Abstract
This study has examined the stimulatory and costimulatory effects of IL-18 on two subsets of murine small intestinal intraepithelial lymphocytes (IELs) defined by the expression of the CD43 S7 glycoform. Data from gene array studies and real-time PCR indicated that S7+ IELs had significantly higher levels of gene expression for the IL-18 receptor and the IL-18R accessory protein than S7− IELs. IL-18 costimulation of IELs in conjunction with CD3-induced activation resulted in significantly greater proliferation than CD3 stimulation alone. In CFSE dilution experiments, IL-18 costimulation favored the S7+ IEL population. IL-18 costimulation did not affect apoptosis of either S7− or S7+ IELs compared with CD3 stimulation alone. Although IL-18 costimulation did not alter the total number of IFN-γ-producing cells relative to CD3 stimulation alone, twice as many S7+ IELs were IFN-γ-secreting cells than S7− IELs in both CD3-stimulated and IL-18-costimulated cultures. Notably, direct IL-18 stimulation in the absence of CD3 activation induced an IFN-γ response that was predominantly directed to the S7+ population, indicating that IL-18 is itself an IFN-γ activational signal for intestinal T cells. In contrast, direct IL-18 stimulation of IELs did not generate TNF-α-producing cells, indicating a differential response in the activation of proinflammatory cytokines following IL-18 exposure. These findings point to distinctly different activational effects of IL-18 on IELs, both with regard to the type of functional responses elicited and with respect to the IEL subsets affected.
Keywords: costimulation, cytokine, mucosa, T cells
INTRODUCTION
Because of their strategic location throughout the intestinal epithelium, it is generally believed that intestinal intraepithelial lymphocytes (IELs) are important in the defense against intestinal infection [1] and that they also contribute to disease processes of intestinal autoimmune pathologies [2–4]. Moreover, IELs in mice [5, 6] and rats [7] differ from other peripheral T cells in that a high proportion of IELs develop extra-thymically within intestinal anatomical sites referred to as cryptopatches. Difficulties in ascribing functional activities to IELs have been confounded, in part, by their extensive heterogeneity [8–10]. IELs express cytotoxic activity in freshly-isolated preparations [11–15]; however, they normally do not produce cytokines until a stimulatory signal has been received through the TCR/CD3 complex or following mitogen activation [15, 16]. Moreover, with the exception of CD69 [15, 17], most IELs do not express markers commonly associated with activated T cells, such as OX40, ICOS, or Ly-6C, and when isolated from naïve mice, they are primarily CD44low and CD45RBhigh [15]. Following CD3-mediated activation or virus infection, IELs acquire OX40, ICOS, and Ly-6C expression and become CD44high and CD45RBlow [15, 17]. In general, IELs have a low proliferative potential in vitro [18–20]. Hence, murine small intestinal IELs exhibit some but not all properties of activated T cells, a characteristic of IELs that is supported by gene array studies [21, 22].
Factors that regulate IEL immune function are poorly understood, although they are central to elucidating the underlying mechanisms of immunity and inflammation at the level of the intestinal mucosa. As part of our studies aimed at gaining a better understanding of the functional properties of IELs, we reported that murine small intestinal IELs consist of two cell populations based on whether they express a CD43 glycoform identified by reactivity with anti-CD43 mAb S7. Thus, although >95% of all IELs express the CD43 core molecule recognized by mAb R2/60 [11], only about half of the IELs are S7+ cells [11]. That difference appears to be specific for intestinal T cells since nearly all T cells in other immunological compartments are S7+ cells [23]. Of particular interest, S7 expression on IELs does not correlate with the type of TCR (αβ vs. γδ) or CD8 (αα vs. αβ) expression [11], both of which have commonly been used to differentiate IELs functionally and developmentally [24]. S7+ IELs are more cytolytic in redirected cytotoxicity assays; they express higher levels of Th1 and Th2 cytokines following CD3 stimulation; and they are the primary IFN-γ-producing cell in the terminal ileum of IL-10−/− mice [11]. Gene-array studies indicate that S7+ IELs are more typical of activated T cells and have properties of adaptive immunity, whereas S7− cells express genes associated with innate immunity [11].
IL-18 is a powerful proinflammatory cytokine that has diverse biological effects that are primarily, though not exclusively, associated with Th1 responses [25–28]. IL-18 has been shown to induce IFN-γ synthesis [29] and to augment human IEL proliferation, which can act synergistically with IL-2, IL-7, or IL-15, either alone or in combination with CD3 stimulation [30]. IL-18 synthesis has been linked to many disease conditions, including the pathology of inflammatory bowel disease [31–39], graft vs. host disease [40], and spontaneous autoimmune diseases [41], and it contributes to the immune defense mechanism in response to infectious agents [25, 42, 43].
On the basis of the above observations, we hypothesized that S7+ and S7− IELs would differ according to their functional responsiveness to IL-18 and that activation via IL-18 would be most pronounced in the S7+ population. As reported here, S7+ IELs had significantly higher levels of gene expression for the IL-18 receptor (IL-18R) and the IL-18R accessory protein (IL-18RAcP) than S7− IELs. IL-18 differentially influenced the responsiveness of IELs with regard to CD3-mediated proliferation and IFN-γ synthesis, thus further documenting the value of using CD43 expression to operationally define intestinal T cell subsets.
MATERIALS AND METHODS
Mice
Adult female C57BL/6 mice, 6–8 wk of age, were purchased from Harlan (Indianapolis, IN, USA). Animals were used in accord with The University of Texas Animal Welfare Guidelines.
IEL isolation, antibodies, and flow cytometry
Small intestinal IELs were isolated according to published protocols [44]. Antibodies and staining reagents used in this study were: purified anti-mouse CD16/32 (2.4G2); NA/LE anti-CD3 (145-2C11); purified NA/LE anti-hamster Ig (G94-56); biotin-anti-CD43 (S7); FITC-annexin V; propidium iodide; monensin (BD PharMingen; San Diego, CA, USA); PE-anti-IFN-γ (XMG1.2); PE-anti-TNF-α (MP6-XT22); streptavidin-APC (eBioscience; San Diego, CA, USA); CFSE (Invitrogen-Molecular Probes; Carlsbad, CA, USA). Flow cytometric analysis was done using a FACSCalibur flow cytometer using Cell Quest software (BD Biosciences). Recombinant IL-15 was purchased from Sigma-Aldrich (St. Louis, MO, USA).
For intracellular IFN-γ and TNF-α staining, IELs were cultured at a density of 1×106 cells/ml in 24-well tissue culture plates (Costar; Corning, NY, USA) coated with 1 μg/ml anti-hamster mAb followed by 1 μg/ml anti-CD3 mAb with or without 50, 100, or 200 ng/ml IL-18 (R&D Systems; St. Paul, MN, USA). After 18 h, 1 μl/ml monensin was added for 4–6 h. Intracellular IFN-γ and TNF-α staining were done according to previously described protocols [11], using a commercial cell staining kit (BD PharMingen) and the manufacturer’s reagents and protocols, with surface staining using biotin-labeled anti-CD43 S7 mAb plus streptavidin-APC.
In vitro proliferation and CFSE staining
Wells of 96-well microtiter plates (Corning #3595) were coated by incubating overnight at 4°C with 50 μl of 1 μg/ml anti-hamster Ig in PBS. The following day, wells were washed with PBS and 50 μl of 1μg/ml anti-CD3 mAb was added to appropriate wells for 2 h at 37°C. Wells were washed with PBS prior to the addition of cells. IELs were added at 1 × 105 cells in 200 μl of RPMI 1640 supplemented with FCS (10% v/v), 100 U/ml penicillin-streptomycin, 2 mM L-glutamine, and 5 × 10−5 M 2-mercaptoethanol (all reagents; Sigma-Aldrich, St. Louis, MO, USA). IL-18 was added at a final concentration of 100 or 200 ng/ml to appropriate wells. Plates were cultured for 42 h at 37°C. 1 μCi 3H-thymidine was added in 20 μl of unsupplemented RPMI-1640 for 6 h; cells were collected using a semiautomated cell harvester (Skatron Instruments; Sunnyvale, CA, USA), and 3H-thymidine levels were measured with a Beckman-Coulter Model LS3801 β-counter (Fullerton, CA, USA).
For CFSE staining, IELs were stained with 2 ml of 5 μM CFSE (Invitrogen; Carlsbad, CA, USA) prepared in DMSO. Cells were incubated for 15 min at 37°C in 5% CO2 environment, pelleted to remove unbound stain, resuspended in 2 ml of supplemented RPMI-1640 (37°C), and cultured for 30 min at 37°C/5% CO2 to ensure complete modification of probe. Cells were washed by centrifugation and cultured in 24-well plates at a density of 1 × 106 cells/ml in supplemented RPMI-1640 without stimulation, with plate-bound anti-CD3 (as above), or with plate-bound anti-CD3 plus 100 or 200 ng/ml of IL-18. After 72 h, cells were harvested, stained with biotin-labeled anti-CD43 S7 plus streptavidin-APC, and analyzed by flow cytometry.
Gene expression analysis
Gene expression microarray studies of purified CD43 S7+ and S7− IELs were done as described previously by our laboratory [11]. Real-time PCR was done using S7+ and S7− IELs purified by autoMACS sorting (Miltenyi Biotec; Auburn, CA, USA). Total RNA was isolated using an RNeasy Protect Mini Kit-50; samples were treated with DNase using an RNase-Free DNase Set-50 (Qiagen; Valencia, CA, USA), according to the manufacturer’s protocols. RNA concentrations were estimated electrophotometrically at A260. Primer sets for mouse IL-18R, GenBank Accession No. NM_008365 for a 174 bp fragment with reference position 1645–1665 of IL-18R mRNA; mouse IL-18RAcP, GenBank Accession No. NM_010553 for a 191-bp fragment with reference position 1866–1888 of IL-18RAcP mRNA; and mouse GAPDH, GenBank Accession No. NM_001001, were purchased from SuperArray Bioscience Corp. (Frederick, MD, USA). Quantitative real-time PCR was performed on 100 ng total RNA using an iScript One-Step RT-PCR kit with SYBR Green (Bio-Rad; Hercules, CA, USA). A blank sample with RNase-free water was used for primer controls. Amplification was done in 96-well thin-wall plates sealed with optical quality film (Bio-Rad) in a Mini-Opticon (Bio-Rad) with a program of 10 min at 50°C for cDNA synthesis, 5 min at 95°C for reverse transcriptase inactivation, followed by 45 cycles of 95°C for 10 s and 55°C for 30 s for data collection. A melt curve was performed using a protocol of 1 min at 95°C, 1 min at 55°C, increasing the temperature in 0.5°C increments for 80 cycles of 10 s each. Real-time PCR data were quantified using the 2−ΔΔCt method of Livak and Schmittgen with each sample normalized to its GAPDH value [45] with a Gene Expression Macro Version 1.1 program (Bio-Rad). For each gene evaluated, the lowest expressing sample was assigned a value of one; the value of the other sample was expressed relative to that. The amplified gene products were electrophoresed through a 2% agarose gel followed by staining with ethidium bromide.
Statistical analyses
Comparisons of multiple culture group combinations were done using two-way factorial analysis of variance (ANOVA). In the event of statistical significance (P<0.05) by ANOVA, post hoc analysis between groups was done using a t-test with unequal variances; a P value of <0.05 was considered to be statistically significant.
RESULTS
The IL-18R and IL-18RAcP genes are preferentially expressed in S7+ IELs
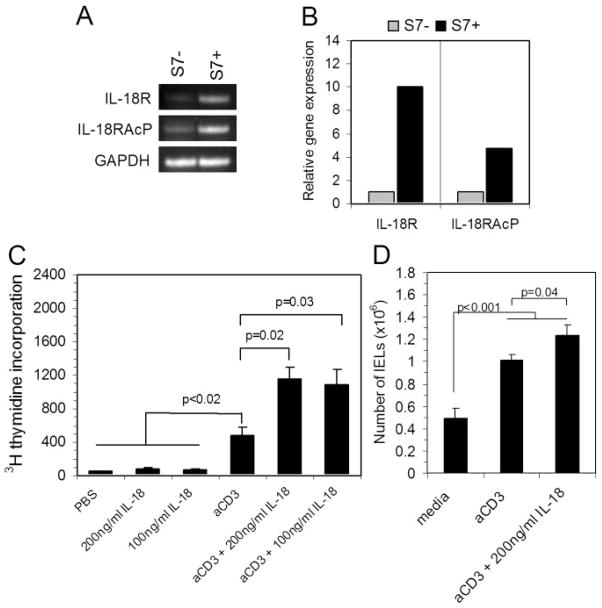
As part of a continuing analysis of data obtained from gene array studies of S7+ and S7− IELs [11], we observed that the IL-18R and the IL-18RAcP genes, both of which are required for IL-18 signaling [46], were expressed at significantly higher levels in the S7+ IEL population. Those differences are shown in Table 1, which shows ≥3-fold excess at a statistically significant level (P<0.05) for the expression of both genes in the S7+ population compared with S7− IELs. This consisted of analyses of three independent isolates of S7+ IELs and two independent isolates of S7− IELs [11]. Those findings were confirmed by conventional (Fig. 1A) and real-time PCR (Fig. 1B) using IELs purified by autoMACS sorting, which indicated higher levels of gene expression for IL-18R and IL-18RAcP in the S7+ population compared with S7− cells.
TABLE 1.
IL-18R and IL-18RAcP Gene Expression in S7+ IELs Relative to S7− IELs
Fig. 1.
Effect of IL-18 costimulation on IEL proliferation. (A) PCR analysis using RNA from S7− and S7+ sorted IELs amplified with primer sets for the murine IL-18R and the IL-18RAcP. Note the difference in PCR product in the S7+ sample compared with the S7−sample. (B) PCR quantification was determined by real-time PCR, which indicated higher gene expression levels for IL-18R and IL-18RAcP in S7+ vs. S7− IELs. (C) 3H-thymidine incorporation after 48 h of in vitro stimulation using the conditions indicated in the graph. Note the enhancement of proliferation in IL-18 co-stimulated cultures, and the lack of proliferation due to direct IL-18 stimulation alone. On the basis of two-way factorial ANOVA, there was a highly significant difference between all six groups (P<0.001), and between the anti-CD3, and anti-CD3 plus IL-18 costimulation groups (P<0.01). Post hoc analysis between individual groups using t-test with unequal variances resulted in the significance levels shown in the graph. Values are numbers of triplicate cultures; data are representative of 2 experiments. (D) Numbers of IELs in cultures after 48 h stimulation. Note the increase in the number of IELs due to IL-18 costimulation. On the basis of two-way factorial ANOVA, there was a highly significant difference between the three groups (P<0.001). Post hoc analysis of individual groups using t-test with unequal variances resulted in significance levels shown in the graph. Data are derived from 4 experiments.
S7+ IELs are more responsive to proliferation mediated by IL-18 costimulation than S7− IELs
Studies of human IELs indicate that IL-18 is an activational signal for proliferation in conjunction with CD3-mediated stimulation [30]. Additionally, IL-18 has been reported to contribute to the proliferation of mucosal lymphocytes in Crohn’s disease [47]. Thus, we were interested in determining the extent to which IL-18 influences the proliferative response of murine IELs and, more importantly, to determine which CD43 IEL population is most responsive to IL-18-mediated proliferation. Although, as expected, CD3-mediated stimulation resulted in greater IEL proliferation compared with nonstimulated IELs based on in vitro 3H-thymidine incorporation 48 h post-CD3 stimulation, there was a statistically significant increase in 3H-thymidine uptake among IL-18 costimulated IELs compared with cells stimulated via CD3 alone; this was true for two concentrations of IL-18 (Fig. 1C). In the absence of CD3 stimulation, IL-18 did not induce cell proliferation (Fig. 1C). IL-18 costimulation also increased the number of total IELs recovered after 48 h of proliferation compared with CD3 stimulation alone (Fig. 1D), further demonstrating the proliferation-inducing effects of IL-18 in combination with CD3-driven activation.
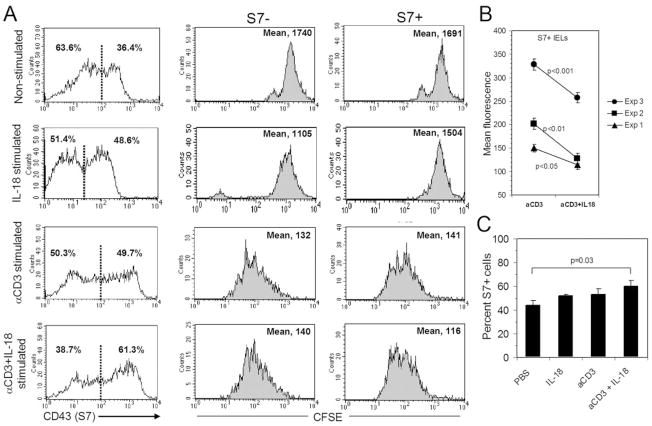
To determine which CD43 population was most responsive to IL-18 costimulation, CFSE-labeled cells were used to track cell proliferation profiles across time. IELs were labeled with CFSE and cultured for 72 h without stimulation, with IL-18 alone, with CD3 stimulation, or with CD3 stimulation in the presence of 100 ng/ml of IL-18. Cells were recovered from cultures and stained for surface expression of CD43 S7 and analyzed by flow cytometry. IL-18 costimulation had a slight but not statistically significant effect on the proliferation of the S7− IEL population (mean 140 vs. 132) (Fig. 2A). In contrast, on the basis of CFSE dilution experiments, IL-18 costimulation augmented the proliferation of S7+ IELs relative to S7+ cells stimulated via CD3 alone (mean 116 vs. 141) (Fig. 2A). These findings were consistent in three independent experiments as shown in Fig. 2B. Differences in the levels of CFSE dilution between experiments likely reflect variations in the initial labeling of cells with CFSE; however, mean fluorescence values of IL-18 costimulated S7+ IELs in each experiment were significantly lower than that of S7+ IELs stimulated via CD3 alone. Additionally, proportionally, more cells were S7+ in the IL-18 costimulation population after 72 h of culture (Fig. 2A, C). These findings collectively indicate that the effect of IL-18 costimulation is principally targeted to the S7+ cell population.
Fig. 2.
(A) Effects of CFSE dilution in S7− and S7+ IELs during 72 h of culture with stimulation by PBS (nonstimulated), IL-18 alone, anti-CD3 stimulation, or anti-CD3 plus 100 ng/ml IL-18 costimulation. Note the increase in proliferation in the S7+ population with IL-18 costimulation compared with S7+ cells stimulated via CD3 alone. (B) Comparison of data from three independent experiments for mean fluorescence values of S7+ IELs. On the basis of two-way factorial ANOVA and pos hoc analysis by t-test with unequal variance, S7+ IELs differed significantly between CD3-stimulated cultures and IL-18 costimulated cultures as shown in the graph. (C) IL-18 costimulation also resulted in a statistically greater (P=0.03) numbers of S7+ IELs between the nonstimulated cultures and the IL-18 costimulated cultures after 72 h, based on the proportion of S7+ cells calculated from the CD43 S7 histograms, in panel A, as determined by t-test analyses with unequal variances for data from individual groups. Demarcation between S7− and S7+ populations was determined from the average distance between mean peaks of each group. Other combinations were not statistically significant. Data are representative of 3 experiments.
IL-18 costimulation does not alter activation-induced cell death compared with CD3 stimulation alone
In mice with induced colitis, IL-18 has been shown to suppress apoptosis of intestinal lymphoid cells [48]. To determine whether IL-18 would alter the pattern of apoptosis following the activation of S7− and S7+ IELs, annexin-V/PI profiles were assessed following CD3 stimulation or with IL-18 costimulation. For both S7− and S7+ IELs, a lack of stimulation or stimulation with IL-18 alone resulted in minimal apoptosis (Fig. 3A, annexin-V+/PI−). CD3-mediated stimulation or IL-18 costimulation resulted in high level of apoptosis for both S7− and S7+ IELs, which was roughly equivalent in both groups (Fig. 3A, B). Thus, IL-18 costimulation had no significant effect on the apoptosis pattern of IELs in either a positive or negative manner.
Fig. 3.
(A) Annexin-V/PI staining of IELs cultures for 24 h in PBS (nonstimulated), with IL-18 alone, with CD3 stimulation (αCD3), or with IL-18 costimulation (αCD3 + IL-18). (B) Both anti-CD3 stimulation and IL-18 costimulation resulted in significantly more apoptosis as determined by ANOVA and post hoc t-test with unequal variances for S7− and S7+ IELs compared with nonstimulated or direct IL-18 stimulated populations. No statistically significant difference was observed between cells stimulated with CD3 or costimulated with IL-18. Data are representative of 3–5 experiments.
Differential effects of IL-18 on IFN-γ synthesis directed to S7− and S7+ subsets
IL-18 has been shown to promote IFN-γ synthesis [27], which can be augmented by IL-12 [49, 50]. To understand how IL-18 might influence the synthesis of IFN-γ in IELs, we examined the effect of IL-18 as an inductive signal for IFN-γ production by IELs in the presence or absence of CD3 stimulation. In 24-h cultures, IL-18 costimulation did not significantly alter the number of intracellular IFN-γ-producing cells in either the S7+ or the S7− population compared with CD3 stimulation alone; however, there were consistently more IFN-γ-secreting S7+ than S7− IELs when stimulated via CD3 both in the presence or absence of IL-18 (Fig. 4A). This held true across a range of IL-18 concentrations from 50 to 200 ng/ml (data not shown). Hence, IL-18 costimulation did not lead to more IFN-γ producing IELs. The most significant finding from these studies was the observation that direct IL-18 stimulation, i.e., in the absence of CD3 stimulation, resulted in an IFN-γ response in both the S7+ and S7− populations, which once again favored the S7+ population (Fig. 4A). Interestingly, IL-18 stimulation without CD3 stimulation consistently had brighter fluorescence than IELs stimulated with IL-18 plus CD3 stimulation (Fig. 4A). The significance of this is not yet evident, however. IELs did not produce IFN-γ in the absence of stimulation by either CD3 or IL-18 stimulation (Fig. 4A).
Fig. 4.
Analyses of effects of IL-18 on intracellular IFN-γ and TNF-α in S7+ and S7− IELs. (A) Stimulation of IELs with anti-CD3 alone and anti-CD3 plus 200 ng/ml IL-18 resulted in equivalent numbers of IFN-γ producing cells in the S7− and S7+ populations. Direct stimulation of IELs with IL-18 resulted in significantly greater IFN-γ-producing cells compared with non-stimulated cultures, but significantly less than that of either CD3 stimulation alone or IL-18 costimulation. This was true for both S7− and S7+ IELs. Statistical evaluation consisted of two-way factorial ANOVA for all four culture conditions followed by post hoc analysis using a t-test with unequal variances. Data are from 5–8 experiments. (B) Stimulation of IELs with anti-CD3 alone and anti-CD3 plus 200 ng/ml IL-18 resulted in equivalent numbers of TNF-α-producing cells in the S7− and S7+ populations. However, direct stimulation of IELs with IL-18 resulted in no significant increase in TNF-α-producing cells compared with nonstimulated cultures. This was true for both S7− and S7+ IELs. Statistical evaluation consisted of two-way factorial ANOVA for all four culture conditions followed by post-hoc analysis using a t-test with unequal variances. All cultures used for TNF-α staining were supplemented with 50 ng/ml recombinant IL-15, which we have previously determined is required for the activation of a TNF-α response in IELs. IL-15 is not needed for the induction of an IFN-γ response from IELs with or without IL-18 (data not shown). (C) Cell size analysis of nonstimulated IELs, CD3-stimulated IELs, and IELs stimulated with IL-18 in the absence of CD3 stimulation. Note that IL-18 stimulation did not induce a blastogenic state, even though they produced IFN-γ (data not shown) similar to that shown in panel A. Data are representative of a total of 2–6 experiments.
To determine whether the effect of IL-18 stimulation resulted in the activation of other cytokine responses, TNF-α-producing cells were studied following the stimulation protocols used for IFN-γ analysis, except that cells were cultured in the presence of 50 ng/ml recombinant IL-15. Previous unpublished studies from our laboratory revealed that IL-15 is required as a cofactor for TNF-α synthesis by IELs when stimulated via CD3. We found this to be the case in the present study as well, since IELs cultured without IL-15 failed to generate a TNF-α response under any of the stimulatory conditions, whereas the addition of IL-15 did not alter IFN-γ activity (data not shown). Most important, in contrast to the IFN-γ response elicited by IL-18, direct stimulation of IELs by IL-18 in the absence of CD3 stimulation did not result in the generation of TNF-α-secreting cells (Fig. 4, panel B). Stimulation of IELs by CD3 or by IL-18 costimulation resulted in equivalent numbers of TNF-α producing cells, indicating that as with the IFN-γ response, IL-18 costimulation did not augment the activity of TNF-α. These findings thus point to a differential response of direct IL-18 stimulation with regard to IFN-γ vs. TNF-α. Finally, direct stimulation of IELs by IL-18 did not result in cells becoming blastogenic. Rather, IL-18-stimulated IELs remained typical of nonstimulated cells (Fig. 4C), even though they produced IFN-γ following IL-18 stimulation (Fig. 4A).
DISCUSSION
Several aspects of this study were particularly noteworthy. From the point of view of IEL proliferation, IL-18 functioned as a strict costimulatory molecule that augmented proliferation only in conjunction with a CD3-mediated signal. That finding was supported by CFSE-staining studies, which demonstrated that there was more dilution of CFSE in the S7+ population following IL-18 costimulation compared with S7+ cells stimulated via CD3 alone.
The ability of IL-18 to induce an IFN-γ response differed from its ability to induce proliferation in that IL-18 by itself was a strong inductive signal for IFN-γ synthesis. In repeated experiments, we found little enhancing effect of IL-18 on the synthesis of IFN-γ when delivered with a CD3-mediated signal across a range of IL-18 concentrations (50–200 ng/ml). In contrast, IFN-γ synthesis was evident within 24 h in both the S7+ and S7− subset following direct IL-18 stimulation. As with proliferation, the S7+ IEL population was the predominant IFN-γ source following direct IL-18 stimulation. These findings collectively indicate that 1) IL-18 operates independently of CD3 signaling as an activational signal to induce IFN-γ synthesis in small intestinal IELs; 2) IELs do not need to undergo proliferation for IFN-γ production; and 3) S7+ IELs are the predominant IFN-γ-producing cell population following IL-18 stimulation.
Although IL-18 has been linked to conditions of chronic intestinal inflammation, the mechanistic basis for that remains poorly understood. The findings reported here indicate that IL-18-driven IFN-γ production, particularly by the S7+ subset, may be an important factor in perpetuating intestinal inflammation in mice. Indeed, studies from our laboratory demonstrated that S7+ IELs in the ileum of IL-10−/− mice secrete 10-fold more IFN-γ than S7− IELs [11]. Consequently, S7+ IELs, which preferentially express the IL-18R (Table 1, and Fig. 1A, B), would be capable of synthesizing IFN-γ in the absence of immune activation and without proliferation, as documented by IFN-γ synthesis from nonblastogenic IELs (Fig. 4C). Thus, factors that lead to IL-18 dysregulation, e.g., diminished IL-10 or TGF-β1 activity, would activate a cascade of events leading to IFN-γ synthesis. Importantly, this also could occur following exposure to enteric infectious agents that promote IL-18 production [42, 43].
Other ancillary cytokine effects could be expected to accompany IL-18 production as inferred from studies in a murine trinitrobenzene sulfonic acid (TNBS)-induced colitis model in which blockade of IL-18 using IL-18 binding protein resulted in suppression of colonic inflammation and decreased levels of intestinal TNF-α, IL-6, and IL-1β, all of which normally accompany TNBS-mediated inflammation [51]. Treatment of mice with dextran sulfate sodium (DSS)-induced colitis using either anti-IL-18 antibody or IL-18 binding protein not only decreased intestinal pathology but lowered local cytokine activity, including TNF-α and IFN-γ [52, 53]. Similar beneficial effects were observed using adenovirus anti-sense IL-18 in a model of CD4 T cell-induced colitis [54]. Interestingly, our studies revealed no evidence for the activation of a TNF-α response by IL-18. Nor did we observe activational effects of IL-18 on IL-2 or IL-17 (data not shown). Differences between our studies and in vivo models of inflammation [51–54] may have to do with the fact that, unlike the latter, it is possible to more precisely control and evaluate the effects of stimulation under in vitro conditions. It also should be noted that TNBS-, DSS-, and CD4-induced inflammation are principally associated with colonic tissues, whereas our studies were restricted to small intestinal IELs.
Finally, in view of our present findings regarding an association between IL-18R expression and IFN-γ production following IL-18 exposure, it will be of interest to determine whether depletion of S7+ IELs will ameliorate intestinal inflammation in an animal model such as IL-10−/− mice. These studies will require considerably more experimental work; however, given that depletion of the S7+ population in vivo cannot readily be achieved through gene inactivation methods since S7 is a carbohydrate determinant of the CD43 molecule. Experiments are currently under way using in vivo treatment with anti-S7 antibody as a treatment protocol.
Acknowledgments
This work was supported by National Institutes of Health grant DK-035566. We thank Dr. Charles Streckfus for assistance with statistical analyses.
References
- 1.Kunisawa J, Kiyono H. A marvel of mucosal T cells and secretory antibodies for the creation of first lines of defense. Cell Mol Life Sci. 2005;62:1308–1321. doi: 10.1007/s00018-005-5035-1. [DOI] [PubMed] [Google Scholar]
- 2.Beagley KW, Elson CO. Cells and cytokines in mucosal immunity and inflammation. Gastroenterol Clin North Am. 1992;21:347–366. [PubMed] [Google Scholar]
- 3.Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol. 2002;20:495–549. doi: 10.1146/annurev.immunol.20.100301.064816. [DOI] [PubMed] [Google Scholar]
- 4.Nancey S, Holvoet S, Graber I, Joubert G, Philippe D, Martin S, Nicolas JF, Desreumaux P, Flourie B, Kaiserlian D. CD8+ cytotoxic T cells induce relapsing colitis in normal mice. Gastroenterology. 2006;131:485–496. doi: 10.1053/j.gastro.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 5.Saito H, Kanamori Y, Takemori T, Nariuchi H, Kubota E, Takahashi-Iwanaga H, Iwanaga T, Ishikawa H. Generation of intestinal T cells from progenitors residing in gut cryptopatches. Science. 1998;280:275–278. doi: 10.1126/science.280.5361.275. [DOI] [PubMed] [Google Scholar]
- 6.Nonaka S, Naito T, Chen H, Yamamoto M, Moro K, Kiyono H, Hamada H, Ishikawa H. Intestinal gamma delta T cells develop in mice lacking thymus, all lymph nodes, Peyer’s patches, and isolated lymphoid follicles. J Immunol. 2005;174:1906–1912. doi: 10.4049/jimmunol.174.4.1906. [DOI] [PubMed] [Google Scholar]
- 7.Hitotsumatsu O, Hamada H, Naganuma M, Inoue N, Ishii H, Hibi T, Ishikawa H. Identification and characterization of novel gut-associated lymphoid tissues in rat small intestine. J Gastroenterol. 2005;40:956–963. doi: 10.1007/s00535-005-1679-8. [DOI] [PubMed] [Google Scholar]
- 8.Maloy KJ, Mowat AM, Zamoyska R, Crispe IN. Phenotypic heterogeneity of intraepithelial T lymphocytes from mouse small intestine. Immunology. 1991;72:555–562. [PMC free article] [PubMed] [Google Scholar]
- 9.Lefrancois L. Phenotypic complexity of intraepithelial lymphocytes of the small intestine. J Immunol. 1991;147:1746–1751. [PubMed] [Google Scholar]
- 10.O’Keeffe J, Doherty DG, Kenna T, Sheahan K, O’Donoghue DP, Hyland JM, O’Farrelly C. Diverse populations of T cells with NK cell receptors accumulate in the human intestine in health and in colorectal cancer. Eur J Immunol. 2004;34:2110–2119. doi: 10.1002/eji.200424958. [DOI] [PubMed] [Google Scholar]
- 11.Wang HC, Montufar-Solis D, Teng BB, Klein JR. Maximum immunobioactivity of murine small intestinal intraepithelial lymphocytes resides in a subpopulation of CD43+ T cells. J Immunol. 2004;173:6294–6302. doi: 10.4049/jimmunol.173.10.6294. [DOI] [PubMed] [Google Scholar]
- 12.Viney JL, Kilshaw PJ, MacDonald TT. Cytotoxic α/β+ and γ/δ+ T cells in murine intestinal epithelium. Eur J Immunol. 1990;20:1623–1626. doi: 10.1002/eji.1830200734. [DOI] [PubMed] [Google Scholar]
- 13.Ernst PB, Clark DA, Rosenthal KL, Befus AD, Bienenstock J. Detection and characterization of cytotoxic T lymphocyte precursors in the murine intestinal intraepithelial leukocyte population. J Immunol. 1986;136:2121–2126. [PubMed] [Google Scholar]
- 14.Goodman T, Lefrancois L. Expression of the γδ T-cell receptor on intestinal CD8+ intraepithelial lymphocytes. Nature. 1988;333:855–858. doi: 10.1038/333855a0. [DOI] [PubMed] [Google Scholar]
- 15.Wang HC, Zhou Q, Dragoo J, Klein JR. Most murine CD8+ intestinal intraepithelial lymphocytes are partially but not fully activated T cells. J Immunol. 2002;169:4717–4722. doi: 10.4049/jimmunol.169.9.4717. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto M, Fujihashi K, Beagley KW, McGhee JR, Kiyono H. Cytokine synthesis by intestinal intraepithelial lymphocytes. Both γ/δ T cell receptor-positive and α/β T cell receptor-positive T cells in the G1 phase of cell cycle produce IFN-γ and IL-5. J Immunol. 1993;150:106–114. [PubMed] [Google Scholar]
- 17.Montufar-Solis D, Garza T, Teng BB, Klein JR. Upregulation of ICOS on CD43+ CD4+ murine small intestinal intraepithelial lymphocytes during acute reovirus infection. Biochem Biophys Res Commun. 2006;342:782–790. doi: 10.1016/j.bbrc.2006.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sydora BC, Mixter PF, Holcombe HR, Eghtesady P, Williams K, Amaral MC, Nel A, Kronenberg M. Intestinal intraepithelial lymphocytes are activated and cytolytic but do not proliferate as well as other T cells in response to mitogenic signals. J Immunol. 1993;150:2179–2191. [PubMed] [Google Scholar]
- 19.Viney JL, MacDonald TT. Selective death of T cell receptor γ/δ+ intraepithelial lymphocytes by apoptosis. Eur J Immunol. 1990;20:2809–2812. doi: 10.1002/eji.1830201242. [DOI] [PubMed] [Google Scholar]
- 20.Mosley RL, Whetsell M, Klein JR. Proliferative properties of murine intestinal intraepithelial lymphocytes (IEL): IEL expressing TCRαβ or TCRγδ are largely unresponsive to proliferative signals mediated via conventional stimulation of the CD3-TCR complex. Int Immunol. 1991;3:563–569. doi: 10.1093/intimm/3.6.563. [DOI] [PubMed] [Google Scholar]
- 21.Fahrer AM, Konigshofer Y, Kerr EM, Ghandour G, Mack DH, Davis MM, Chien YH. Attributes of γδ intraepithelial lymphocytes as suggested by their transcriptional profile. Proc Natl Acad Sci USA. 2001;98:10261–10266. doi: 10.1073/pnas.171320798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shires J, Theodoridis E, Hayday AC. Biological insights into TCRγδ+ and TCRαβ+ intraepithelial lymphocytes provided by serial analysis of gene expression (SAGE) Immunity. 2001;15:419–434. doi: 10.1016/s1074-7613(01)00192-3. [DOI] [PubMed] [Google Scholar]
- 23.Bagriacik EU, Armstrong MD, Okabe M, Klein JR. Differential expression of CD43 isoforms on murine T cells and their relationship to acute intestinal graft versus host disease: studies using enhanced-green fluorescent protein transgenic mice. Int Immunol. 1999;11:1651–1662. doi: 10.1093/intimm/11.10.1651. [DOI] [PubMed] [Google Scholar]
- 24.Cheroutre H. IELs: enforcing law and order in the court of the intestinal epithelium. Immunol Rev. 2005;206:114–131. doi: 10.1111/j.0105-2896.2005.00284.x. [DOI] [PubMed] [Google Scholar]
- 25.Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 regulates both Th1 and Th2 responses. Annu Rev Immunol. 2001;19:423–474. doi: 10.1146/annurev.immunol.19.1.423. [DOI] [PubMed] [Google Scholar]
- 26.Xu D, Chan WL, Leung BP, Hunter D, Schulz K, Carter RW, McInnes IB, Robinson JH, Liew FY. Selective expression and functions of interleukin 18 receptor on T helper (Th) type 1 but not Th2 cells. J Exp Med. 1998;188:1485–1492. doi: 10.1084/jem.188.8.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okamura H, Tsutsi H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K, et al. Cloning of a new cytokine that induces IFN-γ production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 28.Kinoshita M, Kuranaga N, Matsumoto A, Ono S, Shinomiya N, Hiraide H, Seki S. Multiple interleukin-18 injections promote both mouse Th1 and Th2 responses after sublethal Escherichia coli infection. Clin Exp Immunol. 2006;143:41–49. doi: 10.1111/j.1365-2249.2005.02973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maxwell JR, Yadav R, Rossi RJ, Ruby CE, Weinberg AD, Aguila HL, Vella AT. IL-18 bridges innate and adaptive immunity through IFN-γ and the CD134 Pathway. J Immunol. 2006;177:234–245. doi: 10.4049/jimmunol.177.1.234. [DOI] [PubMed] [Google Scholar]
- 30.Okazawa A, Kanai T, Nakamaru K, Sato T, Inoue N, Ogata H, Iwao Y, Ikeda M, Kawamura T, Makita S, et al. Human intestinal epithelial cell-derived interleukin (IL)-18, along with IL-2, IL-7 and IL-15, is a potent synergistic factor for the proliferation of intraepithelial lymphocytes. Clin Exp Immunol. 2004;136:269–276. doi: 10.1111/j.1365-2249.2004.02431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lochner M, Forster I. Anti-interleukin-18 therapy in murine models of inflammatory bowel disease. Pathobiology. 2002;70:164–169. doi: 10.1159/000068149. [DOI] [PubMed] [Google Scholar]
- 32.Siegmund B, Zeitz M. Therapeutic approaches in inflammatory bowel disease based on the immunopathogenesis. Rocz Akad Med Bialymst. 2004;49:22–30. [PubMed] [Google Scholar]
- 33.Dotan I, Mayer L. Immunopathogenesis of inflammatory bowel disease. Curr Opin Gastroenterol. 2002;18:421–427. doi: 10.1097/00001574-200207000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Wiercinska-Drapalo A, Flisiak R, Jaroszewicz J, Prokopowicz D. Plasma interleukin-18 reflects severity of ulcerative colitis. World J Gastroenterol. 2005;11:605–608. doi: 10.3748/wjg.v11.i4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gordon JN, Di Sabatino A, Macdonald TT. The pathophysiologic rationale for biological therapies in inflammatory bowel disease. Curr Opin Gastroenterol. 2005;21:431–437. [PubMed] [Google Scholar]
- 36.Reuter BK, Pizarro TT. Commentary: the role of the IL-18 system and other members of the IL-1R/TLR superfamily in innate mucosal immunity and the pathogenesis of inflammatory bowel disease: friend or foe? Eur J Immunol. 2004;34:2347–2355. doi: 10.1002/eji.200425351. [DOI] [PubMed] [Google Scholar]
- 37.Kanai T, Uraushihara K, Totsuka T, Okazawa A, Hibi T, Oshima S, Miyata T, Nakamura T, Watanabe M. Macrophage-derived IL-18 targeting for the treatment of Crohn’s disease. Curr Drug Targets Inflamm Allergy. 2003;2:131–136. doi: 10.2174/1568010033484250. [DOI] [PubMed] [Google Scholar]
- 38.Ludwiczek O, Kaser A, Novick D, Dinarello CA, Rubinstein M, Tilg H. Elevated systemic levels of free interleukin-18 (IL-18) in patients with Crohn’s disease. Eur Cytokine Netw. 2005;16:27–33. [PubMed] [Google Scholar]
- 39.Monteleone G, Fina D, Caruso R, Pallone F. New mediators of immunity and inflammation in inflammatory bowel disease. Curr Opin Gastroenterol. 2006;22:361–364. doi: 10.1097/01.mog.0000231808.10773.8e. [DOI] [PubMed] [Google Scholar]
- 40.Min CK, Maeda Y, Lowler K, Liu C, Clouthier S, Lofthus D, Weisiger E, Ferrara JL, Reddy P. Paradoxical effects of interleukin-18 on the severity of acute graft-versus-host disease mediated by CD4+ and CD8+ T-cell subsets after experimental allogeneic bone marrow transplantation. Blood. 2004;104:3393–3399. doi: 10.1182/blood-2004-02-0763. [DOI] [PubMed] [Google Scholar]
- 41.Esfandiari E, McInnes IB, Lindop G, Huang FP, Field M, Komai-Koma M, Wei X, Liew FY. A proinflammatory role of IL-18 in the development of spontaneous autoimmune disease. J Immunol. 2001;167:5338–5347. doi: 10.4049/jimmunol.167.9.5338. [DOI] [PubMed] [Google Scholar]
- 42.Liu B, Mori I, Hossain MJ, Dong L, Takeda K, Kimura Y. Interleukin-18 improves the early defence system against influenza virus infection by augmenting natural killer cell-mediated cytotoxicity. J Gen Virol. 2004;85:423–428. doi: 10.1099/vir.0.19596-0. [DOI] [PubMed] [Google Scholar]
- 43.Culshaw S, Leung BP, Gracie JA, Campbell CC, Thomson D, Gemmell C, Liew FY, McInnes IB. Prior elevation of IL-18 promotes rapid early IFN-γ production during staphylococcal infection. Eur J Immunol. 2005;35:1438–1444. doi: 10.1002/eji.200425661. [DOI] [PubMed] [Google Scholar]
- 44.Montufar-Solis D, Klein JR. An improved method for isolating intraepithelial lymphocytes (IELs) from the murine small intestine with consistently high purity. J Immunol Methods. 2006;308:251–254. doi: 10.1016/j.jim.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 45.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 46.Born TL, Thomassen E, Bird TA, Sims JE. Cloning of a novel receptor subunit, AcPL, required for interleukin-18 signaling. J Biol Chem. 1998;273:29445–29450. doi: 10.1074/jbc.273.45.29445. [DOI] [PubMed] [Google Scholar]
- 47.Kanai T, Watanabe M, Okazawa A, Nakamaru K, Okamoto M, Naganuma M, Ishii H, Ikeda M, Kurimoto M, Hibi T. Interleukin 18 is a potent proliferative factor for intestinal mucosal lymphocytes in Crohn’s disease. Gastroenterology. 2000;119:1514–1523. doi: 10.1053/gast.2000.20260. [DOI] [PubMed] [Google Scholar]
- 48.Maerten P, Shen C, Colpaert S, Liu Z, Bullens DA, van Assche G, Penninckx F, Geboes K, Vanham G, Rutgeerts P, et al. Involvement of interleukin 18 in Crohn’s disease: evidence from in vitro analysis of human gut inflammatory cells and from experimental colitis models. Clin Exp Immunol. 2004;135:310–317. doi: 10.1111/j.1365-2249.2004.02362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ahn HJ, Maruo S, Tomura M, Mu J, Hamaoka T, Nakanishi K, Clark S, Kurimoto M, Okamura H, Fujiwara H. A mechanism underlying synergy between IL-12 and IFN-γ-inducing factor in enhanced production of IFN-γ. J Immunol. 1997;159:2125–2131. [PubMed] [Google Scholar]
- 50.Robinson D, Shibuya K, Mui A, Zonin F, Murphy E, Sana T, Hartley SB, Menon S, Kastelein R, Bazan F, et al. IGIF does not drive Th1 development but synergizes with IL-12 for interferon-γ production and activates IRAK and NF-κB. Immunity. 1997;7:571–581. doi: 10.1016/s1074-7613(00)80378-7. [DOI] [PubMed] [Google Scholar]
- 51.Ten Hove T, Corbaz A, Amitai H, Aloni S, Belzer I, Graber P, Drillenburg P, van Deventer SJ, Chvatchko Y, Te Velde AA. Blockade of endogenous IL-18 ameliorates TNBS-induced colitis by decreasing local TNF-α production in mice. Gastroenterology. 2001;121:1372–1379. doi: 10.1053/gast.2001.29579. [DOI] [PubMed] [Google Scholar]
- 52.Siegmund B, Fantuzzi G, Rieder F, Gamboni-Robertson F, Lehr HA, Hartmann G, Dinarello CA, Endres S, Eigler A. Neutralization of interleukin-18 reduces severity in murine colitis and intestinal IFN-γ and TNF-α production. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1264–R1273. doi: 10.1152/ajpregu.2001.281.4.R1264. [DOI] [PubMed] [Google Scholar]
- 53.Sivakumar PV, Westrich GM, Kanaly S, Garka K, Born TL, Derry JM, Viney JL. Interleukin 18 is a primary mediator of the inflammation associated with dextran sulphate sodium induced colitis: blocking interleukin 18 attenuates intestinal damage. Gut. 2002;50:812–820. doi: 10.1136/gut.50.6.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wirtz S, Becker C, Blumberg R, Galle PR, Neurath MF. Treatment of T cell-dependent experimental colitis in SCID mice by local administration of an adenovirus expressing IL-18 antisense mRNA. J Immunol. 2002;168:411–420. doi: 10.4049/jimmunol.168.1.411. [DOI] [PubMed] [Google Scholar]