Abstract
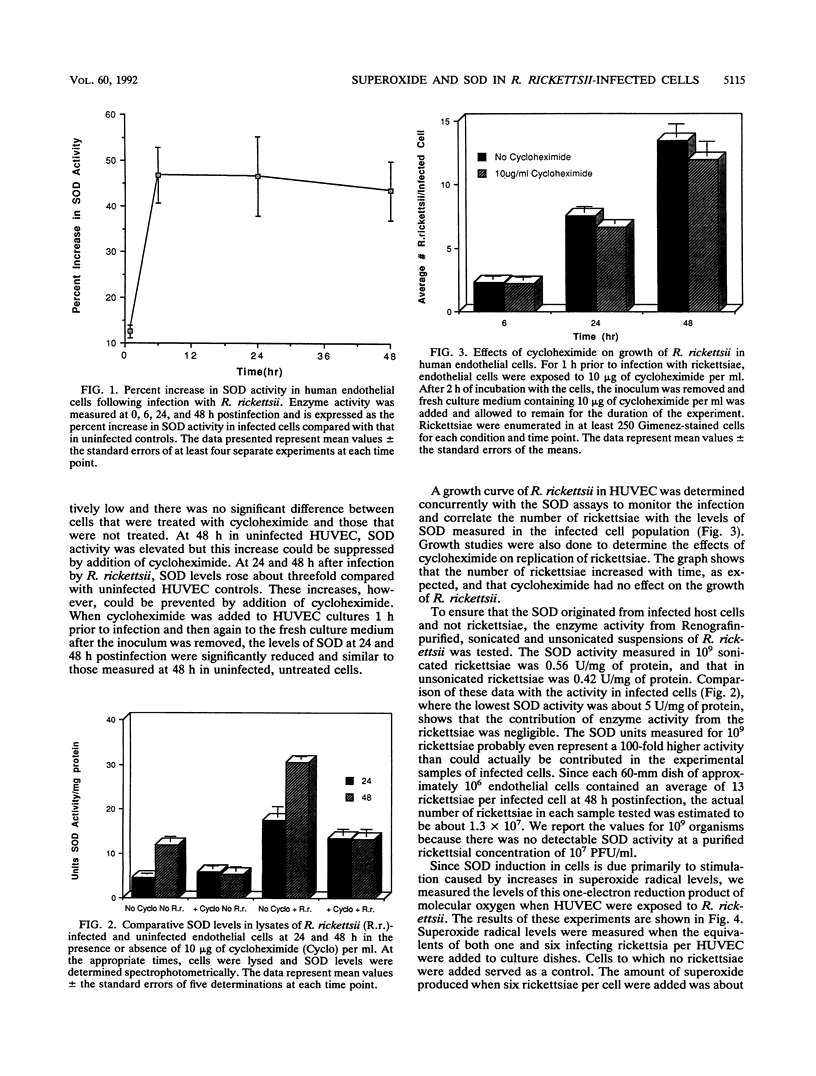
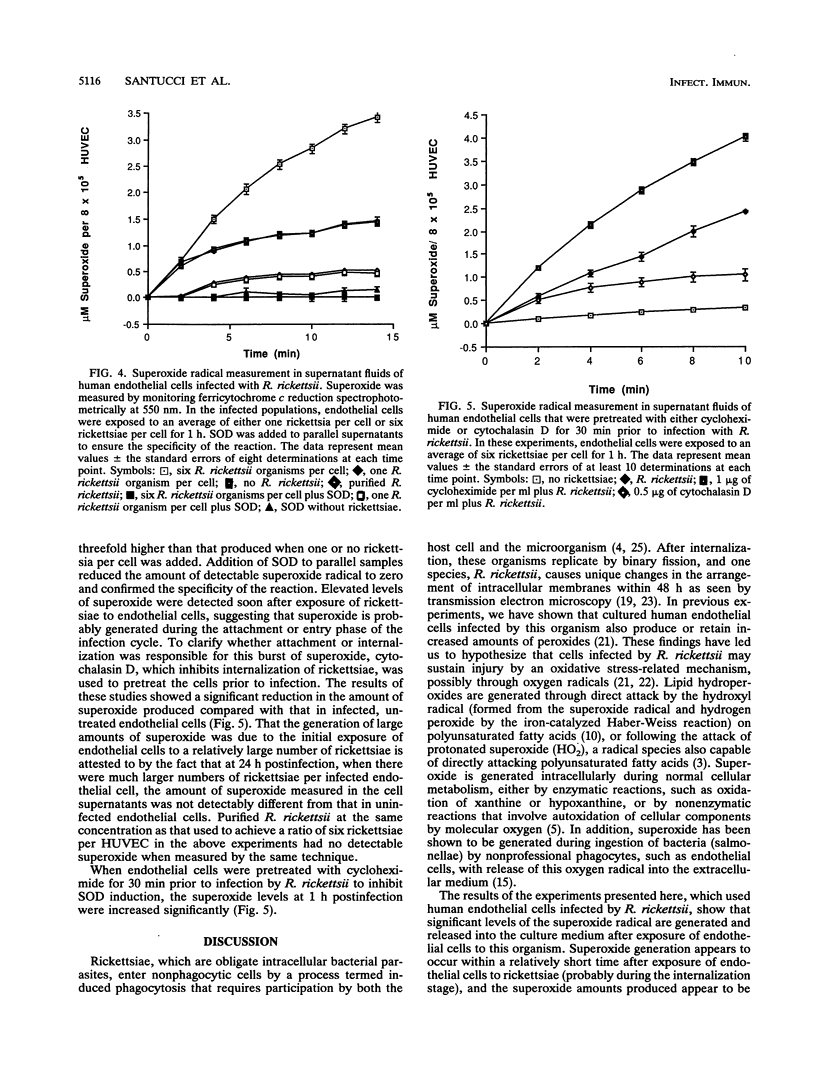
Human endothelial cells infected with Rickettsia rickettsii, the etiological agent of Rocky Mountain spotted fever, undergo striking morphological changes to the endoplasmic reticulum-outer nuclear envelope complex. These changes are accompanied by concurrent accumulation of intracellular peroxides. Both of these findings are consistent with the notion that cells undergo some form of oxidative stress. Since oxidant injury is often initiated or mediated through oxygen radicals, we examined superoxide radical generation when endothelial cells were exposed to R. rickettsii. We also examined the levels of superoxide dismutase, an enzyme induced in response to increased superoxide formation. The levels of both superoxide and superoxide dismutase increased when endothelial cells were exposed to R. rickettsii. These results, together with our previous findings, support our hypothesis that cells infected by this intracellular bacterium experience oxidant-mediated injury that may eventually contribute to cell death.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azzi A., Montecucco C., Richter C. The use of acetylated ferricytochrome c for the detection of superoxide radicals produced in biological membranes. Biochem Biophys Res Commun. 1975 Jul 22;65(2):597–603. doi: 10.1016/s0006-291x(75)80188-4. [DOI] [PubMed] [Google Scholar]
- Bielski B. H., Arudi R. L., Sutherland M. W. A study of the reactivity of HO2/O2- with unsaturated fatty acids. J Biol Chem. 1983 Apr 25;258(8):4759–4761. [PubMed] [Google Scholar]
- COHN Z. A., BOZEMAN F. M., CAMPBELL J. M., HUMPHRIES J. W., SAWYER T. K. Study on growth of Rickettsia. V. Penetration of Rickettsia tsutsugamushi into mammalian cells in vitro. J Exp Med. 1959 Mar 1;109(3):271–292. doi: 10.1084/jem.109.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIMENEZ D. F. STAINING RICKETTSIAE IN YOLK-SAC CULTURES. Stain Technol. 1964 May;39:135–140. doi: 10.3109/10520296409061219. [DOI] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr Culture of vascular endothelium. Prog Hemost Thromb. 1976;3:1–28. [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen free radicals and iron in relation to biology and medicine: some problems and concepts. Arch Biochem Biophys. 1986 May 1;246(2):501–514. doi: 10.1016/0003-9861(86)90305-x. [DOI] [PubMed] [Google Scholar]
- Hanson B. A., Wisseman C. L., Jr, Waddell A., Silverman D. J. Some characteristics of heavy and light bands of Rickettsia prowazekii on Renografin gradients. Infect Immun. 1981 Nov;34(2):596–604. doi: 10.1128/iai.34.2.596-604.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontos H. A., Wei E. P., Ellis E. F., Jenkins L. W., Povlishock J. T., Rowe G. T., Hess M. L. Appearance of superoxide anion radical in cerebral extracellular space during increased prostaglandin synthesis in cats. Circ Res. 1985 Jul;57(1):142–151. doi: 10.1161/01.res.57.1.142. [DOI] [PubMed] [Google Scholar]
- Ryan U. S., Vann J. M. Endothelial cells: a source and target of oxidant damage. Basic Life Sci. 1988;49:963–968. doi: 10.1007/978-1-4684-5568-7_157. [DOI] [PubMed] [Google Scholar]
- Salo D. C., Lin S. W., Pacifici R. E., Davies K. J. Superoxide dismutase is preferentially degraded by a proteolytic system from red blood cells following oxidative modification by hydrogen peroxide. Free Radic Biol Med. 1988;5(5-6):335–339. doi: 10.1016/0891-5849(88)90105-0. [DOI] [PubMed] [Google Scholar]
- Salo D. C., Pacifici R. E., Lin S. W., Giulivi C., Davies K. J. Superoxide dismutase undergoes proteolysis and fragmentation following oxidative modification and inactivation. J Biol Chem. 1990 Jul 15;265(20):11919–11927. [PubMed] [Google Scholar]
- Shingu M., Yoshioka K., Nobunaga M., Yoshida K. Human vascular smooth muscle cells and endothelial cells lack catalase activity and are susceptible to hydrogen peroxide. Inflammation. 1985 Sep;9(3):309–320. doi: 10.1007/BF00916279. [DOI] [PubMed] [Google Scholar]
- Silverman D. J., Bond S. B. Infection of human vascular endothelial cells by Rickettsia rickettsii. J Infect Dis. 1984 Feb;149(2):201–206. doi: 10.1093/infdis/149.2.201. [DOI] [PubMed] [Google Scholar]
- Silverman D. J. Rickettsia rickettsii-induced cellular injury of human vascular endothelium in vitro. Infect Immun. 1984 Jun;44(3):545–553. doi: 10.1128/iai.44.3.545-553.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman D. J., Santucci L. A. A potential protective role for thiols against cell injury caused by Rickettsia rickettsii. Ann N Y Acad Sci. 1990;590:111–117. doi: 10.1111/j.1749-6632.1990.tb42213.x. [DOI] [PubMed] [Google Scholar]
- Silverman D. J., Santucci L. A. Potential for free radical-induced lipid peroxidation as a cause of endothelial cell injury in Rocky Mountain spotted fever. Infect Immun. 1988 Dec;56(12):3110–3115. doi: 10.1128/iai.56.12.3110-3115.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman D. J., Wisseman C. L., Jr In vitro studies of rickettsia-host cell interactions: ultrastructural changes induced by Rickettsia rickettsii infection of chicken embryo fibroblasts. Infect Immun. 1979 Nov;26(2):714–727. doi: 10.1128/iai.26.2.714-727.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Walker T. S., Winkler H. H. Penetration of cultured mouse fibroblasts (L cells) by Rickettsia prowazeki. Infect Immun. 1978 Oct;22(1):200–208. doi: 10.1128/iai.22.1.200-208.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbourn C. C., Stern A. Human red cells scavenge extracellular hydrogen peroxide and inhibit formation of hypochlorous acid and hydroxyl radical. J Clin Invest. 1987 Nov;80(5):1486–1491. doi: 10.1172/JCI113230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisseman C. L., Jr, Edlinger E. A., Waddell A. D., Jones M. R. Infection cycle of Rickettsia rickettsii in chicken embryo and L-929 cells in culture. Infect Immun. 1976 Oct;14(4):1052–1064. doi: 10.1128/iai.14.4.1052-1064.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]



