Abstract
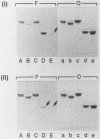
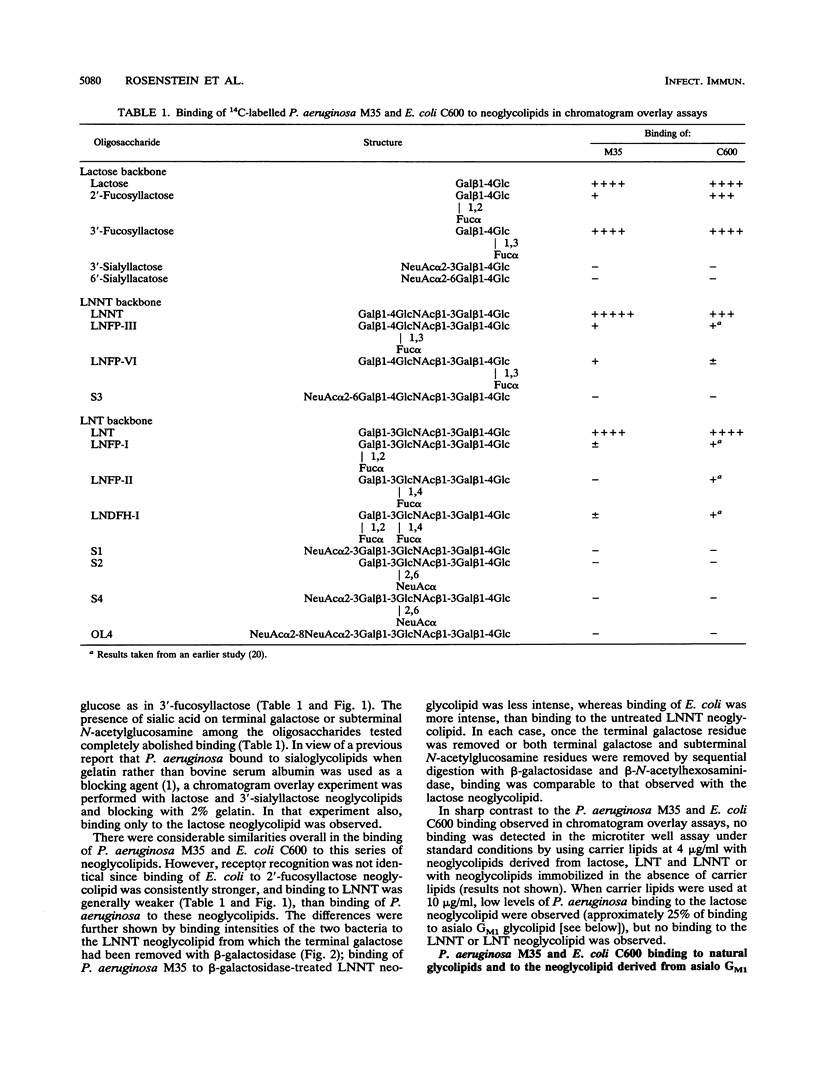
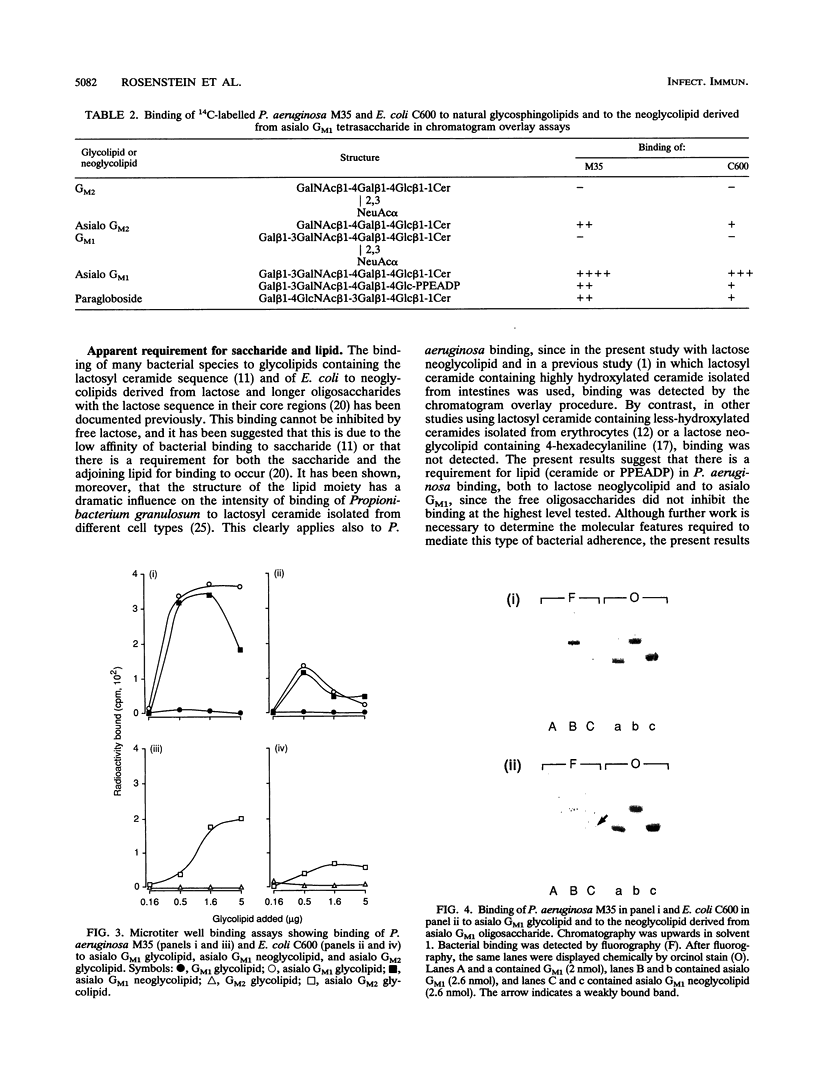
Membrane glycolipids contain the lactose sequence (galactose linked to glucose), and the oligosaccharide is variously extended such that there is a cell-type-specific repertoire. In this study, binding of Pseudomonas aeruginosa M35 to lipid-linked lactose (Gal beta 1-4Glc [structure 1]), lacto-N-neotetraose (Gal beta 1-4GlcNAc beta 1-3Gal beta 1-4Glc [structure 2]), lacto-N-tetraose (Gal beta 1-3GlcNAc beta 1-3Gal beta 1-4Glc [structure 3]), and asialo GM1 (Gal beta 1-3GalNAc beta 1-4Gal beta 1-4Glc [structure 4]) was evaluated and compared with binding of Escherichia coli C600 to these compounds. Oligosaccharides were linked to the lipid phosphatidylethanolamine dipalmitoate, and the resulting neoglycolipids were resolved on thin-layer chromatograms or coated onto plastic microtiter wells. Lipid-linked structures 1 to 4 were bound by P. aeruginosa and E. coli in the chromatogram assay, but only structure 4 was bound in the microtiter well assay. As shown previously for E. coli binding to lipid-linked structures 1 to 3, binding to lipid-linked structure 4 was not inhibited with oligosaccharide, indicating a requirement for lipid and oligosaccharide. With few exceptions, sialylation and fucosylation of structures 1 to 4 resulted in impaired or abolished binding. Comparisons of binding intensities in the chromatogram assay indicated that recognition by P. aeruginosa and recognition by E. coli are not identical. Presence of the additional disaccharide unit, as in structure 2, resulted in enhanced binding of P. aeruginosa but diminished binding of E. coli relative to lactose binding; fucosylation at galactose of lactose resulted in markedly diminished binding of P. aeruginosa only. In the microtiter well assay, binding of E. coli to asialo GM1 was much weaker than P. aeruginosa binding. The saccharide-plus-lipid-dependent adhesion may be an important factor in increased susceptibility to infection of epithelia already damaged by microbial and chemical agents; the differing strengths of adhesion to the structural variants may relate to tissue tropism.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker N. R., Minor V., Deal C., Shahrabadi M. S., Simpson D. A., Woods D. E. Pseudomonas aeruginosa exoenzyme S is an adhesion. Infect Immun. 1991 Sep;59(9):2859–2863. doi: 10.1128/iai.59.9.2859-2863.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker N., Hansson G. C., Leffler H., Riise G., Svanborg-Edén C. Glycosphingolipid receptors for Pseudomonas aeruginosa. Infect Immun. 1990 Jul;58(7):2361–2366. doi: 10.1128/iai.58.7.2361-2366.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D. E., Pitt T. L. An immunological study of the pili of Pseudomonas aeruginosa. J Hyg (Lond) 1975 Jun;74(3):419–430. doi: 10.1017/s0022172400046933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deal C. D., Krivan H. C. Lacto- and ganglio-series glycolipids are adhesion receptors for Neisseria gonorrhoeae. J Biol Chem. 1990 Aug 5;265(22):12774–12777. [PubMed] [Google Scholar]
- Feizi T. Glycoprotein oligosaccharides as recognition structures. Ciba Found Symp. 1989;145:62-74, discussion 74-9. doi: 10.1002/9780470513828.ch5. [DOI] [PubMed] [Google Scholar]
- Firon N., Ofek I., Sharon N. Interaction of mannose-containing oligosaccharides with the fimbrial lectin of Escherichia coli. Biochem Biophys Res Commun. 1982 Apr 29;105(4):1426–1432. doi: 10.1016/0006-291x(82)90947-0. [DOI] [PubMed] [Google Scholar]
- Friend P. A. Pulmonary infection in cystic fibrosis. J Infect. 1986 Jul;13(1):55–72. doi: 10.1016/s0163-4453(86)92325-x. [DOI] [PubMed] [Google Scholar]
- Høiby N., Koch C. Cystic fibrosis. 1. Pseudomonas aeruginosa infection in cystic fibrosis and its management. Thorax. 1990 Nov;45(11):881–884. doi: 10.1136/thx.45.11.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagannatha H. M., Sharma U. K., Ramaseshan T., Surolia A., Balganesh T. S. Identification of carbohydrate structures as receptors for localised adherent enteropathogenic Escherichia coli. Microb Pathog. 1991 Oct;11(4):259–268. doi: 10.1016/0882-4010(91)90030-e. [DOI] [PubMed] [Google Scholar]
- Karlsson K. A. Animal glycosphingolipids as membrane attachment sites for bacteria. Annu Rev Biochem. 1989;58:309–350. doi: 10.1146/annurev.bi.58.070189.001521. [DOI] [PubMed] [Google Scholar]
- Krivan H. C., Ginsburg V., Roberts D. D. Pseudomonas aeruginosa and Pseudomonas cepacia isolated from cystic fibrosis patients bind specifically to gangliotetraosylceramide (asialo GM1) and gangliotriaosylceramide (asialo GM2). Arch Biochem Biophys. 1988 Jan;260(1):493–496. doi: 10.1016/0003-9861(88)90473-0. [DOI] [PubMed] [Google Scholar]
- Neeser J. R., Koellreutter B., Wuersch P. Oligomannoside-type glycopeptides inhibiting adhesion of Escherichia coli strains mediated by type 1 pili: preparation of potent inhibitors from plant glycoproteins. Infect Immun. 1986 May;52(2):428–436. doi: 10.1128/iai.52.2.428-436.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orø H. S., Kolstø A. B., Wennerås C., Svennerholm A. M. Identification of asialo GM1 as a binding structure for Escherichia coli colonization factor antigens. FEMS Microbiol Lett. 1990 Nov;60(3):289–292. doi: 10.1016/0378-1097(90)90319-l. [DOI] [PubMed] [Google Scholar]
- Paruchuri D. K., Seifert H. S., Ajioka R. S., Karlsson K. A., So M. Identification and characterization of a Neisseria gonorrhoeae gene encoding a glycolipid-binding adhesin. Proc Natl Acad Sci U S A. 1990 Jan;87(1):333–337. doi: 10.1073/pnas.87.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramphal R., Carnoy C., Fievre S., Michalski J. C., Houdret N., Lamblin G., Strecker G., Roussel P. Pseudomonas aeruginosa recognizes carbohydrate chains containing type 1 (Gal beta 1-3GlcNAc) or type 2 (Gal beta 1-4GlcNAc) disaccharide units. Infect Immun. 1991 Feb;59(2):700–704. doi: 10.1128/iai.59.2.700-704.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramphal R., Houdret N., Koo L., Lamblin G., Roussel P. Differences in adhesion of Pseudomonas aeruginosa to mucin glycopeptides from sputa of patients with cystic fibrosis and chronic bronchitis. Infect Immun. 1989 Oct;57(10):3066–3071. doi: 10.1128/iai.57.10.3066-3071.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramphal R., Pyle M. Adherence of mucoid and nonmucoid Pseudomonas aeruginosa to acid-injured tracheal epithelium. Infect Immun. 1983 Jul;41(1):345–351. doi: 10.1128/iai.41.1.345-351.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstein I. J., Stoll M. S., Mizuochi T., Childs R. A., Hounsell E. F., Feizi T. New type of adhesive specificity revealed by oligosaccharide probes in Escherichia coli from patients with urinary tract infection. Lancet. 1988 Dec 10;2(8624):1327–1330. doi: 10.1016/s0140-6736(88)90868-9. [DOI] [PubMed] [Google Scholar]
- Stoll M. S., Hounsell E. F., Lawson A. M., Chai W. G., Ten F. Z. Microscale sequencing of O-linked oligosaccharides using mild periodate oxidation of alditols, coupling to phospholipid and TLC-MS analysis of the resulting neoglycolipids. Eur J Biochem. 1990 May 20;189(3):499–507. doi: 10.1111/j.1432-1033.1990.tb15515.x. [DOI] [PubMed] [Google Scholar]
- Stoll M. S., Mizuochi T., Childs R. A., Feizi T. Improved procedure for the construction of neoglycolipids having antigenic and lectin-binding activities, from reducing oligosaccharides. Biochem J. 1988 Dec 1;256(2):661–664. doi: 10.1042/bj2560661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromberg N., Deal C., Nyberg G., Normark S., So M., Karlsson K. A. Identification of carbohydrate structures that are possible receptors for Neisseria gonorrhoeae. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4902–4906. doi: 10.1073/pnas.85.13.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strömberg N., Karlsson K. A. Characterization of the binding of propionibacterium granulosum to glycosphingolipids adsorbed on surfaces. An apparent recognition of lactose which is dependent on the ceramide structure. J Biol Chem. 1990 Jul 5;265(19):11244–11250. [PubMed] [Google Scholar]
- Strömberg N., Nyholm P. G., Pascher I., Normark S. Saccharide orientation at the cell surface affects glycolipid receptor function. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9340–9344. doi: 10.1073/pnas.88.20.9340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishwanath S., Ramphal R. Adherence of Pseudomonas aeruginosa to human tracheobronchial mucin. Infect Immun. 1984 Jul;45(1):197–202. doi: 10.1128/iai.45.1.197-202.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]