the time of angioplasty induced by lysis,31 which could be limited by the administration of Gp IIb–IIIa inhibitors. These benefits may, however, be counterbalanced by a larger incidence of bleeding complications, particularly in elderly patients.32 Several randomised trials have been conducted to investigate the benefits from the early administration of Gp IIb–IIIa inhibitors in patients undergoing primary angioplasty.13–23,27–29 As the adjunctive use of Gp IIb–IIIa inhibitors, mostly abciximab, has been shown to reduce mortality among patients undergoing primary angioplasty,6 7 further benefits would be expected by an early reperfusion achieved by early drug administration.
A subanalysis of the Abciximab before Direct Angioplasty and Stenting in Myocardial Infarction Regarding Acute and Long-Term Follow-up (ADMIRAL) trial showed that early abciximab administration (in the emergency department or in the ambulance) did improve clinical outcome compared with late administration.33
In a recent meta-analysis performed on pharmacological facilitation in primary angioplasty, no overall benefits in short-term (30 days) mortality were observed with inhibitors of Gp IIb–IIIa inhibitors,34 and their use was discouraged by the authors in daily clinical practice, unless in randomised trials.
That meta-analysis did not include all currently available trials, however, and analysed only a restricted number of endpoints with a limited duration of follow-up. Furthermore, no prespecified subanalysis was performed according to the type of molecule. In a recent smaller meta-analysis, restricted to abciximab and including randomised trials and non-randomised subgroup analyses, early abciximab did result in significant benefits in terms of myocardial perfusion and an increased number of aborted infarctions but without significant impact on mortality compared with late administration.35
Although significantly improved preprocedural recanalisation was the major benefit of early Gp IIb–IIIa inhibitors, there were also suggestions of improved myocardial reperfusion. Most notably, complete ST-segment resolution was significantly better overall. Several studies have reported an association between this marker and mortality. Early abciximab, but not the small molecules, was also associated with increased MBG 3 compared with late use. The underlying mechanisms for these beneficial effects may be the diminished distal embolisation of platelet aggregates (as observed in the current meta-analysis) or inhibition of the direct interaction of platelets with the reperfused endothelium by abciximab.36 37
The survival benefits of early Gp IIb–IIIa inhibitors did not significantly change across most of the subgroups analysed.
Disappointing results have been observed in the Facilitated Intervention with Enhanced Reperfusion Speed to Stop Events (FINESSE) trial, recently presented at the 2007 annual meeting of the European Society of Cardiology.29 The trial was prematurely stopped due to slow recruitment, with the inclusion of up to 2500 STEMI patients. No advantages in terms of clinical outcome were observed at 3-month follow-up with facilitation by either combotherapy (abciximab and half-dose reteplase) or abciximab, compared with late periprocedural abciximab administration, despite higher patency rates, mainly with combotherapy. It must be remarked that the FINESSE trial did include several centres with large variability in experience and skills, whereas our meta-analysis included trials mainly conducted at high-volume and highly experienced primary PCI centres. In addition, the slow recruitment rate observed in the FINESSE trial (approximately a mean of 10 patients a year enrolled per centre over 4 years) may have led to a selection bias. Finally, even though the aim of the trial was to investigate facilitation, more than 50% of patients were enrolled and randomly assigned in primary PCI centres. Longer follow-up data and a more extensive analysis will certainly provide important additional information before final conclusions can be drawn from that trial.
The Ongoing Tirofiban in Myocardial Infarction Evaluation 2 (On-TIME-2) trial,38 investigating the early administration of high-dose tirofiban, will certainly provide additional important data on this relevant issue.
Limitations
We were unable to obtain individual patient data from three randomised trials,27–29 whereas the ADMIRAL trial was not included, because it did not compare early versus late administration of Gp IIb–IIIa inhibitors.
Even though the meta-analysis was based on individual patient data, this can not overcome the potential heterogeneity among trials caused by different inclusion and exclusion criteria and the fact that angiographic and ECG data were not analysed by a central core laboratory.
Enzymatic infarct size was estimated by peak creatine kinase levels, whereas the use of scintigraphic techniques would have potentially improved the results of the meta-analysis. The beneficial effects observed in terms of preprocedural recanalisation might have translated into benefits in terms of left ventricular remodelling and larger survival benefits at long-term follow-up, such as up to 3–5 years, which unfortunately were unavailable from current randomised trials.
On the basis of their prognostic implications and availability, we analysed major but not minor bleeding complications.
High-dose tirofiban has been demonstrated to provide higher inhibition of platelet aggregation, compared with a standard bolus dose, as used in trials included in the current meta-analysis and abciximab.39 40 Whether the early administration of this therapy may provide benefits is currently tested in the ongoing On-TIME 2 trial.38
Several factors may have hampered the potential benefits of early eptifibatide administration, such as the restricted number of patients and trials included in the current meta-analysis, the relatively short-term follow-up (available in the vast majority of patients only at 30 days) and the shorter duration of drug administration before angioplasty, compared with other Gp IIb–IIIa inhibitors.
Moreover, it has to be pointed out that this meta-analysis was primarily performed to evaluate early versus late use of Gp IIb–IIIa inhibitors with respect to surrogate markers and clinical endpoints. Given the nature of the randomised studies included in this meta analysis, head-to-head comparisons suggesting significant benefit for early abciximab may reflect larger differences between early versus late abciximab and cannot be interpreted as reflecting the superiority of abciximab over other agents.
Finally, as the patients enrolled in the current randomised trials have for the most part been highly selected, caution should be exercised in extending the conclusion of this meta-analysis to the vast majority of STEMI patients undergoing primary angioplasty. As a result of the higher risk profile, however, at least similar benefits might potentially be expected in trial-ineligible compared with trial-eligible patients.41
CONCLUSIONS
This meta-analysis shows that pharmacological facilitation with Gp IIb–IIIa inhibitors is associated with significant benefits in terms of preprocedural epicardial recanalisation. Despite these beneficial effects, however, early Gp IIb–IIIa inhibitors did translate into non-significant benefits in survival, except for abciximab, explained by the improved myocardial perfusion and less distal embolisation. Therefore, until the results of additional large randomised trials with long-term follow-up data become available, pharmacological facilitation with Gp IIb–IIIa inhibitor administration, particularly abciximab, may be considered in patients undergoing primary angioplasty for STEMI.
Acknowledgments
The authors are indebted to all institutions and investigators involved in the randomised trials included in this meta-analysis. GDL, AWJvH and GB-Z had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Competing interests: GDL received lecture fees from Merck Sharp & Dohme and Eli Lilly; MG received lecture fees from Schering Plough; UZ received research grants and lecture fees from ESSEX Pharma, GSK and Eli Lilly; DD received lecture fees from Eli Lilly; H-RA received an unrestricted grant from Eli Lilly and lecture fees from Boerhinger Ingelheim and Sanofi Aventis; GB-Z is a consultant to Boston Scientific, Cordis and Mediolanum Cardio Research and received lecture fees from Bristol Myers Squibb; AWJvH received lecture fees from Merck Sharp & Dohme.
Contributors: GDL: Conception and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; statistical analysis. AWJvH: Conception and design; analysis and interpretation of data; drafting of the manuscript; statistical analysis. GB-Z: statistical analysis; critical revision of the manuscript; supervision. CMG, FB, SM, MM, MN, UZ, DD, H-RA, SZ, HMG, AE, DC, GB-Z, TR, MG, PM, KH: administrative, technical or material support; critical revision of the manuscript; supervision. All authors declare that they participated in the study and that they have seen and approved the final version.
REFERENCES
- 1.Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet 2003;361:13–20 [DOI] [PubMed] [Google Scholar]
- 2.Nallamothu BK, Bates ER, Herrin J, et al. , and the NRMI Investigators Times to treatment in transfer patients undergoing primary percutaneous coronary intervention in the United States: National Registry of Myocardial Infarction (NRMI)-3/4 analysis. Circulation 2005;111:761–7 [DOI] [PubMed] [Google Scholar]
- 3.De Luca G, Suryapranata H, Ottervanger JP, et al. Time-delay to treatment and mortality in primary angioplasty for acute myocardial infarction: every minute delay counts. Circulation 2004;109:1223–5 [DOI] [PubMed] [Google Scholar]
- 4.De Luca G, van’t Hof AW, de Boer MJ, et al. Time-to-treatment significantly affects the extent of ST-segment resolution and myocardial blush in patients with acute myocardial infarction treated by primary angioplasty. Eur Heart J 2004;25:1009–13 [DOI] [PubMed] [Google Scholar]
- 5.Tarantini G, Cacciavillani L, Corbetti F, et al. Duration of ischemia is a major determinant of transmurality and severe microvascular obstruction after primary angioplasty: a study performed with contrast-enhanced magnetic resonance. J Am Coll Cardiol 2005;46:1229–35 [DOI] [PubMed] [Google Scholar]
- 6.De Luca G, Suryapranata H, Stone GW, et al. Adjunctive abciximab to reperfusion therapy in patients with acute ST-segment elevation myocardial infarction: a meta-analysis of randomized trials. JAMA 2005;193:1759–65 [DOI] [PubMed] [Google Scholar]
- 7.Montalescot G, Antoniucci D, Kastrati A, et al. Abciximab in primary coronary stenting of ST-elevation myocardial infarction: a European meta-analysis on individual patients’ data with long-term follow-up. Eur Heart J 2007;28:443–9 [DOI] [PubMed] [Google Scholar]
- 8.Stone GW, Cox D, Garcia E, et al. Normal flow (TIMI-3) before mechanical reperfusion therapy is an independent determinant of survival in acute myocardial infarction: analysis from the primary angioplasty in myocardial infarction trials. Circulation 2001;104:636–41 [DOI] [PubMed] [Google Scholar]
- 9.De Luca G, Ernst N, Zijlstra F, et al. Preprocedural TIMI flow and mortality in patients with acute myocardial infarction treated by primary angioplasty. J Am Coll Cardiol 2004;43:1363–7 [DOI] [PubMed] [Google Scholar]
- 10.The TIMI Study Group The Thrombolysis in Myocardial Infarction (TIMI) trial. N Engl J Med 1985;312:932–6 [DOI] [PubMed] [Google Scholar]
- 11.van’t Hof AW, Liem A, Suryapranata H, et al. Angiographic assessment of myocardial reperfusion in patients treated with primary angioplasty for acute myocardial infarction: myocardial blush grade. Zwolle Myocardial Infarction Study Group. Circulation 1998;97:2302–6 [DOI] [PubMed] [Google Scholar]
- 12.Henriques JP, Zijlstra F, Ottervanger JP, et al. Incidence and clinical significance of distal embolization during primary angioplasty for acute myocardial infarction. Eur Heart J 2002;23:1112–17 [DOI] [PubMed] [Google Scholar]
- 13.Gyöngyösi M, Domanovits H, Benzer W, et al. , and the ReoPro-BRIDGING Study Group Use of abciximab prior to primary angioplasty in STEMI results in early recanalization of the infarct-related artery and improved myocardial tissue reperfusion—results of the Austrian multi-centre randomized ReoPro-BRIDGING Study. Eur Heart J 2004;25:2125–33 [DOI] [PubMed] [Google Scholar]
- 14.Maioli M, Bellandi F, Leoncini M, et al. Randomized early versus late abciximab in acute myocardial infarction treated with primary coronary intervention (RELAx-AMI Trial). J Am Coll Cardiol 2007;49:1517–24 [DOI] [PubMed] [Google Scholar]
- 15.Rakowski T, Zalewski J, Legutko J, et al. Early abciximab administration before primary percutaneous coronary intervention improves infarct-related artery patency and left ventricular function in high-risk patients with anterior wall myocardial infarction: a randomized study. Am Heart J 2007;153:360–5 [DOI] [PubMed] [Google Scholar]
- 16.Gabriel HM, Oliveira JA, da Silva PC, et al. Early administration of abciximab bolus in the emergency department improves angiographic outcome after primary PCI as assessed by TIMI frame count: results of the early ReoPro administration in myocardial infarction (ERAMI) trial. Catheter Cardiovasc Interv 2006;68:218–24 [DOI] [PubMed] [Google Scholar]
- 17.Zorman S, Zorman D, Noc M. Effects of abciximab pretreatment in patients with acute myocardial infarction undergoing primary angioplasty. Am J Cardiol 2002;90:533–6 [DOI] [PubMed] [Google Scholar]
- 18.Arntz HR, Schroder J, Schwimmbeck P, et al. Is early prehospital administration of abciximab superior to periprocedural theapy in patients with STEMI and planned PCI? Early and late results from the randomized REOMOBILE Pilot study. JACC 2004;43Suppl A [Google Scholar]
- 19.Cutlip DE, Ricciardi MJ, Ling FS, et al. Effect of tirofiban before primary angioplasty on initial coronary flow and early ST-segment resolution in patients with acute myocardial infarction. Am J Cardiol 2003;92:977–80 [DOI] [PubMed] [Google Scholar]
- 20.van’t Hof AW, Ernst N, de Boer MJ, et al. , and the On-TIME study group Facilitation of primary coronary angioplasty by early start of a glycoprotein 2b/3a inhibitor: results of the ongoing tirofiban in myocardial infarction evaluation (On-TIME) trial. Eur Heart J 2004;25:837–46 [DOI] [PubMed] [Google Scholar]
- 21.Emre A, Ucer E, Yesilcimen K, et al. Impact of early tirofiban on myocardial salvage in patients with acute myocardial infarction undergoing infarct-related artery stenting. Cardiology 2006;106:264–9 [DOI] [PubMed] [Google Scholar]
- 22.Zeymer U, Zahn R, Schiele R, et al. Early eptifibatide improves TIMI 3 patency before primary percutaneous coronary intervention for acute ST elevation myocardial infarction: results of the randomized integrilin in acute myocardial infarction (INTAMI) pilot trial. Eur Heart J 2005;26:1971–7 [DOI] [PubMed] [Google Scholar]
- 23.Gibson CM, Kirtane AJ, Murphy SA, et al. , and the TIMI Study Group Early initiation of eptifibatide in the emergency department before primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: results of the Time to Integrilin Therapy in Acute Myocardial Infarction (TITAN)-TIMI 34 trial. Am Heart J 2006;152:668–75 [DOI] [PubMed] [Google Scholar]
- 24.Demets DL. Methods for combining randomized clinical trials: strengths and limitations. Stat Med 1987;6:341–50 [DOI] [PubMed] [Google Scholar]
- 25.Boersma E, Harrington RA, Moliterno DJ, et al. Platelet glycoprotein IIb/IIIa inhibitors in acute coronary syndromes: a meta-analysis of all major randomised clinical trials. Lancet 2002;359:189–98 [DOI] [PubMed] [Google Scholar]
- 26.Steyerberg EW, Bossuyt PM, Lee KL. Clinical trials in acute myocardial infarction: should we adjust for baseline characteristics? Am Heart J 2000;139:745–51 [DOI] [PubMed] [Google Scholar]
- 27.Bolognese L. Effects of pre-treatment with abciximab on coronary artery patency and microcirculation in high risk patients with acute myocardial infarction eligible for primary angioplasty: results of the Abciximab Patients Evaluation (APE) randomized pilot study. Circulation 2000October;150Suppl A [Google Scholar]
- 28.Lee DP, Herity NA, Hiatt BL, et al. TIrofiban Given in the Emergency Room before Primary Angioplasty. Adjunctive platelet glycoprotein IIb/IIIa receptor inhibition with tirofiban before primary angioplasty improves angiographic outcomes: results of the TIrofiban Given in the Emergency Room before Primary Angioplasty (TIGER-PA) pilot trial. Circulation 2003;107:1497–501 [DOI] [PubMed] [Google Scholar]
- 29.Ellis S. The FINESSE Trial (Facilitated INtervention with Enhanced Reperfusion Speed to Stop Events). http://www.escardio.org/congresses/esc_congress/esc2007/news/HLIIFinesseEllisVDWerf.htm (accessed 6 Oct 2007). [Google Scholar]
- 30.Assessment of the Safety and Efficacy of a New Treatment Strategy with Percutaneous Coronary Intervention (ASSENT-4 PCI) investigators Primary versus tenecteplase-facilitated percutaneous coronary intervention in patients with ST-segment elevation acute myocardial infarction (ASSENT-4 PCI): randomised trial. Lancet 2006;367:569–78 [DOI] [PubMed] [Google Scholar]
- 31.Eisenberg PR, Sobel BE, Jaffe AS. Activation of prothrombin accompanying thrombolysis with recombinant tissue-type plasminogen activator. J Am Coll Cardiol 1992;19:1065–9 [DOI] [PubMed] [Google Scholar]
- 32.Savonitto S, Armstrong PW, Lincoff AM, et al. Risk of intracranial haemorrhage with combined fibrinolytic and glycoprotein IIb/IIIa inhibitor therapy in acute myocardial infarction. Dichotomous response as a function of age in the GUSTO V trial. Eur Heart J 2003;24:1807–14 [DOI] [PubMed] [Google Scholar]
- 33.Montalescot G, Barragan P, Wittenberg O, et al. , and the ADMIRAL Investigators Abciximab before direct angioplasty and stenting in myocardial infarction regarding acute and long-term follow-up. Platelet glycoprotein IIb/IIIa inhibition with coronary stenting for acute myocardial infarction. N Engl J Med 2001;344:1895–903 [DOI] [PubMed] [Google Scholar]
- 34.Keeley EC, Boura JA, Grines CL. Comparison of primary and facilitated percutaneous coronary interventions for ST-elevation myocardial infarction: quantitative review of randomised trials. Lancet 2006;367:579–88 [DOI] [PubMed] [Google Scholar]
- 35.Gödicke J, Flather M, Noc M, et al. Early vs periprocedural administration of abciximab for primary angioplasty: a pooled analysis of six studies. Am Heart J 2005;150:1015.e11–17 [DOI] [PubMed] [Google Scholar]
- 36.Coller BS. Potential non-glycoprotein IIb/IIIa effects of abciximab. Am Heart J 1999;138Suppl:S1–5 [DOI] [PubMed] [Google Scholar]
- 37.Schwarz M, Nordt T, Bode C, et al. The GP IIb/IIIa inhibitor abciximab (c7E3) inhibits the binding of various ligands to the leukocyte integrin Mac-1 (CD11b/CD18, alphaMbeta2). Thromb Res 2002;107:121–8 [DOI] [PubMed] [Google Scholar]
- 38.van’t Hof AWJ. A randomized, controlled trial evaluating the benefits of early up-front-loaded high dose tirofiban in the treatment of patients with ST-segment elevation myocardial infarction, who are candidates for primary angioplasty. On-TIME 2 trial. http://www.trialregister.nl/trialreg/admin/rctview.asp?TC = 74. [Google Scholar]
- 39.Schneider DJ, Herrmann HC, Lakkis N, et al. Enhanced early inhibition of platelet aggregation with an increased bolus of tirofiban. Am J Cardiol 2002;90:1421–3 [DOI] [PubMed] [Google Scholar]
- 40.Ernst NM, Suryapranata H, Miedema K, et al. Achieved platelet aggregation inhibition after different antiplatelet regimens during percutaneous coronary intervention for ST-segment elevation myocardial infarction. J Am Coll Cardiol 2004;44:1187–93 [DOI] [PubMed] [Google Scholar]
- 41.Dabbous HO, Anderson FA, Gore JM, et al. , for the GRACE Investigators Outcomes with the use of glycoprotein IIb/IIIa inhibitors in non-ST-segment elevation acute coronary syndromes. Heart 2008;94:159–65 [DOI] [PubMed] [Google Scholar]