Abstract
The structure of the title compound, C7H14O6, one natural myo-inositol derivative has been determinaded. Average atom distances, bond lengths and dihedral angles are similar to myo-inositol.
Comment
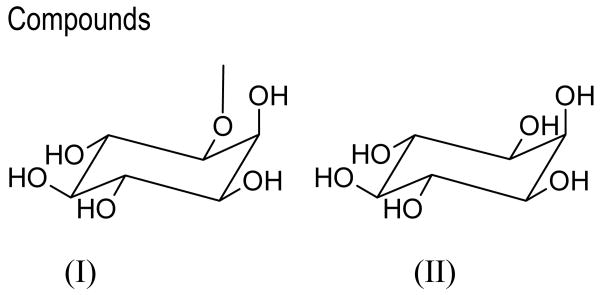
Bornesitol (I) is a myo-inositol methyl derivative found in several plant species (Girard, 1871; Nishibe et al., 2001), its chemical structure has been previously described (Foster & Stacey, 1953, Bien & Ginsburg, 1958). myo-Inositol (II) is a cyclitol which has only one axial-oriented hydroxyl group (C2) and has mirror symmetry (Rabinowitz & Kraut, 1964, Bonnet et al., 2006).
As part of our study of plant products, we report here the absolute structure for one of bornesitol enantiomers. The analyzed crystal belongs to the dextrorotatory enantiomer, with [α]D= +20.7 ± 3.5°, a value similar to those previously described for bornesitol (Angyal & Bender, 1961). Bornesitol crystallizes in the orthorhombic space group P212121. The dimension of (I) is similar to myo-inositol (II) (Rabinowitz & Kraut, 1964), but it has non-centrosymmetric structures (Fig. 1). The intermolecular O⋯H distances range from 1.78 to 2.03 Å (Table 1) and are less than the intramolecular O⋯O distances (from 2.67 to 2.89 Å), in contrast to other cyclitols that present similar values (Rabinowitz & Kraut, 1964; Hosomi et al., 2000, Bonnet et al., 2006). The angle between O3—H3⋯O1 is ca 10° less than O6—H6⋯O4, and a minor difference is noted between O2—H2⋯O6 and O4—H4⋯H2 (Table 1).
Figure 1.
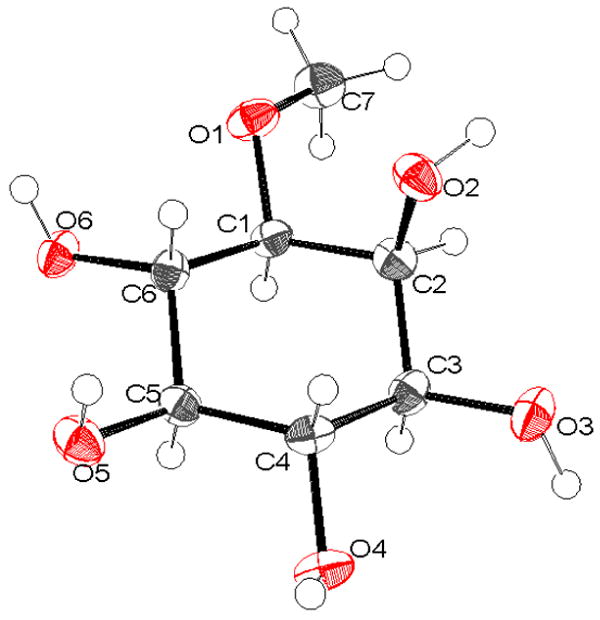
ORTEP plot of L-(+)-bornesitol, showing atomic notation and thermal ellipsoids. The molecule is viewed approximately normal to the center plane of the chair-shaped cyclohexane ring: C2 and C5 are below and above the plane, respectively. Thermal ellipsoids are shown at the 50% probability level for non-H atoms.
Table 1.
Hydrogen-bond geometry (A% , °)
| D | H | A | D-H | A-H | D-A | D-H-A |
|---|---|---|---|---|---|---|
| O2 | H2 | O6 | 0.86 (3) | 1.82 (2) | 2.667 (3) | 170 (3) |
| O3 | H3 | O1 | 0.95 (3) | 1.80 (3) | 2.723 (3) | 162 (3) |
| O4 | H4 | O2 | 0.87 (3) | 1.84 (3) | 2.695 (3) | 167 (3) |
| O5 | H5 | O3 | 0.89 (3) | 2.03 (3) | 2.892 (3) | 163 (3) |
| O6 | H6 | O4 | 0.90 (3) | 1.80 (3) | 2.688 (2) | 171 (3) |
The absolute structure of (+)-bornesitol was estimated based on the known absolute configuration of the title compound (Angyal; Gilman, 1957), as (1R)-O-methyl-myo-inositol. According to IUPAC recommendations for nomenclature of inositol derivatives, which names L-bornesitol as 1-O-methyl-myo-inositol, with clockwise numbering, (+)-bornesitol should be denominated (1R)-1-L-O-methyl-myo-inositol (Angyal et al., 1992).
Experimental
Compound (I) was obtained from the EtOAc:MeOH (4.5:5.5) fraction of Hancornia speciosa leaves (Apocynaceae). The crude fraction was dissolved in methanol:water (9:1) and the solution was kept at room temperature. Crystals of (I) grew as colourless prisms from this solution by slow evaporation. Optical rotation was determined for a water solution (0.11 g/100 ml) of compound (I), in a Perkin Elmer polarimeter-341 at 589 nm and 20° C, using a 100 mm path length cell.
| Crystal data | |
| C7H14O6 | Dx = 1.521 Mg m−3 |
| Mr = 194.19 | Mo - Kα radiation |
| Orthorhombic, P212121 | Cell parameters from 5715 reflections |
| a = 6.5756 (4) Å | θ = 3–27° |
| b = 11.0565 (7) Å | μ = 0.13 mm−1 |
| c = 11.6622 (9) Å | T = 150 K |
| V = 847.88 (10) Å3 | Plate, colourless |
| Z = 4 | 0.48 × 0.44 × 0.23 mm |
| Data collection | |
| Nonius KappaCCD diffractometer | 1151 independent reflections |
| ω scans | 974 reflections with > 2.0σ(I) |
| Absorption correction:
multi-scan (Otowinwski & Minor, 1997) |
Rint = 0.035 |
| Tmin = 0.926, Tmax = 0.972 | θmax = 27.5° |
| 5715 measured reflections | |
| Refinement | |
| Refinement on F2 | 1/[σ2(Fo2) + (0.0543P)2]
where P = (Fo2 + 2Fc2)/3 |
| R[F2 > 2σ(F2)] = 0.034 | (Δ/σ)max < 0.001 |
| wR(F2) = 0.085 | Δρmax = 0.19 e Å−3 |
| S = 1.05 | Δρmin = −0.20 e Å−3 |
| 1151 reflections | Extinction correction: SHELXL97 (Sheldrick 1997) |
| 140 parameters | Extinction coefficient: 0.036 (5) |
| H atoms treated by a mixture of independent and constrained refinement |
The structure was solved by direct methods using SIR2004 (Burla et al., 2005). The remaining atoms were located in succeeding difference Fourier syntheses. Hydrogen atoms were included in the structure factor calculation in idealized positions using the riding model but they were not refined. The structure was refined in full-matrix least-squares where the function minimized was ©w(|Fo|2-|Fc|2)2 and the weight w is defined as 1/[ ⎧2(Fo2)+(0.0461P)2+0.0000P] where P=(Fo2+2Fc2)/3. Scattering factors were taken from the “International Tables for Crystallography”.
Acknowledgments
CNPq/Brazil (Conselho Nacional de Desenvolvimento Científico e Tecnológico) is acknowledged for undergraduate (C.M.S.) and research fellowships (F.C.B.). CAPES/Brazil (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) supported this project with a Ph.D. fellowship (D.C.E.). The work was also supported by program project grant P01 CA48112 awarded by the National Cancer Institute. Dr. Júlio Antônio Lombardi is acknowledged for collecting the plant material.
Footnotes
Data collection: Collect (Nonius, 1998); cell refinement: DENZO/SCALEPACK (Otwinowski & Minor, 1996); data reduction: DENZO/SCALEPACK (Otwinowski & Minor, 1996); program(s) used to solve structure: Direct methods (SIR2004, Burla et al., 2005); program(s) used to refine structure: SHELX97 (Sheldrick, 1997); molecular graphics: ORTEP (Johnson, 1976) PLATON (Spek, 1997); software used to prepare material for publication: SHELX97(Sheldrick, 1997) and local programs.
References
- Angyal SL, Anderson L, Cahn RS, Dawson RMC, Hoffmann-Ostenhof O, Klyne W, Posternak T. Biochemical nomenclature and related documents. 2nd. IUPAC; London: 1992. [Google Scholar]
- Angyal SL, Bender V. J Chem Soc. 1961:4718–4720. [Google Scholar]
- Angyal SL, Gilham PT. J Chem Soc. 1957:3691–3699. [Google Scholar]
- Bien S, Ginsburg D. J Chem Soc. 1958:3189–3194. [Google Scholar]
- Bonnet A, Jones W, Samuel Motherwell WD. Acta Cryst. 2006;E62:o2902–o2904. [Google Scholar]
- Burla MC, Caliandro M, Camalli M, Carrozzini B, Cascarano GL, De Caro L, Giacovazzo C, Polidori G, Spagna R. J Appl Cryst. 2005;38:381–388. [Google Scholar]
- Foster AS, Stacey M. Chem Ind (London) 1953:279. [Google Scholar]
- Girard A. Compt Rend Heb Acad Sci. 1871;73:426–432. [Google Scholar]
- Hosomi H, Ohba S, Ogawa S, Takahashi A. Acta Cryst. 2000;C56:e584–e585. doi: 10.1107/S0108270100003371. [DOI] [PubMed] [Google Scholar]
- Johnson CK. ORTEPII, Report ORNL-5138. Oak Ridge National Laboratory; Tennessee: 1976. [Google Scholar]
- Nishibe S, Sakushima A, Takemura H, Takenaka T, Noguchi Y. Nature Med. 2001;55:268–271. [Google Scholar]
- Nonius. Collect Users Manual, Nonius Delft. The Netherlands: 1998. [Google Scholar]
- Otwinowski Z, Minor W. Methods Enzymol. 1997;276:307–327. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- Rabinowitz I, Kraut J. Acta Cryst. 1964;17:159–168. [Google Scholar]
- Sheldrick GM. SHELXL97, Program for the Refinement of Crystal Structures. Univ. of Göttingen; Germany: 1997. [Google Scholar]
- Spek AL. PLATON. Program. Univ. of Ultrecht; The Netherlands: 1997. [Google Scholar]