Abstract
Restoration of the tumor-suppression function by gene transfer of the melanoma differentiation-associated gene 7 (MDA7)/interleukin 24 (IL-24) successfully induces apoptosis in melanoma tumors in vivo. To address the molecular mechanisms involved, we previously revealed that MDA7/IL-24 treatment of melanoma cells down-regulates interferon regulatory factor (IRF)-1 expression and concomitantly up-regulates IRF-2 expression, which competes with the activity of IRF-1 and reverses the induction of IRF-1–regulated inducible nitric oxide synthase (iNOS). Interferons (IFNs) influence melanoma cell survival by modulating apoptosis. A class I IFN (IFN alfa) has been approved for the treatment of advanced melanoma with some limited success. A class II IFN (IFN gamma), on the other hand, supports melanoma cell survival, possibly through constitutive activation of iNOS expression. We therefore conducted this study to explore the molecular pathways of MDA7/IL-24 regulation of apoptosis via the intracellular induction of IFNs in melanoma. We hypothesized that the restoration of the MDA7/IL-24 axis leads to upregulation of Class I IFNs and induction of the apoptotic cascade. We found that MDA7/IL-24 induces the secretion of endogenous IFN beta, another class I IFN, leading to the arrest of melanoma cell growth and apoptosis. We also identified a series of apoptotic markers that play a role in this pathway, including the regulation of tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) and Fas-FasL. In summary, we described a novel pathway of MDA7/IL-24 regulation of apoptosis in melanoma tumors via endogenous IFN beta induction followed by IRF regulation and TRAIL/FasL system activation.
Keywords: MDA7, IL24, Melanoma, IFN, TRAIL
Introduction
Melanoma differentiation-associated gene 7 (Mda-7)/interleukin 24 (IL-24), an IL-10 cytokine family member, has a growth-inhibitory effect on many human cancers, including melanoma. The overexpression of MDA7/IL-24 via adenovirus-mediated gene (Ad-mda7/IL-24) delivery has been reported to induce apoptosis selectively in cancer cells but not in normal cells [1–4]. The mechanism of cancer cell apoptosis in response to the viral transduction of MDA7/IL-24 is partly understood and has been reported in cell lines derived from breast cancer, melanoma, lung cancer, and other solid tumors [1–3, 5–7]
Briefly, Ad-mda7 transduction of tumor cells results in profound changes in posttranscriptionally regulated signaling pathways. In melanoma cells, Ad-mda7 transduction of tumor cells results in up-regulation of growth arrest and DNA damage (GADD) proteins, and the proapoptotic protein BAX [8]. This might further induce down-regulation of inducible nitric oxide synthase (iNOS) via modulation of the interferon regulatory factor (IRF)-1/IRF-2 balance [9]. In lung cancer cells, Ad-mda7 activates protein kinase regulated by RNA (PKR), signal transducers and activator of transcription 3 (STAT3), Jun N-terminal kinase (JNK), and p38 mitogen-activated protein kinase (p38 MAPK). In ovarian cancer cells, Fas activation is a critical mediator of tumor cell death, and the inhibition of this pathway blocks apoptosis [10]. In melanoma cells, p38 MAPK inhibitors reduce Ad-mda7-mediated cell killing (4]. Together these findings indicate that the pathways mediating Ad-mda7 toxicity vary from one tumor cell type to another.
In more recent studies [11–13], transfection with specific receptor subunits and binding assays with MDA7/IL-24 protein identified two distinct receptors: IL-20R1/IL-20R2 (i.e., type 1 IL-20 receptors) and IL-22R1/IL-20R2 (i.e., type 2 IL-20 receptors). The maximum binding activity for MDA7/IL-24 and the activation of MDA7/IL-24 signaling pathways were detected only when cells were transfected with a combination of type 1 and type 2 IL-20 receptors, indicating that these receptor complexes can mediate MDA7/IL-24 signal transduction [11–13].
A particularly active area of research is the molecular pathways used by MDA7/IL-24 protein for the induction of apoptosis. We previously reported that MDA7/IL-24 treatment of melanoma cells down-regulates IRF-1 expression and concomitantly up-regulates IRF-2 expression, which competes with the activity of IRF-1 and reverses the induction of IRF-1–regulated iNOS expression [9]. The transduction of active forms of IRFs modulates apoptotic and antitumor properties, possibly leading to novel antitumor effector functions. It is now known that IRFs are directly involved in the transcriptional induction of tumor necrosis factor (TNF)–related apoptosis-inducing ligand (TRAIL), a key player in the apoptosis pathway [14].
Apoptosis could be executed via two main pathways that lead to the activation of caspases: the death receptor (DR) and the mitochondrial pathways [15]. The DR pathway is activated by ligation of members of the TNF family, such as the Fas ligand (FasL), TNF-α, and TRAIL, to DRs on the plasma membrane. The receptors of these ligands, Fas, TNF receptor 1, and DR4/DR5, are members of the TNF receptor superfamily, characterized by similar cysteine-rich extracellular domains and homologous cytoplasmic death domains [16]. Binding of these DRs by their respective ligands recruits the adaptor proteins Fas-associated death domain (FADD) and caspase-8 to the death-inducing signaling complex, triggering the proteolytic activation of caspase-8. Caspase-8 in turn activates the effector caspases, such as caspase-3, and triggers the proteolytic targeting of key apoptotic or cell-cycle regulatory proteins.
On the whole, interferons (IFNs) influence cell survival by modulating apoptosis. The biologic effects of IFNs are primarily mediated via the activation of the Janus kinase (JAK)/STAT pathway and the subsequent induction of IFN-stimulated genes. IFNs were originally identified by their role in viral infections. A wide variety of effects have subsequently been described, such as antiviral, antitumor, immunomodulatory, and antiproliferative activities.
The three main types of IFNs are IFN alfa, IFN beta, and IFN gamma. IFN alfa and IFN beta are class I IFNs, and mitogen-induced IFN gamma is a class II IFN, which uses distinct receptors to mediate cellular responses. Since antitumor activity is the major function of class I IFNs, they have been used extensively against various types of cancer in clinical trials, either as monotherapy or combined with chemotherapeutic agents. However, the exact mechanisms of class I IFNs, including the induction of apoptosis, are not yet fully understood.
Early events in class I IFN signaling include tyrosine phosphorylation of the receptor subunits and activation of the receptor-associated Tyk-2 and Jak-1 Janus kinases, followed by the phosphorylation of STAT proteins (JAK/STAT pathway). The activation of signaling cascades downstream from these proteins includes IRFs. Recent extensive studies have demonstrated that IRFs play an important role in a wide spectrum of IFN responses [17]. IRF-1 and IRF-2 have been involved in most studies because IRF-1 acts as a transcriptional activator for class II IFNs and IFN-inducible genes, whereas IRF-2 represses the action of IRF-1 and competes with its actions.
We previously reported that MDA7/IL-24 treatment of melanoma cells down-regulates IRF-1 expression and concomitantly up-regulates IRF-2 expression [9]. We therefore conducted the current study to explore the molecular pathway regulated by IFNs and its role in melanoma cell survival. We hypothesized that the regulation of gene expression in melanoma cells activated by class I IFNs results in antitumor effects MDA7/IL-24 is the key cytokine to trigger the up-regulation of class I IFNs. To gain insight into these mechanisms, we investigated the differential IFN signaling pathways, focusing particularly on apoptosis-inducing pathways by MDA7/IL-24 in melanoma cell lines.
Material and Methods
Cell Culture
We obtained two metastatic melanoma cell lines, A375 and MeWo, from the American Type Culture Collection (Rockville, MD). Normal human epidermal melanocytes (NHEM) were obtained from Clonetics (San Diego, CA) and cultured with the manufacturer’s human melanocyte growth medium. Melanoma cell lines used in this study were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum, 100 U/mL of penicillin, 100 mg/mL of streptomycin, 2 mM L-glutamine, and HEPES buffer (all from Life Technologies, Inc., Grand Island, NY). Cells were either treated with purified MDA7/IL-24 at 100 to 200 ng/mL or cocultured with stably transfected HEK 293 cells at 1:1 or 1:2 dilutions with the target melanoma cells.
Reagents
Anticaspase-3, -8, -9; PARP; PI3K p85 rabbit polyclonal antibodies (Cell Signaling Technology, Inc, Beverly, MA) were used for Western blotting. Affinity-purified polyclonal rabbit antibodies to MDA7/IL-24 were provided by Introgen Therapeutics (Houston, TX). Anti-Fas and FasL mouse monoclonal antibodies were purchased from BD BioSciences (San Jose, CA). Goat polyclonal antibody (anti-DR5) to TRAIL–receptor 2 (TRAIL-R2 or DR5) was from Alexis Biochemicals (San Diego, CA), and rabbit polyclonal antibody (anti-DR4) to TRAIL–receptor 1 (TRAIL-R1 or DR4) was from ProSci Inc. (Poway, CA). The same antibodies were used for both Western blotting and immunohistochemistry studies. Preimmune normal mouse IgG (Vector Laboratories, Burlingame, CA) was used as a negative control. Antivimentin antibody (BioGenex Laboratories, San Ramon, CA) was used as a positive control for all melanoma staining. Recombinant proteins of IFN alfa and IFN gamma were purchased from eBioscience (San Diego, CA), and IFN beta-1a was purchased from PBL Biomedical Laboratories (Piscataway, NJ).
Purification of Human MDA7/IL-24
As we described in a recent publication [18], the full-length cDNA of mda7/IL-24 was cloned into the pCEP4 FLAG vector (Invitrogen Corporation, Carlsbad, CA) containing the CMV promoter. The plasmid was transfected into HEK 293 cells, and stable subclones were isolated using 0.4 mg/mL of hygromycin. The supernatant containing the secreted MDA7/IL-24 was supplemented with protease inhibitors (1 mg/mL of leupeptin, 1 mg/mL of pepstatin, and 0.5 mM phenylmethylsulfonyl fluoride) and 0.05% sodium azide and was concentrated 10-fold with an Amicon stirred cell (Amicon, Beverly, MA) on a YM10 membrane. Ten-milliliter aliquots of concentrated supernatant were separated over an S200 Superdex prep grade column (Amersham Pharmacia Biotech, Piscataway, NJ) in 1× PBS; pH 7.4, and fractions containing MDA7/IL-24 on Western blotting and enzyme-linked immunosorbent assay (ELISA) were pooled.
After buffer exchange on an Amicon stirred cell to 50 mM 4 morpholinepropanesulfonic acid (pH 6), a second purification step was performed using an Bio-Rad S column (BioRad Lab, Hercules, CA). Column conditions consisted of a 0–to 90-mM NaCl gradient, a 5-min hold at 90 mM NaCl, a 30-min 90- to 250-mM gradient at 1 mL/min, and a 5-min hold at 250 mM NaCl. The entire purification process was conducted at 4°C, and MDA7/IL-24 was identified using ELISA and Western blotting. The final samples contained at least 300 ng/mL of MDA7/IL-24 as determined by ELISA. Individual lots of partially purified MDA7/IL-24 were tested for endotoxin using the QCL-1000 quantitative chromogenic limulus amebocyte lysate (LAL) kit (BioWhittaker, Inc., Walkersville, MD). In some cases, further purification of IL-24 was performed with immunoaffinity and cation exchange purification. Two affinity-enriched lots, approximately 50 mg each and at 30% to 40% purity, were exchanged into 0.1 M NaPO4 (pH 5.0) containing 0.5 M NaCl. The semipure protein was bound to a cation exchange column (BioRad Lab) and then eluted at 1 M NaCl. The MDA7/IL-24–containing fractions were pooled, and bovine serum albumin was added to a final concentration of 0.1 mg/mL, to lots used in the functional assays.
Coculturing of Melanoma Cells with MDA7/IL-24-Transfected HEK 293 Cells
Stably transfected HEK 293 cells used for production were also used for Transwell experiments so that the effect of protein manipulation could be eliminated. These HEK 293 cells were plated into the top wells of Transwell inserts in 6-well plates (BD Labware, Franklin Lakes, NJ) at the same concentration, and a 5× concentration of melanoma cells was placed in the bottom chambers. The inserts were incubated for 1 to 5 days. Cell concentrations were based on the length of incubation time and ranged from 2.5 × 103 to 1 × 105.
Cell Viability and Apoptosis Assays
Cell viability was determined by the trypan blue exclusion assay. At previously designated times, cells were harvested by trypsinization, and a small aliquot was suspended in a 1:10 volume with 0.1% trypan blue (Gibco BRL, Grand Island, NY). Total cell numbers and cell viability counts were assessed by using a hemacytometer under light microscopy. We used the ApoAlert Annexin V–FITC kit (Clontech, Mountain View, CA) to analyze cells for apoptosis. Briefly, melanoma cells (105 to 106 cells total) were washed extensively in PBS and then incubated with Annexin V–FITC reagent for 30 min on ice. Cells were then washed with PBS and processed by flow cytometric analysis with use of a FACScan flow cytometer (BD Biosciences). Assays were performed two or three times.
Cell-Cycle Analysis
We performed cell-cycle staging by using propidium iodide (PI) staining to evaluate DNA content. For identifying cells at different stages of the cell cycle, untreated, immunoaffinity-purified, and immunoaffinity/cation exchange–enriched MDA7/IL-24–treated melanoma cells were prepared as a single-cell suspension of 1 × 106 cells/mL in PBS. After the cells were fixed with cold 70% ethanol for 2 h, they were centrifuged and then washed twice with PBS. They were next stained with PI solution (Boehringer Mannheim, Indianapolis, IN) to a final concentration of 5 mg/mL of PI to 10 mg/mL of RNase. We analyzed DNA content and cell-cycle phases with a FACScan flow cytometer (BD Biosciences).
ELISA
MeWo cells were cultured in supplemented RPMI-1640 medium (Invitrogen) at 5 × 105 cells/mL, with MDA7/IL-24 added at designated concentrations. At indicated times, we harvested supernatants and determined the IFN beta content with an ELISA test kit (R&D Systems, Inc., Minneapolis, MN) according to the manufacturer’s protocol. Reference standards for ELISA were resuspended in a cell culture medium. The amounts of IFN beta were quantified on the basis of a standard curve after optical density was measured at 450 nm with a reference of 550 nm on an ELISA reader (Dynex Technologies, Chantilly, VA). The mean values at each time point were then used directly for the analysis reported.
Cytochemical Analysis
Cytochemical labeling was performed on the slides of the melanoma and normal control cells previously prepared as Cytospins. Cells were prepared as single-cell suspensions of 1 × 106 cells/mL in PBS with 1% bovine serum albumin, and 1 × 105 cells were used for each slide. Next, slides were quickly fixed (within seconds) in acetone at room temperature. For staining, slides were fixed again in −20°C acetone for 20 min. Next, slides were washed with distilled water for 5 min and covered with 3% hydrogen peroxide (Sigma Chemical Co., St. Louis, MO) in methanol to block endogenous peroxidase activity. All incubations were carried out at room temperature in a humidified covered slide chamber.
We washed the slides in PBS before incubating them in PBS containing 0.05% Triton X-100 (Sigma Chemical Co.) for 15 min to permeabilize the cells. We then used an avidin-biotinperoxidase complex kit (Vectastain; Vector Laboratories) to detect staining. After the slides were incubated for 30 min with the blocking serum, we added primary antibodies at various dilutions (1:100 to 1:200) and then incubated the slides for 60 min at room temperature. The slides were then washed, incubated for 30 min with secondary biotinylated antibody, washed again, and then incubated for 30 min with the avidin-biotinperoxidase complex reagent. After the slides were washed in PBS, the immunostaining was developed by using 3-amino-9-ethylcarbazole as a chromogen for 15 min. Slides were counterstained with hematoxylin (Vector Laboratories) and mounted with Aqua-Mount (Lerner Laboratories, Pittsburgh, PA). For each sample, vimentin and its isotype-matched control IgG served as positive and negative primary antibody controls, respectively.
Immunoblotting Assays
We rinsed 2 × 106 of the indicated melanoma cells twice in ice-cold PBS and lysed them for 10 min on ice in 60 mL of lysis buffer (25 mM Tris, 140 mM NaCl, and 1% NP40 [pH 7.5]) containing 5 mM EDTA, 0.2 mM orthovanadate, 10 mM NaF, leupeptin, aprotinin, and phenylmethylsulfonyl fluoride. Equal amounts of total protein (measured with DC Protein Assay Reagent; BioRad Lab) were loaded on a standard-percentage SDS polyacrylamide gel, and fractionated proteins were electroblotted onto a nitrocellulose membrane. These membranes were blocked for 1 h at room temperature using 5% dry milk in 1× PBS and washed three times for 5 min each in PBS containing 0.05% Tween 20 at room temperature. The membranes were incubated overnight at 4°C in a sealed bag with a 1:1000 dilution of all antibodies in 10 mL of 5% dry milk/0.1% Tween 20 in 1× PBS. The membranes were then washed three times for 5 min each in PBS containing 0.05% Tween 20 and then incubated with peroxidase-conjugated antirabbit IgG secondary antibody (BD Biosciences) at a 1:2000 dilution in PBS with 5% dry milk and 0.1% Tween 20 for 45 min at room temperature. The blots were visualized using an enhanced chemiluminescence detection kit (Amersham Pharmacia Biotech).
Gene Expression Array Analysis
To screen major molecular differences between MDA7/IL-24–treated and nontreated melanoma, we used the GEArray Q Series for Apoptosis and the Signal Transduction Pathways gene array kits (SuperArray Bioscience Corp., Frederick, MD). Genes identified by the array technology were also confirmed by Western blot analysis. RNA samples from the MeWo cells were obtained by standard methods and by a new probe-labeling method, TrueLabeling-RT, performed according to manufacturer’s guidelines (SuperArray Bioscience Corp.). This new method reduces false-positive results (reportedly up to 90%) by eliminating RNA self-priming, which often occurs in conventional RT labeling. The array required 1 to 5 mg of RNA, and probes were labeled with phosphorus 32–labeled deoxycytidine 5'-triphosphate for radioactive detection. After the overnight hybridization procedure with the membrane, the x-ray film was washed, blocked, and exposed. The raw data were collected from the raw image by using a scanner and ImageQuant software (Molecular Dynamics, Inc., Sunnyvale, CA) and analyzed with use of GEArray analyzer software (SuperArray Bioscience Corp.) for preparing data tables and graphs.
Statistical Analysis
We determined means and SDs for the variables and used the Student’s t test to evaluate the statistical significance of the experimental results. All experiments were performed at least three times unless otherwise indicated. Statistical significance was set at P < 0.05.
Results
Immunoaffinity and Cation Exchange Purification of MDA7/IL-24
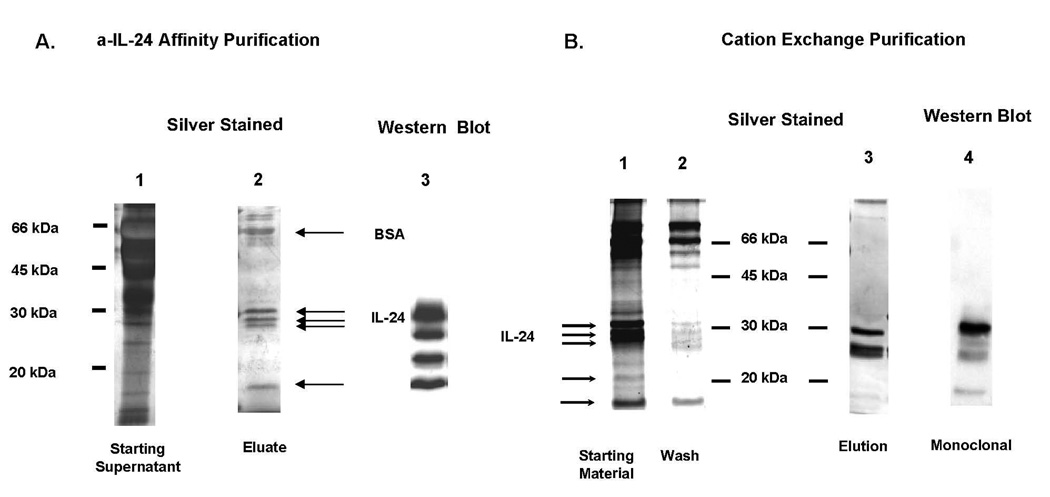
Immunoaffinity purification of MDA7/IL-24 with monoclonal anti–MDA7/IL-24, followed by silver staining of SDS-PAGE analysis, detected proteins at 18.5, 23, 27, 29, and 32 kDa and a larger positive area at ~66 kDa (Fig. 1A, lane 2). Four of these bands [18.5, 23, 27, and 32 kDa) were consistent with those in the MDA7/IL-24 immunoblots. After cation exchange purification of this affinity-purified material, only two of these proteins were further enriched and identified by immunoblotting and silver staining (Fig. 1B, lanes 1–4). We used both immunoaffinity and immunoaffinity/cation exchange–enriched MDA7/IL-24 protein preparations to perform MDA7/IL-24 function studies as indicated in the specific experiments.
Figure 1.
A, Purification of MDA7/IL-24 by immunoaffinity chromatography. All samples were resolved by 12% SDS-PAGE. Lane 1, Heat-denatured, reduced-starting supernatant. Lane 2, Heat-denaturation and reduced-affinity purification of MDA7/IL-24 performed with resolving gel (pH 8.9) 0.5 M Tris-HCl. An analysis of MDA7/IL-24 by ELISA showed a 3000-fold increase in purity over the supernatant. BSA = bovine serum albumin. Lane 3, Western blot with anti–N-terminal monoclonal of the elution fraction. B, Further purification of MDA7/IL-24 by immunoaffinity and cation exchange purification. All samples were resolved by 12% SDS-PAGE (pH 8.32) 1.5 M Tris-HCl. Affinity-purified MDA7/IL-24 was further purified by cation exchange chromatography. Lane 1, MDA7/IL-24 affinity purified (starting material loaded onto column). Lane 2, Wash from the column. Lane 3, Elution at 1 M NaCl. Lane 4, Western blot with anti–N-terminal monoclonal of the elution fraction.
Affinity-Purified MDA7/IL-24 Induced Cell Death and Apoptosis in Melanoma Cells via Ligand Binding to Type 1 IL-20R
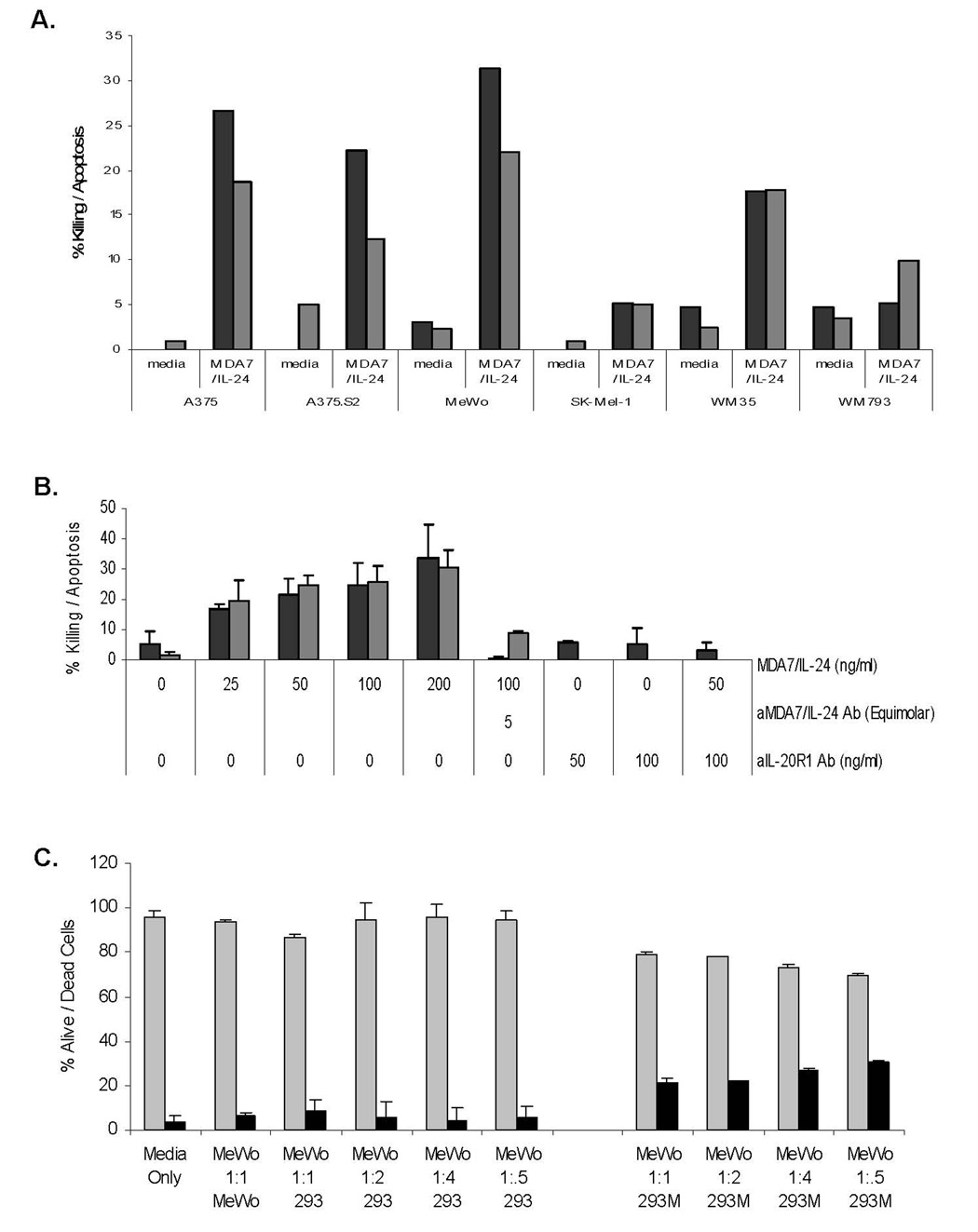
To evaluate the biologic effects of MDA7/IL-24 treatment on melanoma cells, we investigated cell killing by using trypan blue exclusion assays and apoptosis by Annexin V staining in various melanoma lines (Fig. 2A). MDA7/IL-24 treatment induced cell killing and apoptosis at various levels in all cell lines. However, the most notable killing and apoptosis were observed in MeWo cells (53.5%) and A375 cells (45%) (Fig. 2A). Both killing and apoptosis occurred in a dose-dependent fashion (Fig. 2B), and the treatment of melanoma cells with neutralizing monoclonal anti–MDA7/IL-24 antibody significantly inhibited killing and apoptosis in MeWo cells (P =0.01). No significant decrease in cell death was observed in MeWo cells treated with nonspecific IgG (data not shown).
Figure 2.
A, Affinity-purified MDA7/IL-24 induces cell death in melanoma cells. A panel of melanoma cells (A375, A375.S2, MeWo, SK-Mel-1, WM35, WM793) was exposed to a dose titration of affinity-purified MDA7/IL-24 and assessed for cell death by trypan blue exclusion after 5 days. Cells were analyzed for apoptosis via Annexin V–FITC staining followed by fluorescence-activated cell sorter analysis. Black bars = percentage of dead cells (trypan blue positive) and gray bars = percentage of apoptotic cells based on total cells. B, MDA7/IL-24 kills melanoma cells via ligand binding to type 1 IL-20R. MeWo melanoma cells were exposed to a dose increase of affinity-purified MDA7/IL-24 and assessed for cell death (cytotoxicity and apoptosis) after 5 days. Black bars = percentage of dead cells (trypan blue positive) and gray bars = percentage of apoptotic cells (by Annexin-V-FITC staining) based on total cells. .Cells were also treated with antibodies to MDA7/IL-24, IL-20R1 antibody, or IL-22R1 antibody. MDA7/IL-24 protein was added to antibodies, and cell killing was assessed. Data were plotted as mean + SD. Error bars represent one standard deviation of the mean. C, MDA7/IL-24–transfected HEK-293 cells (293M) kill melanoma cells. MeWo melanoma cells were cocultured with MDA7/IL-24–transfected 293 cells and assessed for cell death after 5 days. Black bars = percentage of dead cells (trypan blue positive) and gray bars = percentage of living cells based on total cells. Cells were also cocultured with nontransfected HEK-293 cells and MeWo cells. Data were plotted as mean + SD. Error bars represent one standard deviation of the mean.
These results indicate that our purified MDA7/IL-24 preparation induced a direct and specific cytotoxic effect in melanoma cells. To determine whether MDA7/IL-24 induced cell killing and apoptosis regulated by IL-20R1 (common receptor), we first treated MeWo cells with 50 to 100 ng/mL of anti–IL-20R1 antibody. After the cells were incubated with anti–IL-20R1 antibody for 1 h, we added 50 to 200 ng/mL of recombinant MDA7/IL-24 and cultured the cells for up to 5 days.
No significant cell death was induced by cells exposed to anti–IL-20R1 (Fig. 2B), but up to 34% of the cells died after treatment with 200 ng/mL of recombinant MDA7/IL-24. Moreover, cotreatment of MeWo cells with anti–IL-20R1 antibody followed by MDA7/IL-24 protein inhibited both killing and apoptosis. Our previously published results showed that MDA7/IL-24–induced apoptosis correlated with the expression of MDA7/IL-24 receptors (IL-20R1/IL-20R2 or IL-22R1/IL-20R2) in these cells [19]. Together, these data strongly suggest that MDA7/IL-24 induces cytotoxicity through IL-20R1 engagement, which eventually leads to the death of melanoma cells.
Secreted MDA7/IL-24 Protein from Transfected HEK-293 Cells Induced Death in Melanoma Cells
Secreted MDA7/IL-24 protein from stably transfected HEK-293 (293M) cells was used in Transwell studies as an independent protein source. To determine whether coculturing of melanoma cells with 293M cells induces cell death at levels comparable to those seen with MDA7/IL-24 protein treatment, we cocultured 293M cells or nontransfected HEK-293 (293) cells with MeWo cells (Fig. 2C). We evaluated the cytotoxic effects of 293M treatment on melanoma cells using trypan blue exclusion assays. The 293M treatment killed up to 31% of the MeWo cells, which was comparable to purified MDA7/IL-24 protein killing at various levels. When MeWo cells were cocultured with 293 cells, only background killing was observed.
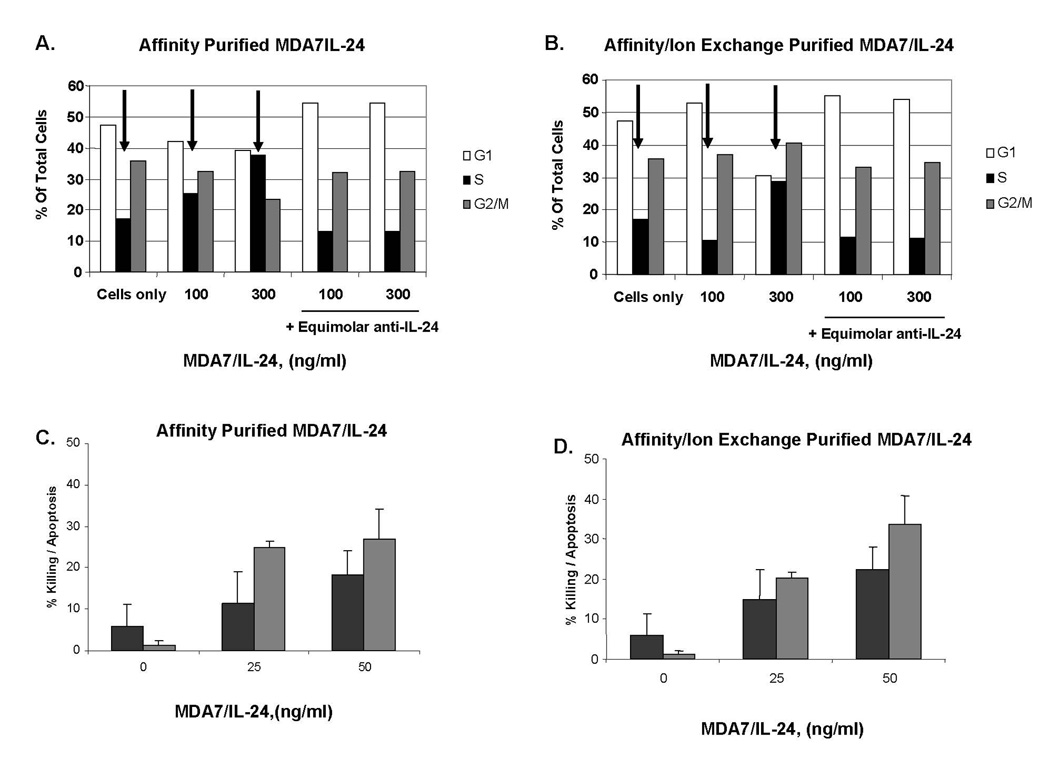
Affinity-Purified and Affinity/Ion Exchange–Purified MDA7/IL-24 Was Cytotoxic and Induced the Accumulation of Cells in the S phase
We used both affinity-purified and affinity/ion exchange–enriched MDA7/IL-24 protein preparations to perform functional studies (Fig. 3). We first showed that both affinity-purified and affinity/ion exchange–enriched MDA7/IL-24 considerably increased the number of cells arrested in the S phase (Fig. 3A, 3B). Blockade of both affinity-purified and affinity/ion exchange–purified MDA7/IL-24 with an anti–MDA7/IL-24 neutralizing antibody inhibited this effect. We further analyzed whether this effect correlated with cytotoxicity in melanoma cells. We then demonstrated that affinity/ion exchange–purified MDA7/IL-24, compared with a similar concentration of affinity-purified MDA7/IL-24, was cytotoxic to melanoma cells (Fig. 3C, 3D). Although affinity-purified MDA7/IL-24 induced up to 18% killing and 27% apoptosis in melanoma cells, affinity/ion exchange–purified MDA7/IL-24 induced up to 22% killing and 34% apoptosis in MeWo cells.
Figure 3.
A and B, Purified MDA7/IL-24 induces accumulation of cells in the S phase. A, MeWo melanoma cells were exposed to a dose titration of affinity-purified MDA7/IL-24 and assessed by propidium iodide staining for distribution of cells in each cell cycle. B, MeWo melanoma cells were exposed to a dose titration of affinity/ion exchange–purified MDA7/IL-24 and assessed by propidium iodide for distribution of cells in each cell cycle. Each arrow indicates S phase population bars. C and D, Affinity-purified and affinity/ion exchange–purified MDA7/IL-24 is cytotoxic. C, MeWo melanoma cells were exposed to a dose titration of affinity-purified MDA7/IL-24 and assessed for cell death by trypan blue exclusion after 5 days. Cells were analyzed for apoptosis via Annexin V–FITC staining followed by fluorescence-activated cell sorter analysis. For (C) and (D), black bars = percentage of dead cells (trypan blue positive) and gray bars = percentage of apoptotic cells based on total cells. Error bars represent one standard deviation of the mean. D, MeWo melanoma cells were exposed to a dose increase of affinity/ion-purified MDA7/IL-24 and assessed for cell death after 5 days. Error bars represent one standard deviation of the mean.
MDA7/IL-24 Induced IFN Beta Secretion in Melanoma Cells, and IFN Beta Blockade Partially Diminished MDA7/IL-24–Induced Cell Death
We then explored the mechanism by which MDA7/IL-24 induced cell death in melanoma cells. We previously reported that MDA7/IL-24 down-regulates inducible nitric oxide synthase (iNOS) in melanoma cells. iNOS has been characterized as a poor prognosis marker for patients with metastatic disease [9, 20]. Our findings demonstrated that MDA7/IL-24 causes an inverse modulation of IRF-1 and IRF-2, the expression of which is directly regulated by exogenous IFNs. We also reported previously that MDA7/IL-24 induces phosphorylation of STAT3 [19], which is one of the essential signaling molecules of IFNs in response to extracellular stimuli.
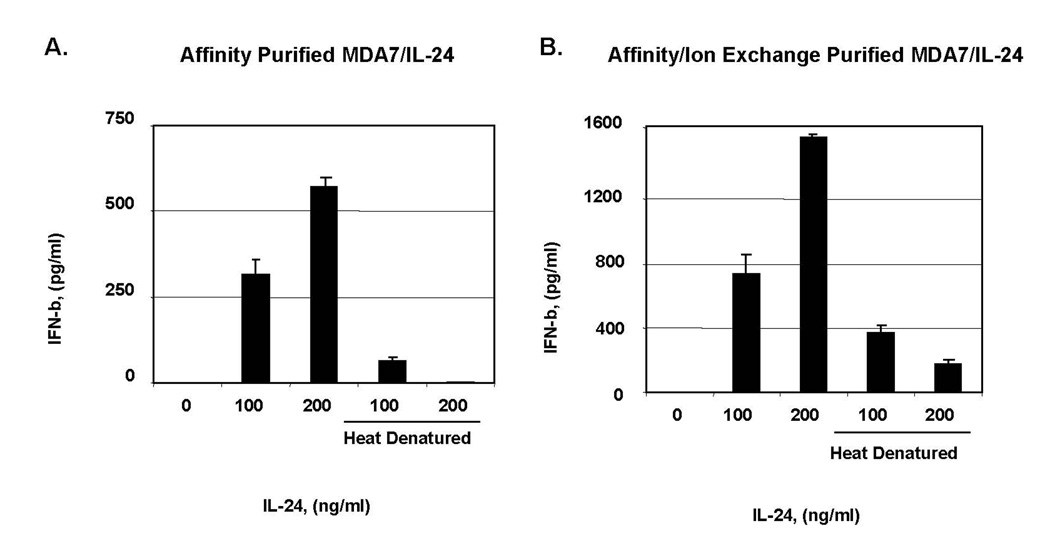
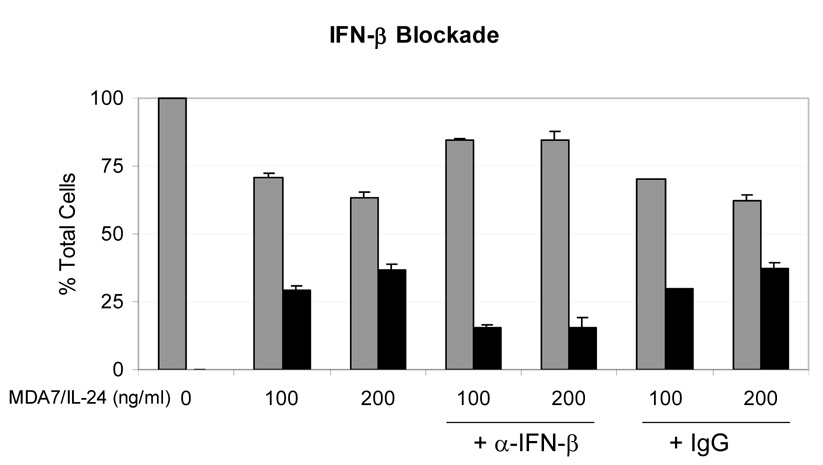
We next initiated studies of the role of IFNs in the MDA7/IL-24 pathway. IFN beta induces apoptosis in melanoma [21,22] and causes prolongation of cell-cycle progression via the accumulation of cells in the S phase [23] and up-regulation of TRAIL [23,24]. To test our hypothesis that the regulation of gene expression in melanoma cells activated by class I IFNs results in antitumor effects, we exposed melanoma cells to both affinity-enriched and affinity/ion exchange–enriched MDA7/IL-24 protein preparations and measured the IFN beta in the culture supernatant by ELISA. We showed that both affinity-purified and affinity/ion exchange–purified MDA7/IL-24 induced IFN beta secretion from MeWo cells in a dose-dependent fashion (Fig. 4A, 4B). We then tested whether the neutralization of IFN beta reduces MDA7/IL-24–induced death of melanoma cells. Figure 5 shows that the cotreatment of MeWo cells with neutralizing antibody to IFN beta, which also exposed to MDA7/IL-24, inhibited cell death by at least 50%,p<0.01 (Fig. 5).
Figure 4.
MDA7/IL-24-induced interferon (IFN) beta secretion from melanoma cells. A, MeWo cells exposed to affinity; B, affinity/ion-purified MDA7/IL-24 and supernatants from melanoma cells treated with affinity-purified and affinity/ion exchange–purified MDA7/IL-24, respectively, were tested by ELISA after 5 days for IFN beta. Error bars represent one standard deviation of the mean.
Figure 5.
Interferon (IFN) beta blockade partially diminishes MDA7/IL-24-induced cell death. MeWo melanoma cells were exposed to a dose titration of affinity-purified MDA7/IL-24 in the presence of neutralizing antibodies to IFN beta and assessed for cell death after 5 days. Gray bars = percentage of living cells, and black bars = percentage of dead cells (trypan blue positive) based on total cells. Anti-IFN beta–neutralizing antibody was used at 5× molar excess for predicting the level of IFN beta in supernatant with the IgG control added at the same concentration. Error bars represent one standard deviation of the mean.
Class I IFNs Were Cytotoxic to Melanoma Cells
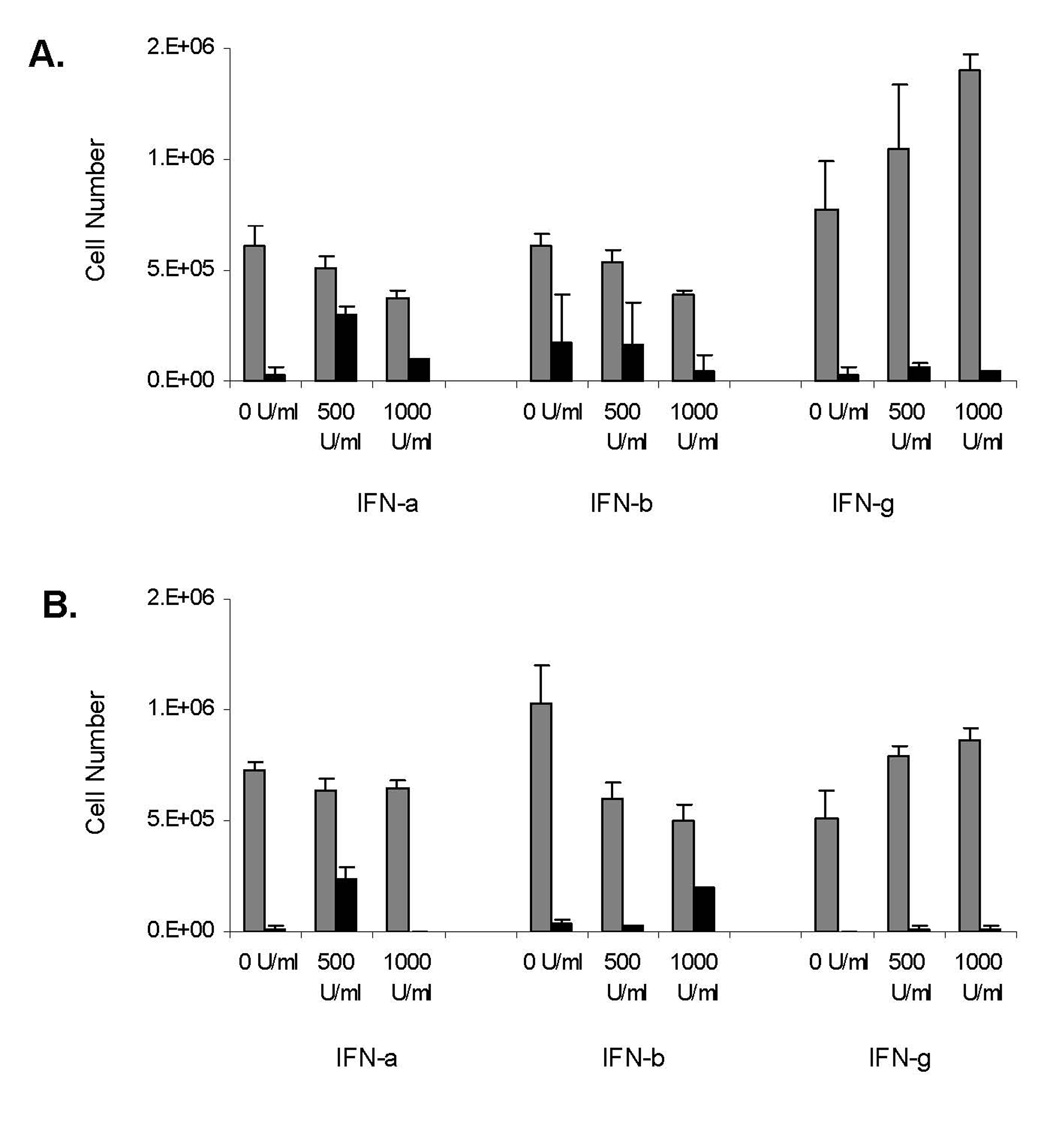
The roles of IRFs and IFN beta up-regulation in MDA7/IL-24 signaling were the focus of studies on melanoma response to treatment with this group of cytokines. We therefore exposed A375 and MeWo cells to both class I and class II IFNs at concentrations of 500 to 1000 U/mL for up to 24 h. To evaluate the proliferative effects of IFN treatment on melanoma cells, we investigated the killing of melanoma cells by using trypan blue exclusion assays (Fig. 6) and showed that class I IFNs induced cell killing and inhibited proliferation in A375 cells (Fig. 6A). Figure 6B shows that MeWo cells were more resistant to IFN alfa–induced killing and/or inhibition of proliferation, whereas IFN beta successfully induced cell killing. Moreover, we were able to compare the biologic effects of IFN gamma with those of IFNs alfa and beta in melanoma cell lines. To this end, we exposed melanoma cell lines to IFN gamma and evaluated the proliferative effects of the treatment. Our results demonstrated that melanoma cell proliferation is greatly induced by IFN gamma, as opposed to the cell death induced by IFNs alfa and beta (P=0.007, P=0.001, respectively).
Figure 6.
Class I (alfa [a] and beta [b]) interferons (IFNs) are cytotoxic to melanoma cells. A, A375 melanoma cells were exposed to a dose titration of both class I and class II (gamma [g]) IFNs and assessed for cell death after 24 h. For (A) and (B), gray bars = total number of live cells and black bars = total number of dead cells, trypan blue positive. Error bars represent one standard deviation of the mean. B, MeWo melanoma cells were exposed to a dose titration of both class I and II IFN sand assessed for cell death after 24 h. Error bars represent one standard deviation of the mean.
MDA7/IL-24 Differentially Regulated Gene Signaling and Apoptosis
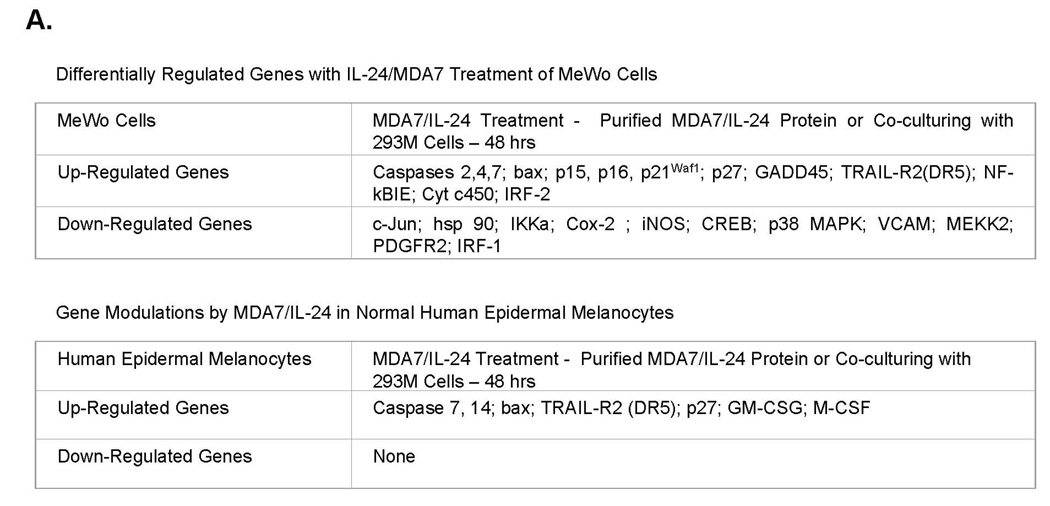
We performed microarray analyses in three pathways (apoptosis, MAPK, and signal transduction, including 96 genes each) to evaluate gene regulation by MDA7/IL-24. These analyses revealed that apoptotic pathway markers, including caspases and TRAIL, along with cell-cycle regulators were up-regulated in both normal human epidermal melanocytes and MeWo cells (Fig. 7A). Down-regulated genes included tumor growth markers such as iNOS, Cox-2, p38 MAPK, and IRF-1. We repeated these experiments five times in both MeWo and A375 cells after treatment with MDA7/IL-24. Moreover, cells were either treated with affinity-purified proteins or cocultured with 293M cells to compare gene regulation using the same pathway arrays. All treatment conditions identified the same genes that were regulated by MDA7/IL-24 independent of the protein source. However, treatment of normal human epidermal melanocytes with these preparations did not identify any genes that were down-regulated in MeWo cells, such as IRF-1, iNOS, Cox-2, and hsp90.
Figure 7.
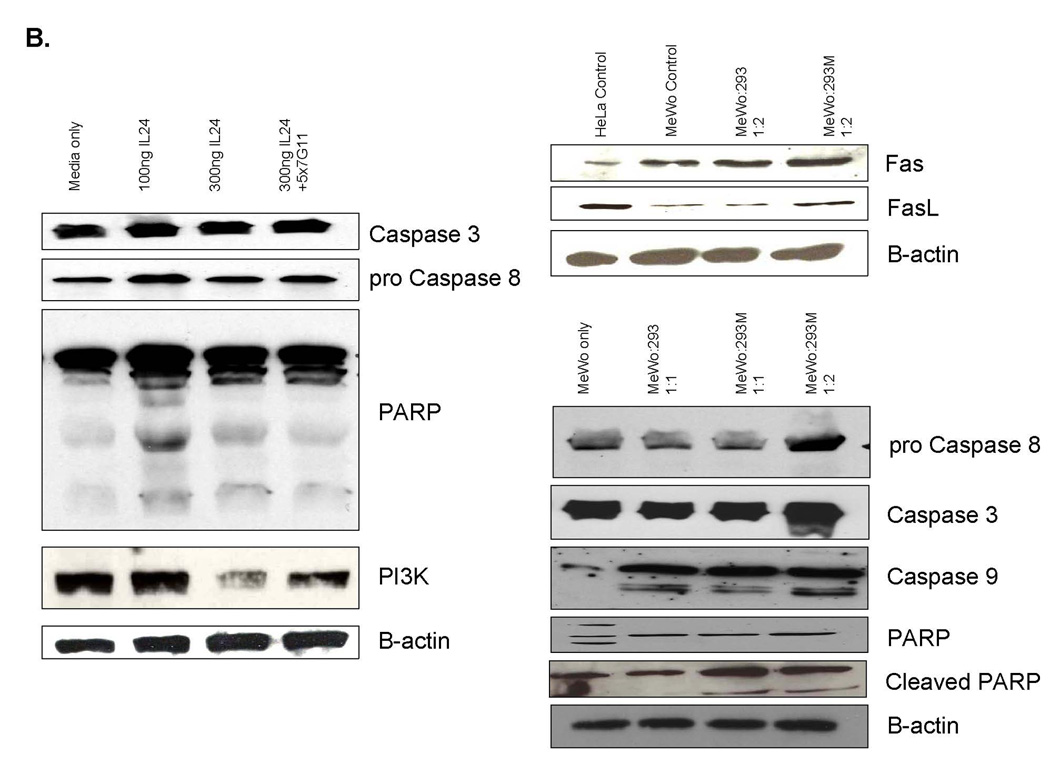
MDA7/IL-24 differentially regulates genes on signaling and apoptosis. A, MeWo melanoma cells were exposed to either a dose titration of affinity-purified MDA7/IL-24 or cocultured with MDA7/IL-24–transfected HEK-293 cells. After 48 h of treatment, cells were assessed for their apoptotic and signaling pathways in gene regulation with commercially available gene array analysis. B, MeWo melanoma cells were exposed to either a dose titration of affinity-purified MDA7/IL-24 or cocultured with MDA7/IL-24–transfected HEK-293 cells. After 48 h of treatment, lysates were taken and proteins were precipitated and washed in 1× PBS, resolved by SDS-PAGE, transferred to nitrocellulose, and Western blotted for apoptotic and signaling pathway markers
MDA7/IL-24 Induced TRAIL-R2 in Human Melanoma Cells
We confirmed our findings from the gene array studies with immunoblotting experiments. Figure 7B shows a group of apoptotic markers after treatment with MDA7/IL-24 protein. Initial experiments with affinity-purified MDA7/IL-24 protein revealed caspase-3 and procaspase-8 up-regulation with 100 ng/mL of protein rather than with a higher concentration (300 ng/mL). We observed a similar effect on PARP cleavage, but PI3K inhibition occurred at higher concentrations of MDA7/IL-24. We showed that the neutralizing antibody to MDA7/IL-24 reversed this effect. Our coculturing experiments of MeWo cells with 293M cells showed both Fas and FasL up-regulation and PARP cleavage in the cells cocultured with 293M cells compared with MeWo controls. Caspase-3 and -9 and procaspase-8 up-regulation in treated 293M cocultures was considerable compared with that in control 293 cells.
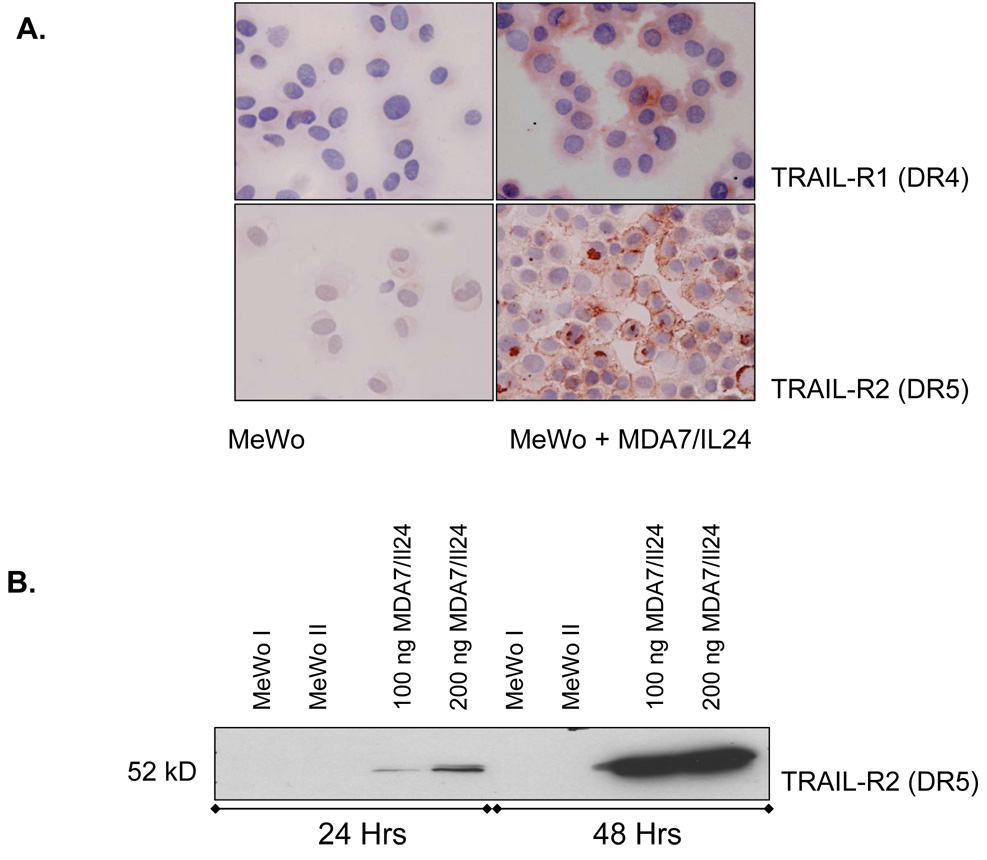
We further analyzed TRAIL-R1 (DR4) and TRAIL-R2 (DR5) expression in melanoma cells treated or not treated with MDA7/IL-24. Figure 8A shows that both DR4 and DR5 expression were up-regulated in MeWo cells after treatment with affinity-purified MDA7/IL-24. We confirmed our results on TRAIL-R1 and -R2 expression by cytochemical analysis, which we performed concurrently with the immunoblotting experiments. DR4 up-regulation was modest and resulted in increased expression primarily in the cytosol. In contrast, DR5 expression was strongly up-regulated and localized to plasma membrane.
Figure 8.
MDA7/IL-24 induces tumor necrosis factor–related apoptosis-inducing ligand (TRAIL)–receptor 2 (TRAIL-R2 or DR5) in human melanoma cells. A, MeWo melanoma cells were exposed to a dose titration of affinity-purified MDA7/IL-24. After 48 h of treatment, cells were collected and cytospins prepared for cytochemical assessment of their TRAIL receptor (R1 and R2) expression. (anti-DR4 or anti-DR5, AEC, hematoxylin. Original magnification X400). B, A second set of identical component were reserved in culture for 48 h of treatment, lysates were taken, and proteins were precipitated and washed in 1× PBS, resolved by SDS-PAGE, transferred to nitrocellulose and Western blotted for TRAIL-R1 and R2. MeWo melanoma cells were exposed to media only (MeWo I), protein preparation media only (MeWo II), and a dose titration of affinity-purified MDA7/IL-24. After 48 h of treatment, lysates were taken, and proteins were precipitated and washed in 1× PBS, resolved by SDS-PAGE, transferred to nitrocellulose and Western blotted for TRAIL-R2.
Discussion
Here, we described a novel pathway of MDA7/IL-24 regulation of apoptosis in melanoma tumors via endogenous IFN beta induction followed by IRF regulation and TRAIL/FasL system activation.
The treatment of metastatic melanoma remains a major clinical challenge. Malignant melanomas are characterized by high intrinsic resistance to chemotherapy and thus, single-agent or combination chemotherapies do not improve overall survival rates. In recent years, biochemotherapeutic approaches have resulted in overall higher responses, but their effect on overall survival has been disappointing. Research to increase our knowledge of the cellular mechanisms precipitating and modulating neoplasia is critical for the treatment of melanoma. The detection of cellular targets and pathways for individualized treatment modalities is therefore crucial.
We previously showed that melanocytes endogenously express MDA7/IL-24 protein, although its expression is down-regulated during the progression of melanoma [25, 26]. Initial studies with Ad-mda7/IL-24 showed potent growth suppression and apoptosis induction in many different tumor types, including melanoma, glioblastoma, osteosarcoma, and carcinomas of the breast, colon, lung, cervix, kidney, and prostate, but not in any normal cells [27, 28]. Subsequent mechanistic studies demonstrated that MDA7/IL-24 can induce apoptosis at various levels such as activating the caspase cascade and up-regulating p53, Bax, TRAIL, and cell growth arrest [27]. We and others also showed that additional mechanisms for MDA7/IL-24–mediated cell killing include the regulation of iNOS and MAPK in melanoma [4, 9]. Thus, MDA7/IL-24 exerts its tumor-suppressive effects via different signaling pathways in melanoma, which could also initiate a secondary line of cytokine activation or interactions.
Early events in class I IFN signaling include tyrosine phosphorylation of the IFN receptor subunits and activation of signaling cascades including IRFs. Recent studies have suggested that apoptosis is very important for the direct antitumor effect observed after IFN beta treatment in melanoma cells [24]. However, the precise molecular mechanisms that regulate the direct effect of IFN beta, including apoptosis, are still only partially understood.
One possible approach to this pathway in melanoma, which we showed previously, is that MDA7/IL-24 protein activates phosphorylation of STAT3 and down-regulates IRF-1 while up-regulating IRF-2. Thus, we hypothesized that receptor engagement by MDA7/IL-24 activates STAT3 to bind to the IRF-2 promoter, which in turn competes with IRF-1 binding to IFN gamma activation cascade molecules, blocking iNOS gene expression. It is well known that IFN gamma is a major inducer of iNOS in various systems and that the induction of IRF-1 activity by IFN gamma mediates the binding to the iNOS promoter [29]. This pathway positively cooperates with STAT-1a activation for the induction of the iNOS promoter [30]. In murine macrophages, discovering the essential role of the IRF-1 binding site for the induction of the iNOS promoter revealed that the binding of IRF-1 in protein complexes takes place only after IFN gamma incubation [29]. Of note, Coccia et al. [31] reported that the inhibitory effect of IL-4 on IFN gamma–stimulated iNOS expression in murine macrophages occurs because of the potentiation of IRF-2 expression and reduced binding of IRF-1 to the ISRE (Interferon response sequence element). Because we previously showed that iNOS expression in melanoma correlates with poor survival, we did not evaluate this particular pathway for any positive effect by IFN gamma.
In this study, we specifically targeted class I IFNs because of their effect on Fas/FasL- and TRAIL-induced apoptotic pathways in melanoma. TRAIL was originally identified as an IFN-stimulated gene. Once TRAIL binds to its receptors TRAIL-R1 (DR4) and TRAIL-R2 (DR5), proapoptotic pathways are activated [32]. In several tumor types, including melanoma, the activity of TRAIL can be enhanced by IFNs, both in vitro and in vivo [33–34]. In melanomas, IFN beta preferentially induces TRAIL, and this induction is poor in response to IFN gamma [24]. Strong induction of TRAIL and/or Fas/FasL in response to IFN leads to recruitment and activation of FADD. FADD has a death-effector domain sequence that activates procaspase-8 and then execution caspases, which results in cell death. The intrinsic pathway is also activated by caspase-8, which cleaves Bid, leading to Bax oligomerization. This in turn leads to cytochrome C release from mitochondria and the activation of caspase-9 [34]
In this study, we showed the induction of TRAIL-R2 (DR5) and then subsequent activation of both intrinsic and extrinsic caspases (first procaspase-8 followed by caspase-3 and then caspase-9) and then PARP cleavage. This effect was stronger in coculturing experiments with MeWo cells and MDA7/IL-24–transfected HEK 293 cells than it was with the affinity-purified MDA7/IL-24 protein exposure.
However, low doses of protein exposure stimulated the caspases and PARP cleavage at comparable levels that may indicate dose-dependent activation of the pathway. Our results on this particular pathway correlates with recently published results by Zhao et al. [35] that demonstrated the antitumor activity of TRAIL and MDA7/IL-24 combined treatment of colorectal cancer lines with replicating oncolytic adenovirus system. This combination treatment induces growth suppression and apoptosis in colorectal cancer lines via the activation of caspases and the release of cytochrome C.
In addition, our coculturing experiment results showed that accompanying Fas/FasL up-regulation induced an apoptotic cascade in MeWo cells. On the other hand, PI3K inhibition, which leads to a balanced signal between antiapoptotic and proapoptotic pathways, required a high amount of protein. Melanoma cells reportedly express constitutively active Akt because of the loss of the lipid phosphatase gene PTEN, a negative regulator of PI3K [36]. This could determine TRAIL sensitivity in these cells, which is dependent on the level of Akt. Cells expressing the highest level of Akt are the most resistant to TRAIL [37]. However, a direct correlation between Akt activity and resistance to TRAIL signaling remains unsolved in melanoma. At this point, we believe that MDA7/IL-24–mediated IFN beta induction of the TRAIL pathway could be controlled by Akt and/or PI3K levels in melanoma cells.
MDA7/IL-24 protein binds two distinct type II cytokine heterodimeric receptor complexes, IL-20R1/IL-20R2 (type 1 IL-20R) and IL-22R1/IL-20R2 (type 2 IL-20R). In our previous study [19], we found that although MDA7/IL-24 induces STAT3 activation and cytotoxicity in melanoma cells, these activities are disengaged, and that STAT3 phosphorylation is not required for MDA/7/IL-24 receptor–mediated killing. Thus, we concluded that human MDA7/IL-24 protein kills melanoma cells via an IL-20R–dependent but STAT3-independent mechanism. In our current study, we confirmed that IL-20R1 is a required signaling receptor for killing and apoptosis of melanoma cells by MDA7/IL-24.
In conclusion, this study, to the best of our knowledge, is the first to show that MDA7/IL-24 induces endogenous IFN beta production and secretion leading to melanoma cell growth arrest and apoptosis. We also identified a series of apoptotic markers, including TRAIL and Fas-FasL regulation that may mediate this pathway. We thus propose a pathway of MDA7/IL-24 regulation of melanoma apoptosis via IFN beta induction followed by TRAIL/FasL system activation. We emphasize that the IFNs and their signal transduction pathways could characterize some of the therapeutic target sites for the MDA7/IL-24–mediated regulation of cell growth in melanoma.
Acknowledgments
Supported by NIH Grant R41 CA 89778 and P50 CA 93459 (to E.A.G. and S.E.), and The Cancer Foundation for Melanoma Research (to S.E.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jiang H, Su ZZ, Lin JJ, Goldstein NI, Young CS, Fisher PB. The melanoma differentiation associated gene mda-7 suppresses cancer cell growth. Proc Natl Acad Sci USA. 1996;93:9160–9165. doi: 10.1073/pnas.93.17.9160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Madireddi MT, Su ZZ, Young CS, Goldstein NI, Fisher PB. Mda-7, a novel melanoma differentiation associated gene with promise for cancer gene therapy. Adv Exp Med Biol. 2000;465:239–261. doi: 10.1007/0-306-46817-4_22. [DOI] [PubMed] [Google Scholar]
- 3.Mhashilkar AM, Schrock RD, Hindi M, Liao J, Sieger K, Kourouma F, Zou-Yang XH, Onishi E, Takh O, Vedvick TS, Fanger G, Stewart L, Watson GJ, Snary D, Fisher PB, Saeki T, Roth JA, Ramesh R, Chada S. Melanoma differentiation associated gene-7 (mda-7): a novel anti-tumor gene for cancer gene therapy. Mol Med. 2001;7:271–282. [PMC free article] [PubMed] [Google Scholar]
- 4.Sarkar D, Su ZZ, Lebedeva IV, Sauane M, Gopalkrishnan RV, Valerie K, Dent P, Fisher PB. mda-7 (IL-24) Mediates selective apoptosis in human melanoma cells by inducing the coordinated overexpression of the GADD family of genes by means of p38 MAPK. Proc Natl Acad Sci USA. 2002;99:10054–10059. doi: 10.1073/pnas.152327199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramesh R, Mhashilkar AM, Tanaka F, Saito Y, Branch CD, Sieger K, Mumm JB, Stewart AL, Boquoi A, Dumoutier L, Grimm EA, Renauld JC, Kotenko S, Chada S. Melanoma differentiation-associated gene 7/ interleukin (IL)-24 is a novel ligand that regulates angiogenesis via the IL-22 receptor. Cancer Res. 2003;63:5105–5113. [PubMed] [Google Scholar]
- 6.Ramesh R, Ito I, Gopalan B, Saito Y, Mhashilkar AM, Chada S. Ectopic production of MDA-7/IL-24 inhibits invasion and migration of human lung cancer cells. Mol Ther. 2004;9:510–518. doi: 10.1016/j.ymthe.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 7.Saito Y, Miyahara R, Gopalan B, Litvak A, Inoue S, Shanker M, Branch CD, Mhashilkar AM, Roth JA, Chada S, Ramesh R. Selective induction of cell cycle arrest and apoptosis in human prostate cancer cells through adenoviral transfer of the melanoma differentiation-associated-7 (mda-7)/interleukin-24 (IL-24) gene. Cancer Gene Ther. 2005;12:238–247. doi: 10.1038/sj.cgt.7700780. [DOI] [PubMed] [Google Scholar]
- 8.Lebedeva IV, Su ZZ, Chang Y, Kitada S, Reed JC, Fisher PB. The cancer growth suppressing gene mda-7 induces apoptosis selectively in melanoma cells. Oncogene. 2002;21:708–718. doi: 10.1038/sj.onc.1205116. [DOI] [PubMed] [Google Scholar]
- 9.Ekmekcioglu S, Ellerhorst JA, Mumm JB, Zheng M, Broemeling L, Prieto VG, Stewart AL, Mhashilkar AM, Chada S, Grimm EA. Negative association of melanoma differentiation associated gene (mda-7) and inducible nitric oxide synthase (iNOS) in human melanoma: MDA-7 regulates iNOS expression in melanoma cells. Mol Cancer Ther. 2003;2(1):9–17. [PubMed] [Google Scholar]
- 10.Gopalan B, Litvak A, Sharma S, Mhashilkar AM, Chada S, Ramesh R. Activation of the Fas-FasL Signaling Pathway by MDA-7/IL-24 Kills Human Ovarian Cancer Cells. Cancer Res. 2005;65:3017–3024. doi: 10.1158/0008-5472.CAN-04-3758. [DOI] [PubMed] [Google Scholar]
- 11.Dumoutier L, Leemans C, Lejeune D, Kotenko SV, Renauld JC. Cutting edge: STAT activation by IL-19, IL-20 and mda-7 through IL-20 receptor complexes of two types. J Immunol. 2001;167:3545–3549. doi: 10.4049/jimmunol.167.7.3545. [DOI] [PubMed] [Google Scholar]
- 12.Wang M, Tan Z, Zhang R, Kotenko SV, Liang P. Interleukin 24 (MDA-7/MOB-5) signals through two heterodimeric receptors, IL-22R1/IL-20R2 and IL-20R1/IL-20R2. J Biol Chem. 2002;277:7341–7347. doi: 10.1074/jbc.M106043200. [DOI] [PubMed] [Google Scholar]
- 13.Parrish-Novak J, Xu W, Brender T, Yao L, Jones C, West J, Brandt C, Jelinek L, Madden K, McKernan PA, Foster DC, Jaspers S, Chandrasekher YA. Interleukins 19, 20, and 24 signal through two distinct receptor complexes. Differences in receptor-ligand interactions mediate unique biological functions. J Biol Chem. 2002;277:47517–47523. doi: 10.1074/jbc.M205114200. [DOI] [PubMed] [Google Scholar]
- 14.Chawla-Sarkar M, Lindner DJ, Liu YF, Williams BR, Sen GC, Silverman RH, Borden EC. Apoptosis and interferons: role of interferon-stimulated genes as mediators of apoptosis. Apoptosis. 2003;8(3):237–249. doi: 10.1023/a:1023668705040. [DOI] [PubMed] [Google Scholar]
- 15.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 16.Almasan A, Ashkenazi A. Apo2L/TRAIL: apoptosis signaling, biology, and potential for cancer therapy. Cytokine Growth Factor Rev. 2003;14:337–348. doi: 10.1016/s1359-6101(03)00029-7. [DOI] [PubMed] [Google Scholar]
- 17.Mamane Y, Heylbroeck C, Génin P, Algarté M, Servant MJ, LePage C, DeLuca C, Kwon H, Lin R, Hiscott J. Interferon regulatory factors: the next generation. Gene. 1999;237(1):1–14. doi: 10.1016/s0378-1119(99)00262-0. [DOI] [PubMed] [Google Scholar]
- 18.Mumm JA, Ekmekcioglu S, Poindexter NJ, Chada S, Grimm EA. Soluble human MDA-7/IL-24: characterization of the molecular form(s) inhibiting tumor growth and stimulating monocytes. J Interferon Cytokine Res. 2006;26(12):877–886. doi: 10.1089/jir.2006.26.877. [DOI] [PubMed] [Google Scholar]
- 19.Chada S, Mhashilkar AM, Ramesh R, Mumm JB, Sutton RB, Bocangel D, Zheng M, Grimm EA, Ekmekcioglu S. Bystander Activity of Ad-mda7: Human MDA-7 Protein Kills Melanoma Cells via an IL-20 Receptor-Dependent but STAT3-Independent Mechanism. Mol Ther. 2004;10(6):1085–1095. doi: 10.1016/j.ymthe.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 20.Ekmekcioglu S, Ellerhorst JA, Prieto VG, Johnson MM, Broemeling LD, Grimm EA. Tumor iNOS predicts poor survival for stage III melanoma patients. Int J Cancer. 2006;119:861–866. doi: 10.1002/ijc.21767. [DOI] [PubMed] [Google Scholar]
- 21.Yoshida J, Mizuno M, Wakabayashi T. lnterferon-β gene therapy for cancer: Basic research to clinical application. Cancer Sci. 2004;95:858–865. doi: 10.1111/j.1349-7006.2004.tb02194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagatani T, Okazawa H, Kambara T, Satoh K, Nishiyama T, Tokura H, Yamada R, Nakajima H. Effect of natural interferon-beta on the growth of melanoma cell lines. Mel Res. 1998;8:295–299. doi: 10.1097/00008390-199808000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Vannucchi S, Chiantore MV, Fiorucci G, Percario ZA, Leone S, Affabris E, Romeo G. TRAIL is a key target in S-phase slowing-dependent apoptosis induced by interferon-b in cervical carcinoma cells. Oncogene. 2005;24:2536–2546. doi: 10.1038/sj.onc.1208403. [DOI] [PubMed] [Google Scholar]
- 24.Chawla-Sarkar M, Leaman DW, Borden EC. Preferential Induction of Apoptosis by Interferon (IFN)-β Compared with IFN-α2: Correlation with TRAIL/Apo2L Induction in Melanoma Cell Lines. Clin Cancer Res. 2001;7:1821–1831. [PubMed] [Google Scholar]
- 25.Ekmekcioglu S, Ellerhorst JA, Mhashilkar AM, Sahin AA, Read CM, Prieto VG, Chada S, Grimm EA. Down-Regulated Melanoma Differentiation Associated Gene (Mda-7) Expression In Human Melanomas. Int J Cancer. 2001;94:54–59. doi: 10.1002/ijc.1437. [DOI] [PubMed] [Google Scholar]
- 26.Ellerhorst JA, Prieto VG, Ekmekcioglu S, Broemeling L, Yekell S, Chada S, Grimm EA. Loss of MDA-7 Expression With Progression of Melanoma. J Clin Oncol. 2002;20(4):1069–1074. doi: 10.1200/JCO.2002.20.4.1069. [DOI] [PubMed] [Google Scholar]
- 27.Chada S, Sutton RB, Ekmekcioglu S, Ellerhorst J, Mumm JB, Leitner WW, Yang HY, Sahin AA, Hunt KK, Fuson KL, Poìndexter N, Roth JA, Ramesh R, Grimm EA, Mhashilkar AM. MDA-7/IL-24 is a unique cytokine-tumor suppressor in the IL-10 family. Int Immunopharmacol. 2004;4:649–667. doi: 10.1016/j.intimp.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 28.Fisher PB, Gopalkrishnan RV, Chada S, Ramesh R, Grimm EA, Rosenfeld MR, Curiel DT, Dent P. Mda-7/IL-24, a novel cancer selective apoptosis inducing cytokine gene. Cancer Bio Ther. 2003;2:24–38. [PubMed] [Google Scholar]
- 29.Kleinert H, Schwarz PM, Forstermann U. Regulation of the expression of inducible nitric oxide synthase. Biol Chem. 2003;384(10–11):1343–1364. doi: 10.1515/BC.2003.152. [DOI] [PubMed] [Google Scholar]
- 30.Darville MI, VEizirik DL. Regulation by cytokines of the inducible nitric oxide synthase promoter in insulin-producing cells. Diabetologia. 1998;41:1101–1108. doi: 10.1007/s001250051036. [DOI] [PubMed] [Google Scholar]
- 31.Coccia EM, Stellacci E, Marziali G, Weiss G, Battistini A. IFNg and IL-4 differently regulate inducible NO synthase gene expression through IRF-1 modulation. Int Immunol. 2000;12:977–985. doi: 10.1093/intimm/12.7.977. [DOI] [PubMed] [Google Scholar]
- 32.Ashkenazi A, Dixit VM. Death receptors: Signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 33.Chawla-Sarkar M, Leaman DW, Jacobs BS, Borden EC. IFN-beta pretreatment sensitizes human melanoma cells to TRAIL/Apo2 ligand-induced apoptosis. J Immunol. 2002;169(2):847–855. doi: 10.4049/jimmunol.169.2.847. [DOI] [PubMed] [Google Scholar]
- 34.Younes A, Kadin ME. Emerging applications of the tumor necrosis factor family of ligands and receptors in cancer therapy. J Clin Oncol. 2003;21(18):3526–3534. doi: 10.1200/JCO.2003.09.037. [DOI] [PubMed] [Google Scholar]
- 35.Zhao L, Dong A, Gu Z, Liu Z, Zhang Y, Zhang W, Wang Y, He L, Qian C, Qian Q, Liu X. The antitumor activity of TRAIL and IL-24 with replicating oncolytic adenovirus in colorectal cancer. Cancer Gene Therapy. 2006;13:1011–1022. doi: 10.1038/sj.cgt.7700969. [DOI] [PubMed] [Google Scholar]
- 36.Li G, Kalabis J, Xu X, Meier F, Oka M, Bogenrieder T, Herlyn M. Reciprocal regulation of MelCAM and AKT in human melanoma. Oncogene. 2003;22(44):6891–6899. doi: 10.1038/sj.onc.1206819. [DOI] [PubMed] [Google Scholar]
- 37.Bhojani MS, Rossu BD, Rehemtulla A. TRAIL and anti-tumor responses. Cancer Biology and Therapy. 2003;2(4):S71–S78. [PubMed] [Google Scholar]